1. Introduction
In the aftermath of the COVID pandemic, post-COVID-19 syndrome (PCS) or ‘long’ COVID-19 remains a challenge and may continue to represent a major health problem in the future. The symptoms range from chronic fatigue and dyspnea to cognitive problems. Obesity, even being overweight and diabetes have been global health problems and are key risk factors for various infections, including COVID-19. Zhou et al. reviewed the relationship between obesity, diabetes, and COVID-19 infection. They concluded that despite all the facts regarding altered immunity, aggravation of inflammatory storm, and abnormalities in lung physiology in diabetes patients, we still lack evidence of whether diabetes increases susceptibility to COVID-19 infection [
1]. Obesity and being overweight are associated with altered pulmonary mechanics and physiology and increased angiotensin-converting enzyme 2 (ACE2) expression, which affects the acute phase of infection and progression to respiratory failure [
1]. Diabetes and obesity alter pulmonary and lung function. Diabetes predominantly affects central and peripheral airway function and the dissipative and elastic properties of respiratory tissues [
2]. The most consistently reported effect of obesity on lung function is a reduction in the functional residual capacity (FRC). Moreover, spirometric variables, such as forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC), tend to decrease with increasing body mass index (BMI) [
3,
4,
5]. However, this effect is small, and both FEV1 and FVC are usually within the normal range in healthy obese adults [
4,
5].
Several studies have revealed that overweight patients and those with diabetes mellitus have an increased risk of developing PCS [
6,
7,
8,
9]. PCS can be classified into two subtypes: post-acute COVID-19 with persistent symptoms lasting beyond four weeks after infection (PACS) and ‘long’ COVID-19 with irreversible tissue damage lasting beyond 12 weeks [
8].
The influence of type 2 diabetes mellitus on PCS via various pathophysiological mechanisms has also been confirmed by Raveendran and Misra [
10]. Lacavalerie et al. [
11] found that more than half of obese patients with PCS had a significant alteration in aerobic exercise capacity and a marked reduction in oxygen pulse. Moreover, these patients were consequently more prone to pathological pulmonary limitations and pulmonary gas exchange impairment [
12]. Picone et al. [
13] showed that nutrition is closely linked to PCS due to inter-systemic homeostasis involving the endocrine, neurological, and immune–metabolic systems, particularly in obese and diabetes patients. Similar results, especially in patients older than 65 years, have been reported by Paneni and Patrono [
14] and Shang et al. [
15].
The purpose of our study was to determine whether similar PCS patterns also occur in Slovenia, especially in its northern region. This study included patients who were hospitalized at the University Clinical Centre of Maribor and then followed up in the Community Healthcare Center dr. Adolf Drolc. In particular, we focused on patients with diabetes and overweight patients and assessed how they differ from the reference population of patients without those conditions. Our preliminary aimed to examine the differences and anomalies but not to explain them. However, we want to encourage such research and plan to carry it out in future studies.
2. Materials and Methods
2.1. Study population and research design
This retrospective observational study was conducted at the Community Healthcare Center dr. Adolf Drolc Maribor (HCM), Slovenia, from October 2020 to July 2022. All patients had previously been infected with SARS-CoV-2 and developed COVID-19 pneumonia that required hospitalization. They were regularly checked by physicians at the HCM within 3-4 weeks after discharge from the hospital and then every 3 months until post-COVID-19 symptoms disappeared.
All patients with persistent symptoms after 1 month were selected as the study population for our analysis. Clinical and demographic data collected during hospitalization and the first HCM visit were analyzed. Physicians reviewed and collected data, including patient demographics, comorbidities, home medications, vital signs at hospital admission, duration of hospitalization, maximum oxygen requirement at hospitalization, treatment with remdesivir and dexamethasone (doses and protocols used were standard for all patients), radiological findings, admission to the intensive care unit (ICU), duration of ICU hospitalization, mechanical or non-invasive ventilation, bacterial superinfection, and vaccination status after vaccination was available. At regular checks after discharge from the hospital, the physicians performed the clinical evaluation and completed a questionnaire about post-COVID-19 symptoms (dyspnea, cough, chest pain, and fatigue), chest X-ray results, and lung function tests.
