3. Results and Discussion
It is often assumed that the quality of a density functional can be assessed by comparing its results to experimental values or to the results of post-Hartree-Fock calculations. However, this is not always possible, as experimental data may not be available for the systems being studied, or the molecules may be too large for accurate calculations to be feasible.
To address this issue, a protocol called KID (Koopmans in DFT) has been developed [
35,
36,
37,
38,
39]. This protocol aims to validate a density functional by assessing its internal consistency. The KID protocol has been previously shown to be effective for peptides, but it is worth further validating for the molecules considered in this study.
The KID protocol was implemented using the in-house developed CDFT software tool. The results of this analysis are shown in
Table 1:
The J and J descriptors consider differences between the HOMO and ionization energy (I), and between the LUMO and electron affinity (A) respectively. According to the Ionization Energy Theorem, A can be predicted by the SOMO, which is equivalent to the anion’s HOMO. To align with Koopmans’ theorem, we introduced the SL descriptor, comparing SOMO and LUMO values. The J descriptor verifies the difference between the H-L gap and the I-A gap calculated from energy differences of neutral, positive, and negative species.
The results in
Table 1 show that the KID descriptor values are nearly 0, indicating that the MN12SX/Def2TZVP/H2O model chemistry is effective for verifying Janak and Ionization Energy theorems. Although further calculations involve energy differences, accurate predictions for HOMO, LUMO, and H-L gap provide confidence in our estimations of chemical reactivity properties. These estimations are theoretically grounded, not accidental as often seen with other density functionals.
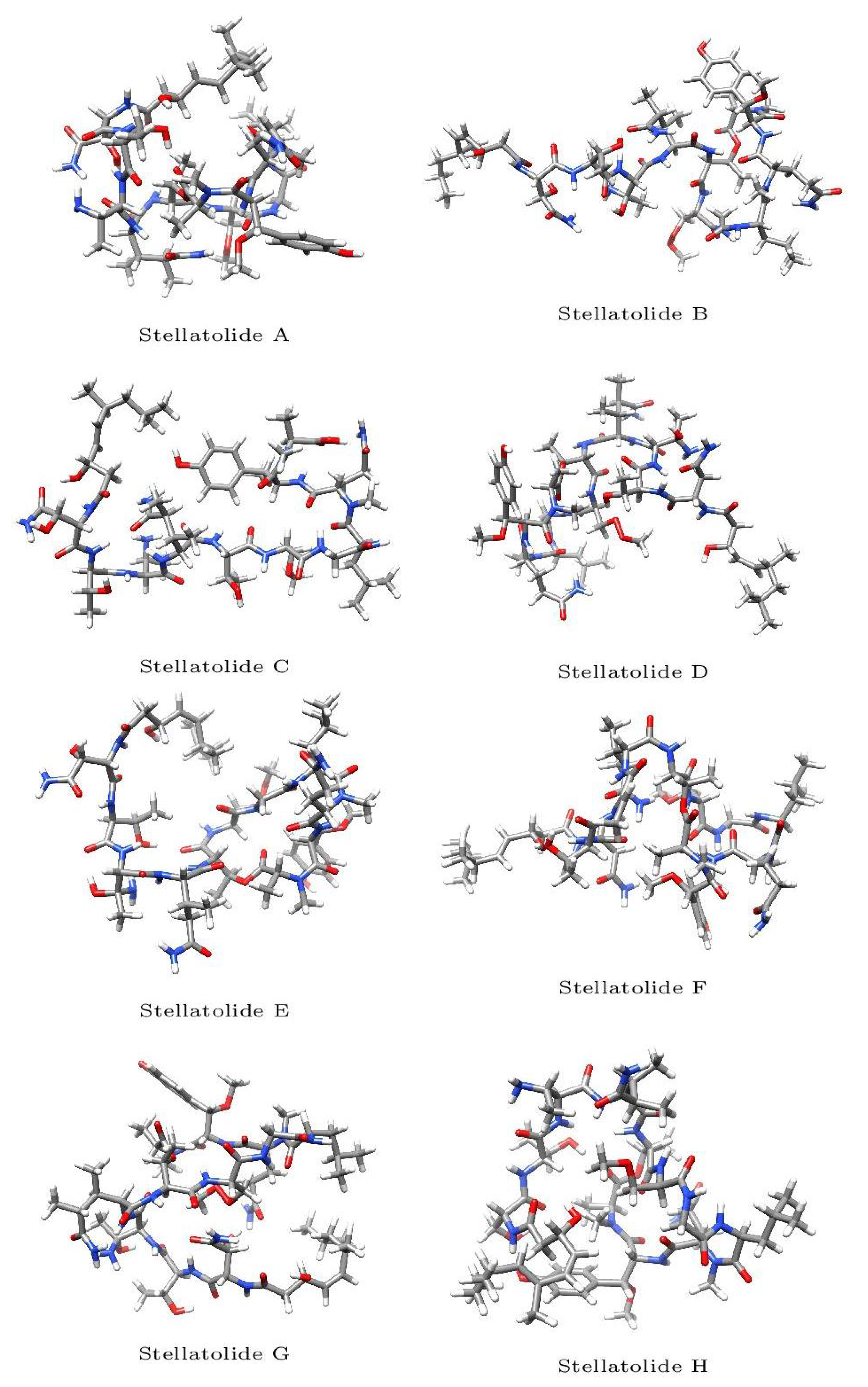
The optimized molecular structures of the eight members of the Stellatolides A-H family of cylopeptides are displayed in
Figure 2. These optimized structures have been obtained by resorting to the procedure described in sub
Section 2.1 of the Materials and Methods section.
The usual way of analyzing the chemical reactivity of molecular systems through the consideration of Conceptual DFT is by reporting the values of the global and local descriptors that arise from this theory [
