Introduction
Melanoma is a highly aggressive type of skin cancer produced by malignant transformation of melanocytes and it is usually associated with a poor prognosis. Clinically, melanoma has several stages associated with migration and invasion of the cells through the skin layers, rapid cell spreading and formation of tumors in multiple organs. Nowadays, active development of novel targeted drugs is underway, but the main problem is the emergence of resistance of melanoma to the applied methods of treatment [
1,
2]. Therefore, it is of primary importance to find more crucial signaling pathways that control the progression of this type of cancer. Mechanosensitive ion channels and molecules, especially those that mediate Ca
2+ influx into the cell, regulate cancer development, metabolism and progression [
3,
4]. Recently, it was shown that mechanosensitive TRPV2 channel regulates melanoma invasiveness and metastatic potential [
3]. The high expression of Piezo1, another important mechanosensitive Ca
2+- permeable ion channel, was detected in melanoma [
3], and was shown to control its malignant progression [
4]. The main life-threatening stage of melanoma is metastasis formation, and 3D spheroids are better suited for the study of this process due to imitation
in vivo conditions that are observed during melanoma progression [
5]. Here, we evidenced functional activity of Piezo1 channels in aggressive melanoma SK-MEL-2 cell line. We found that chemical activation of Piezo1 by its selective agonist Yoda1 completely prevents melanoma spheroid formation and thus could be considered as a novel targeted approach for the prevention of melanoma development.
Materials and Methods
Cells and reagents
SK-MEL-2 human malignant melanoma cell line (ATCC® HTB-68™, RRID: CVCL_0069) was purchased from Biolot, St. Petersburg, Russia. Cells were cultured in RPMI 1640 medium (Biolot, Russia) containing 10% fetal bovine serum (Biowest, Rui de Caille, France) and antibiotic gentamicin (Biolot, Russia) in humidified incubator at 37o C and 5% CO2. For patch-clamp studies, fluorescent staining and Ca2+-imaging, the cells were plated on glass coverslips in 2-3 days before the experiments. Selective chemical Piezo1 activator Yoda1 was purchased from Tocris Bioscience (Bristol, United Kingdom). Ca2+ ionophore ionomycin was purchased from Thermo Fischer Scientific (Waltham, MA, USA).
RT-PCR
Total RNA and cDNA were obtained as described in previous studies [
6,
7]. The primer sequences for hPIEZO1 were: 3’-CCAGAACAGGTATCGGAAG-5’ (reverse) and 5’-TGCTGTACCAGTACCTGCTG-3’ (forward). The cycling conditions were as in previous studies [
8]. The number of PCR cycles was 30, and the optimal annealing temperature for hPIEZO1 primers is 57 °C.
Electrophysiology
The detailed description of the whole-cell mode of patch-clamp technique used to record single ion channels in the plasma membrane is available in our publications [
8,
9]. Cytosol-like solution contained (in mM): 140 KAsp, 5 NaCl, 1 MgCl
2, 2 EGTA, 20 HEPES/TrisOH and CaCl
2 to establish intracellular Ca2+ concentration ([Ca
2+]
i) at 10 nM. The extracellular chamber solution contained: 145 NaCl, 2 CaCl
2, 1 MgCl2, and 10 HEPES/TrisOH. pH of all solutions was set at 7.3. Yoda1 was applied to the extracellular solution.
Immunofluorescence
Polyclonal primary antibodies against extracellular domain of Piezo1 were used (KD/KO validated, from Proteintech, Manchester, United Kingdom, Cat No 15939-1-AP, RRID: AB_2231460). Cells were fixed with 4% paraformaldehyde (15 min at room temperature, RT), blocked with 10% Goat serum (1 h, RT) and incubated with 1:100 Anti-Piezo1 antibodies (overnight, at 4o C). Then the cells were incubated with 1:200 secondary goat anti-rabbit-Cy3 fluorescent antibodies (Santa Cruz, TX, USA, for 1 h, RT). Cell nuclei were counterstained with DAPI (0.05 μg/ml, 30 min, RT). Cells were mounted on glass slides using Vectashield mounting media (Vector Laboratories, Newark, CA, USA) and visualized using Olympus FV3000 (Olympus Corporation, Shinjuku, Tokyo, Japan) confocal microscope.