Before conducting the study, we obtained the consent of the research participants and carefully instructed them about the goals and purposes of the research. We also explained to them that participation is voluntary and that they can withdraw from the research at any time without giving a reason. Additionally, we explained that only anonymized data will be used and will be carefully protected by the principal researcher. The researchers of this study also obtained the consent of the HCM Ethics Commission.
2.2. Statistical analysis
Based on the study objectives, the patient population was divided into four groups:
Patients with neither diabetes nor overweight (None group (NG));
Overweight patients (Overweight group (OWG));
Patients with type 2 diabetes (Diabetes group (DMG));
Overweight patients with type 2 diabetes (Diabetes + Overweight group (DM+OWG)).
Being overweight was determined based on BMI. All patients with a BMI greater than 25 kg/m2 were classified as overweight. Most of the characteristics associated with PCS consisted of dichotomous information; therefore, the exact chi-square test with each group as a grouping variable was applied. The 16 analyzed variables (discretized lung function, symptoms, complications, and treatment) were coded as 1 or 0, corresponding to the presence or absence of the patient’s features. The eight continuous variables (demographics and clinical assessment) were analyzed using the Kruskal-Wallis test with each group as the variable. The duration of the PCS was analyzed using the Kaplan-Meier survival analysis.
Statistical analyses were performed using SPSS Statistics for Windows (version 28.0; IBM Corp., Armonk, NY, USA). A p-value less than 0.05 is deemed to be statistically significant.
3. Results
Our sample consisted of 466 patients with post-COVID-19 syndrome. Among them, there were 269 males and 197 females. The males were between 30 and 92 years old, with a median age of 65. The females were between 19 and 98 years old, with a median age of 67. There were 77 patients in the NG (41 males, 36 females), 274 in the OWG (144 males, 130 females), 17 (12 males, five females) in the DMG, and 98 (72 males, 26 females) in the DM + OWG.
The Kruskal-Wallis test showed that in the male population, body mass index (BMI) (p<0.001), intensive care unit (ICU) duration (p=0.031), and age differed significantly among the four groups. However, FVC%, FEV1%, FEV1/FVC%, and duration of PCS hospitalization did not differ significantly. In the female population, the BMI differed significantly between the groups (p<0.001).
The differences between the groups are shown in
Table 1. The longest median PCS duration for males (5 months) was observed in the OWG and the DM+OWG for females (6 months). The longest median hospitalization was observed in the DMG for males (13 days) and the NG and DM+OWG for females. The shortest median hospitalization was observed in the OWG for males (8 days) and in the DMG for females (6 days). The median PCS duration was generally shorter for males than for females. The DM+OWG had the highest median BMI in males (BMI=31.00) and females (BMI=33.9). Moreover, the lowest median BMI was observed in the NG for males (BMI=23.40) and DMG for females (BMI=22.8). The longest ICU median duration in males (6 days) was observed in the DM+OWG, OWG, and NG and the NG in females (11 days). The shortest ICU median duration was observed in the DMG for both males (0 days) and females (4 days). In general, the median ICU stay for females was longer than for males.
The lowest FVC% value was observed in the DM+OWG for males (85.5%) and in the DMG for females (90%). Overall, FVC% values were lower in males than in females. The lowest median FEV1% values were observed in the DMG for males (87.5%) and the OWG for females (93%). The lowest median FEV1/FVC% value was observed in the DM+OWG for males (82%) and the DMG for females (78%). In general, female patients were older than male patients. Furthermore, the chi-square test for categorical variables revealed significant differences in frequencies between the four groups defined above in females only, namely FEV1<85% (p=0.049), dexamethasone (DEXA) (p=0.012), and secondary bacterial infection (p=0.019).