27,
28,
29,
30,
31,
32,
33,
34]. The three basic descriptors are the electronegativity
, the global hardnesss
, and the global electrophilicity
. The first one, is a measure of the tendency of an atom to attract electrons. It is a fundamental property of atoms that affects their chemical bonding and reactivity. In CDFT, electronegativity is defined as the Lagrange multiplier associated with the constraint that the total electronic chemical potential of a system is equal to a constant. This means that electronegativity
is a measure of the resistance of an atom to losing electrons, and it can be estimated through the operational formula
[
27,
28,
29,
30,
31,
32,
33,
34].
Global hardness
is a crucial concept within the realm of CDFT, which imeasures the resistance of a system to changes in its electron density when subjected to external perturbations. The global hardness can be used to predict the reactivity of a molecule. A molecule with a high global hardness
is less reactive than a molecule with a low global hardness. This is because a molecule with a high global hardness
is more difficult to polarize, and therefore less likely to react with other molecules. The formula
is used for the evaluation of
, either considering energy differences or frontier orbitals [
27,
28,
29,
30,
31,
32,
33,
34].
The third basic descriptor, gobal electrophilicity
[
64] is particularly useful in predicting reactions and interactions between molecules. It can be expressed as a relation between
and
as
=
. A more electrophilic species tends to react with more nucleophilic ones, as electron flow will be from the nucleophile to the electrophile. This concept aids in understanding reaction mechanisms, molecular binding, and site-selectivity in chemical processes [
27,
28,
29,
30,
31,
32,
33,
34].
In addition to the global reactivity descriptors originating from Conceptual Density Functional Theory (DFT), Domingo and his colleagues [
65,
66,
67,
68,
69] introduced a Nucleophilicity Index labeled as N. This index is established by evaluating the HOMO energy using the KS approach, wherein the reference point is shifted arbitrarily, centered around the tetracyanoethylene (TCE) molecule.
Newer developed global reactivity descriptors are the electrodonating and electroaccepting powers:
=
,
=
, respectively [
29], and the net electrophilicity
[
30] as a comparison of the former two.
