1. Introduction
The ability to extract energy from food is essential for animals. Many obligate herbivores obtain their energy from angiosperms whose recalcitrant cell wall constituents, cellulose and lignin, retard digestion and which may additionally contain antifeedants such as latex, resins, gums, silicates, phenolics and alkaloids. Obligate herbivores include nematodes, the larvae and adults of many insects, the tadpoles of some anurans, certain fish, the hoatzin bird, reptiles, and odd- and even-toed ungulates and primates among mammals. This variety obscures similar behavioral adaptations such as avoidance of noxious plants and parts, and physiological adaptations such as the presence of salivary proteins for detoxification and digestion [
1] and fermentation chambers with symbionts to assist the digestive process [
2].
Among the many deterrents that plants produce (see
Supplementary Table S1), latex is considered to be primarily an antifeedant [
3]. Latex is an emulsion with suspended particles including rubber, and may additionally contain a slew of other deterrent metabolites. Rubber is a megapolymer of terpene units which is sequestered in particles with a protein-rich membrane. When treated with acid, the membrane is removed and the rubber inside is released like strands of spaghetti to form insoluble tangles. Latex remains liquid in neutral and alkaline solutions because the membrane is not disrupted. The exact timeline of the evolution of latex is uncertain, but the major precursor for latex is the common isoprene molecule whose use spans across the spectrum from the synthesis of fatty acids for essential structural membrane components all the way to secondary metabolites that provide evolutionary advantage, to terpene compounds with antifungal, anthelminthic, antibacterial, cytotoxic, and insect-repellent properties [
4].
Studies focused on selection pressures on the evolution of herbivory have noted that herbivore pressure on plants increases towards the Equator, and plants respond with a higher density of defenses towards generalist herbivores [
5]. That angiosperms radiated in the Cretaceous is well accepted [
6] and it is accepted that “Because some of the oldest and most diverse angiosperm floras are found in Africa near the Equator, followed by low-latitude, angiosperm-dominated floras in North America, angiosperms are thought to have radiated from the Equator and spread to either pole.” [
7]. Complementary studies have identified latex as a deterrent for herbivory. A study of common dandelion (
Taxaracum officinale) showed that latex fouls chewing mouthparts and a constituent secondary metabolite, the sesquiterpene lactone taraxinic acid β-D-glucopyranosyl lactone (TA-G), has a marked effect on the fitness of larvae of the Coleopteran May bug beetle, (
Melolontha melolontha), which preferentially feeds on the roots of
T. officinale [
8]. Since latex has varied composition, presumably due to selection pressures the plants experience, the hypothesis that latex is a herbivory deterrent has gained wide acceptance [
3].
Latex-containing plants span 43 families and 20,000 species, and make up a large proportion (>10%) of angiosperms, 1 fern (
Regnellidium diphyllum) and 1 gymnosperm (
Gnetum gnemon) [
9]. They overlap with areas with enormous herbivore diversity in the tropics and have a variety of uses for humans as well. Some are cultivated for food [
3], rubber [
10], pharmacologically active molecule production [
5] and herbivore-deterrent fencing [
11]. Even animals such as Monarch butterfly (
Danaus plexippus) caterpillars which have specialized to feed on milkweed (
Ascelpias species), avoid consuming latex, severing latex-carrying tubes and feeding on the latex-depleted portions of leaves [
12]. In a separate experiment, washing off latex from fig and other leaves made the leaves more palatable to herbivores (cited in Ref. 9). Despite this clear evidence of latex’s deterrence of herbivory, and the abundance and diversity of plants that produce latex, the question of how the two major categories of herbivores tackle the large or small amounts of latex that must inevitably enter their diet has not been explored, even by the venerable
The Ecology of Browsing and Grazing II, in which the word “latex” does not even appear [
13].
