1. Introduction
The Low Cardiac Output Syndrome (LCOS) is a clinical condition caused by a transient decrease in systemic perfusion secondary to a myocardial dysfunction. The result is an oxygen delivery (DO
2) /oxygen consumption (VO2) uncoupling, eventually leading to metabolic acidosis. [
1]
This syndrome is defined by a cardiac index (CI) lower than 2 L/min/m
2, in association with signs of tissue hypoperfusion (cold extremities, mottling, oliguria, high lactate levels) but with no signs of hypovolemia, requiring inotropic drugs or mechanical circulatory support, i.e. extracorporeal membrane oxygenation (ECMO). [
2,
3]
LCOS is most frequently observed in patients undergoing cardiopulmonary bypass (CPB) during cardiac surgery for correction of a congenital heart disease. Literature reports a 25-60% incidence of LCOS in pediatric cardiac surgery patients. [
3,
4,
5]
Causes of LCOS can include myocardial ischemia due to aortic cross-clamping, residual effects of cardioplegia, CPB-induced myocardial disfunction and inflammatory pathway activation due to the exposure of blood to foreign surfaces during CPB. [
6]
Early recognition and treatment of LCOS are crucial in order to minimize perioperative related morbidity and mortality.
CI measurement can result difficult in pediatric patients through thermodilution due to both the device size and the peculiar cardiovascular pathophysiology of little patients with congenital heart disease, hence the interest to define a reliable marker of LCOS in this subgroup of patients. CI measurement can result difficult in pediatric patients through thermodilution due to both the device size and the peculiar cardiovascular pathophysiology of little patients with congenital heart disease, hence the interest to define a reliable marker of LCOS in this subgroup of patients. Another aspect that must be consider in this type of patients, which adds difficulties in CI measurement, is that the definition “pediatric heart defects” encompass a large spectrum of pathologies with different physiopathological implications. These are normally divided in two big categories considering the clinical presence of cyanosis and the characteristics of pulmonary circulation: cyanotic and acyanotic defects.
Table 1 summarizes the principal defects.
A few recent studies [
7,
8] have identified veno-arterial carbon dioxide difference/arterial-venous oxygen difference ratio [P(v-a)CO
2/C(a-v)O
2] as an index of tissue hypoxia caused by acute circulatory failure. The physiological rationale is that, according to Fick’s equation, oxygen consumption (VO2) and CO2 production (VCO2) are proportional to cardiac output. Under normal conditions VCO2, VO2 and venous to arterial CO2 content (CvCO2-CaCO2) and arterial to venous O2 content (CaO2-CvO2) are comparable. Hence, VCO2 should not exceed O2 production, and the respiratory exchange ratio should not exceed 1. The relationship appears as follows: CO x (CvCO2 – CaCO2)/CO x (CaO2 – CvO2).
Since cardiac output is present in both the numerator and denominator the ratio is: C(v-a)CO2/C(a-v)O2. In steady state conditions is it possible to replace CO2 concentrations with partial pressure obtaining the P(v-a)CO2/C(a-v)O2 ratio.
It has been speculated that this ratio could be used as a marker of global tissue hypoxia in adult patients undergoing cardiac surgery. The Authors demonstrated that this ratio might be used as a prognostic tool with better relevance when compared to other commonly used perfusion indexes (lactates, central venous oxygen saturation [ScVO
2], Oxygen Extraction Ratio [ERO
2], P(v-a)CO
2, anion gap, prolonged capillary refill time). A ratio higher of 1.6 mmHg/mL is considered predictive of oxygen supply dependency [
7,
8].
The aim of this study was to evaluate the possible use of P(v-a)CO2/C(a-v)O2 ratio as a marker of tissue hypoxia in pediatric cardiac patients undergoing cardiac surgery and/or requiringECMO.
2. Materials and Methods
This study included pediatric patients (0-14 years of age) having undergone cardiac surgery with or without postcardiotomy mechanical support between June 2018 and October 2020 and being subsequently admitted to Cardiothoracic Surgery Intensive Care Unit at the University Hospital Integrated Trust of Verona, Italy. All subjects belonged to the REINSURE-ARDS registry, a prospective registry of patients requiring intensive care unit (ICU) admission for any form of respiratory failure (Prog 1946CESC, Prot 72485 12/11/2018, amendment 26/02/2021). Patient identification remained anonymous, all participants’ legal representative provided informed consent before inclusion to the registry and for the use of their clinical and biological data.
