Introduction
In the context of COVID-19, patients with severe or critical illness may be more susceptible to developing secondary bacterial infections. Several meta-analysis studies have found a prevalence of bacterial coinfection in hospitalized patients ranging from 3.5% to 12% (1)(2). The risk of coinfection increases in patients requiring admission to critical care units, reaching up to 14%-23% (2)(3). In most published observational studies and systematic reviews, the timing of bacterial infection diagnosis is not reported, so the term "coinfection" does not discriminate between community-acquired or nosocomial acquisition in these studies. However, in a study describing the time since admission (4) of the rate of nosocomial bacterial superinfections found was similar to those published by other studies that did not consider this factor.
The immunosuppressive treatment used for managing hospitalized COVID-19 patients has been considered a potential risk factor for developing nosocomial-acquired infections. In the current management of patients admitted with COVID-19, dexamethasone and tocilizumab have a strong recommendation level in guidelines (5). The association of tocilizumab treatment with the occurrence of bacterial infections was previously known (6). The available data to the date regarding the influence of tocilizumab on the risk of superinfection in COVID-19 are controversial. Stone et al (7) did not find a higher incidence of superinfections in patients treated with tocilizumab, but in their series, most of these patients did not receive steroids. In the cohort by Ripa et al (8), in the subgroup of patients who received immunosuppressive biological therapy, 14% presented at least one secondary infection compared to 9% of the overall population, with a median time from the start of therapy of 9 days; although in this cohort, only 22% of the included patients had received steroids. However, the meta-analysis by Tleyjeh IM. et al (9) and the series by Narain et al. (10) associate the use of the combination of tocilizumab and steroids with a higher incidence of superinfections. In the meta-analysis published by Peng et al., a significant increase in fungal infections was noted after the use of tocilizumab (11), although it was not associated with an increase in bacterial infections.
Given that there are no consistent data to support an appropriate recommendation regarding the use of antibiotics in patients without a clinically suspected or documented infection (5), antibiotic prophylaxis is not routinely recommended in hospitalized patients with COVID-19. However, it could be considered in patients with risk factors for secondary bacterial infections.
In this study, we examined the relationship between the use of prophylactic antibiotic therapy and bacterial or fungal isolates following the administration of tocilizumab in a cohort of hospitalized COVID-19 patients who had previously received steroids during the first and second waves of the pandemic in Spain.
Study design and patients
This retrospective observational study was performed at Hospital Central de la Defensa “Gómez Ulla”,Madrid (Spain), a 500-bed university center that provides broad and specialized medical, surgical and intensive care for an urban population of 120,000 individuals. We included all patients of 14 years or older, hospitalized in the conventional ward and/or Intensive Care Unit (ICU) between 31 January and 6 December 2020 (first and second waves of the pandemic in Spain) with a clinical diagnosis of COVID-19,confirmed by real-time reverse transcription PCR for SARS-CoV-2 in respiratory samples, who received tocilizumab during hospitalization. The study was approved by the Ethics and Research Committee of the Study Hospital with code 25/20. Participants did not sign informed consent as it was a retrospective study, and there was no temporal overlap between their admission and data collection.
Data collection and outcomes
Using the off-guide drug dispensing software of the Hospital Pharmacy Department, we obtained the number of medical records of all patients who had been treated with at least one 400 mg dose of tocilizumab during the study period.
For all patients hospitalized with COVID-19 who met the inclusion criteria, data concerning demographics (age, gender), epidemiology, comorbidities, laboratory tests, microbiologic results (blood and urine cultures, respiratory samples, urinary antigen tests and antimicrobial susceptibility), treatment and outcomes (intensive care unit admission, length of hospital stay and mortality) were collected directly from electronic health records and drug dispensing software.
The records of all patients with positive microbiologic results were reviewed by two clinics with specific training in infectious diseases researchers (MEM and JMN) to assess clinical significance. Microbiological isolates considered contaminants by microbiological or clinical criteria were excluded.