By considering the mentioned CDFT software tool as well as the MultiWFN package [
70,
71] applied to the results of the calculation of the electronic properties of the studied cyclopeptides, the values of the defined global reactivity descriptors could be obtained and they are displayed in
Table 2:
From
Table 2, the values of the electronegativity
range from 3.2610 eV for Stellatolide A to 3.9451 eV for Stellatolide F. A little inferior result is obtained for Stellatolide G, while the other considered peptides have similar intermediate values for this descriptor. If we now turn our attention to global hardness
, then it can be appreciated that the lowest value of this property is for the case of Stellatolide F, with 4.6145 eV. The results from both reactivity descriptors agree in that Stellatolide F will be the most reactive cyclopeptide of this family. The other members display larger values of
, meaning that they will be less reactive than the H species. Notwithstanding, there are differences between Stellatolides F and G, that will be a bit more reactive than Stellatolides A-E. As mentioned earlier, the global electrophilicity
represents a balance between
and
. The results for this descriptor from
Table 2 ranges bteween 1.1759 eV for Stellatolide C to 1.6864 eV for Stellatolide F. Stellatolides A-E together with Stellatolide H display the lowest values of
, while Stellatolide G presents a bit greater number. The analysis of the first three basic chemical reactivity descriptors lead us to the conclusion that Stellatolide F will the most reactive of all the cyclopeptides considred in this research.
As mentioned earlier, the nucleophilic index N is another important quantity that serves as a descriptor of chemical reactivity. From
Table 2, their values ranges from 2.8066 eV for Stellatolide C to 3.1565 eV for Stellatolide H. As a matter of fact, all the member of this family have similar values for the nucleophilicity index N, with the exception of stellatolide H which is a bit larger. According to the clasification proposed by Domingo et al [
65,
66,
67,
68,
69], while Stellatolide H may be regarded as a strong nucleophile, the others can be considered as moderated ones.
The last three descriptors in
Table 2 are interrelated between them. As it is usual for this kind of molecules, the electrodonating power
is large that the corresponding electroaccepting power
. This behavior could be attributed to their cyclic structure and the large number of conjugated bonds, as well as the presence of several O and N atoms. Stellatolide F possesses the larges value of
, followed by Stellatolide G, while the others display almost the same values for this chemical reactivity descriptor. For the case of the electroaccepting power
, again Stellatolide F is the most important among the whole family of cyclopeptides, with significative differences with the other molecules, with the exception of Stellatolide G. As expected, the net electrophilicity
, being defines as a way to compare
with
, shows the same behavior for the cyclopeptides as for the other two reactivity descriptors, allowing the conclusion that Stellatolide F will be the most reactive cyclopeptide of all those considered in this work.
The chemical reactivity descriptors that have been reported are classified as global indicators, as they play a crucial role in predicting the overall properties of molecules. Nevertheless, there are instances where delving into the characteristics of specific regions within molecular systems becomes intriguing. By recognizing that distinct chemical reactivity is confined to various zones within these molecules, we gain insights into their behavior. This prompts the use of what are known as local reactivity descriptors, which stem from the principles of Conceptual DFT (CDFT) [
27,
28,
29,
30,
31,
32,
33,
34].
The Fukui functions (FF), cited in references such as [
27,
28,
29,
30,
31,
32,
33,
34], stand out as the most prevalent local descriptors in Conceptual Density Functional Theory (CDFT). These functions can be categorized into nucleophilic, electrophilic, and radical Fukui functions, their classification contingent on the incoming reagent and the specific attack site. This classification enables FF to meticulously characterize individual atoms within a molecule where reactions might take place.
While this approach has proven incredibly valuable and achieved significant success in the context of small molecules, its application to larger molecular systems poses challenges. This is due to the tendency for the resulting values to become exceedingly minute, making it arduous to differentiate among them.
In CDFT, the dual descriptor, often abbreviated as DD, stands as a powerful tool utilized for the examination of molecule reactivity [
72,
73]. This descriptor furnishes a quantification of a molecule’s nucleophilic and electrophilic traits, thus establishing its worth as a predictive instrument in anticipating chemical reactivity.