No exclusion criteria were identified.
Cardiac surgery procedures include either full correction operations and temporary or long-term palliative solutions.
The following data were collected:
Age and anthropometric measurements (weight and lenght)
Type of congenital heart disease (both cyanotic and acyanotic) and type of surgery
Pre- and post-surgery laboratory tests (serum creatinine and hemoglobin)
Intraoperative data (length of CPB, need for transfusions, renal and cerebral near infrared spectroscopy [NIRS] and body temperature)
Post operative data (hemodynamic instability, renal and cerebral near infrared spectroscopy [NIRS] and body temperature, vasopressor/inotrope infusion, need for mechanical circulation support)
At anesthesia induction (pre-CPB).
Arterial and central venous blood gas samples were analyzed using a point of care gas analyzer (GEM 4000,Instrumentation Laboratory, Bedford, MA, USA)
5 markers of tissue perfusion were taken into account: lactate, ScVO2, ERO2, ΔP(v-a)CO2and P(v-a)CO2/C(a-v)O2 ratio.
The value of[P(v-a)CO2/C(a-v)O2] is calculated from the O2-derived and CO2-derived variables. The calculation formulas are as follows:
CaO2= SaO2 x Hb x 1.34 + PaO2 x 0.0031
CVO2= ScVO2 x Hb x 1.34 + PVO2 x 0.0031
C(a-v)O2= CaO2 – CvO2
P(v-a)CO2= PvCO2 – PaCO2
P(v-a)CO2/C(a-v)O2= (PcvCO2 – PaCO2)/(CaO2 – CcVO2)
The primary outcome was to assess the ability of the P(v-a)CO2/C(a-v)O2 ratioto predict tissue hypoxia in the pediatric cardiac population. Secondary outcome was to evaluate the relationship of other 5 different perfusion markers at different sampling times with the development of LCOS.
The incidence of LCOS was evaluated anddefined by hemodynamic instability with the need of high dose of vasopressors/inotropes (defined as milrinone >0.25 mcg/kg/min, dopamine >3 mcg/kg/min, adrenaline >0.05 mcg/kg/min, dobutamine >5 mcg/kg/min, noradrenaline >0.1 mcg/kg/min).
Statistically significant differences were evaluated with Fisher’s exact test for nominal variables.
A Wilcoxon signed-rank test (Mann-Whitney) was also used to evaluate the relationship between markers and LCOS development. A non-parametric test was chosen due to the asymmetrical distribution of variables. Main effects and interaction plot graphs were used to complete the analysis. The statistical analysis was performed with Minitab 19.
3. Results
The study included 98 pediatric patients undergoing corrective cardiac surgery for congenital heart defects.
Table 2 shows the demographic data of the population.
Mean age, expressed in months, was 32.0 ± 48.0 SD (standard deviation).
All congenital heart defects (CHD) were included in the study. In our sample 35 patients were affected by a cyanotic heart defect and 63 patients by acyanotic heart defect (
Table 3).
LCOS was detected in 46.9% cases. The incidence of LCOS was significantly higher in cyanotic heart defects compared to non-cyanotic ones (74.2 % vs 31.7%, p value <0.05).
The concentrations of 5 different perfusion markers at the different sampling times were evaluated so as their relationship with the development of LCOS, both in the whole studied population and in the two sub-groups (cyanotic and acyanotic CHD).