Table 2 presents the percentages of patients with abnormal lung function, in-hospital treatment, complications during hospitalization, and PCS.
Table 2 also reveals that the significant difference in FEV1<85% in females was due to the low number of female patients in the DMG (FEV1% was 60.0%; five female patients) compared to other groups. The significant difference in DEXA was due to the fact that in the NG, only 52.1% of females were treated with DEXA. The most common secondary bacterial infections were found in females in the DMG (33.3%) and DM+OWG (28.6%) and males in the NG (15.4%) and OWG (12.3%). The chi-square test on categorical variables revealed significant differences in the frequencies between males and females only in FVC<85% (p=0.049), with more females having FVC% lower than 85% than males. Additionally, more females significantly claimed fatigue (p=0.038) than males did.
Overall, our study showed that within the treatment variables, most patients were treated with DEXA, and the fewest patients underwent rehabilitation. Males were more likely to be treated with all forms of therapy except rehabilitation. The most frequently treated group with DEXA for females was the DMG (87.5%) and for males was the DMG (84.6%). Furthermore, most female patients treated with remdesivir (REMDE) were in the DM+OWG (44.1%) and males in NG (39.2%). Inhalation corticosteroids (ICS) and antibiotics (ANT) treatment were mostly used in males in the NG (33.3% (ICS), 38.0% (ANT)) and in females in the DM+OWG (32.4% (ICS); 39.4% (ANT)). In all groups, we found that the percentage of patients underwent rehabilitation was very low. In both sexes, the highest DMG frequencies were 13.3% in males and 11.1% in females.
Generally, hospital complications occur more frequently in females than in males. In males, we found that more myopathies (NG (25%); OWG (23.3%)) were observed than other complications during hospitalization. Among complications in females, bacterial superinfections (DMG (33.3%); DM+OWG (28.6%) prevailed.
The most prevalent PCS symptoms were dyspnea and rare chest pain. Dyspnea was most prevalent in the female NG (59.2%); however, the groups did not differ significantly. Moreover, chest pain (33.3%) and coughing (55.6%) in the female DMG and fatigue in the female DM+OWG (40%) were the highest. In males, the most prevalent symptom was dyspnea (49%), but the highest was in the DMG (53.3%).
Our study showed that, among all groups, most patients treated in the ICU belonged to the DMG for females (20%) and DM+OWG for males (19.4%). However, the lowest number of patients in the NG were treated in the ICU (9.8% males; 5.6% females). Additionally, we found that more men had a lower FVC% in the DM+OWG than in the other groups.
Summarizing the median values for our two target groups (DMG and DM+OWG), we can conclude that males in the DMG had the highest hospitalization values and FEV1% than females. On the other hand, males and females in the DM+OWG had the highest BMI, and females had the longest PCS duration. Males in the DMG had the lowest ICU and PCS duration and FVC%. Women in the DMG had the lowest ICU, hospitalization, and PCS durations, FVC%, FEV1/FVC%, and BMI.
Regarding frequencies, males in the DMG were the prevalent group for DEXA treatment, undertaking rehabilitation, and experiencing dyspnea. The females in the DMG were the prevalent group for DEXA treatment, undertaking rehabilitation, bacterial infections, chest pain, coughing, and experiencing fatigue. The least prevalent group in males included REMDE treatment, time spent in the ICU, antibiotic consumption, and complications such as embolia, thrombosis, myopathy, bacterial infection, and PCS fatigue. The least prevalent group in females also included REMDE treatment, time spent in the ICU, and additional complications, such as embolia, thrombosis, myopathy, and PCS fatigue. On the other hand, the results for males in the DM+OWG did not stand out except that it was the least prevalent group regarding undertaking rehabilitation. However, females in the DM+OWG were the most prevalent group for REMDE treatment, time spent in the ICU, consuming antibiotics, and fatigue as PCS. The group was also the least prevalent in complication thromboses and PCS, such as dyspnea, chest pain, and cough.