The derivation of the dual descriptor DD emanates from the frontier molecular orbitals, namely, the HOMO and LUMO of the molecule. çVia the HOMO and LUMO, one can obtain the corresponding electrophilic and nucleophilic Fukui functions, which in turn allow for the computation of the dual descriptor DD [
72,
73,
74,
75].
The dual descriptor DD boasts an array of chemistry applications, encompassing catalyst design, chemical reaction prognosis, and chemical reactivity analysis. This tool serves as a valuable asset for comprehending the electronic structure of molecules, enabling the prediction of their conduct in chemical reactions.
By harnessing the dual descriptor DD, one can foresee a molecule’s reactivity toward nucleophilic or electrophilic assaults. A markedly positive value of this descriptor signifies the molecule’s proficiency as an electrophile, while a notably negative value suggests its competence as a nucleophile [
72,
73,
74,
75].
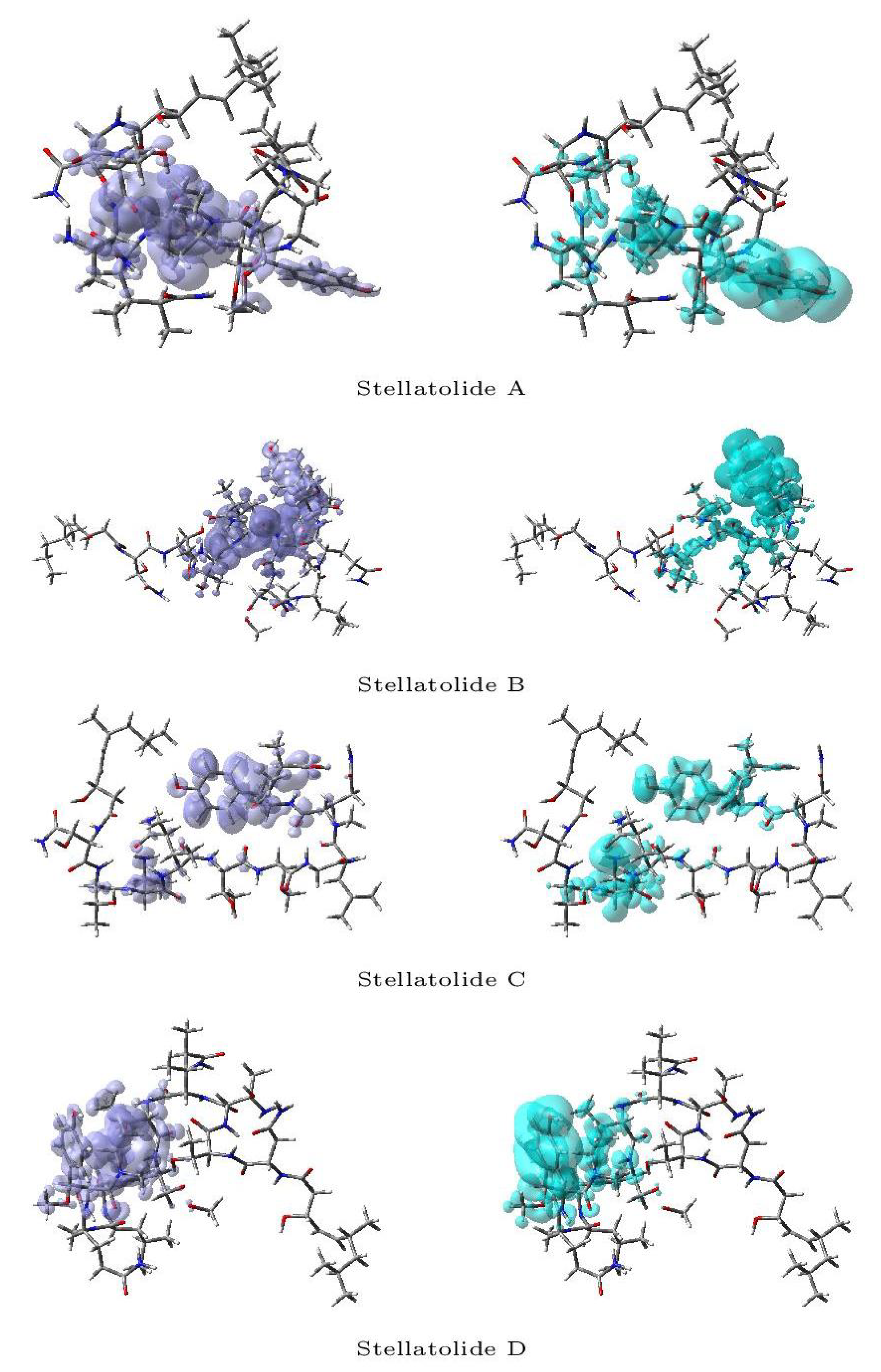
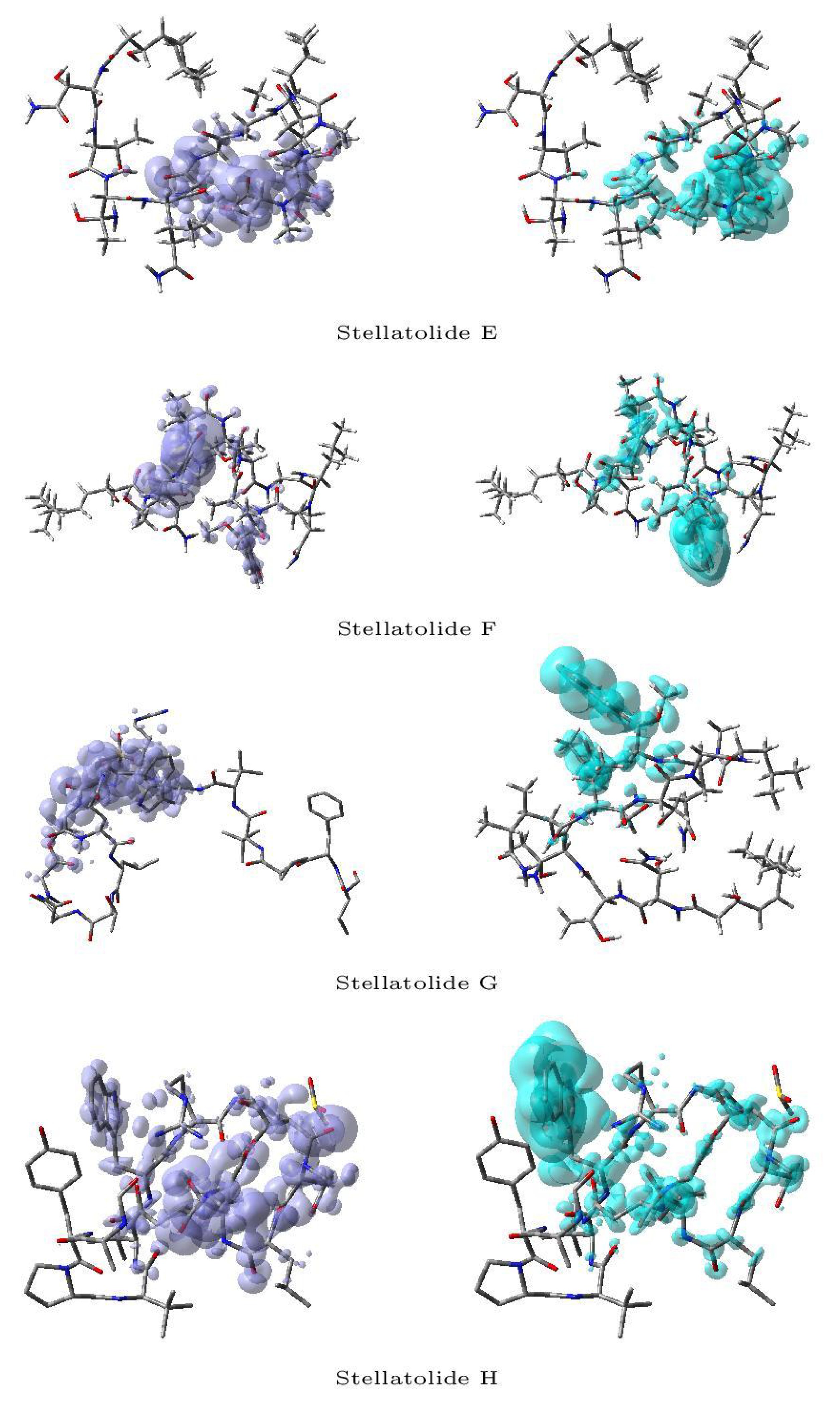
Figure 3 and
Figure 4 display a graphical representation of the dual descriptor DD of the Stellatolides A-H in a separate way: the left pictures indicates the zones where DD > 0, while the right ones corresponds to the areas where DD < 0.
