1. Introduction
Spinal cord injury (SCI) induces local neural cell death and the disruption of axonal pathways, which potentially causes devastating neurological damage to patients. However, neurological recovery is considered to be limited after SCI because the central nervous system (CNS) environment does not generally permit regeneration.[
1,
2] To compensate for local spinal cord damage, cell transplantation has emerged as a potential therapeutic approach.[
3] Among many cell sources for regenerative medicine, the unique characteristics of multilineage-differentiating stress-enduring (Muse) cells, which are endogenous pluripotent stem cells, have attracted attention.[
4] Muse cells exist in mesenchymal tissues, including bone marrow and adipose and dermal tissues. Since Muse cells express the pluripotent surface marker, stage-specific embryonic antigen-3, they may be harvested and cultured as a source for regenerative medicine.[
5] Muse cells exhibit low telomerase activity and are rarely tumorigenic.[
6]
We recently performed experimental SCI studies using CL2020 (Life Science Institute, Inc., Tokyo, Japan), which is a Muse cell-based product produced from human mesenchymal stem cells. When CL2020 was intravenously administered one day after SCI, spinally injured animals showed better functional recovery from their neurological deficits.[
7] We demonstrated that Muse cells in CL2020 recognized and migrated to the injured site. Furthermore, Muse cells differentiated into neuronal cells and exerted neuroprotective and regenerative effects in acute SCI.
In the present study, we administered CL2020 2 weeks after SCI, which we called the subacute SCI model. We examined the behavior of Muse cells following their intravenous administration in the subacute stage after SCI. Behavioral and histological evaluations were performed to confirm the therapeutic effects of CL2020 against subacute SCI.
2. Results
2.1. Intravenous CL2020 improves hindlimb locomotor functions after subacute SCI
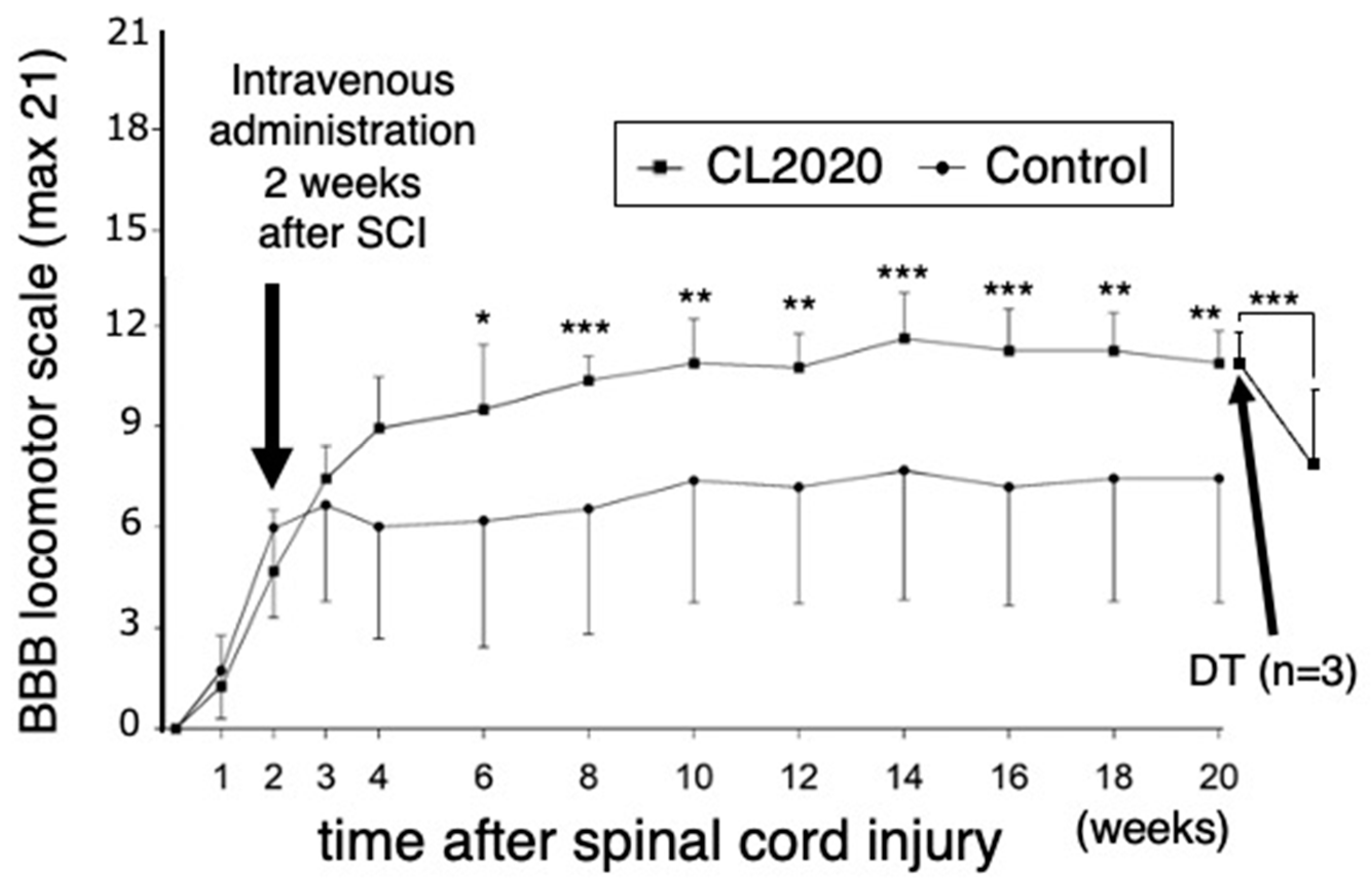
Rats received CL2020 two weeks after SCI. BBB locomotor scores for the CL2020 and Control groups were determined each week for 4 weeks after injury and every other week thereafter for 20 weeks. Improvements in hind limb motor function were significantly greater in CL2020 group than in Control group after 6 weeks (p < 0.05) and over 8-20 weeks (p < 0.01 or 0.001, repeated measures ANOVA followed by Bonferroni post hoc test,
Figure 1). Twenty weeks after the injury, average BBB scores were 10.9 ± 0.96 and 7.4 ± 3.1 in the CL2020 and Control groups, respectively (p < 0.01,
Figure 1); treated animals could support its weight on its hindlimbs and take plantar steps. There were also occasional instances of coordination between the forelimbs and hindlimbs, signaling a progression in the recovery process.