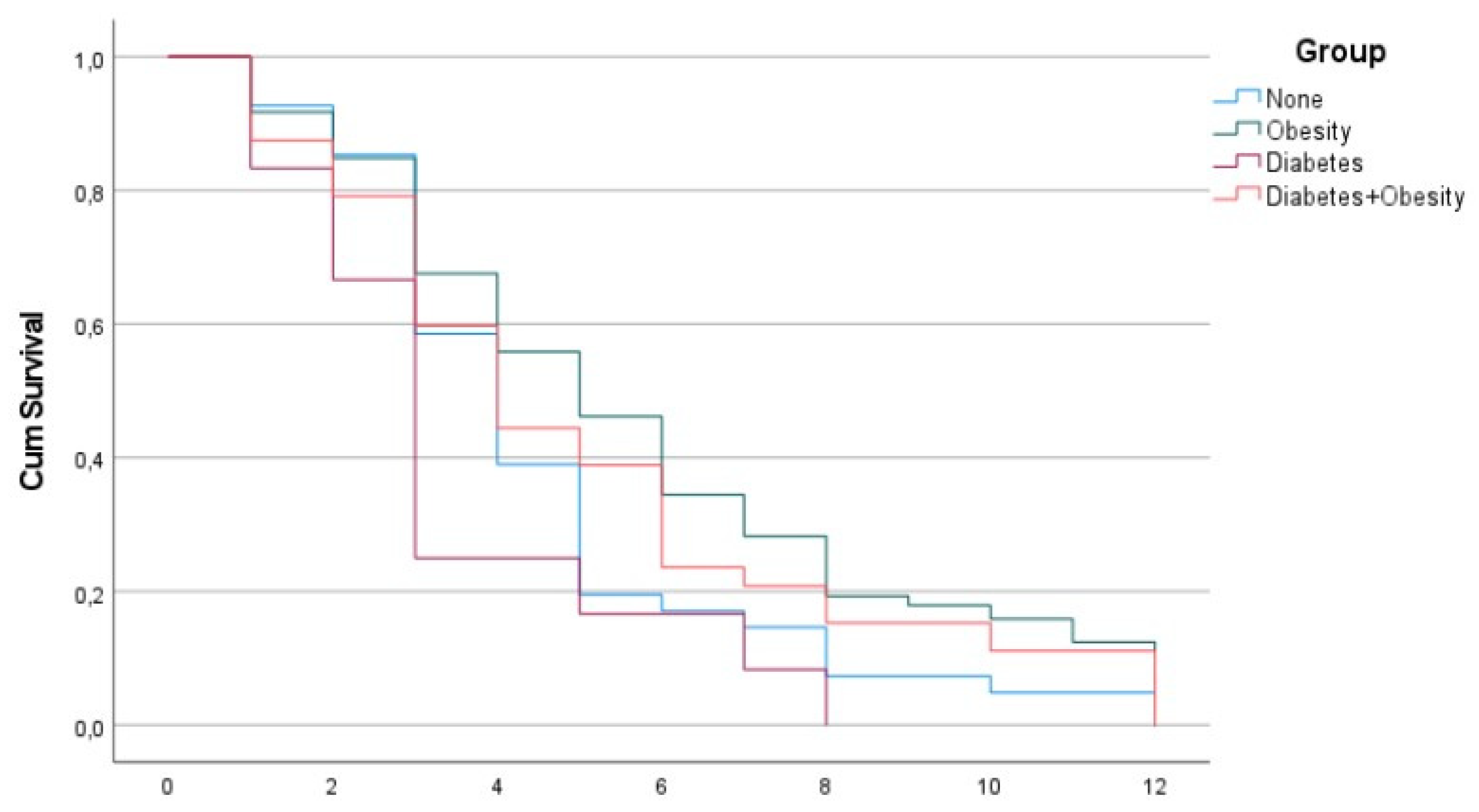
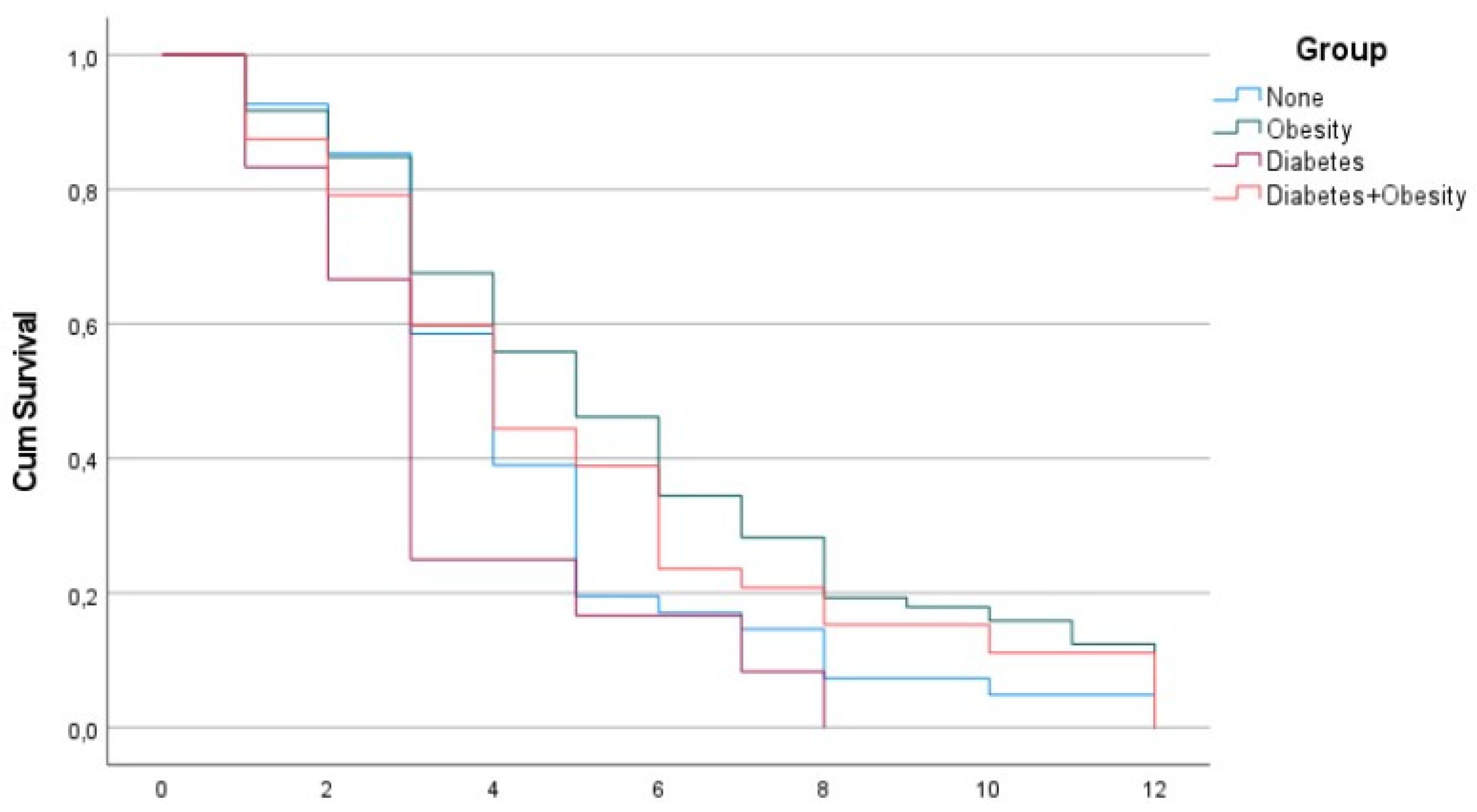
Figure 1 and
Figure 2 reveal the surprising fact that both males and females with diabetes had the shortest PCS duration, while the other groups had a PCS duration of 12 months, which is possibly beyond the current study.