Upon reviewing
Figure 3 and
Figure 4, it becomes evident that it is indeed possible to discern specific regions within the peptides that are prone to susceptibility towards either nucleophilic or electrophilic attacks. This capability proves to be immensely valuable, as it lays the groundwork for the prospective evaluation of these molecular systems as potential therapeutic agents.
The online platform for open bioactivity prediction, such as Molinspiration by Molinspiration Cheminformatics in Slovensky Grob, Slovak Republic, has proven instrumental in assessing molecular properties and bioactivity scores. This is achieved through an analysis of the chemicals in question, utilizing the Simplified Molecular Input Line Entry Specification (SMILES) notation. Molinspiration serves as an adept system for calculating drug-likeness information, while SwissTargetPrediction also contributes to this endeavor [
61]. Notably, SwissTargetPrediction aids in predicting various targets, including g-protein coupled receptors (GPCR) ligands, ion channel modulators, kinase inhibitors, nuclear receptor ligands, protease inhibitors, and other enzyme targets (
http://www.molinspiration.com/cgi-bin/properties).
In line with the information garnered from a CDFT analysis to gauge the chemical reactivity of the studied marine cyclopeptides,
Table 3 furnishes bioactivity scores for these peptides across a spectrum of targets. These encompass GPCR ligands, ion channel modulators, nuclear receptor ligands, kinase inhibitors, protease inhibitors, and enzyme inhibitors. These scores play a pivotal role in predicting the peptides’ corresponding biological targets, an indispensable facet of drug development that offers profound insights into the potential therapeutic applications of these compounds [
46].
The bioactivity scores associated with the analyzed cyclopeptides can be comprehended in the following manner: considered active when the bioactivity score exceeds 0, categorized as moderately active if the score falls within the range of -5.0 to 0.0, and deemed inactive when the bioactivity score is below -5.0. This categorization system allows for a clear interpretation of the cyclopeptides’ bioactivity levels.
ADMET properties, standing for Absorption, Distribution, Metabolism, Excretion, and Toxicity, play a pivotal role in the realm of drug discovery. These properties collectively determine how a potential drug candidate interacts within the human body, influencing its efficacy and safety profile [
43,
44,
46].
Computational prediction of ADMET properties has emerged as an indispensable tool in the drug discovery process. Traditional experimental methods for assessing these properties are time-consuming, costly, and often carried out in later stages of drug development. In contrast, computational models utilize data-driven algorithms to swiftly evaluate a compound’s ADMET characteristics, even in the early stages of drug design.
By harnessing computational approaches, researchers can rapidly analyze vast chemical libraries to identify promising candidates with favorable ADMET profiles. This accelerates the drug discovery timeline and reduces the likelihood of investing resources into compounds that might encounter significant hurdles in clinical trials due to unfavorable ADMET properties.
Furthermore, computational prediction assists in identifying potential toxicity issues, enabling researchers to eliminate compounds with a higher risk of adverse effects. This proactive strategy not only minimizes the chances of late-stage failures but also enhances patient safety.
In summary, the computational prediction of ADMET properties revolutionizes the drug discovery landscape by providing timely insights into a compound’s behavior within the body. This predictive power not only expedites the identification of viable drug candidates but also contributes to more efficient, cost-effective, and safer drug development processes.