2.2. CL2020 prevented spinal cord damage and contributed to structural preservation
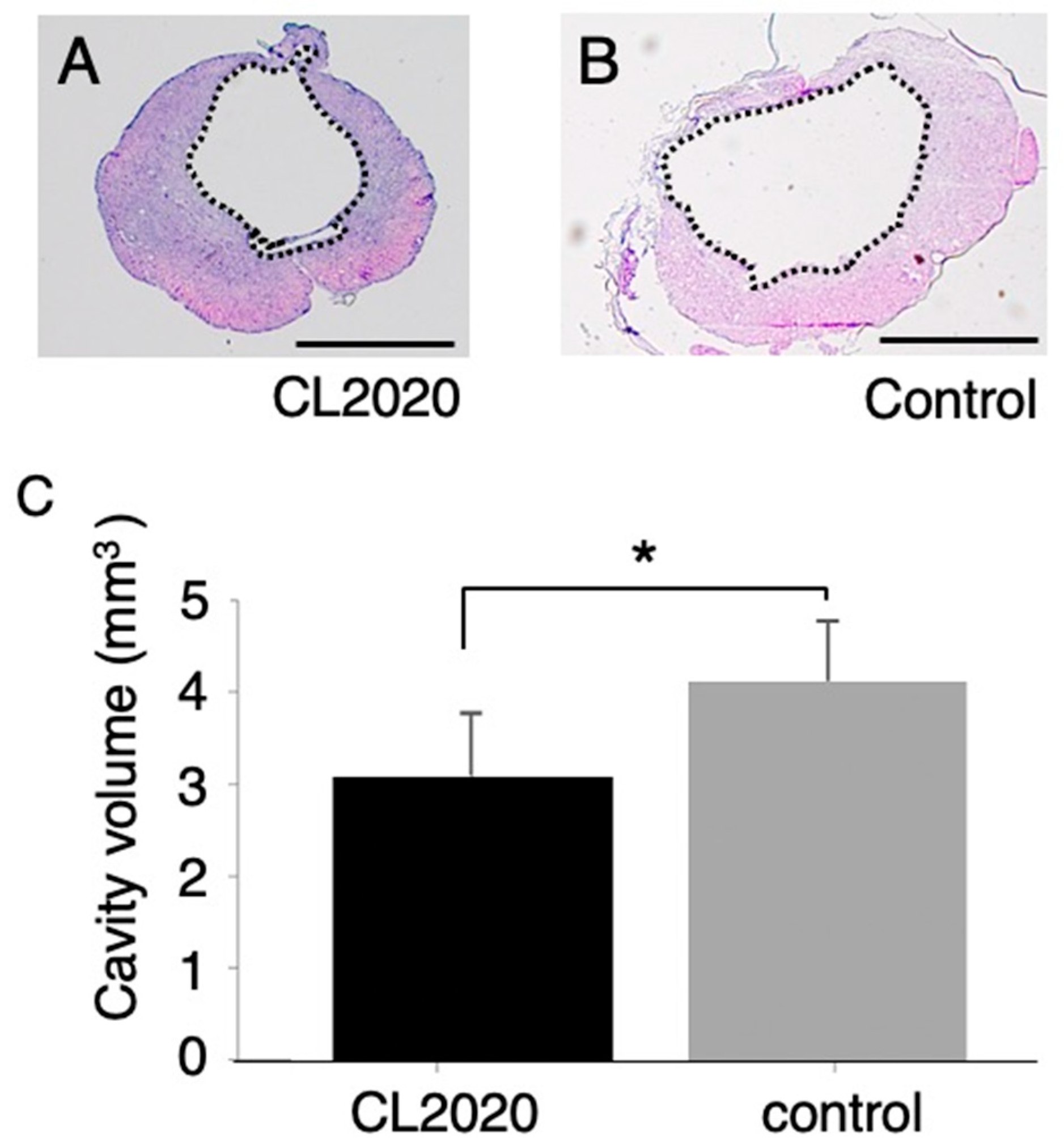
The volumes of the cystic cavity in rats treated with CL2020 were significantly smaller than those in the Control group (p < 0.05,
Figure 2). The average volumes of the cavity were 3.1 ± 0.69 and 4.1 ± 0.64 mm
3 in the CL2020 and Control groups, respectively.
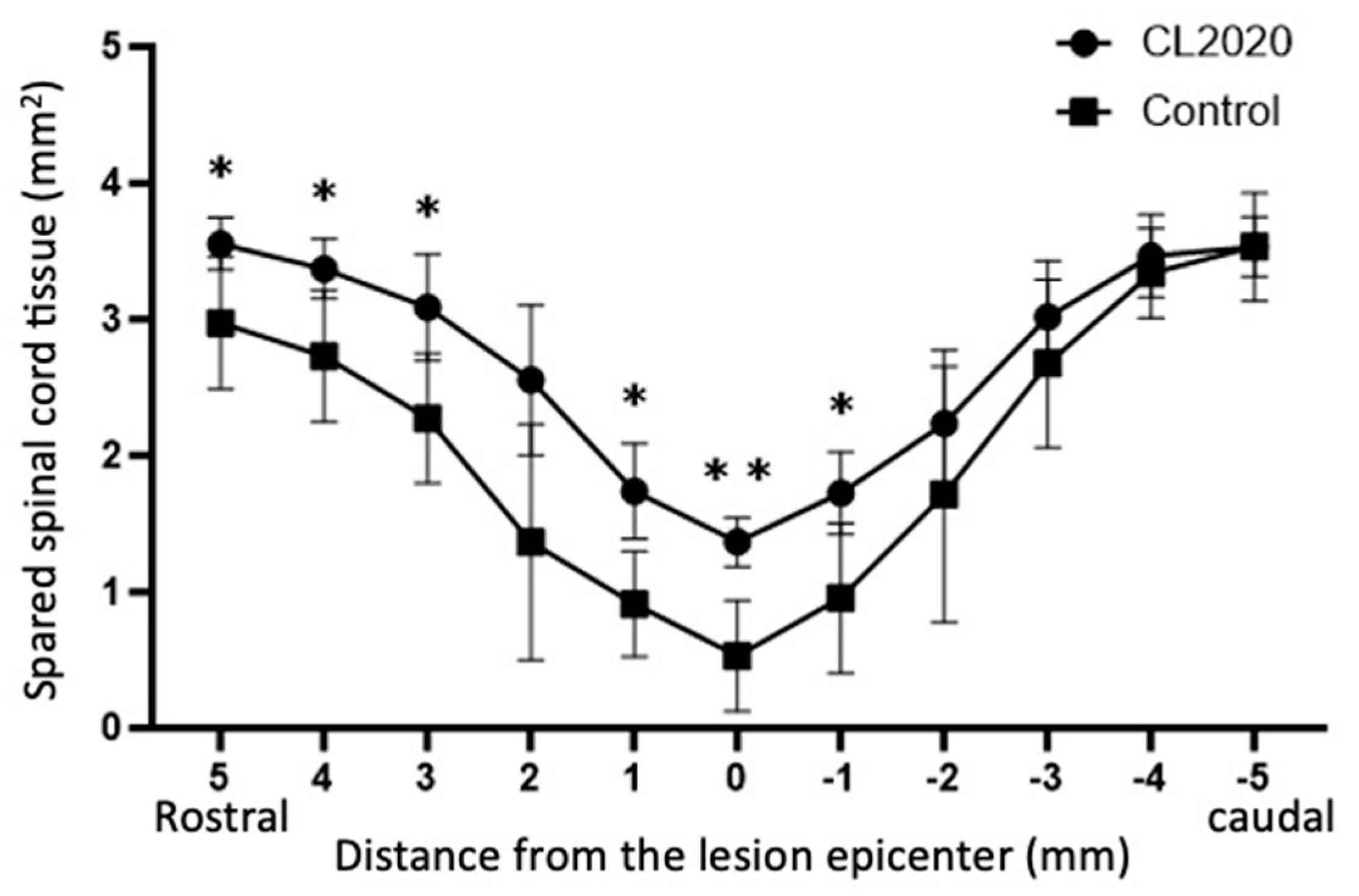
The areas of the spared spinal cord tissue were significantly larger than those of the Control group 20 weeks after the injury (
Figure 3). It was noted that the larger areas of the spinal cord were preserved in the CL2020 group than in the control group in 5, 4, 3 and 1 mm rostral and 1mm caudal to the lesion epicenter.
