Submitted:
28 August 2023
Posted:
29 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
Flaws in Lipinski’s Rule of Five and Quantifying Druglikeness
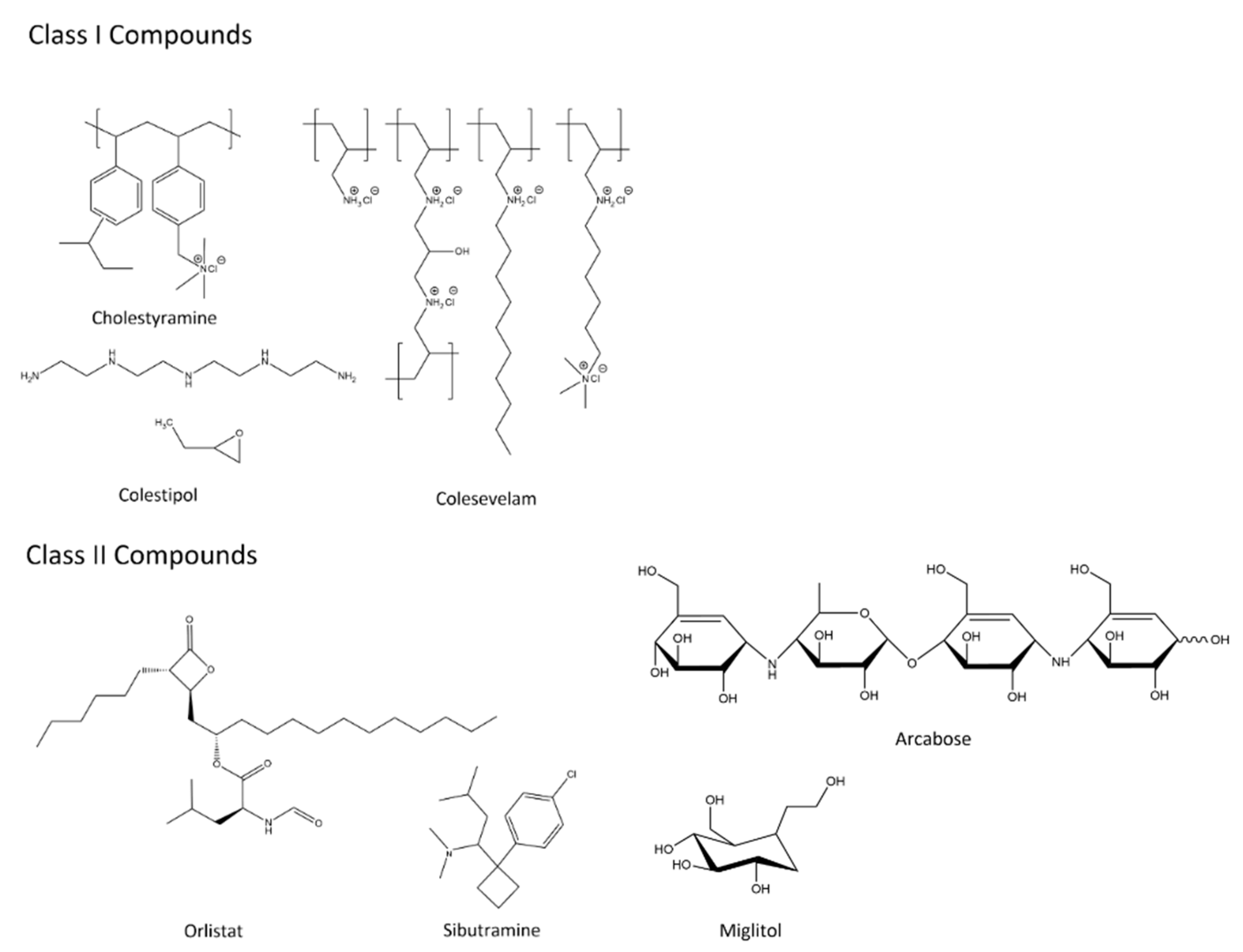
Design of non-systemic compounds
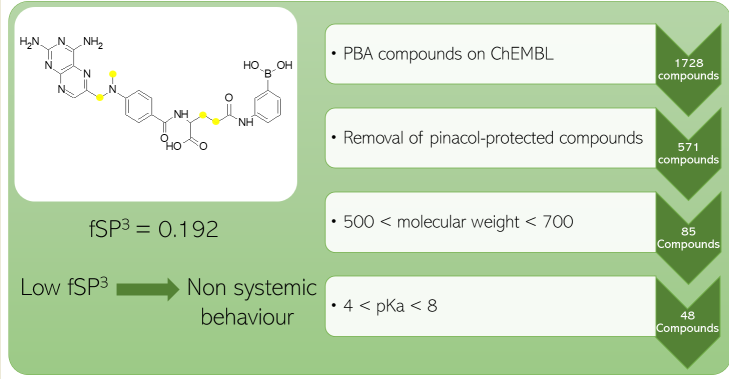
Case study – Phenylboronic acid Fructose Scavengers
Conclusion
Supplementary Materials
Funding
Author Contributions:
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hingorani AD, Kuan V, Finan C, Kruger FA, Gaulton A, Chopade S, et al. Improving the odds of drug development success through human genomics: modelling study. Sci Rep 2019;9:18911. [CrossRef]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 1997;23:3–25. [CrossRef]
- Ghose AK, Viswanadhan VN, Wendoloski JJ. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J Comb Chem 1999;1:55–68. [CrossRef]
- Muegge I, Heald SL, Brittelli D. Simple Selection Criteria for Drug-like Chemical Matter. J Med Chem 2001;44:1841–6. [CrossRef]
- Veber DF, Johnson SR, Cheng H-Y, Smith BR, Ward KW, Kopple KD. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J Med Chem 2002;45:2615–23. [CrossRef]
- Egan WJ, Merz Kenneth M., Baldwin JJ. Prediction of Drug Absorption Using Multivariate Statistics. J Med Chem 2000;43:3867–77. [CrossRef]
- DeGoey DA, Chen H-J, Cox PB, Wendt MD. Beyond the Rule of 5: Lessons Learned from AbbVie’s Drugs and Compound Collection. J Med Chem 2018;61:2636–51. [CrossRef]
- Hann MM. Molecular obesity, potency, and other addictions in drug discovery. Medchemcomm 2011;2:349–55. [CrossRef]
- Wong FYK, Desmarchelier PM. VIBRIO | Vibrio Cholerae. In: Robinson RK, editor. Encyclopedia of Food Microbiology, Oxford: Elsevier; 1999, p. 2242–8. [CrossRef]