4. Discussion
In our study, we found that PCS was shorter in patients who only had type 2 diabetes (DMG) when compared to the other three groups. The type 2 diabetes group (DMG), both in men and women, had the shortest duration of PCS at 3 and 4 months, respectively, and was significantly shorter than overweight males and overweight females with diabetes, which had the longest duration of PCS at 5 and 6 months, respectively. The shorter duration of PCS in patients with type 2 diabetes than in the other investigated groups in our study was unexpected.
While the vast majority of researchers mention diabetes and obesity as risk factors for the onset of PCS or its prolonged duration, some researchers disagree. Su et al. [
16] associated pre-existing type 2 diabetes with PCS in their study. Multiple early factors predict post-acute COVID-19 sequelae. Patients with diabetes have significantly more fatigue as part of PCS. Moreover, diabetes and metabolic diseases increase the risk of developing PCS [
17]. Additionally, a small study from India showed that patients with type 2 diabetes have significantly higher levels of fatigue than those with pre-diabetes [
18].
In contrast, a Spanish study that analyzed cough in PCS 1 year after COVID-19 pneumonia did not find any association with diabetes. The authors concluded that the metabolic impact of diabetes was more important in the acute phase of COVID-19 [
19]. Moreover, a meta-analysis by Sudhakar et al. [
20] collected 11 studies that linked diabetes and acute post-COVID-19 for at least 4 weeks and 14 studies that did not. Therefore, there is no clear answer to whether diabetes is an important risk factor for PCS.
The average length of treatment in the ICU in our study was 6 days for DMG-females, but DMG-males in the same group were not admitted to the ICU. This is in contrast to the general finding in our study that ICU treatment was longer in men. The lack of treatment in the ICU for the DMG-males was surprising and could indicate the possibility of survival bias, which we cannot refute with the current data. Secondary bacterial infections are common in DMG-females. There were practically no complications (myocarditis, embolisms, and thrombosis) in the type 2 diabetes group of both sexes, despite complications occurring in the other groups.
The lowest average FVC% value was found in DMG-females. This is also in contrast to the higher average FVC% values in females. A high median FEV 1% value was observed in DMG-females, but the lowest was observed in DMG-males, which agrees with the average higher general FEV 1% value for females. Additionally, the lowest FEV1/FVC% value was observed in DMG-females. However, it is difficult to explain these observations. The DMG was small, with five females and 12 males. The age of females in this group was the lowest on average (66 years), but the age of males in the type 2 diabetes group was the second highest (74.5 years. Women with type 2 diabetes were the youngest and spent the longest time in the ICU, which may indicate a higher morbidity of women in our group.
In addition, women with type 2 diabetes (DMG-females) had one of the lowest FVC% and FEV1/FVC% values, with higher FEV1% values as part of lung function monitoring. Men in the type 2 diabetes group (DMG-males) did not differ significantly in lung function parameters.
Based on analyzing data in the field of treatment, our study showed that type 2 diabetes patients had fewer chronic lung diseases such as asthma and chronic obstructive pulmonary disease (COPD) compared to the other groups. Therefore, they also needed fewer days of treatment in the ICU. We also found that type 2 diabetes patients had a more difficult course of treatment and longer hospitalization; therefore, they needed more rehabilitation time than other groups. Moreover, we found that more patients with type 2 diabetes were treated with dexamethasone compared to other groups, and the difference was especially evident in female patients. According to these results, type 2 diabetes could have more influence on the acute phase of COVID-19 disease and its early complications, including pneumonia, and less influence on PCS. However, more intensive treatment and monitoring by physicians for type 2 diabetes patients during hospitalization and rehabilitation after COVID-19 pneumonia may influence the PCS duration and shorten it compared to other groups of patients.