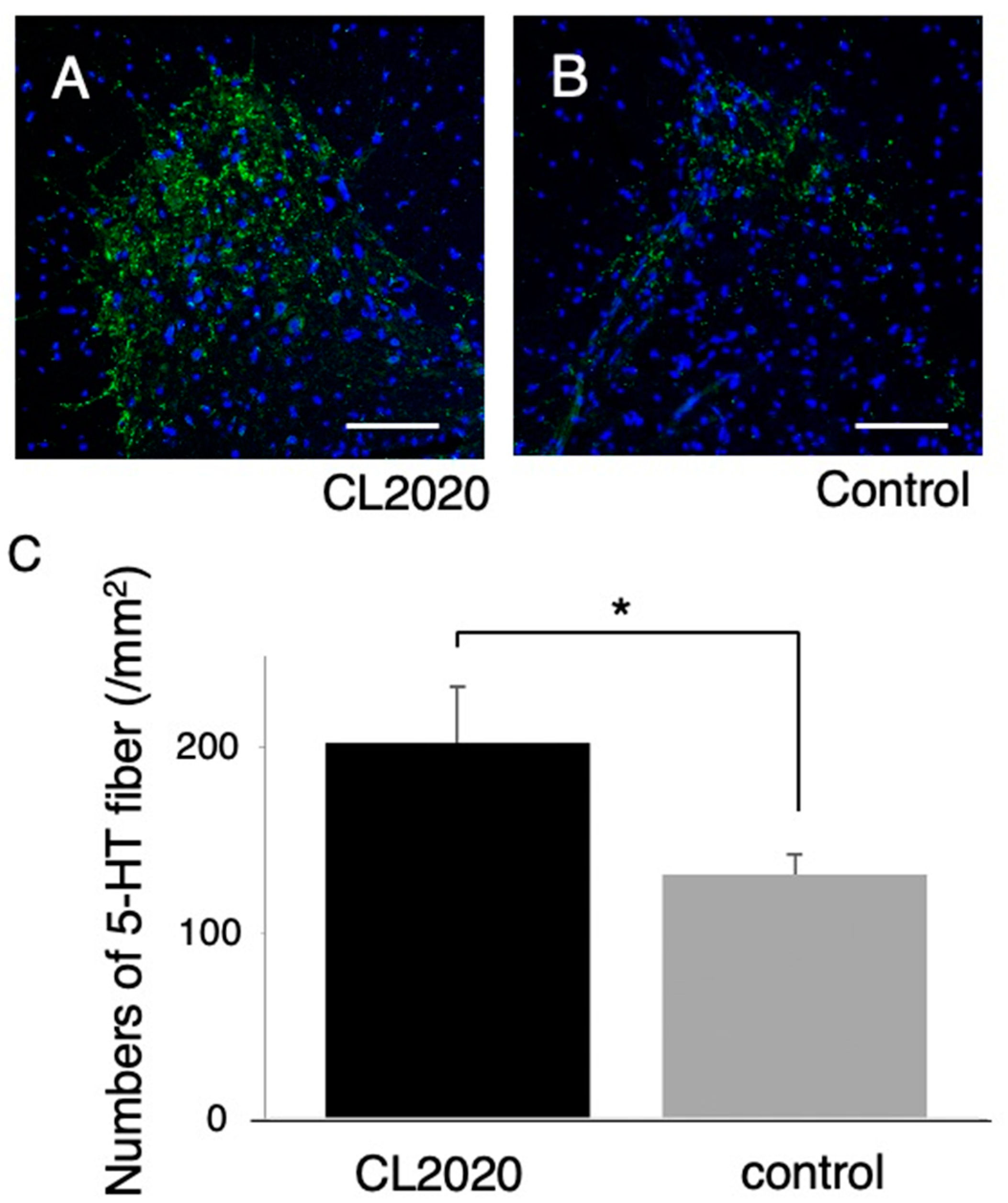
Furthermore, larger numbers of 5-HT-positive axons were preserved in ventral horn of the caudal spinal cord in the CL2020 group (p <0.05, Figure 4). These results suggest that Muse cells in CL2020 exerted neuroprotection in the injured spinal cord.
Figure 4.
5-HT immunostaining. A and B, Representative figures of axial spinal cord sections 3 mm distal to the injury in the CL2020 (A) and Control groups (B) in the ventral horn of the spinal cord. 5-HT-positive fibers are stained green. Scale bars = 100 µm. C. A graph showing that the number of preserved 5-HT-positive fibers was larger in the CL2020 group (black) than in the Control group (gray) (*: p < 0.05). The figure is available in color online only.
Figure 4.
5-HT immunostaining. A and B, Representative figures of axial spinal cord sections 3 mm distal to the injury in the CL2020 (A) and Control groups (B) in the ventral horn of the spinal cord. 5-HT-positive fibers are stained green. Scale bars = 100 µm. C. A graph showing that the number of preserved 5-HT-positive fibers was larger in the CL2020 group (black) than in the Control group (gray) (*: p < 0.05). The figure is available in color online only.