- Percival SL, Williams DW. Chapter Eleven - Shigella. In: Percival SL, Yates M V, Williams DW, Chalmers RM, Gray NF, editors. Microbiology of Waterborne Diseases (Second Edition), London: Academic Press; 2014, p. 223–36. [CrossRef]
- Poolman JT. Escherichia coli. In: Quah SR, editor. International Encyclopedia of Public Health (Second Edition), Oxford: Academic Press; 2017, p. 585–93. [CrossRef]
- Charmot D. Non-systemic drugs: a critical review. Curr Pharm Des 2012;18:1434–45. [CrossRef]
- Benet LZ, Hosey CM, Ursu O, Oprea TI. BDDCS, the Rule of 5 and Drugability. Adv Drug Deliv Rev 2016;101:89. [CrossRef]
- Gale AR, Wilson M. Clostridioides difficile Infection. Gastrointestinal Emergencies: Evidence-Based Answers to Key Clinical Questions 2023:353–6. [CrossRef]
- Mullish BH, Williams HRT. Clostridium difficile infection and antibiotic-associated diarrhoea. Clinical Medicine 2018;18:237. [CrossRef]
- Cho JC, Crotty MP, Pardo J. Ridinilazole: a novel antimicrobial for Clostridium difficile infection. Ann Gastroenterol 2019;32:134–40. also see Summit Therapeutics press release. Available online: https://www.globenewswire.com/news-release/2015/11/23/789529/10157064/en/Summit-Therapeutics-Announces-Novel-Antibiotic-Ridinilazole-SMT19969-Achieves-Statistical-Superiority-Over-Vancomycin-in-CoDIFy-Phase-2-Clinical-Trial-for-C-difficile-Infection.html (accessed on 24 August 2023). [CrossRef]
- Zhanel GG, Walkty AJ, Karlowsky JA. Fidaxomicin: A novel agent for the treatment of Clostridium difficile infection. The Canadian Journal of Infectious Diseases & Medical Microbiology 2015;26:305. [CrossRef]
- Alagga AA, Gupta V. Drug Absorption. 2023.