Procedures
Investigation of bacterial and fungal pathogens in blood, normally sterile fluids, sputum and other samples was performed with standard microbiologic procedures during hospitalization, as requested by the attending physician. Microbiological isolates within 14 days following the administration of tocilizumab were considered. Antibiotic prophylaxis was considered in patients who had received an antibiotic prescribed before or following the tocilizumab infusion with the aim of prophylaxis against bacterial superinfection. Concomitant treatment with steroids was considered in patients who had received, at least one dose of dexamethasone equal to or greater than 6 mg/day or its equivalent before the tocilizumab infusion. Coinfection was considered if the microbiological identification occurred within the first 48 hours of admission. Superinfection was considered if the isolate corresponded to a sample obtained at least 48 hours after hospital admission, and if, according to the clinical history data, there was clinical suspicion of infection prior to the communication of the isolate, and/or the responsible clinician had prescribed targeted antibiotic therapy against the isolated microorganism. The appearance of multidrug-resistant (MDR) organisms was analyzed: carbapenem-resistant Acinetobacter baumannii (CRAB), carbapenem-resistant Enterobacteriaceae (CRE), extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, vancomycin-resistant enterococci (VRE), methicillin-resistant Staphylococcus aureus (MRSA), and carbapenem-resistant Pseudomonas aeruginosa (CPE).
Charlson comorbidity index (12) and SEIMC score (13), a prognostic scale that evaluates the risk of 30-day mortality based on parameters measured upon admission to the Emergency Department, were calculated for all patients included.
Statistical analysis
Median and interquartile range were used for quantitative variables. Absolute frequencies and relative frequencies in percentages (%) were used for qualitative variables. Hypothesis testing was conducted using Fisher's exact test and the Mann-Whitney test. A p-value of less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS® version 25 software.
Results
During the study period, a total of 2,069 COVID-19 patients were admitted to our hospital. Among these patients, 76 had a prescription for tocilizumab in the drug dispensation records of the Hospital Pharmacy Service. After reviewing the medical records, 6 patients who finally didn’t receive the drug were excluded, resulting in a final study population of 70 patients.
Comorbidities and risk at admission
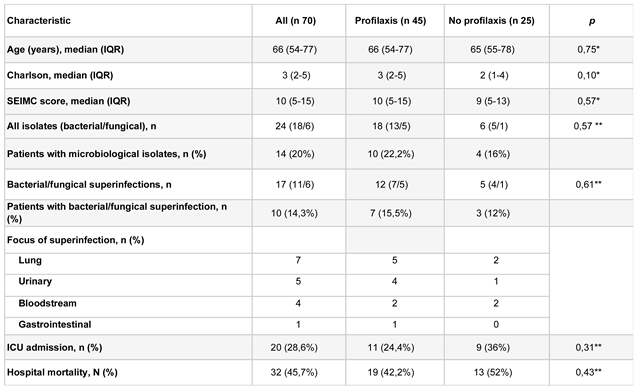
The 70 patients had a confirmed diagnosis of COVID-19 with a positive SARS-CoV-2 PCR test and were receiving steroid treatment at the time of tocilizumab administration. The median age was 66 years (IQR 54 - 77 years). The median Charlson index was 2 (IQR 2 - 5), and the median SEIMC score was 10 (interquartile range 5 - 15). Twenty patients (28.6%) required admission to the ICU, and 32 (45.7%) died during hospitalization. All patients received tocilizumab either in the Emergency Department or on the joint COVID-19 standard hospitalization wards. A total of 20 patients (28.5%) were subsequently admitted to the intensive care unit.
Forty-five patients (64.3%) received antibiotic prophylaxis. Age and SEIMC score at admission were analyzed, and no statistically significant differences were found in relation to receiving antibiotic prophylaxis. However, there was a tendency for patients who received antibiotic prophylaxis to have a higher Charlson index (
Table 1).
A 24.4% of patients who required antibiotic prophylaxis ended up being admitted to the ICU compared to 36% of patients who did not receive prophylaxis. However, no statistically significant differences were found. There were also no statistically significant differences in mortality between the two subgroups (
Table 1).
Prophylaxis used and microbiological results
Out of the 45 patients who received prophylaxis, 10 patients (22%) had significant microbiological isolates interpreted as colonization or infection. Out of the 25 patients who did not receive prophylaxis, 4 patients (16%) had significant microbiological isolates (
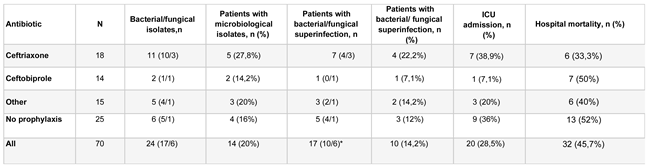
Table 1). The most commonly used antibiotic prophylaxis were ceftriaxone and ceftobiprole. Other antibiotics used alone or in combination included piperacillin plus tazobactam, teicoplanin, meropenem, linezolid, ciprofloxacin, amoxicillin plus clavulanic acid, and cefepime. Subgroups with the two most commonly used prophylaxes, ceftriaxone and ceftobiprole, were analyzed independently (
Table 2). No patient had a documented coinfection or superinfection prior to the administration of tocilizumab.