2.3. CL2020 engrafts and differentiates into neuronal cells in the injured spinal cord
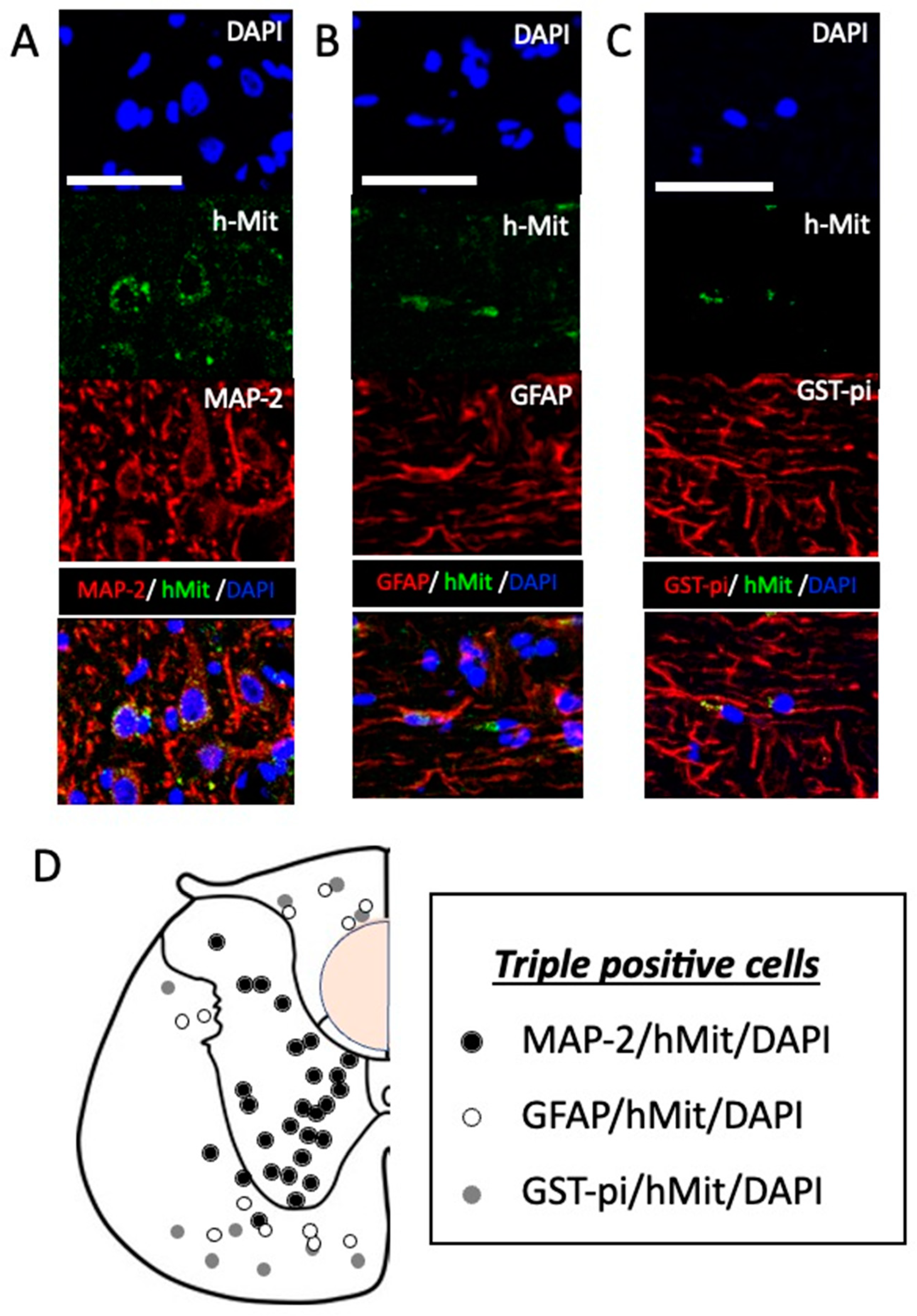
Twenty weeks after the administration of CL2020, human cells in the injured spinal cord were identified by positive immunostaining using hMit in 5 CL2020-treated animals. Positive cells located in the lesion center and rostral and caudal perilesional areas in the spinal cord. In triple staining, hMit-positive cells expressing MAP-2, GFAP, or GST-pi were identified in the lesion center and rostral and caudal spinal cord. The percentages of these cell types in different locations of the spinal cord are listed in Table 1 (n=5 in the CL2020 group). No significant differences were observed in the percentages of these three cell types. However, MAP-2/hMit/DAPI-positive cells were predominantly located in the gray matter and GFAP/hMit/DAPI- and GST-pi/hMit/DAPI-positive cells in the white matter (Figure 5). These results suggest that intravenously administered CL2020 homed into the injured spinal cord and predominantly differentiated into neuronal marker-positive cells.
Figure 5.
Differentiation of intravenously administered CL2020 in the injured spinal cord. A-C. Higher-magnification histological images of engrafted cells 20 weeks after the spinal cord injury. Human cells were identified as hMit-positive cells (green). Nuclei were counterstained by DAPI (blue). In panel A, cells positive for MAP-2 (red) indicate neuronal differentiation. Cells positive for GFAP (red) and GST-pi (red) were also identified, indicating the astrocytic and oligocytic differentiation of hMit-positive cells in panels B and C, respectively. Bars = 50 μm. D. The locations of MAP-2/hMit/DAPI- (black circles), GFAP/hMit/DAPI- (white circles), and GST-pi/hMit/DAPI- (gray circles) triple-positive cells are indicated in original illustrations (drawn by T.K.) of axial sections of the unilateral spinal cord. MAP-2/hMit/DAPI-positive cells are predominantly located in the gray matter and GFAP/hMit/DAPI and GST-pi/hMit/DAPI-positive cells in the white matter. An orange circle indicating where the spinal cord was injured.
Figure 5.
Differentiation of intravenously administered CL2020 in the injured spinal cord. A-C. Higher-magnification histological images of engrafted cells 20 weeks after the spinal cord injury. Human cells were identified as hMit-positive cells (green). Nuclei were counterstained by DAPI (blue). In panel A, cells positive for MAP-2 (red) indicate neuronal differentiation. Cells positive for GFAP (red) and GST-pi (red) were also identified, indicating the astrocytic and oligocytic differentiation of hMit-positive cells in panels B and C, respectively. Bars = 50 μm. D. The locations of MAP-2/hMit/DAPI- (black circles), GFAP/hMit/DAPI- (white circles), and GST-pi/hMit/DAPI- (gray circles) triple-positive cells are indicated in original illustrations (drawn by T.K.) of axial sections of the unilateral spinal cord. MAP-2/hMit/DAPI-positive cells are predominantly located in the gray matter and GFAP/hMit/DAPI and GST-pi/hMit/DAPI-positive cells in the white matter. An orange circle indicating where the spinal cord was injured.