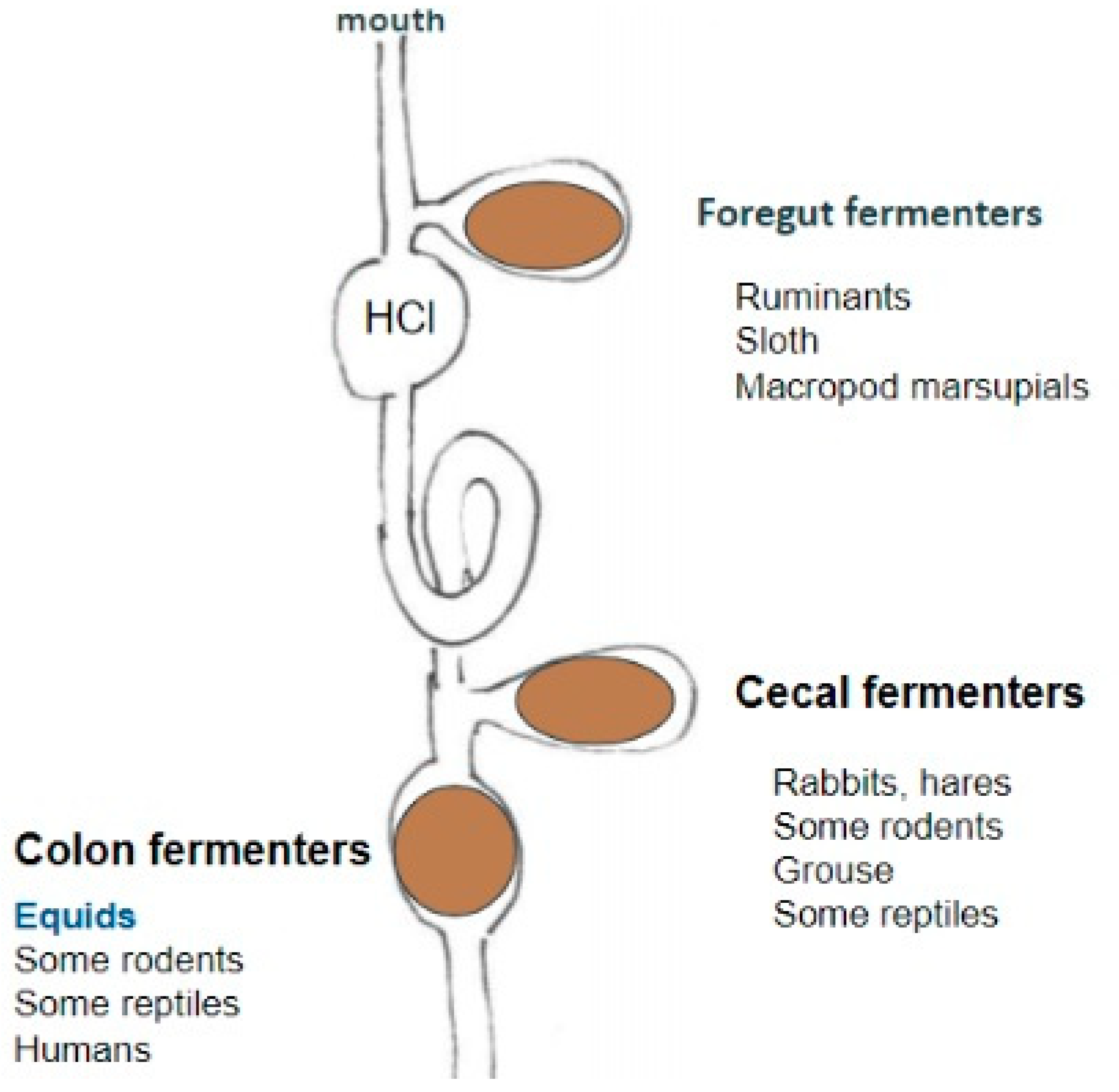
I propose that the answer lies in the behavior of rubber in alkaline and acidic pH conditions encountered in the digestive system of foregut and hindgut fermenters Foregut-fermenting herbivores have pre-stomach neutral-to-alkaline chambers and browse on vegetation including latex-containing shrubs, forbs, leaves and latex-free grasses. By contrast, hindgut-fermenting herbivores are largely grazers with the cecum or colon where fermentation occurs posterior to the acidic stomach (
Figure 1).