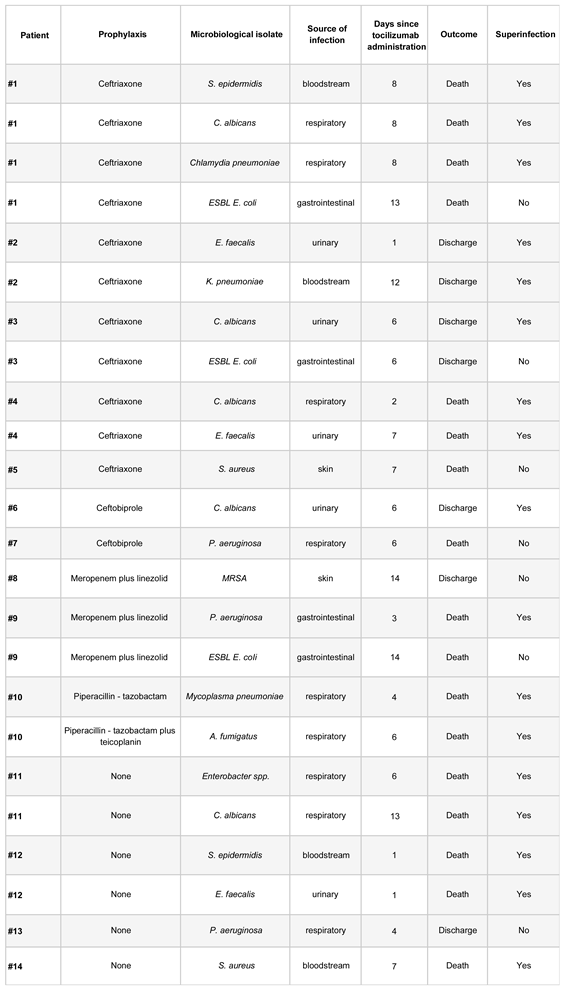
Four samples with growth of multidrug-resistant organisms were identified, and all of these samples were from patients who had received antibiotic prophylaxis (
Table 3). The four isolates with growth of multidrug-resistant organisms were interpreted as colonization.
The most frequently isolated microorganisms were Candida albicans (n 5), Enterococcus faecalis (n 3), Staphylococcus aureus (n 3), and Staphylococcus epidermidis (n 2). No statistically significant differences were found in isolates, colonization, ICU admissions, or mortality among the different studied prophylaxis groups.
Discussion
In this study, we describe the incidence and microbiological characteristics of bacterial and fungal colonizations/infections identified in patients hospitalized for COVID-19 and treated with steroids, following tocilizumab treatment, based on the use of antibiotic prophylaxis. In our cohort, 60.3% of the patients received antibiotic prophylaxis, mainly with ceftriaxone and ceftobiprole. There were no significant clinical differences between patients based on the use of prophylaxis, with a trend towards a higher number of comorbidities in the subgroup that received antibiotic prophylaxis. There were no significant differences between the two patient subgroups regarding the number of clinically diagnosed infections. However, in almost half of the patients who received antibiotic prophylaxis, colonization by a multidrug-resistant organism was identified, compared to no isolation with growth of a multidrug-resistant organism in the subgroup that did not receive antibiotic prophylaxis.
This work, to our knowledge, represents the first published study on the effects of prior antibiotic therapy before the administration of the combination of tocilizumab and steroids in COVID-19 patients, aiming to prevent the development of clinical infections or promote colonization. One of the likely reasons for the lack of literature on this topic is the difficulty in obtaining a significant number of patients, as evidenced in this study, where only 3.4% of the patients admitted during the study period met the inclusion criteria. 64.3% of the included patients received prophylaxis, with 11 different antibiotics, some of them in variable combinations, making it difficult to draw statistically significant differences. However, precisely because of the difficulty and lack of published data on this clinical question that arises in the daily practice of infectious disease units and intensive care units worldwide, the analysis of this cohort is relevant for two reasons. First, to attempt to draw conclusions that help discern the approach to prescribing antibiotic therapy or not in these patients. Second, to serve as a basis and stimulus for scientific societies and international study groups to analyze existing multicenter cohorts of COVID-19 patients, in search of a larger number of patients to identify statistically significant trends.