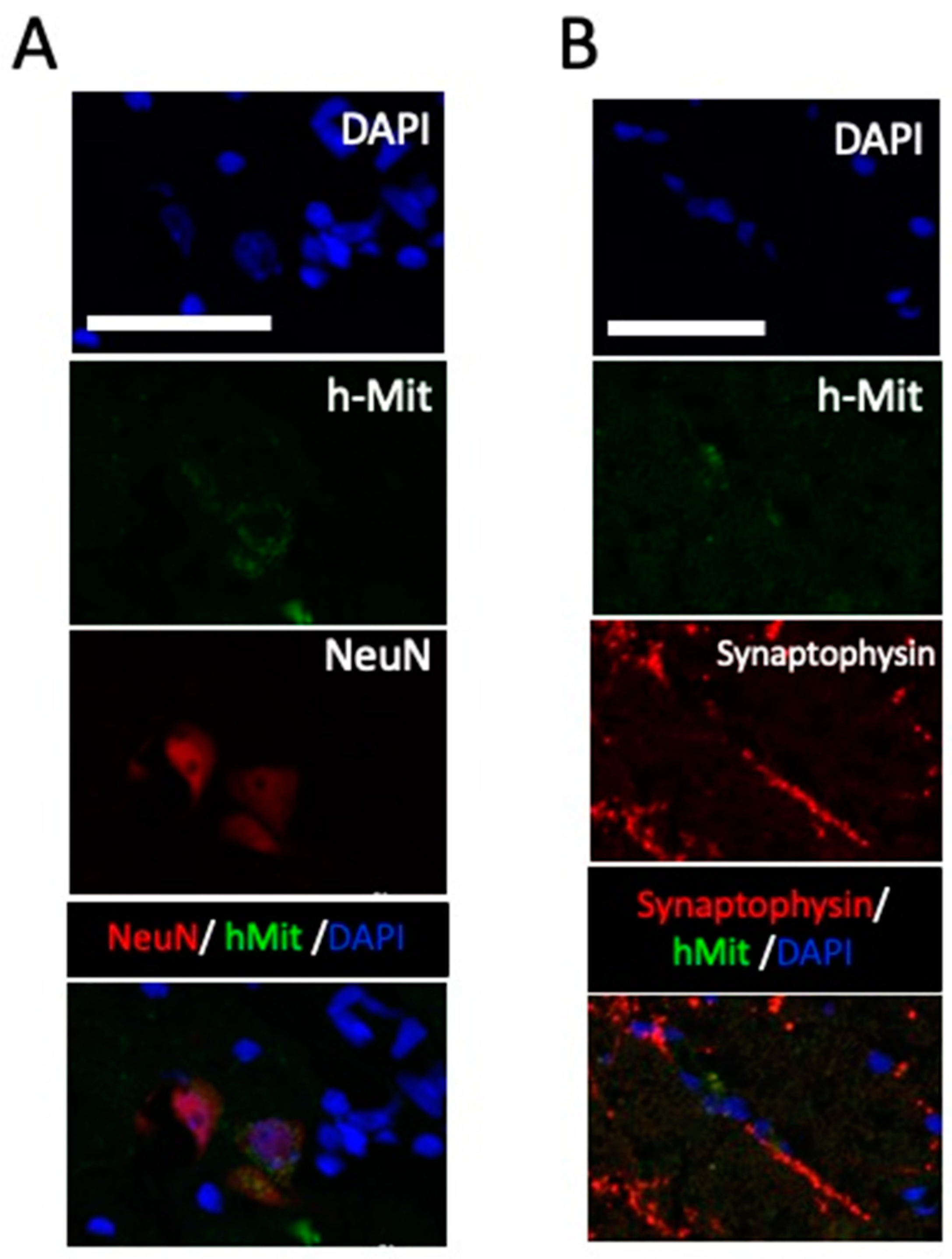
Additionally, in the grey matter close to the lesion center, positive NeuN staining were recognized in hMit-positive cells. In the same area, synaptophysin positivity was noted in close relation to the h-Mit positive cells. These data support neuronal differentiation of the engrafted cells (
Figure 6).
Figure 6.
Higher-magnification histological images of engrafted cells 20 weeks after the spinal cord injury. A. Differentiation of intravenously administered CL2020 to NeuN positive cells in the injured spinal cord. Photomicrographs showing triple positive cells with DAPI (blue), hMit (green), and NeuN (red). B. Synaptophysin (red) was expressed around hMit-positive engrafted cells (green). Photomicrographs of a merged image was presented in a bottom panel. Scale bars = 50 μm.
Figure 6.
Higher-magnification histological images of engrafted cells 20 weeks after the spinal cord injury. A. Differentiation of intravenously administered CL2020 to NeuN positive cells in the injured spinal cord. Photomicrographs showing triple positive cells with DAPI (blue), hMit (green), and NeuN (red). B. Synaptophysin (red) was expressed around hMit-positive engrafted cells (green). Photomicrographs of a merged image was presented in a bottom panel. Scale bars = 50 μm.
2.4. Deterioration of hindlimb functions after the administration of diphtheria toxin
The loss of function study was performed using diphtheria toxin to selectively cancel the functional improvements provided by CL2020 in spinally injured animals. Five days after the administration of diphtheria toxin, BBB scores in the CL2020 group (n = 3) significantly decreased (p <0.001, Figure 1). No significant differences were observed in BBB scores in the Control group(n=3) even after the administration of diphtheria toxin. Namely, 5 days after the diphtheria toxin, BBB scores dropped to 71.5 ± 12.8 % (p <0.001) in CL2020 group, while it was 92.7 ± 4.2% in Control group. These results suggested CL2020 administration led the BBB score improvement in the treatment group.
3. Discussion
The present study demonstrated that the administration of CL2020 significantly ameliorated neurological deficits in spinally injured experimental animals. When CL2020 was intravenously administered 2 weeks after SCI, Muse cells were considered to migrate into the injured spinal cord and differentiated into neuronal and neural lineage cells. Engrafted human Muse cells facilitated improvements in hindlimb motor functions over 20 weeks. When diphtheria toxin selectively ablated human Muse cell functions, the BBB scores of spinally injured animals in the CL2020 group significantly deteriorated. The direct contribution of Muse cells to the functional recovery of hindlimb functions after SCI is supported by these results as well as histological evidence of a smaller cystic cavity and larger numbers of 5-HT fibers preserved distal to the injury.
3.1. Muse cells migrate into the injured spinal cord
In this experimental model, hMit-positive cells were detected in the injured spinal cord 20 weeks after the intravenous administration of CL2020. This result
suggested that Muse cells recognized the spinal cord as a damaged site and migrated there. As observed in many other organs, including the heart, liver, kidney, and brain,[
8,
9,
10] the homing phenomenon was confirmed in the spinal cord. In the current experimental protocol, we newly demonstrated Muse cell engraftment in the subacute SCI model after the intravenous administration of CL2020. Importantly, Muse cells survived in the injured spinal cord for as long as 20 weeks.
Using the same SCI model, we recently reported the migration of Muse cells into the damaged spinal cord when CL2020 was administered 1 day after the injury.[
7] Collectively, these findings and the present results suggest that the intravenous administration of CL2020 is a reasonable approach for the delivery of Muse cells at different stages following SCI, including acute and subacute SCI. The long-term survival of intravenously administered Muse cells in the injured spinal cord reinforces the therapeutic potential of the administration of CL2020.
3.2. Mechanisms underlying Muse cell migration and homing
The mechanisms underlying the migration and homing of Muse cells following their intravenous administration are related to sphingosine-1-phosphate (S1P).[
10] S1P is a potent chemoattractant for neural stem/progenitor cells.[
11] When cell membranes are injured, S1P is generated and functions as a signal of acute inflammation. In SCI, S1P concentrations reached maximum levels 7 days after the injury.[
11] Since Muse cells express S1P receptor 2,[
12] they sense the trigger and migrate to the site of injury. These findings indicate that the S1P-S1P receptor 2 system is efficient in subacute treatment protocols. Hori et al., evaluated systemic distribution of the human Muse cells after intravenous injection for hindlimb ischemia animal model.[
13] They utilized human genomic DNA to detect the engrafted Muse cells which led identification of the Muse cells in the injured limb, the lung and the spleen, but not int the uninjured limb.