I additionally propose that browsers can occupy a diversity of habitats where latex is abundant, from tree canopies to woodland fringe to pasture, whereas grazers are typically found on grassland or eat latex-free plants in regionally restricted habitats. Significantly, carnivores, omnivores and herbivores with hindgut-fermentation and acidic stomachs are largely capable of pivoting to new diets, provided the new diet is latex-free. This was seen in a population of Italian wall lizards (
Podarcis sicula) of which a few breeding pairs were transported from the island of Pod Kopište where they had an insectivorous diet to the island of Pod Mrčaru where, in a mere 36 years, they adapted to a largely herbivorous diet [
14]. This habitat pivot, according to my hypothesis, would not have been possible had the forage consisted of mostly latex-bearing plants.
There are three correlations that support my hypothesis that it was the selective pressure of latex that shaped and continue to shape the evolution of digestive systems. Direct evidence will have to be collected to assess these correlations for latex tolerance, but there is some circumstantial evidence for the following that support latex-based selection.
1. Foregut-fermenting browsers have higher first chamber pH to tolerate latex than hindgut-fermenting grazers.
2. Plasticity of the gut to accommodate the change from herbivory to carnivory in animals which undergo metamorphosis.
3. Areas where latex-containing plants are more abundant correlate with browsers, who have greater forage adaptability.
These are developed further in the analysis.
2. Materials and Methods
Using the Google Scholar search engine, I searched the literature for three key topics:
1. The stomach pHs of various herbivores.
2. Plant deterrents against herbivory, including latex.
3. The action of Bacillus thuringiensis and related toxins in animals with alkaline gut pH.
Following finding data on the stomach pHs of a wide variety of animals, I classified them into polygastric ruminants or monogastric non-ruminant categories. Based on available information across the internet, I further classified them as browsers (capable of eating forbs and leaves in addition to grass) or grazers (largely subsisting on grass or living in grassland habitats).
Being aware of the action of Bacillus thuringiensis and related insecticidal toxins which are activated by alkaline gut pHs, I further explored the literature for impact of crystal toxins on ruminants and metamorphosing herbivores such as tadpoles which have alkaline gut pH.
3. Results
1. Foregut-fermenting browsers have higher first chamber pH than hindgut-fermenting grazers, indicating higher latex tolerance.
Fresh latex from plants has a pH between 6-7.5 [
15]. The guts of foregut herbivores (many of which are ruminants) may chew the cud to break up food, contain symbionts to digest recalcitrant molecules, have a higher gut pH which keeps latex from coagulating, and a large rumen providing the dilution factor which makes any rubber particles less cohesive and less likely to occlude the system. Browsers eat leaves and forbs as well as grasses, and appear to be able to handle small amounts of latex in their mixed forage without adverse effects.
Table 1 shows the pH levels of an assortment of foregut fermenters. Of all the foregut fermenters, the most likely to encounter substantial amounts of latex on a daily basis are sloths which feed on the latex-containing
Cecropia tree and colobus monkeys which feed on unripe fruit. Both these animals have relatively high pH in the first chamber, whereas hippos and sheep are largely grazers and have a lower pH.
Table 2 shows the first chamber pH of hindgut-fermenting grazers which primarily eat latex-free forage, including seeds, fruits, nuts, and sometimes carrion. The presence of an acidic stomach would hinder hindgut fermenters from tolerating latex consumption in the diet [
19]. It seems that Perissodactyls evolved in North America, Europe and Asia (all parts of the Laurasia supercontinent, rather than Gondwanaland where latex-bearing plants probably evolved about 200 mya at the end of the Jurassic period). These animals would have been less exposed to latex in their diet since latex-bearing plants probably originated and spread from the Amazon-Congo nexus as they separated into South America and Africa during the Cretaceous period. Their presence in Africa and South America are due to migration from Europe and North America [
20].