The ADMET parameters for the eight members of the Stellatolides A-H family estimated by considering the SwissADME [
62] and pkCSM [
63] software are displayed in
Table 4,
Table 5,
Table 6,
Table 7 and
Table 8:
From
Table 4 it can be seen that the values of the excretion parameters are the same for the eight cyclopeptides with the exception of Caco-2 Permeability.
The Caco-2 cell line plays a pivotal role in the field of ADMET within the realm of drug development. Caco-2 cells are derived from human colon carcinoma and are widely used as an in vitro model to simulate the behavior of the intestinal epithelium, specifically for drug absorption studies [
43,
44,
46]. These cells form a monolayer that mimics the intestinal lining, allowing researchers to assess how well a drug can be absorbed through the gastrointestinal tract. This information is crucial because a drug’s bioavailability, or the extent to which it reaches systemic circulation, heavily depends on its ability to be absorbed by the intestines. In drug development, Caco-2 assays are employed to predict a drug’s potential for oral absorption thus allowing the estimation of the likelihood of a drug being absorbed efficiently or encountering obstacles due to efflux transporters. This information guides medicinal chemists and pharmaceutical scientists in optimizing drug structures to enhance their bioavailability. In this regards, Stellatolides C, D and G excels over the others, while Stellatolide H presents a very different small value.
Distribution is a critical aspect of ADMET. Once a drug is absorbed into the bloodstream, it needs to be effectively distributed to its target tissues and organs to exert its therapeutic effects. Understanding a drug’s distribution properties is essential for optimizing its efficacy and minimizing potential side effects. Distribution can also be influenced by factors like tissue perfusion rates and the presence of drug transporters. Tissues with high blood flow receive more drug, while those with low blood flow receive less [
43,
44,
46].
Volume of Distribution at Steady State (VDss) is a pharmacokinetic parameter used in the field of clinical pharmacology to describe the theoretical volume into which a drug would need to distribute in order to account for its total amount in the body at steady state concentration. VDss provides valuable insights into a drug’s distribution characteristics within the human body. The concept of VDss is particularly useful for understanding how extensively a drug distributes beyond the bloodstream into various tissues and compartments. It helps determine whether a drug tends to stay predominantly within the blood plasma or if it has a tendency to accumulate in specific tissues or organs. VDss is a crucial parameter in drug dosing calculations. It aids in determining the appropriate dosage needed to achieve the desired therapeutic effect based on the drug’s distribution characteristics. Drugs with high VDss may require larger loading doses to rapidly establish therapeutic levels, while drugs with low VDss may need lower loading doses due to their tendency to remain in the bloodstream [
44].
Fraction unbound, also known as the free fraction or unbound fraction, is a crucial concept in the field of ADMET within drug development. It refers to the proportion of a drug that exists in its pharmacologically active, unbound form within the bloodstream. In pharmacology, only the unbound fraction of a drug is capable of interacting with its target receptors and exerting therapeutic effects. The fraction unbound is particularly relevant in understanding a drug’s distribution, as only the unbound drug can pass through cell membranes and diffuse into tissues to reach its target site.
Blood-Brain Barrier (BBB) permeability is a fundamental concept in neuroscience and pharmacology, playing a critical role in understanding how substances, including drugs, interact with the central nervous system (CNS). The BBB is a specialized barrier that separates the bloodstream from the brain and spinal cord, tightly regulating the passage of molecules between these two compartments. Understanding BBB permeability is of utmost importance in drug development, especially for drugs targeting neurological disorders. A drug’s ability to cross the BBB is critical for it to exert therapeutic effects on the brain. Some drugs are designed to have high BBB permeability, while others are engineered to stay outside the CNS to prevent unwanted side effects.
Central Nervous System (CNS) permeability refers to the ability of substances, particularly drugs, to cross the blood-brain barrier (BBB) and gain access to the brain and spinal cord. The CNS is a highly protected and vital area of the body, and its permeability properties significantly influence the efficacy of drugs targeting neurological disorders. CNS permeability is a pivotal consideration in drug development for neurological disorders. Understanding the factors influencing CNS permeability and designing drugs that can effectively cross the blood-brain barrier are essential for developing treatments that target the central nervous system with precision and efficacy.