- Lovering F, Bikker J, Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J Med Chem 2009;52:6752–6. [CrossRef]
- Feher M, Schmidt JM. Property Distributions: Differences between Drugs, Natural Products, and Molecules from Combinatorial Chemistry. J Chem Inf Comput Sci 2003;43:218–27. [CrossRef]
- Clemons PA, Wilson JA, Dančík V, Muller S, Carrinski HA, Wagner BK, et al. Quantifying structure, and performance diversity for sets of small molecules comprising small-molecule screening collections. Proceedings of the National Academy of Sciences 2011;108:6817–22. [CrossRef]
- Sepsey Für C, Bölcskei H. New Spiro[cycloalkane-pyridazinone] Derivatives with Favorable Fsp3 Character. Chemistry (Easton) 2020;2:837–48. [CrossRef]
- Stotani S, Lorenz C, Winkler M, Medda F, Picazo E, Ortega Martinez R, et al. Design and Synthesis of Fsp3-Rich, Bis-Spirocyclic-Based Compound Libraries for Biological Screening. ACS Comb Sci 2016;18:330–6. [CrossRef]
- Wei W, Cherukupalli S, Jing L, Liu X, Zhan P. Fsp3: A new parameter for drug-likeness. Drug Discov Today 2020;25:1839–45. [CrossRef]
- Xie W, Wang F, Li Y, Lai L, Pei J. Advances and Challenges in De Novo Drug Design Using Three-Dimensional Deep Generative Models. J Chem Inf Model 2022;62:2269–79. [CrossRef]
- Bickerton GR, Paolini G V., Besnard J, Muresan S, Hopkins AL. Quantifying the chemical beauty of drugs. Nat Chem 2012;4:90. [CrossRef]
- Ivanenkov YA, Zagribelnyy BA, Aladinskiy VA. Are We Opening the Door to a New Era of Medicinal Chemistry or Being Collapsed to a Chemical Singularity? J Med Chem 2019;62:10026–43. [CrossRef]
- Riaz S, John S. Cholestyramine Resin. Handbook of Nuclear, Biological, and Chemical Agent Exposures 2023:60–1.
- Hameed MH, Farzam K. Colestipol. StatPearls 2022.
- Patel PH, Can AS. Colesevelam. StatPearls 2023.
- Jain SS, Ramanand SJ, Ramanand JB, Akat PB, Patwardhan MH, Joshi SR. Evaluation of efficacy and safety of orlistat in obese patients. Indian J Endocrinol Metab 2011;15:99–104. [CrossRef]
- R. Araujo J, Martel F. Sibutramine Effects on Central Mechanisms Regulating Energy Homeostasis. Curr Neuropharmacol 2012;10:49. [CrossRef]
- McIver LA, Preuss C V., Tripp J. Acarbose. XPharm: The Comprehensive Pharmacology Reference 2022:1–3. [CrossRef]
- Miglitol. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury 2021.