Calcium imaging
Ca
2+-measurements were performed as described earlier [
7]. Briefly, the cells were loaded with 5 μM of Fluo8-AM fluorescent Ca
2+ probe (AAT Bioquest, Pleasanton, CA, USA) for 45 min in Ca
2+-containing solution (at 37
o C and 5% CO
2, in dark), then the cells were incubated for 15 min without the dye. The Ca
2+-containing solution consists of (in mM): 150 NaCl, 4.5 KCl, 2 CaCl
2, 1 MgCl
2 and 10 HEPES/TrisOH. At the start of the experiment, cells were placed in Ca
2+-containing solution, and basal fluorescence signals were recorded for 15 s. After that, 10 µM Yoda1 was applied to the cells. The experiment was performed at least 3 times.
Cell spreading and morphology assays
Cells were trypsinized, resuspended in full medium, and vehicle (0.3% DMSO) or 30 µM Yoda1 were added to the cells. After that, cell suspensions were plated on Petri dishes. At least five fields of view for control or Yoda1-treated cells were captured every 20 min, and a number of spreaded/non-spreaded cells was counted. For morphology analysis, the contours of the cells were traced manually in ImageJ using the “Freehand selection” tool. Cell area and shape descriptors were measured using the “Analyze - Measure” commands. A total number of 100-110 cells was analyzed for each experimental condition at each timepoint.
Statistics
The statistical analysis was performed in GraphPad Prism 8.0 (GraphPad Software, USA), p<0.05 was considered as significant. The statistical tests used for data comparison are indicated in the corresponding figure legends.
Results and Discussion
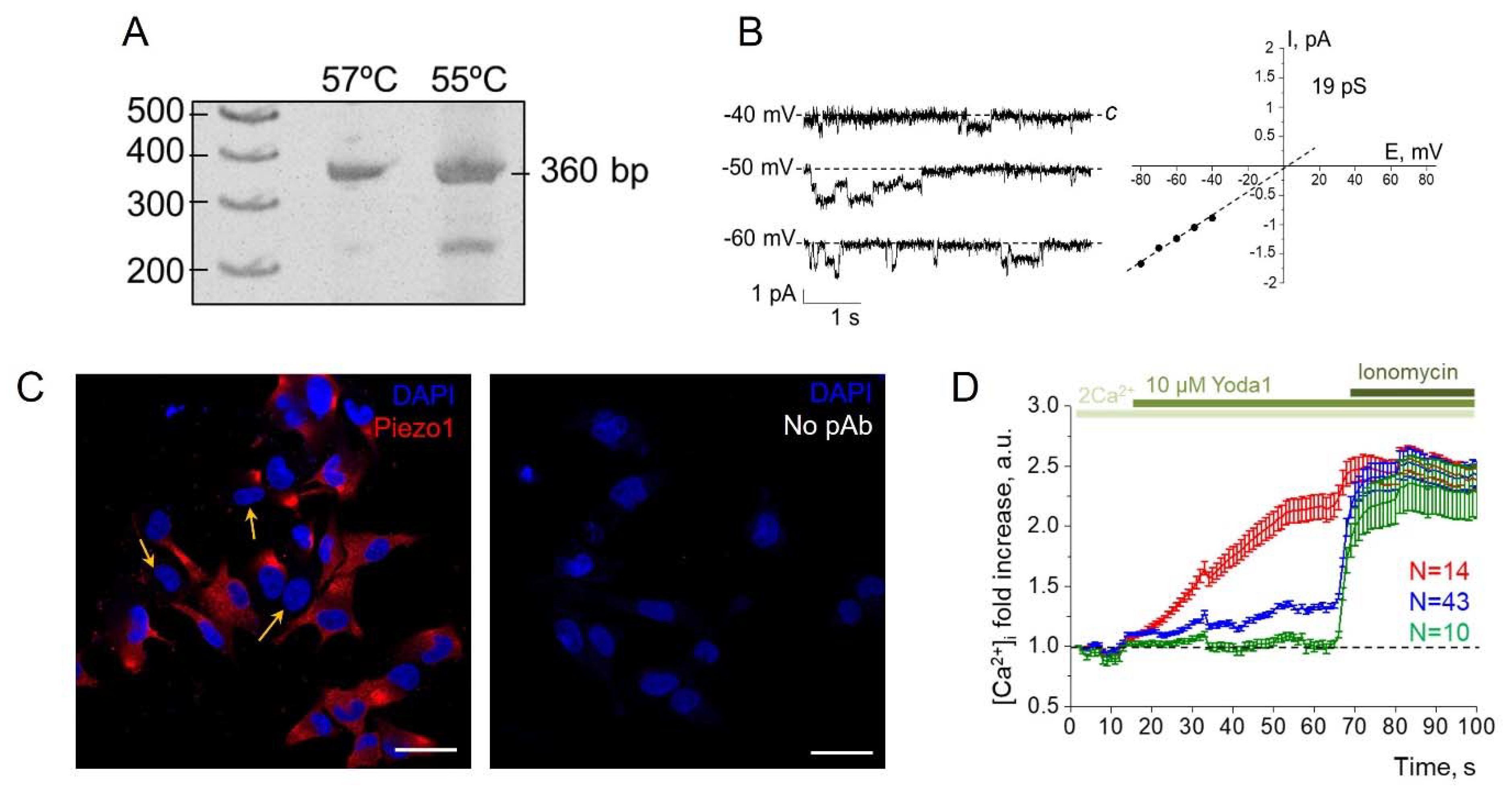
Firstly, we performed RT-PCR to confirm the presence of hPIEZO1 mRNA in SK-MEL-2 cells (