A possible limitation of our study is the small number of patients in the type 2 diabetes group compared to the other three groups. However, the group was statistically large enough, and non-parametric tests were used, which are less sensitive to the sample size. The second possible limitation is that we did not consider the influence of morbidity. However, considering general statistical distributions, it is very unlikely that our DMG was bipolar; that is, on one extreme, having patients who recovered fully from PCS, and on the other hand, having patients who died in the acute phase. Furthermore, our study dealt with PCS; therefore, patients who died before PCS could be detected would not have contributed to the knowledge about the long course. Moreover, our study was observational in nature, and analyzing causalities was beyond its scope [
21]. However, the study revealed some interesting phenomena that require further investigation to explain them.
Additionally, the study was relatively small in terms of collecting data on symptoms, as we collected data only for dyspnea, cough, chest pain, and fatigue. These four symptoms are crucial in PCS treatment and are in the list provided by Ballering et al. [
22].
The advantage of our research is that it is based on clinical practice in a real-world healthcare system environment, which is uniform for practically all citizens of Slovenia; therefore, the follow-up of patients in our center was systematic. Therefore, generalization of the data is probably possible for the entire country because Slovenia is a very small country and, therefore, quite uniform in most health determinants. The data were collected by a small but qualified and dedicated group of medical professionals in one center; therefore, we considered the data to be of high quality regarding their accuracy.
In our study, we found that PCS resolution was shorter in patients who only had type 2 diabetes compared to the other three groups, namely, only overweight and obese, with overweight/obesity and type 2 diabetes, and without these comorbidities. The outcomes of our study are comparable to those of similar studies performed worldwide, which might be because COVID-19 management in Slovenia did not differ from that in other countries. We currently do not know how to explain the above finding, but we would like to draw attention to it and encourage studies that would more precisely define and perhaps explain this observed fact. Thus, further research will be dedicated to the influence of type 2 diabetes treatment and rehabilitation on the duration of PCS as well as other pathological mechanisms that might affect the duration of PCS.
Author Contributions
S.K., H.B.V. and P.K. designed the research methodology, S.K., N.S. and B.A.S. performed the study and collected data, S.K., H.B.V., P.K., J.Z. and M.Z. prepared the manuscript, P.K. performed statistical analysis, all authors contributed to the results interpretation and tables and additionally reviewed the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee Community Healthcare Center dr. Adolf Drolc Maribor (Zdravstveni dom dr. Adolfa Drolca Maribor) – 02/010/03-002/01/22.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to data privacy restrictions.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and diabetes as high-risk factors for severe. Diabetes/Metabolism Research and Reviews 2021, 37, e3377. [Google Scholar] [CrossRef] [PubMed]
- Südy, R.; Peták, F.; Kiss, L.; Balogh, A.L.; Fodor, G.H.; Korsós, A.; et al. Obesity and diabetes: similar respiratory mechanical but different gas exchange defects. Am. J. Physiol. Cell. Mol. Physiol. 2021, 320, L368–L376. [Google Scholar] [CrossRef] [PubMed]