- Somaraju UR, Solis-Moya A. Pancreatic enzyme replacement therapy for people with cystic fibrosis. Cochrane Database Syst Rev 2016;2016. [CrossRef]
- Buchwald P. Soft drugs: design principles, success stories, and future perspectives. Expert Opin Drug Metab Toxicol 2020;16:645–50. [CrossRef]
- Pevtsov A, Kerndt CC, Ahmed I, Fredlund KL. Esmolol. XPharm: The Comprehensive Pharmacology Reference 2023:1–5. [CrossRef]
- Domanovits H, Wolzt M, Stix G. Landiolol: pharmacology and its use for rate control in atrial fibrillation in an emergency setting. Eur Heart J Suppl 2018;20:A1. [CrossRef]
- Levy ES, Samy KE, Lamson NG, Whitehead KA, Kroetz DL, Desai TA. Reversible inhibition of efflux transporters by hydrogel microdevices. European Journal of Pharmaceutics and Biopharmaceutics 2019;145:76–84. [CrossRef]
- Makagiansar IT, Avery M, Hu Y, Audus KL, Siahaan TJ. Improving the Selectivity of HAV-Peptides in Modulating E-Cadherin-E-Cadherin Interactions in the Intercellular Junction of MDCK Cell Monolayers. Pharm Res 2001;18:446–53. [CrossRef]
- Song K-H, Kim S-B, Shim C-K, Chung S-J, Kim D-D, Rhee S-K, et al. Paracellular permeation-enhancing effect of AT1002 C-terminal amidation in nasal delivery. Drug Des Devel Ther 2015:1815. [CrossRef]
- Álvarez R, López Cortés LE, Molina J, Cisneros JM, Pachón J. Optimizing the Clinical Use of Vancomycin. Antimicrob Agents Chemother 2016;60:2601–9. [CrossRef]
- Wang NN, Dong J, Deng YH, Zhu MF, Wen M, Yao ZJ, et al. ADME Properties Evaluation in Drug Discovery: Prediction of Caco-2 Cell Permeability Using a Combination of NSGA-II and Boosting. J Chem Inf Model 2016;56:763–73. [CrossRef]
- Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. Journal of Clinical Investigation 1985;75:1144–52. [CrossRef]
- Little TJ, Horowitz M, Feinle-Bisset C. Role of cholecystokinin in appetite control and body weight regulation. Obesity Reviews 2005;6:297–306. [CrossRef]