Figure 1A). Further, to record the functional activity of endogenous Piezo1 channels in the plasma membrane, we used the patch-clamp technique. Previously, we have shown that 1 µM Yoda1, a selective agonist of Piezo1, could evoke single Piezo1-mediated channel activity in whole-cell experiments [
8]. Consistently, we recorded single-channel Piezo1 currents in melanoma cells in response to the application of 1 µM Yoda1. The single channel properties of Yoda1-induced Piezo1 channels (
Figure 1B) were similar to those reported earlier [
8]. The channels were activated in 7 out of 9 experiments indicating that several cells seem to have no functionally active Piezo1 in the plasma membrane. Consistently, immunofluorescent staining revealed that part of SK-MEL-2 cells was Piezo1-negative (
Figure 1C). As Piezo1 channels provide physiologically relevant pathways for Ca
2+ entry in various cells and tissues, the Ca
2+- measurements were done for additional verification of obtained results (
Figure 1D). We found that Yoda1 application resulted in [Ca
2+]
i increase further confirming the presence of functional Piezo1 channels in SK-MEL-2 cells. Interestingly, Ca
2+ imaging allowed to distinguish three types of cell responses: (1) cells with significant increases in [Ca
2+]
i (about 2-fold, red line), (2) with slow moderate increases (about 1.3 fold, observed in majority of cell population, blue line), and (3) cells with no Ca
2+ entry in response to Yoda1 (green line). The results confirm the variations in Piezo1 expression in SK-MEL-2 cells. Taken together, our data show that SK-MEL-2 cells have functionally active Piezo1 channels in the plasma membrane, whose activity could be evoked by Yoda1, a selective chemical Piezo1 activator. The intriguing result is the presence of a number of the cells in which no Piezo1 staining or activity was detected. This phenomenon could be explained by various factors that could be revealed and analyzed in further studies.