2. Plasticity of digestive morphology based on dietary changes from larvae to adults in animals which undergo metamorphosis can be measured by susceptibility to Bacillus thuringiensis (Bt) crystal toxins.
While direct measurement may be awkward, there is an indirect way to test the pH of the first chamber of the gut by measuring the organism’s susceptibility to
Bacillus thuringiensis (Bt) crystal toxins. Bt toxins are proteins which are activated in an alkaline pH [
21]. When activated, they punch holes in the gut lining, causing leakage and death [
22]. If the proteins encounter acid, they are degraded. Thus, animals who have alkaline guts will both activate Bt toxin and not cause latex to coagulate, whereas animals that have an acidic gut break down crystal toxins, but they also cause latex to coagulate. Death by leakage or by blockage are the options on offer.
Insect larvae of Lepidoptera (moths and butterflies), Coleoptera (beetles), Diptera (flies and mosquitoes), Hemiptera (true bugs) and Hymenoptera (bees, wasps and ants) typically have neutral to alkaline midguts and many of their diets include plant material. The larvae are also susceptible to Bt crystal toxins, which are used as biocontrol agents. Coleoptera larvae, which have a slightly more acidic to neutral midgut (pH 5–7), are less susceptible than Lepidoptera larvae which have a slightly more alkaline midgut (pH > 9). Interestingly, a data point that Huber et al. (2016) could not explain in their study of the Coleopteran larvae of May bugs feeding on dandelion roots was that the larvae gained mass when they fed on latex, even as the concentration of the secondary metabolite TA-G in the latex negatively correlated with
M. melolontha growth [
8]. This can be explained if the latex was solidifying inside the larva due to Coleopterans’ more acidic gut pH, and therefore failing to be digested and eliminated. This has significant consequences for the use of insect larvicidal toxins for biological control. Bt was also claimed to be toxic to cows, goats, buffaloes, and sheep [
23,
24,
25] which would support an alkaline pH of their guts activating the toxin. This correlation has never been addressed before in the scientific literature as a causative agent for Bt-engineered plants being detrimental to foregut-herbivores because of their alkaline gut pH. Silaging appears to remove the toxic effect, consistent with the process breaking down Bt toxin [
26,
27].
In an experiment conducted by Lajmanovich et al., Bt proved toxic to the tadpoles of the South American common frog,
Leptodactylus latrans [
28]. Herbivorous tadpoles have a foregut, midgut and hindgut with neutral pH [
29]. Carnivorous tadpoles have an acidic stomach chamber. But when they metamorphose to adulthood, their diet changes and so does their digestive system pH. Although not all gut pHs have been measured, an indirect measurement can be applied by assessing the toxicity of
Bacillus thuringiensis (Bt) crystal toxins, which requires alkaline pH for activation, to larvae and adults of the same species. However, Lajmanovich et al. did not extend their findings to foregut herbivores.
The point here is to highlight that guts are plastic. Equivalently, infant mammals maturing from a diet of protein- and fat-rich milk to a plant-based diet from also showing gut maturation phenomena with changing first chamber pH through diet-induced plasticity.
3. Areas where latex-containing plants correlate with the presence of browsers, who have greater digestive adaptability.
The presence of copious numbers of plants with latex provided the selection pressure for the evolution of alkaline guts. Since latex-bearing plants are most abundant in the tropics, which correlates with the greatest herbivore pressure [
5], this is not a surprise. Browsers and grazers alike have dispersed due to natural and human-mediated actions, so it is a little less straightforward to assess the pressures extant during evolution of the various species.