3.3. Muse cells differentiate into neuronal lineage cells
Muse cells express pluripotency markers, such as Sox2, Oct3/4, and Nanog.[
4] They spontaneously differentiate into tissue-compatible cells after homing to replenish new functional cells and repair damaged tissues.[
8,
9,
10] In our subacute SCI model, Muse cells differentiated into various cells, including MAP-2-,
NeuN, GFAP-, and GST-pi-positive cells. As shown in
Figure 5D, MAP-2/hMit-positive neuronal cells were predominantly located in the gray matter and GFAP/hMit- and GST-pi/hMit-positive cells in the white matter. Our results suggest that the differentiation of Muse cells is dependent on migration sites even within the spinal cord. When Muse cells were intravenously administered to an acute myocardial infarction model, they spontaneously differentiated into cardiac marker-positive cells, including troponin-I and sarcomeric α-actinin.[
10] Human Muse cells showed neuronal differentiation in the brain.[
14] When Muse cells were transplanted into a rat stroke model, 65 and 30% of surviving engrafted cells were positive for NeuN and MAP-2, respectively. In the current protocol, more than 50% of surviving engrafted cells were positive for MAP-2 in the subacute SCI experiment. As recently reported, we confirmed the predominant neuronal differentiation of Muse cells in the injured spinal cord in the acute SCI experiment.[
7] By comparing these two studies, the percentage of neuronal differentiation from CL2020 did not significantly differ based on the timing of administration. Since we detected larger numbers of surviving cells in the subacute SCI model, the subacute administration appeared to be more beneficial for ensuring higher survival rates of engrafted cells in the injured spinal cord (Data not shown). The present results are consistent with the subacute administration of neural stem cells supporting higher rates of graft survival and accommodation.[
15] Immediately after SCI, a series of pathological and reactive alterations occur in the damaged spinal cord. Ischemia and necrosis make the injured spinal cord a hostile environment for transplanted cells to survive.[
16] Prior to the application of the product CL2020 to future clinical trials on SCI, we consider it important to obtain evidence of its effects in a subacute administration experimental model.
3.4. Muse cells contribute to functional recovery
In the present study, the systemic administration of CL2020 induced significant functional improvements. We intended to selectively ablate transplanted human Muse cells using diphtheria toxin, as previously reported.[
7,
17,
18] Theoretically, this method confirms whether Muse cells in CL2020 directly contribute to functional recovery. BBB scores in the CL2020 group significantly decreased after the administration of diphtheria toxin to the subacute SCI model. This result indicated that Muse cells from CL2020 were directly associated with functional restoration after severe subacute SCI. SCI studies previously demonstrated that grafted neuronal cells make synaptic connections with endogenous neurons and reconstruct neuronal circuits in the spinal cord.[
3,
18] When a stroke model was intravenously administered Muse cells, the anterograde labeling of cortical neurons confirmed the reconstruction of the corticospinal tract.[
14] Oligodendrocytes are also expected to promote recovery through the remyelination of axons.[
19] Although the underlying mechanisms have yet to be elucidated in detail, the present results support the potential of the product CL2020 as a reliable source for regenerative medicine in subacute SCI patients.
3.5. Limitations
The present results suggest the migration and homing of Muse cells into the injured spinal cord following their intravenous administration. We have utilized human mitochondria antibody to identify engrafted human cells in CL2020. While the antibody is expected to be specific for the human mitochondria based on our previous literatures [
7,
14,
17], attention should be paid for the cross reactivity of it to different species including rats, mice, and pigs.[
20] Further, the mechanisms by which these cells reach the injured spinal cord and differentiate to neuronal and neural cells remain unclear. The transfer of differentiation-directing factors via the phagocytosis of apoptotic differentiated cells was proposed as a novel mechanism for Muse cell differentiation into the target cell lineages.[
21]
In this paper, we could observe smaller cystic cavities in the injury center and larger numbers of preserved 5HT fibers in the CL2020 treated animals. However, what we demonstrated was limited to indirect evidence to explain behavioral improvements. Further studies are needed to establish whether the same hypothesis may be applied to SCI.
4. Materials and Methods
4.1. Experimental animals
The Animal Studies Ethics Committee of Tohoku University Graduate School of Medicine approved all animal experiments related to the present study. Efforts were made to minimize the numbers of animals used and decrease animal suffering in experiments. Specifically, our researchers were well trained to minimize variability and ensure the quality of data, which helped to minimize the number of animals. Further, we checked the animals frequently to minimize discomfort of animals by detecting early signs of stress or pain. Twenty adult female Sprague-Dawley rats (body weight, 200-230 g) were used (Japan SLC, Inc., Shizuoka, Japan). Two or three rats were housed per cage and kept at a temperature of 24°C with water and food ad libitum throughout experiments.
4.2. SCI
Rats were anesthetized with 2% isoflurane in 30% oxygen and 70% nitrous oxide. The T9 spinous process was palpated on the top of the back while rats were positioned comfortably. During surgery, rectal temperature was maintained at 37.0 ± 0.5℃ using a feedback-regulated heating pad (BWT-100, Bio Research Center, Nagoya, Japan). Skin above the T9 spinous process was shaved and cleaned in an antiseptic manner. T9 laminectomy was performed after a midline dorsal skin incision, which widely exposed the dorsal spinal cord surface. The Infinite Horizon (IH) impactor (Precision System and Instrumentation, LLC., Lexington, KY, USA) was used to induce compression injury with a force of 200 k dynes and a dwell time of zero seconds to the spinal cord. Care was taken to place rats on the IH impactor table with the spinal cord parallel to it. Following the injury, the delivery of the planned force to the spinal cord was confirmed. Muscles and skin were closed in layers. The urinary bladder was manually emptied twice daily during the first week and once daily thereafter for 20 weeks.
4.3. Intravenous administration of CL2020 (Muse cells)
Two weeks after SCI, the Muse cell-based product CL2020 (CL2020 group, n=8) or Dulbecco’s Phosphate Buffered Saline (D-PBS, Funakoshi Co., Ltd., Tokyo, Japan.) (Control group, n=12) was intravenously administered through the tail vein. A total of 0.3 ml of CL2020 containing 300,000 Muse cells or D-PBS was slowly injected over 1 minute. All rats received a subcutaneous injection of an immunosuppressant (Prograf, 0.5 mg/kg) (Tacrolimus Hydrate, Astellas Pharma, Inc., Tokyo, Japan) every other day for 20 weeks.