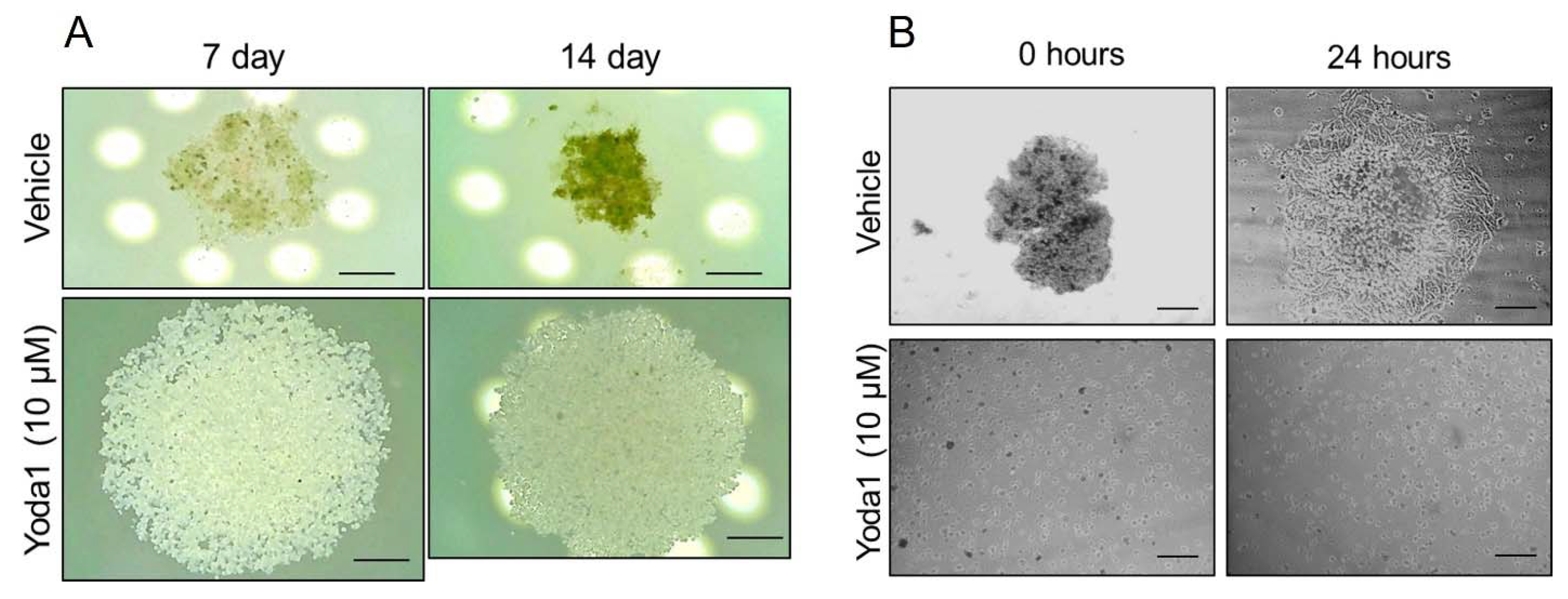
Cell aggregation in 3D spheroids is an important step in melanoma progression where cells primarily have to change their microenvironment and mechanical properties as well as morphology, polarization and cell-cell contacts. These steps could be critically controlled by the functioning of various ion transporters, including mechanosensitive channels. Thus, we probed if selective chemical activation of Piezo1 could have any effect on melanoma spheroids by adding 10 µM Yoda1 to SK-MEL-2 suspension during the formation of spheroids. Importantly, we detected the perturbation of spheroid formation in the presence of Yoda1: firstly, no compaction of the cells was observed (
Figure 2A), whereas control cells became compacted after 7 days from the start of spheroid formation, and the compaction continued (to the 14th day). Further (after 14 days), the control- and Yoda1-containing drops were transferred to the Petri dish to confirm stable spheroid formation. Evidently, no spheroids were formed in the presence of Yoda1, whereas control cells formed distinct compact spheroids (
Figure 2B). After 24 hours on the adherent surface, the cells from the control spheroids have spread out, whereas the Yoda1-treated cells remain round, are unable to spread and are non-viable (
Figure 2B). Thus, the addition of Yoda1 completely blocked the spheroid formation from SK-MEL-2 aggressive melanoma cell line.