- Zerah, F.; Harf, A.; Perlemuter, L.; Lorino, H.; Lorino, A.-M.; Atlan, G. Effects of Obesity on Respiratory Resistance. Chest 1993, 103, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Schachter, L.M.; Salome, C.M.; Peat, J.K.; Woolcock, A.J. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax 2001, 56, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D.; Jones, R.L.; Man, S.F.P. Obesity Is a Risk Factor for Dyspnea but Not for Airflow Obstruction. Arch. Intern. Med. 2002, 162, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Arora, A.; Sharma, P.; Anikhindi, S.A.; Bansal, N.; Singla, V.; Khare, S.; Srivastava, A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 535–545. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Cozma, D.; Kamel, M.; Hamad, M.; Mohammad, M.G.; Khan, N.A.; et al. Long-COVID, Metabolic and Endocrine Disease. Hormone and Metabolic Research 2022, 54, 562–566. [Google Scholar] [CrossRef]
- Menezes, A.S.; Botelho, S.M.; Santos, L.R.; Rezende, A.L. Acute COVID-19 Syndrome Predicts Severe Long COVID-19: An Observational Study. Cureus 2022, 14, e29826. [Google Scholar] [CrossRef]
- Sanoudou, D.; Hill, M.A.; Belanger, M.J.; Arao, K.; Mantzoros, C.S. Editorial: Obesity, metabolic phenotypes and COVID-19. Metabolism Clinical and Experimental 2022, 128, 155121. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Misra, A. Post COVID-19 Syndrome (“Long COVID”) and Diabetes: Challenges in Diagnosis and Management. Diabetes Metabolic Syndrome Clinical Research Reviews 2021, 15, 102235. [Google Scholar] [CrossRef]
- Lacavalerie, M.R.; Pierre-Francois, S.; Agossou, M.; Inamo, J.; Cabie, A.; B, J.L.; et al. Bese patients with long COVID-19 display abnormal hyperventilatory response and impaired gas exchange at peak exercise. Future Cardiology 2022, 18, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Palma, G.; Sorice, G.P.; Genchi, V.A.; Giordano, F.; Caccioppoli, C.; D’oria, R.; Marrano, N.; Biondi, G.; Giorgino, F.; Perrini, S. Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity. Int. J. Mol. Sci. 2022, 23, 7349. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Sanfilippo, T.; Guggino, R.; Scalisi, L.; Monastero, R.; Baschi, R.; Mandalà, V.; Biagio, L.S.; Rizzo, M.; Giacomazza, D.; et al. Neurological Consequences, Mental Health, Physical Care, and Appropriate Nutrition in Long-COVID-19. Cell. Mol. Neurobiol. 2022, 43, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Patrono, C. Increased risk of incident diabetes in patients with long COVID. Eur. Hear. J. 2022, 43, 2094–2095. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wang, L.; Zhou, F.; Li, J.; Liu, Y.; Yang, S. Long-term effects of obesity on COVID-19 patients discharged from hospital. Immunity, Inflamm. Dis. 2021, 9, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895. [Google Scholar] [CrossRef]
- Steenblock, C.; Hassanein, M.; Khan, E.G.; Yaman, M.; Kamel, M.; Barbir, M.; Lorke, D.E.; Rock, J.A.; Everett, D.; Bejtullah, S.; et al. Diabetes and COVID-19: Short- and Long-Term Consequences. Horm. Metab. Res. 2022, 54, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Mittal, J.; Ghosh, A.; Bhatt, S.P.; Anoop, S.; Ansari, I.A.; Misra, A. High prevalence of post COVID-19 fatigue in patients with type 2 diabetes: A case-control study. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102302. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Guijarro, C.; Plaza-Canteli, S.; Hernández-Barrera, V.; Torres-Macho, J. Prevalence of Post-COVID-19 Cough One Year After SARS-CoV-2 Infection: A Multicenter Study. Lung 2021, 199, 249–253. [Google Scholar] [CrossRef]
- Sudhakar, M.; Winfred, S.B.; Meiyazhagan, G.; Venkatachalam, D.P. Mechanisms contributing to adverse outcomes of COVID-19 in obesity. Mol. Cell. Biochem. 2022, 477, 1155–1193. [Google Scholar] [CrossRef]
- Gilmartin-Thomas, J.F.; Liew, D.; Hopper, I. Observational studies and their utility for practice. Aust. Prescr. 2018, 41, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Ballering, A.V.; van Zon, S.K.R.; Hartman, T.C.O.; Rosmalen, J.G.M. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 2022, 400, 452–461. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).