4.4. Behavioral analysis
The hindlimb motor functions of all animals (n = 20) were evaluated using the Basso, Beattie, Bresnahan (BBB) locomotor scale before and following SCI on days 1, 5 and 7 and weekly thereafter for 20 weeks.[
22] BBB scores were recorded by an animal care technician who was blinded to the study and animal groups. BBB scores were compared between the CL2020 and Control groups using a multiple measurement analysis of variance (ANOVA) followed by the Bonferroni post hoc test. All values are shown as means ± standard deviations.
4.5. Immunohistochemical analyses
Twenty weeks after SCI, all rats, except animals undergoing the loss of function study, were anesthetized by aspirating excess isoflurane. Rats were transcardially perfused with saline to remove blood and then with 2% paraformaldehyde (0.1 mol/L). A fixed spinal cord was cut into 10-mm pieces with the injured portion in the center and embedded to be frozen by liquid nitrogen (Thermo ScientificTM ShandonTM M-1 Embedding Matrix. Thermo Fisher Scientific., USA). Using a cryostat, the spinal cord was sectioned at a thickness of 5 μm. Slides were created from spinal cord sections 1, 1.5, 2, 3, 4, and 5 mm in the rostral and caudal directions with the injured epicenter as 0 in 5 and 7 animals in the CL2020 and Control groups, respectively.
4.6. Cystic cavity and spared spinal cord tissue measurement
Hematoxylin and Eosin (HE) staining was used for morphological evaluations. In the spinal cord, areas of the cystic cavity and the spared spinal cord tissue were measured using ImageJ (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA). Cystic cavity volumes were calculated using cavity areas on slides 1, 1.5, 2, 3, 4, and 5 mm in the rostral and caudal directions with the injured epicenter as 0. Cavity volumes were calculated in the CL2020 and Control groups. The results obtained were compared using the Mann-Whitney U test.
Spared spinal cord tissue were also calculated on on slides 1, 1.5, 2, 3, 4, and 5 mm in the rostral and caudal directions with the injured epicenter as 0. Areas in the CL2020 and Control groups were compared using the Mann-Whitney U test.
4.7. Quantification of preserved 5-HT fibers
The fluorescent staining of an anti-5-HT antibody (ab66047, Abcam, Cambridge, England) was used to evaluate preserved 5-HT fibers in the spinal cord. The number of 5-HT-immunolabeled axons 3 mm caudal from the epicenter of the injury was quantified with ImageJ (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA) in a section perpendicular to the rostral-caudal axis of the spinal cord. Number of positive fibers were counted in three different high magnified images from every fifth section. Number of the positive fibers were calculated to represent in a given area of 1 square millimeter in a microscopic analysis. Preserved 5-HT fibers in the CL2020 and Control groups were compared using the Mann-Whitney U test.
4.8. Identification of Muse cells in the injured spinal cord
Immunostaining with antibodies against human mitochondria (hMit) (1:50; ab3298, Abcam, Cambridge, England) was used to identify human Muse cells in CL2020 homing into the injured spinal cord, conjugated with Alexa 488 anti-mouse (Invitrogen) as the secondary antibody. 4',6-Diamidino-2-phenylindole (DAPI) was used to detect nuclei. Microtubule-associated protein-2 (MAP-2) (1:500; ab5392, Abcam) with a secondary antibody (Alexa 568 anti-chicken, Invitrogen), NeuN (1:100; ab177487, Abcam) with a secondary antibody (Alexa 568 anti-rabbit, Invitrogen), glial fibrillary acidic protein (GFAP) (1:200; 80788, cell signaling technology) with a secondary antibody (Alexa 568 anti-rabbit, Invitrogen), and Glutathione S-Transferase (GST)-pi (1:200; 311, Medical & Biological Laboratories) with a secondary antibody (Alexa 568 anti-rabbit, Invitrogen) were used to identify neurons, astrocytes, and oligodendrocytes, respectively. To evaluate synaptic activities, synaptophysin (1:200; MA5-14532, Invitrogen) with a secondary antibody (Alexa 568 anti-rabbit, Invitrogen) was used. Samples were enclosed with mounting agent (Prolong diamond antifade mountant with DAPI: P36962, Thermo Fisher Scientific, MA, USA) and inspected under a laser confocal microscope (FV3000, OLYMPUS Tokyo, Japan).
4.9. Administration of diphtheria toxin
Twenty weeks after SCI, a loss of function study was performed using diphtheria toxin from
Corynebacterium diphtheriae (Sigma-Aldrich Co., LLC., USA). A previous study reported that human cells were 100,000-fold more sensitive to the toxin than rodent cells.[
23] Therefore, diphtheria toxin selectively ablates human cells in rodent models.[
17] Three and five rats from the CL2020 and Control groups were intraperitoneally administered diphtheria toxin (50 μg/kg) twice at a 24-h interval. Hindlimb motor functions were assessed 5 days after the administration of diphtheria toxin. A paired
t-test was used to evaluate BBB scores before and after the administration of diphtheria toxin.
5. Conclusions
Spinally injured animals achieved significant functional recovery following the intravenous administration of the human-derived product CL2020 2 weeks after the injury. In this subacute SCI model, engrafted Muse cells in CL2020 migrated and homed into the spinal cord. They differentiated into neuronal and neural cells and contributed to the restoration of descending spinal tracts. CL2020 offers a feasible treatment option in future clinical trials on SCI patients.
Author Contributions
Conceptualization, Endo, Niizuma, Tominaga.; methodology, Takahashi, Kajitani, Endo; validation, Niizuma, Tominaga; formal analysis, Takahashi, Kajitani, Endo; investigation, Nakayashiki, Inoue; writing—original draft preparation, Takahashi, Kajitani, Endo; writing—review and editing, Inoue, Niizuma, Tominaga; visualization, Nakasyashiki; supervision, Tominaga; project administration, Endo, Niizuma; funding acquisition, Endo, Niizuma, Tominaga. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by MEXT/AMED Translational Research Network Program (J190000613), JSPS Grant-in-Aid for Scientific Research (C, 19K09448)(B, 22H03180), HIROMI Medical Research Foundation, The Ichiro Kanehara Foundation, SENSHIN Medical Research Foundation, AO SPINE international foundation, OKINAKA foundation and co-research expenses with Life Science Institute Inc (LSII: Tokyo, Japan).
Institutional Review Board Statement
The Animal Studies Ethics Committee of Tohoku University Graduate School of Medicine approved all animal experiments related to the present study (2018MdA-204-04).