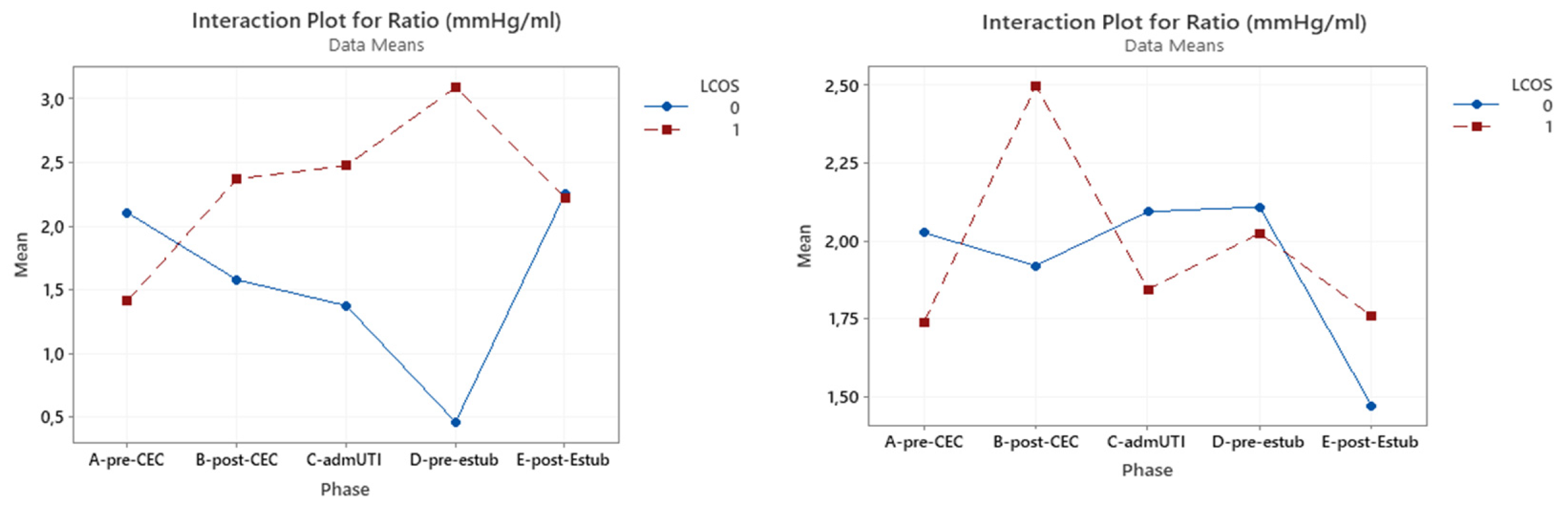
3.1. P(v-a)CO2/C(a-v)O2 ratio
P(v-a)CO
2/C(a-v)O
2 ratio have only a statistical significant effect in the whole group in the pre-CBP phase (p=0.023) (
Figure 1).
Nonetheless, an interaction was found between the onset of LCOS and the pre-CPB and post-CPB phases, with a 31% increase in patients who developed LCOS and a 17% decrease in patients who do not develop LCOS (
Table 4).
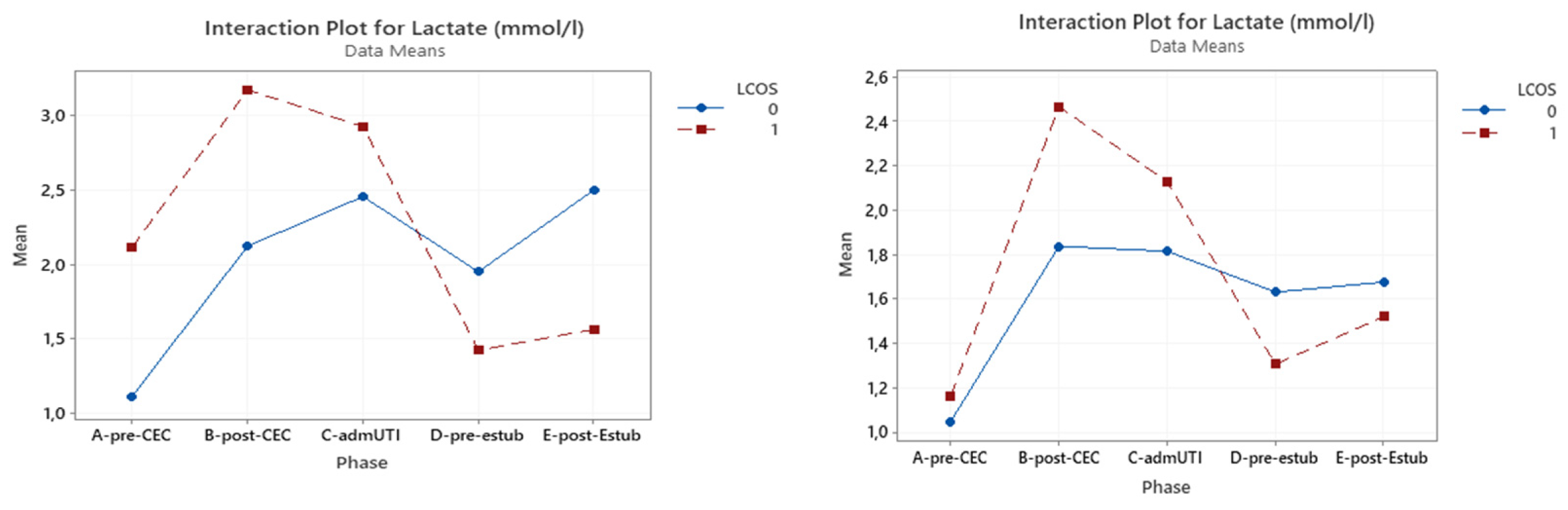
3.2. Serum Lactates
Table S-I (Supplementary Material) illustrates the correlation between serum lactate levels and LCOS development in the whole population, which was significant pre-CPB (p = 0.048), post-CBP (p = 0.001) and at ICU