Most foregut-fermenting browsers (cattle, sheep, goats, insect larvae) have a wide variety of habitats from grasslands to forests, whereas most hindgut-fermenting grazers (elephants, horses, rhino, rabbits) live on grasslands. Browsers have a more adaptable digestive system due to the presence of foregut fermentation as well as cecum fermentation. Grazers are much more limited in their forage diversity, eating grasses which are poor in nutrition and therefore they have to eat voraciously and become extremely large to accommodate the enormous quantity of low-quality forage they eat (elephants, rhinos, horses) or practice coprophagy (rabbits). Old world monkeys (langur, colobus) and New world monkeys (spider) are foregut fermenters and they live in tree canopies and eat leaves. Howler monkeys also live in tree canopies but are omnivores, so their hindgut-digestive system is adequate to their diet. Although this is a scattershot justification rather than prediction, the correlations still apply. Interestingly, the giant panda is an exception to this hueristic. Its forage is extremely protein-rich bamboo shoots and it has carnivore-like digestion with neither foregut nor hindgut fermentation [
30].
4. Discussion
I propose that the neutral pH of the rumen in foregut herbivores has a more straightforward evolutionary purpose: to enable toleration of latex and as a fermentation chamber for cellulosics. Obviously, this does not preclude the adaptation of the chamber to symbiont fermentation in more evolutionarily-recent browsers and larger animals. Additionally, this hypothesis is supported by the presence of neutral-to-alkaline anterior digestive chambers in folivores, which eat leaves with high levels of latex, and frugivores, where latex is a gatekeeper for fruit ripeness. What of the monogastric herbivores which have a posterior cecum where fermentation occurs? These animals are typically grazers which specialize in grasses which contain silicate abrasives as the primary deterrents. Typically, mature leaves and ripe fruit have lower latex levels and are more often browsed than tender shoots, particularly by hindgut fermenters.
The choice of food for animals that are confined, as in zoos, or domesticated and do not range on their own, is critical for their health. In zoos, care is taken to monitor nutrition, and usually zoos follow the precedents of other zoos which have successfully nurtured a picky herbivore. But it is as well to know why so that the diet can be modified to resemble the wild diet. Domesticated animals are also at risk if food is offered without foresight. It is known that horses should not be given a lot of unripe apples, but it is not known why. Apples and other unripe fruit contain latex, and with horses being a foregut-fermenter, latex will coagulate rather than break down in the gut. Regarding cattle, a recent move to silvopasture where animals are released into lightly forested woodland probably resembles the environment in which they are best suited to eat grasses, forbs and leaves. Aurochs, the wild cattle of Europe, were woodland animals. Bison and deer are notorious for eating young trees and preserving grasslands, but they can cope with the latex produced by those very trees. Horses do not forage on trees. Such observations can now be put into a physiological context.
Another interesting consequence of my hypothesis relates to the widespread use of Bt and similar protein biopesticides which are thought of as highly target specific. Rather, by using the metric that these proteins are activated by alkaline gut pH, their target spectrum widens to include any animal which has foregut fermentation as well as metamorphosing insects and amphibians. Indeed, Lajmanovich et al. [
28] show that Bt can target herbivorous tadpoles. This is concerning, given the already stressful environmental conditions that amphibians are encountering. Even those who may be working to diminish chemical pesticides in the environment by moving to more “natural” control may be causing unintended damage. For livestock fed on the remnants of Bt-containing crops, the impact on their digestion may cause morbidity and even mortality. This has been shown in an anecdotal manner by several reports. It seems that silaging may break down the Bt toxin, which is a simple fix for a consequential problem.
There is still a lot of work to be done to clarify the impact of latex on digestive systems. For example, many reptiles are carnivorous, but some are obligate herbivores. The manchineel tree (Hippomane mancinella) is considered the deadliest plant in the world because its latex causes skin to blister on contact, and its apple-like fruit are usually fatal if eaten. But the omnivorous black-spined iguana (Ctenosaura similis) both eats the apple and lives in the tree. This cannot be explained simply by the structure of the digestive system.