Possible biases and confounding factors are also presented. Bacterial superinfections might be underdiagnosed for several reasons: difficulties in sample collection due to overwhelming hospital services during the first wave of the pandemic ((14), a high number of bacterial pneumonias where microbiological diagnosis is not achieved, as is well-known in routine clinical practice (15)(16), and the non-use of PCR diagnostic techniques for bacterial identification in the analyzed samples. Another potential limitation of the study, given its observational nature, is that the risk of developing an infection might have been higher in the group of patients who received prophylaxis, and therefore, despite finding similar results between the two patient subgroups, this could be interpreted as a protective role of antibiotic prophylaxis. Interestingly, although not reaching statistically significant differences, the group of patients who received antibiotic prophylaxis showed a significant trend towards having more pre-existing comorbidities before the onset of the clinical condition (12)(17).
40% of patients who received prophylaxis were treated with ceftriaxone, and 31.3% with ceftobiprole, allowing for some consequential case description. This is particularly important as these are two antimicrobials with different, and in the case of ceftobiprole, broader spectrum of action. Patients who received prophylaxis with ceftriaxone had a higher incidence of superinfection (22.2%) compared to the subgroup of patients who received prophylaxis with ceftobiprole (7.1%), and to the subgroup that did not receive prophylaxis (12%). We could infer that an ineffective empirical antibiotic therapy, without coverage of some bacteria that most frequently superinfect immunosuppressed COVID-19 patients (methicillin-resistant Staphylococcus aureus, Pseudomonas, Enterococcus faecalis) (4)(18)(19)(20), could alter the patients' commensal flora, thereby creating an ecological niche for these pathogens to subsequently infect patients more easily than would have occurred without unnecessary prior antibiotic therapy. This would explain cases like that of patient #1, with up to four isolations, where three of them could be interpreted as colonization, detected within 14 days after tocilizumab infusion. In fact, in this study, we did not find any superinfections caused by Streptococcus pneumoniae, despite it being another of the most frequently identified bacteria causing superinfections in COVID-19 (4)(18)(19)(20).
Reviewing the patients who received other antibiotic therapies, the isolations detected in patients treated with the combination of meropenem and linezolid (#8 and #9) are striking. Despite these patients receiving prophylaxis that, in theory, should have eliminated non-multidrug-resistant Pseudomonas aeruginosa as in the case of meropenem (21)(22), and methicillin-resistant Staphylococcus aureus, including linezolid (23), a colonization by methicillin-resistant Staphylococcus aureus (patient #8) and a superinfection by Pseudomonas aeruginosa were detected. In patients treated with ceftobiprole, no isolates of bacteria included in its microbiological coverage were identified. In the group of patients who received prophylaxis with ceftriaxone, only one patient presented a microbiological isolate that was initially covered by the bacterial spectrum of the chosen antibiotic prophylaxis (patient #4, bacteremia caused by ceftriaxone-sensitive Klebsiella pneumoniae).
Regarding fungal infections, the heterogeneity of the data complicates the interpretation of the results. Fungal isolates (Candida albicans and Aspergillus fumigatus) are found in both patient subgroups analyzed with respect to the use of prophylaxis, with no significant trends observed in either case. Therefore, it is not observed that the alteration of the patient's commensal flora by antibiotic therapy predisposes to fungal colonization and/or superinfection in this aspect.
In conclusion, in our cohort of patients hospitalized for COVID-19, treated with steroids, and receiving tocilizumab, the use of antibiotic prophylaxis did not reduce the incidence of secondary bacterial infections. However, after the administration of prophylaxis, patients showed an increase in the incidence of colonization by multidrug-resistant germs.
Transparency Declaration
Authors report no conflicts of interest relevant to this editorial.
Statement
The results of this manuscript were partially presented at IdWeek 2021.
References
- Calderon M, Gysin G, Gujjar A, McMaster A, King L, Comandé D, et al. Bacterial co-infection and antibiotic stewardship in patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 9 de enero de 2023;23(1):14. [CrossRef]
- Soltani S, Faramarzi S, Zandi M, Shahbahrami R, Jafarpour A, Akhavan Rezayat S, et al. Bacterial coinfection among coronavirus disease 2019 patient groups: an updated systematic review and meta-analysis. New Microbes New Infect. septiembre de 2021;43:100910. [CrossRef]
- Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. agosto de 2020;81(2):266-75. [CrossRef]
- Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. enero de 2021;27(1):83-8. [CrossRef]
- Bartoletti M, Azap O, Barac A, Bussini L, Ergonul O, Krause R, et al. ESCMID COVID-19 living guidelines: drug treatment and clinical management. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. febrero de 2022;28(2):222-38. [CrossRef]
- Morel J, Constantin A, Baron G, Dernis E, Flipo RM, Rist S, et al. Risk factors of serious infections in patients with rheumatoid arthritis treated with tocilizumab in the French Registry REGATE. Rheumatol Oxf Engl. 1 de octubre de 2017;56(10):1746-54. [CrossRef]
- Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 10 de diciembre de 2020;383(24):2333-44. [CrossRef]
- Ripa M, Galli L, Poli A, Oltolini C, Spagnuolo V, Mastrangelo A, et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. marzo de 2021;27(3):451-7.