- Herranz R. Cholecystokinin antagonists: Pharmacological and therapeutic potential. Med Res Rev 2003;23:559–605. [CrossRef]
- Szewczyk J, Laudeman C. CCK1R Agonists: A Promising Target for the Pharmacological Treatment of Obesity. Curr Top Med Chem 2003;3:837–54. [CrossRef]
- Sugg EE, Birkemo L, Gan L-SL, Tippin TK. Orally Active Nonpeptide CCK-A Agonists. Integration of Pharmaceutical Discovery and Development, Boston: Kluwer Academic Publishers; n.d., p. 507–24. [CrossRef]
- Elliott RL, Cameron KO, Chin JE, Bartlett JA, Beretta EE, Chen Y, et al. Discovery of N-benzyl-2-[(4S)-4-(1H-indol-3-ylmethyl)-5-oxo-1-phenyl-4,5-dihydro-6H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepin-6-yl]-N-isopropylacetamide, an orally active, gut-selective CCK1 receptor agonist for the potential treatment of obesity. Bioorg Med Chem Lett 2010;20:6797–801. [CrossRef]
- Evangelista S. Quaternary Ammonium Derivatives as Spasmolytics for Irritable Bowel Syndrome. Curr Pharm Des 2004;10:3561–8. [CrossRef]
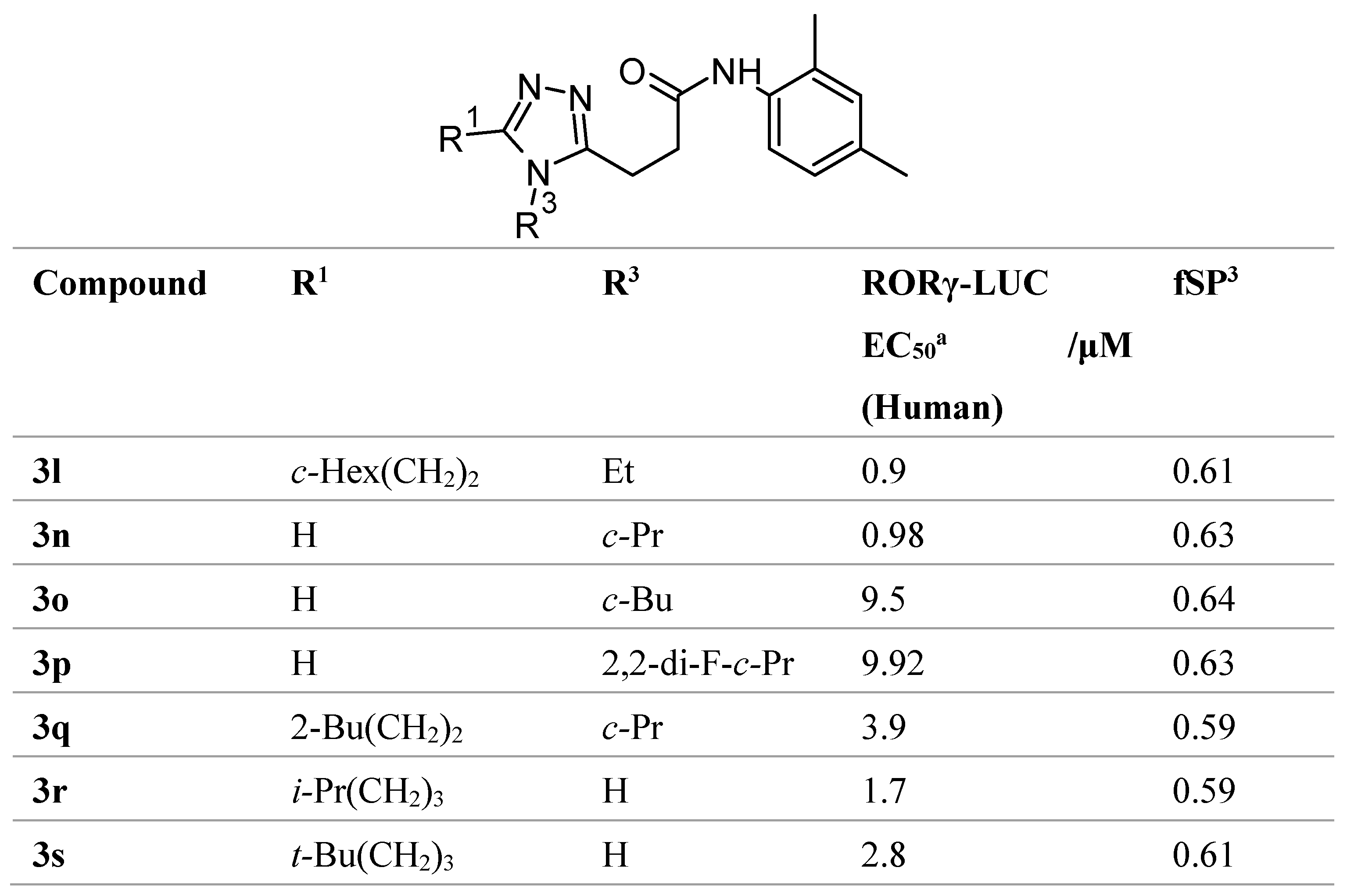
- Hirata K, Kotoku M, Seki N, Maeba T, Maeda K, Hirashima S, et al. SAR Exploration Guided by LE and Fsp(3): Discovery of a Selective and Orally Efficacious RORγ Inhibitor. ACS Med Chem Lett 2015;7:23–7. [CrossRef]
- Iusupov IR, Lukyanenko ER, Altieri A, Kurkin A V. Design and Synthesis of Fsp3-Enriched Spirocyclic-Based Biological Screening Compound Arrays via DOS Strategies and Their NNMT Inhibition Profiling. ChemMedChem 2022;17:e202200394. [CrossRef]
- Dowlut M, Hall DG. An Improved Class of Sugar-Binding Boronic Acids, Soluble and Capable of Complexing Glycosides in Neutral Water. J Am Chem Soc 2006;128:4226–7. [CrossRef]
- Benardout M, Le Gresley A, ElShaer A, Wren SP. Fructose malabsorption: causes, diagnosis, and treatment. British Journal of Nutrition 2022;127:481–9. [CrossRef]