Sixty years ago, Polhamus (1962; cited in Ref. 4) stated: “no one has demonstrated why a plant makes rubber and it does not appear to be a food reserve.” Arbus et al. go on to state that “there is no scientific evidence of the metabolic role of latex in plants.” [
4] This is probably because there is no role for latex in the plant itself, except to prevent browsing by herbivores. In this paper, I concur that latex is primarily as an antifeedant which has been suggested by Agrawal and Konno [
12], and I propose that foregut-fermenting animals’ alkaline chambers evolved as a mechanism to cope with the presence of latex in the environment.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
VR conceived the hypothesis, did the research, and wrote the paper.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Rivera-Vega, L.J.; Acevedo, F.E.; Felton, G.W. Genomics of Lepidoptera Saliva Reveals Function in Herbivory. Curr. Opin. Insect Sci. 2017, 19, 61–69. [Google Scholar] [CrossRef]
- Furness, J.B.; Cottrell, J.J.; Bravo, D.M. Comparative Gut Physiology Symposium: Comparative physiology of digestion. J Anim. Sci. 2015, 93, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A.; Hastings, A.P. Plant Defense by Latex: Ecological Genetics of Inducibility in the Milkweeds and a General Review of Mechanisms, Evolution, and Implications for Agriculture. J. Chem. Ecol. 2019, 45, 1004–1018. [Google Scholar] [CrossRef] [PubMed]
- Abarca, L.F.S.; Kinkhamer, P.G.L.; Choi, Y.H. Plant Latex, from Ecological Interests to Bioactive Chemical Resources. Planta Med. 2019, 85, 856–868. [Google Scholar] [CrossRef]
- Salazar, Diego, and Robert J. Marquis. Herbivore Pressure Increases toward the Equator. Proc. Natl. Acad.
Sci. U.S.A., 2012, 109 (31), 12616–12620. [CrossRef]
- Doyle, James A. Molecular and fossil evidence on the origin of angiosperms. Annu. Rev. Earth Planet. Sci. 2012, 40, 301–326. [Google Scholar] [CrossRef]
- Angiosperm-Classification. n.d. Encyclopedia Britannica. Available online: https://www.britannica.com/plant/angiosperm/Classification (accessed on 24 August 2023).
- Huber, Meret, Janina Epping, Christian Schulze Gronover, Julia Fricke, Zohra Aziz, Théo Brillatz, Michael Swyers et al. A latex metabolite benefits plant fitness under root herbivore attack. PLoS Biol. 2016, 14, e1002332. [Google Scholar]
- Gracz-Bernaciak, J.; Mazur, O.; Nawrot, R. Functional Studies of Plant Latex as a Rich Source of Bioactive Compounds: Focus on Proteins and Alkaloids. Int. J. Mol. Sci. 2021, 22, 12427. [Google Scholar] [CrossRef]
- Hayashi, Yasuyuki. Production of natural rubber from Para rubber tree. Plant Biotechnol. 2009, 26, 67–70. [Google Scholar] [CrossRef]
- Yohanna, C.T.; Onaji, A.I.; Nyam, M.A.; Azila, J.J. Suitability of Latex-Producing Plant Species as Bio-security for some Landed Properties in Jos South, Jos, Plateau State. Int. J. Biol. Sci. 2023, 6, 1–15 http://ijojournalscom/indexphp/bs/article/view/650. [Google Scholar]
- Agrawal, Anurag A. , and Kotaro Konno. Latex: a model for understanding mechanisms, ecology, and evolution of plant defense against herbivory. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 311–331. [Google Scholar] [CrossRef]
- Gordon, I. J., & Prins, H. H. (2019). The ecology of browsing and grazing II (pp. 1-4). Springer International Publishing.