- Tleyjeh IM, Kashour Z, Damlaj M, Riaz M, Tlayjeh H, Altannir M, et al. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. febrero de 2021;27(2):215-27.
- Narain S, Stefanov DG, Chau AS, Weber AG, Marder G, Kaplan B, et al. Comparative Survival Analysis of Immunomodulatory Therapy for Coronavirus Disease 2019 Cytokine Storm. Chest. marzo de 2021;159(3):933-48. [CrossRef]
- Peng J, Fu M, Mei H, Zheng H, Liang G, She X, et al. Efficacy and secondary infection risk of tocilizumab, sarilumab and anakinra in COVID-19 patients: A systematic review and meta-analysis. Rev Med Virol. mayo de 2022;32(3):e2295. [CrossRef]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-83. [CrossRef]
- Berenguer J, Borobia AM, Ryan P, Rodríguez-Baño J, Bellón JM, Jarrín I, et al. Development and validation of a prediction model for 30-day mortality in hospitalised patients with COVID-19: the COVID-19 SEIMC score. Thorax. septiembre de 2021;76(9):920-9. [CrossRef]
- Condes E, Arribas JR, COVID19 MADRID-S.P.P.M. group. Impact of COVID-19 on Madrid hospital system. Enfermedades Infecc Microbiol Clin Engl Ed. mayo de 2021;39(5):256-7. [CrossRef]
- Burman LA, Trollfors B, Andersson B, Henrichsen J, Juto P, Kallings I, et al. Diagnosis of pneumonia by cultures, bacterial and viral antigen detection tests, and serology with special reference to antibodies against pneumococcal antigens. J Infect Dis. mayo de 1991;163(5):1087-93. [CrossRef]
- Reimer LG, Carroll KC. Role of the microbiology laboratory in the diagnosis of lower respiratory tract infections. Clin Infect Dis Off Publ Infect Dis Soc Am. marzo de 1998;26(3):742-8. [CrossRef]
- Vaquero-Herrero MP, Ragozzino S, Castaño-Romero F, Siller-Ruiz M, Sánchez González R, García-Sánchez JE, et al. The Pitt Bacteremia Score, Charlson Comorbidity Index and Chronic Disease Score are useful tools for the prediction of mortality in patients with Candida bloodstream infection. Mycoses. octubre de 2017;60(10):676-85. [CrossRef]
- Westblade LF, Simon MS, Satlin MJ. Bacterial Coinfections in Coronavirus Disease 2019. Trends Microbiol. octubre de 2021;29(10):930-41. [CrossRef]
- Puzniak L, Finelli L, Yu KC, Bauer KA, Moise P, De Anda C, et al. A multicenter analysis of the clinical microbiology and antimicrobial usage in hospitalized patients in the US with or without COVID-19. BMC Infect Dis. 27 de febrero de 2021;21(1):227. [CrossRef]
- Zamora-Cintas MI, López DJ, Blanco AC, Rodriguez TM, Segarra JM, Novales JM, et al. Coinfections among hospitalized patients with covid-19 in the first pandemic wave. Diagn Microbiol Infect Dis. noviembre de 2021;101(3):115416. [CrossRef]
- Unal S, Garcia-Rodriguez JA. Activity of meropenem and comparators against Pseudomonas aeruginosa and Acinetobacter spp. isolated in the MYSTIC Program, 2002-2004. Diagn Microbiol Infect Dis. diciembre de 2005;53(4):265-71. [CrossRef]
- Linden P. Safety profile of meropenem: an updated review of over 6,000 patients treated with meropenem. Drug Saf. 2007;30(8):657-68. [CrossRef]
- Bouza E, Muñoz P. Linezolid: pharmacokinetic characteristics and clinical studies. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2001;7 Suppl 4:75-82.
Table 1.
Characteristics of study population.
Table 1.
Characteristics of study population.
Table 2.
Outcomes according to antimicrobial prophylaxis prior to tocilizumab.
Table 2.
Outcomes according to antimicrobial prophylaxis prior to tocilizumab.
Table 3.
Description of microbiological isolates.
Table 3.
Description of microbiological isolates.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).