Acknowledgments
The authors would like to thank Medical English Service for the English language review. The authors appreciate Ms. Natsumi Konno, and Ms. Marisa Ota for technical support for this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Filbin, M.T. Myelin-Associated Inhibitors of Axonal Regeneration in the Adult Mammalian CNS. Nat Rev Neurosci 2003, 4, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Yiu, G.; He, Z. Glial Inhibition of CNS Axon Regeneration. Nat Rev Neurosci 2006, 7, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Aizawa-Kohama, M.; Endo, T.; Kitada, M.; Wakao, S.; Sumiyoshi, A.; Matsuse, D.; Kuroda, Y.; Morita, T.; Riera, J.J.; Kawashima, R. Transplantation of Bone Marrow Stromal Cell-Derived Neural Precursor Cells Ameliorates Deficits in a Rat Model of Complete Spinal Cord Transection. Cell Transplantation 2013, 22, 1613–1625. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kitada, M.; Wakao, S.; Nishikawa, K.; Tanimura, Y.; Makinoshima, H.; Goda, M.; Akashi, H.; Inutsuka, A.; Niwa, A.; et al. Unique Multipotent Cells in Adult Human Mesenchymal Cell Populations. Proc Natl Acad Sci U S A 2010, 107, 8639–8643. [Google Scholar] [CrossRef]
- Kuroda, Y.; Wakao, S.; Kitada, M.; Murakami, T.; Nojima, M.; Dezawa, M. Isolation, Culture and Evaluation of Multilineage-Differentiating Stress-Enduring (Muse) Cells. Nat Protoc 2013, 8, 1391–1415. [Google Scholar] [CrossRef] [PubMed]
- Young, W. Future of Muse Cells. Adv Exp Med Biol 2018, 1103, 309–315. [Google Scholar] [CrossRef]
- Kajitani, T.; Endo, T.; Iwabuchi, N.; Inoue, T.; Takahashi, Y.; Abe, T.; Niizuma, K.; Tominaga, T. Association of Intravenous Administration of Human Muse Cells with Deficit Amelioration in a Rat Model of Spinal Cord Injury. J Neurosurg Spine 2021, 1–8. [Google Scholar] [CrossRef]
- Iseki, M.; Kushida, Y.; Wakao, S.; Akimoto, T.; Mizuma, M.; Motoi, F.; Asada, R.; Shimizu, S.; Unno, M.; Chazenbalk, G.; et al. Muse Cells, Nontumorigenic Pluripotent-Like Stem Cells, Have Liver Regeneration Capacity Through Specific Homing and Cell Replacement in a Mouse Model of Liver Fibrosis. Cell Transplant 2017, 26, 821–840. [Google Scholar] [CrossRef]
- Katagiri, H.; Kushida, Y.; Nojima, M.; Kuroda, Y.; Wakao, S.; Ishida, K.; Endo, F.; Kume, K.; Takahara, T.; Nitta, H.; et al. A Distinct Subpopulation of Bone Marrow Mesenchymal Stem Cells, Muse Cells, Directly Commit to the Replacement of Liver Components. Am J Transplant 2016, 16, 468–483. [Google Scholar] [CrossRef]
- Yamada, Y.; Wakao, S.; Kushida, Y.; Minatoguchi, S.; Mikami, A.; Higashi, K.; Baba, S.; Shigemoto, T.; Kuroda, Y.; Kanamori, H.; et al. S1P-S1PR2 Axis Mediates Homing of Muse Cells Into Damaged Heart for Long-Lasting Tissue Repair and Functional Recovery After Acute Myocardial Infarction. Circ Res 2018, 122, 1069–1083. [Google Scholar] [CrossRef]
- Kimura, A.; Ohmori, T.; Ohkawa, R.; Madoiwa, S.; Mimuro, J.; Murakami, T.; Kobayashi, E.; Hoshino, Y.; Yatomi, Y.; Sakata, Y. Essential Roles of Sphingosine 1-Phosphate/S1P1 Receptor Axis in the Migration of Neural Stem Cells toward a Site of Spinal Cord Injury. Stem Cells 2007, 25, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Wakao, S.; Dezawa, M. Muse Cells Are Endogenous Reparative Stem Cells. Adv Exp Med Biol 2018, 1103, 43–68. [Google Scholar] [CrossRef]
- Hori, Y.; Kitani, T.; Yanishi, K.; Suga, T.; Kogure, M.; Kusaba, T.; Kushida, Y.; Dezawa, M.; Matoba, S. Intravenous Administration of Human Muse Cells Recovers Blood Flow in a Mouse Model of Hindlimb Ischemia. Front Cardiovasc Med 2022, 9, 981088. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Niizuma, K.; Kushida, Y.; Wakao, S.; Tominaga, T.; Borlongan, C.V.; Dezawa, M. Human Muse Cells Reconstruct Neuronal Circuitry in Subacute Lacunar Stroke Model. Stroke 2017, 48, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Okano, H. Cell Transplantation Therapies for Spinal Cord Injury Focusing on Induced Pluripotent Stem Cells. Cell Res 2013, 23, 70–80. [Google Scholar] [CrossRef]
- Olson, L. Combinatory Treatments Needed for Spinal Cord Injury. Exp Neurol 2013, 248, 309–315. [Google Scholar] [CrossRef]
- Abe, T.; Aburakawa, D.; Niizuma, K.; Iwabuchi, N.; Kajitani, T.; Wakao, S.; Kushida, Y.; Dezawa, M.; Borlongan, C.V.; Tominaga, T. Intravenously Transplanted Human Multilineage-Differentiating Stress-Enduring Cells Afford Brain Repair in a Mouse Lacunar Stroke Model. Stroke 2020, 51, 601–611. [Google Scholar] [CrossRef]
- Abematsu, M.; Tsujimura, K.; Yamano, M.; Saito, M.; Kohno, K.; Kohyama, J.; Namihira, M.; Komiya, S.; Nakashima, K. Neurons Derived from Transplanted Neural Stem Cells Restore Disrupted Neuronal Circuitry in a Mouse Model of Spinal Cord Injury. J Clin Invest 2010, 120, 3255–3266. [Google Scholar] [CrossRef]
- Nistor, G.I.; Totoiu, M.O.; Haque, N.; Carpenter, M.K.; Keirstead, H.S. Human Embryonic Stem Cells Differentiate into Oligodendrocytes in High Purity and Myelinate after Spinal Cord Transplantation. Glia 2005, 49, 385–396. [Google Scholar] [CrossRef]
- Allard, J.; Li, K.; Lopez, X.M.; Blanchard, S.; Barbot, P.; Rorive, S.; Decaestecker, C.; Pochet, R.; Bohl, D.; Lepore, A.C.; et al. Immunohistochemical Toolkit for Tracking and Quantifying Xenotransplanted Human Stem Cells. Regen Med 2014, 9, 437–452. [Google Scholar] [CrossRef]
- Wakao, S.; Oguma, Y.; Kushida, Y.; Kuroda, Y.; Tatsumi, K.; Dezawa, M. Phagocytosing Differentiated Cell-Fragments Is a Novel Mechanism for Controlling Somatic Stem Cell Differentiation within a Short Time Frame. Cell Mol Life Sci 2022, 79, 542. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Pappenheimer, A.M.; Harper, A.A.; Moynihan, M.; Brockes, J.P. Diphtheria Toxin and Related Proteins: Effect of Route of Injection on Toxicity and the Determination of Cytotoxicity for Various Cultured Cells. J Infect Dis 1982, 145, 94–102. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).