admission (p = 0.026). In the cyanotic CHD group a statistical significance was reached only pre-CPB (p = 0.001), while this was found only post-CPB (p = 0.013) in the acyanotic CHD group.
Serum lactates tend to be higher in patients with LCOS and for the cyanotic CHD group. This difference is statistically significant until ICU admission only in the entire population group .
Figure 2 shows the interaction plot of lactate level in relation to LCOS onset, subpopulation (cyanotic CHD and acyanotic CHD) and sampling time.
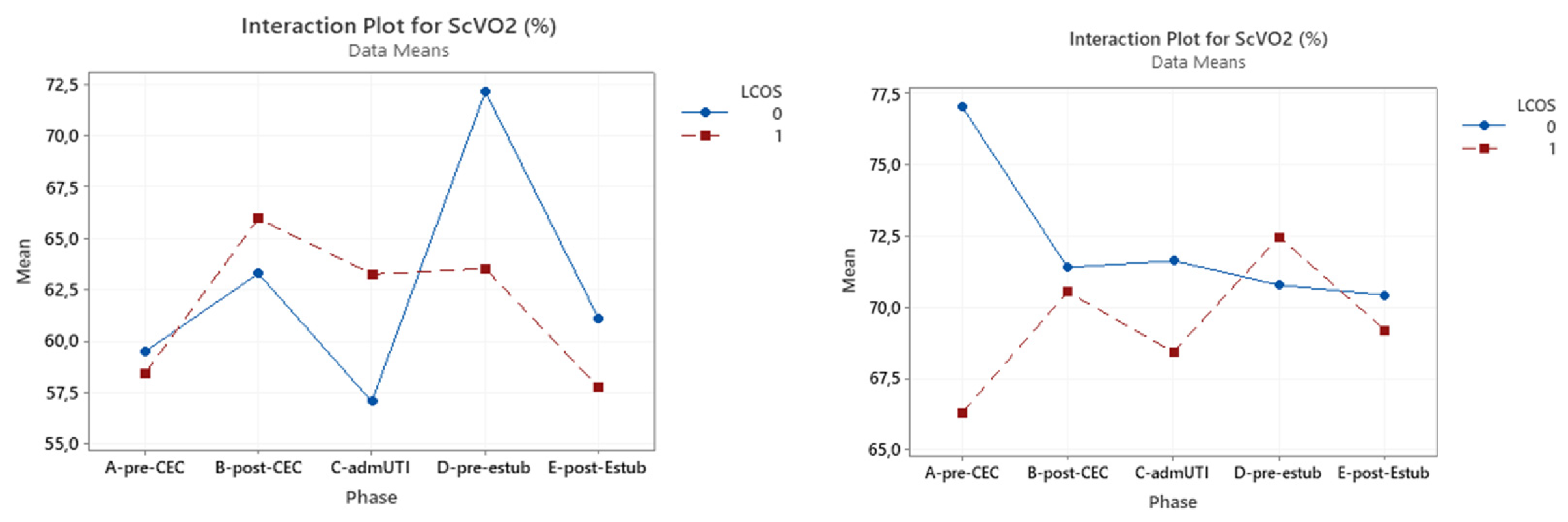
3.3. ScVO2
A significant correlation was found between ScVO2 with LCOS development pre-CPB (p <0.0001) and after extubation (p = 0.05) .