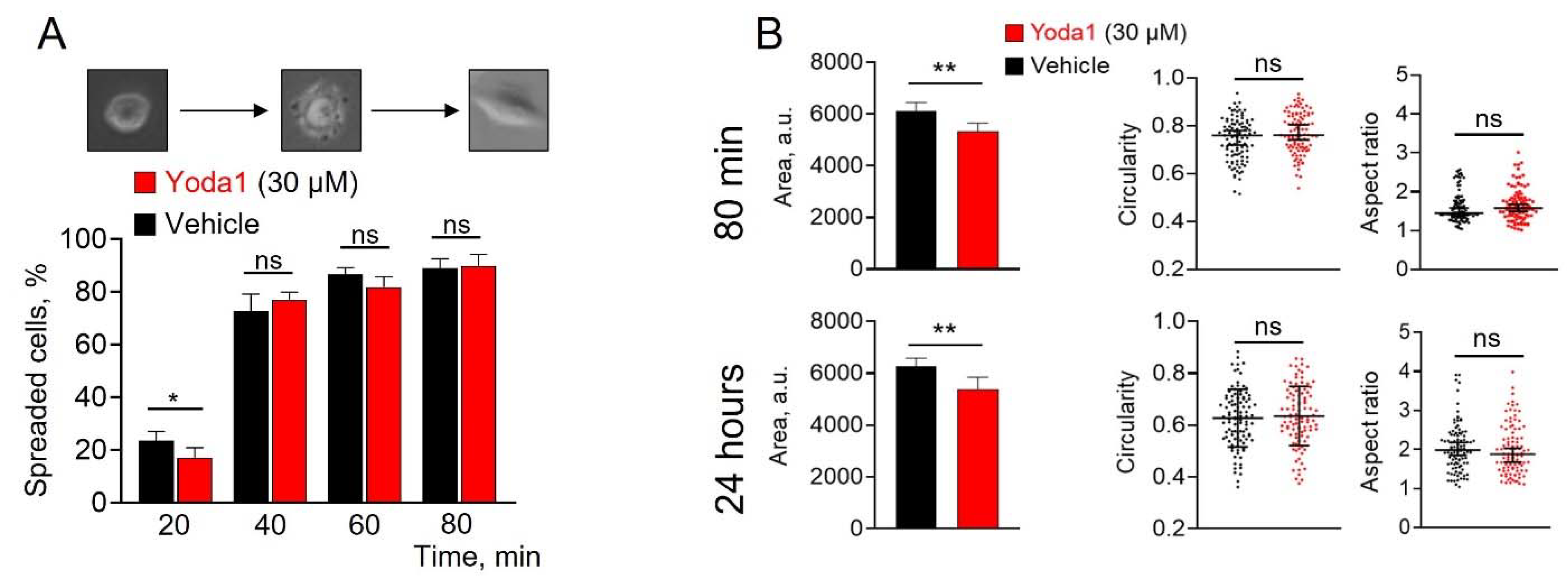
The first key step of spheroid formation is the transfer of the cells to suspension, which significantly affects their shape and plasma membrane curvature compared to adherent cells. As membrane curvature was shown to strongly affect Piezo1 distribution [
10], as well as the activating potential of Yoda1 [
11], we analyzed the action of Yoda1 on the SK-MEL-2 cells in the suspension. We hypothesized, that Yoda1 could have a strong effect on suspension melanoma cells, and this could potentially underlie the prevention of spheroid formation. Thus, we tested if the addition of Yoda1 to cell suspension could affect their spreading and viability (
Figure 3). Our assays showed only a small (or non-significant effect) of 30 µM Yoda1 (note the higher concentration used compared to spheroid formation) on SK-MEL-2 cells. Particularly, the number of spreaded cells in the presence of Yoda1 was slightly lower at the early stage (after 20 minutes), however no significant changes were detected at later time points (
Figure 3A) that also confirms the viability of the cells. Also, Yoda1 had no effect on cell shape, but caused a slight decrease in cell size (
Figure 3B). Thus, the lack of significant effect of Yoda1 on melanoma cell suspension could indicate, that the perturbation of melanoma spheroid formation in the presence of Yoda1 occurs at latter stages of the process. It should be noted that no specific cell-cell interactions occur between the cells in suspension (at least during a short timescale) or during cell spreading, where cell-surface interactions prevail. Thus, we speculate that Piezo1 activation probably affects cell-cell interactions that are observed in 3D culture of SK-MEL-2 cells. It is of specific interest to analyze the mechanisms that underlie the inhibition of melanoma spheroid formation by selective activation of Piezo1 channels. 3D structure formation occurs through the sequential establishment of cell-cell contacts in which adhesion molecules such as integrins and cadherins are the key players [
12]. Interestingly, both cadherins and integrins were shown to interact with or be regulated by Piezo1, and v.v. [
13,
14]. Thus, the study of the possible regulation of integrin/cadherin activity and interactions by Piezo1 during spheroid formation could be a promising experimental goal of future research in the prevention of tumor formation.