- Silva MP, Saraiva L, Pinto M, Sousa ME. Boronic acids and their derivatives in medicinal chemistry: synthesis and biological applications. Molecules 2020, 25(18), 4323. [CrossRef]
- Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, et al. ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Res 2012;40:D1100-7. [CrossRef]
- Bento AP, Gaulton A, Hersey A, Bellis LJ, Chambers J, Davies M, et al. The ChEMBL bioactivity database: an update. Nucleic Acids Res 2014;42:D1083-90. [CrossRef]
- Gaulton A, Hersey A, Nowotka M, Bento AP, Chambers J, Mendez D, et al. The ChEMBL database in 2017. Nucleic Acids Res 2017;45:D945–54. [CrossRef]
- Santos R, Ursu O, Gaulton A, Bento AP, Donadi RS, Bologa CG, et al. A comprehensive map of molecular drug targets. Nat Rev Drug Discov 2017;16:19–34. [CrossRef]
- Mendez D, Gaulton A, Bento AP, Chambers J, De Veij M, Félix E, et al. ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res 2019;47:D930–40. [CrossRef]
- DeGoey DA, Chen H-J, Cox PB, Wendt MD. Beyond the Rule of 5: Lessons Learned from AbbVie’s Drugs and Compound Collection. J Med Chem 2018;61:2636–51. [CrossRef]
- O’ Donovan DH, De Fusco C, Kuhnke L, Reichel A. Trends in Molecular Properties, Bioavailability, and Permeability across the Bayer Compound Collection. J Med Chem 2023;66:2347–60. [CrossRef]
- Yan J, Springsteen G, Deeter S, Wang B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—it is not as simple as it appears. Tetrahedron 2004;60:11205–9. [CrossRef]
- Mansouri K, Grulke CM, Judson RS, Williams AJ. OPERA models for predicting physicochemical properties and environmental fate endpoints. J Cheminform 2018;10:10. [CrossRef]
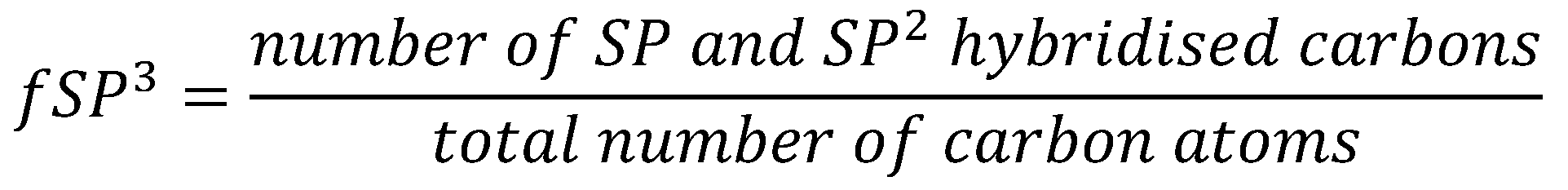
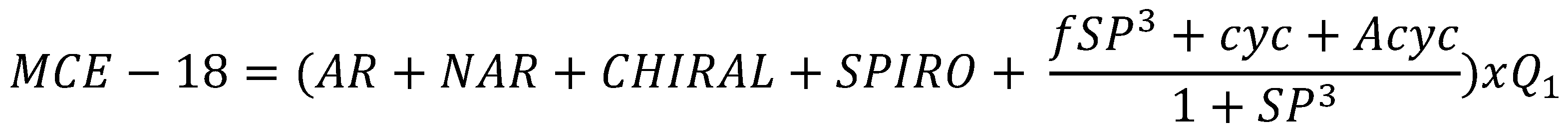
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