In summary, distribution properties play a vital role in determining a drug’s effectiveness and potential side effects. By studying how a drug is distributed within the body, researchers can optimize drug formulations, dosing regimens, and delivery strategies to ensure that the right concentration reaches the intended target while minimizing the risk of adverse effects in non-target tissues. Thus, the results from
Table 5 may be of great when developing therapeutic drugs through the consideration of Stellatolides A-H.
Metabolism properties are a crucial aspect of ADMET studies in the field of drug development [
43,
44,
46]. Metabolism refers to the biochemical processes that transform a drug into different compounds, often resulting in its inactivation and elimination from the body. Understanding a drug’s metabolism properties is essential for optimizing its efficacy, safety, and dosing regimens. Drug metabolism primarily occurs in the liver, where enzymes break down drugs into metabolites that are more water-soluble and can be excreted through urine or bile.
From
Table 6, it can be appreciated that all the studied cyclopeptides will display a negative behavior on being either substrate or inhibitor of different cytochrome enzymes.
Excretion is one of the key properties within ADMET that focuses on the elimination of drugs and their metabolites from the body. This process primarily occurs through the kidneys via urine, but can also involve other routes like bile, sweat, and exhaled air. The excretion property of ADMET plays a pivotal role in determining a drug’s dosing regimen and potential toxicity. Efficient excretion helps maintain appropriate drug levels in the body, preventing accumulation that could lead to adverse effects. Impaired excretion, on the other hand, can lead to drug accumulation, prolonged therapeutic effects, or even harmful side effects [
43,
44,
46].
Clearance is a fundamental pharmacokinetic parameter as it directly influences a drug’s dosing regimen. It helps determine how often a drug needs to be administered to maintain therapeutic levels in the body and avoid toxicity due to drug accumulation. Total clearance takes into account two primary processes: renal clearance and hepatic clearance. Renal clearance refers to the removal of a drug via the kidneys, primarily through urine. Hepatic clearance involves the metabolism and subsequent elimination of a drug by the liver. These two processes are often the dominant contributors to a drug’s total clearance.
Renal excretion is especially crucial, as the kidneys filter the bloodstream to eliminate drug molecules. This filtration process is influenced by factors such as the drug’s size, charge, and lipid solubility. Active transporters in the kidneys can also affect excretion, leading to variations in drug clearance among individuals. Renal OCT2 (Organic Cation Transporter 2) substrate refers to a drug or compound that can be transported into and out of cells in the kidneys by the OCT2 transporter protein. OCT2 is a type of transporter that plays a crucial role in the renal excretion of various organic cations, which are positively charged molecules.
It can be seen from
Table 7, that all the cyclopeptides present a negative behavior for being a Renal OC2 susbtrate. On the contrary, they display different activity under the Total Clearence test, with Stellatolides F and G showing positive values while they are negative for all the others.
Toxicity is a significant aspect of the ADMET framework that focuses on assessing the potential harmful effects of a drug or compound on living organisms [
43,
44,
46]. It plays a critical role in drug development, as understanding and mitigating potential toxicities is essential for ensuring the safety and efficacy of pharmaceutical products. Toxicity can manifest in various ways, including adverse effects on organs, tissues, cells, and biochemical processes. There are different types of toxicity, such as acute toxicity (rapid and severe effects shortly after exposure) and chronic toxicity (long-term and cumulative effects). The toxic effects can be dose-dependent, where higher doses lead to more severe reactions, or idiosyncratic, occurring unpredictably in certain individuals due to genetic variations. In summary, toxicity assessment within the ADMET framework is crucial for identifying and managing potential harmful effects of drugs and compounds. It guides drug development by ensuring the safety of pharmaceutical products and contributing to better patient outcomes. Thus, we believe that the results from
Table 8 will be of great help to know in advance the possible toxicities of the Stellatolides A-H to guide future developments of therapeutic drugs based in the marine cyclopeptides.