- Herrel, A.; Huyghe, K.; Vanhooydonck, B.; Backeljau, T.; Breugelmans, K.; Grbac, I.; Van Damme, R.; Irschick, D.J. Rapid large-scale evolutionary divergence in morphology and performance associated with exploitation of a different dietary resource. Proc. Natl. Acad. Sci. 2008, 105, 4792–4795. [Google Scholar] [CrossRef] [PubMed]
- Ng, Jing Wei, Nadras Othman, and Nurul Hayati Yusof. Various Coagulation Techniques and Their Impacts towards the Properties of Natural Rubber Latex from Hevea Brasiliensis — a Comprehensive Review Related to Tyre Application. Ind. Crops Prod. 2022, 181, 114835. [Google Scholar] [CrossRef]
- Beasley, D.E.; Koltz, A.M.; Lambert, J.E.; Fierer, N.; Dunn, R.R. The Evolution of Stomach Acidity and Its Relevance to the Human Microbiome. PLOS ONE 2015, 10. [Google Scholar] [CrossRef]
- Hofmann, R.R. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologia 1989, 78, 443–457. [Google Scholar] [CrossRef]
- Grajal, A. Structure and Function of the Digestive Tract of the Hoatzin (Opisthocomus hoazin): A Folivorous Bird with Foregut Fermentation. The Auk 1995, 112, 20–28. [Google Scholar] [CrossRef]
- Thirunavukkarasu, K.; Yoheswaran, K. Coagulation of rubber latex in the stomach. Br. Med. J. 1967, 4, 484. [Google Scholar] [CrossRef]
- Evolution | Perissodactyl. Amnh.org. 2014. https://research.amnh.org/paleontology/perissodactyl/evolution/intro.
- Cohen, Z.P. Bacillus thuringiensis, bio-pesticide. Cornell.edu. (2015). Available online: https://biocontrol.entomology.cornell.edu/pathogens/bacillus.php. (accessed on 24 August 2023).
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef]
- Glöckner, G.; Séralini, G.É. Pathology reports on the first cows fed with Bt176 maize (1997–2002). Sch. J. Agric. Sci., 2016, 6, 1–8. [Google Scholar]
- Ramdas, S.R. Bt cotton and livestock: Health impacts, bio-safety concerns and the legitimacy of public scientific research institutions. 2010. In National workshop on Genetically Modified Crops/Foods and Heath Impacts. PDF available at: http://indiaenvironmentportal.org.in/files/bt-cotton-and-livestock-health-impacts-dr-sagari-r-ramdas.pdf.
- Hashim, M.A.; ElObied, G.H.; Adawi, I.A. Respondents Evolution of the Effect of Grazing on Bt-cotton Crop Residues by Ruminants on Health and Milk Characteristics in Gezira State, Sudan. Int. J. Res. Agric. Sci. (IJRAS) PDF available at: http://www.ijras.org/administrator/components/com_jresearch/files/publications/IJRAS_610_FINAL.pdf. 2017, 4, 304–309. [Google Scholar]
- Folmer, J.D.; Grant, R.J.; Milton, C.T.; Beck, J. Utilization of Bt corn residues by grazing beef steers and Bt corn silage and grain by growing beef cattle and lactating dairy cows. J. Anim. Sci. 2002, 80, 1352–61. [Google Scholar] [CrossRef] [PubMed]
- Faust, M.; Smith, B.; Rice, D.; Owens, F.; Hinds, M.; Dana, G.; Hunst, P. Performance of lactating dairy cows fed silage and grain from a maize hybrid with the cry1F trait versus its nonbiotech counterpart. J. Dairy Sci. 2007, 90, 5706–5713. [Google Scholar] [CrossRef] [PubMed]
- Lajmanovich, R.C.; Junges, C.M.; Cabagna-Zenklusen, M.C.; Attademo, A.M.; Peltzer, P.M.; Maglianese, M.; Márquez, V.E.; Beccaria, A.J. Toxicity of Bacillus thuringiensis var. israelensis in aqueous suspension on the South American common frog Leptodactylus latrans (Anura: Leptodactylidae) tadpoles. Environ. Res. 2015, 136, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Bjorndal, K.A. Fermentation in Reptiles and Amphibians. In: Mackie, R.I., White, B.A. (eds) Gastrointestinal Microbiology. Chapman & Hall Microbiology Series. Springer, Boston, MA. 1997. 199-230. [CrossRef]
- Yong, E. The Giant Panda Is a Closet Carnivore. [online] The Atlantic. 2019. Available at:https://www.theatlantic.com/science/archive/2019/05/giant-panda-closet-carnivore/588553 /.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).