In the cyanotic group this marker did not reach statistical significance at any evaluated time, while this was reached in the acyanotic group in the pre-CPB sampling (p = 0.002). (
Table 6)(
Figure 3).
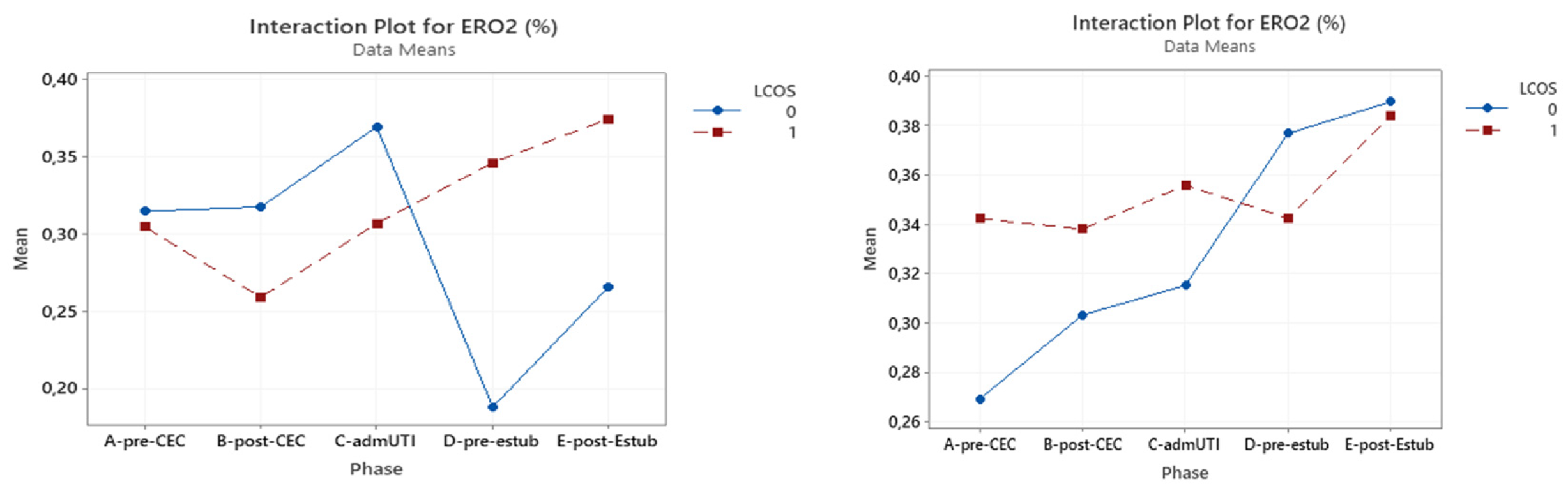
3.4. ERO2
Within the whole studied population, reached statistical significance for prediction of LCOS development only pre-CPB (p = 0.027). This was confirmed in LCOS patients of the acyanotic group (p = 0.013), but ERO
2 did not have a significant impact in the cyanotic CHD group.(
Table 7) (
Figure 4)
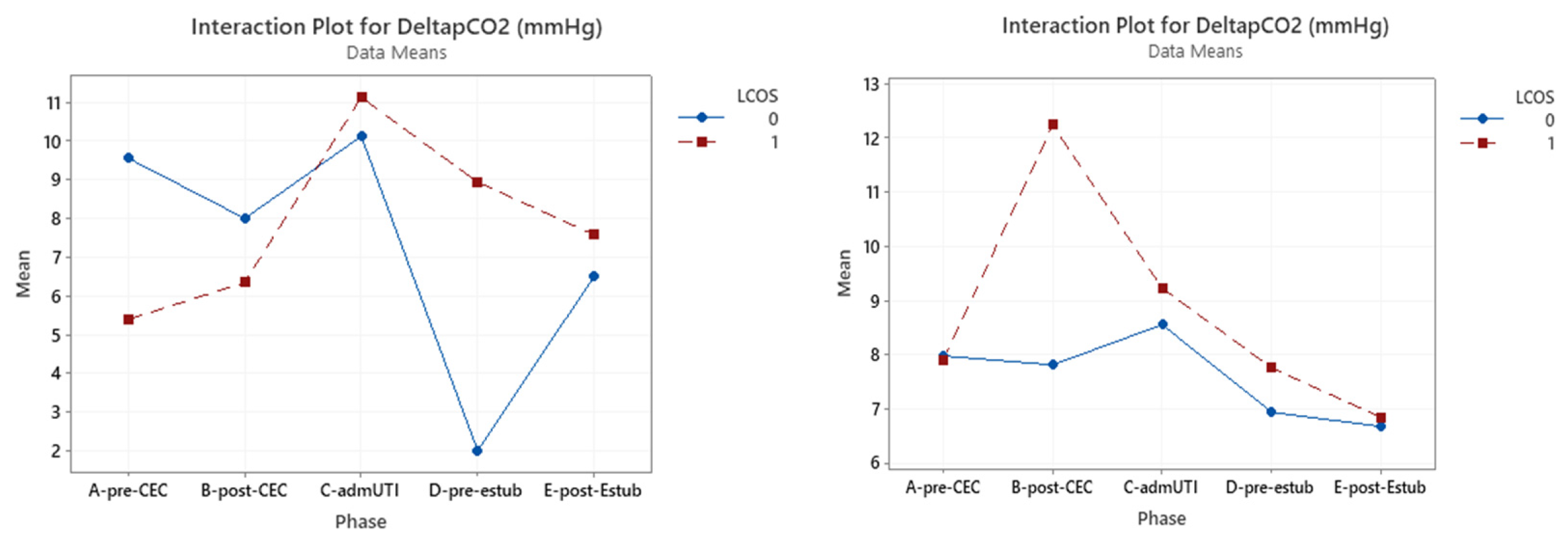
3.5. ΔP(v-a)CO2
ΔP(v-a)CO
2was significant in pre-CBP considering the whole group (p=0.034).In the cyanotic group only in the pre-CBP phase a statistically significance was reached (p=0.018), while in the acyanotic group this result was obtained in the post-CBP phase (p=0.013). (
Table 8)(
Figure 5)
4. Discussion
Organ perfusion monitoring is crucial in the management of acute circulatory failure and its evaluation needs to be both clinical and biological. The ideal marker of organ perfusion should be quick, not invasive and easy to measure. The aim of this study was to assess the possible role of some commonly used perfusion markers (such as serum lactate and ScVO2) and some others more seldom used (ERO2, ΔP(v-a)CO2, ratio ΔP(v-a)CO2/ΔC(a-v)O2 ) to identify the onset of LCOS and of an anaerobic state.
LCOS early recognition and treatment are crucial in order to avoid ischemic tissue damage and multiorgan failure.
Serum lactate concentrations are frequently used to detect an anaerobic state, but their rise is often delayed and therefore inadequate. Lactate levels are often inappropriate in the cardiac surgery setting as hyperlactatemia may occur as a consequence of surgical stress, use of beta-adrenergic drugs [
9] or lactate lung production [
10]. Within surgical stress lactate washout is indeed reduced and production is enhanced by the anaerobic glycolysis pathway. [
11] Moreover, the surgery related blood flow improvement, especially for renal and coronary perfusion, is associated with higher lactate washout with consequent increased serum levels [
12,
13]. Nonetheless, high and persistent lactate levels are not justified by these mechanisms.
This study shows that increased serum lactate concentrations are associated with the development of LCOS in the pre- and after-CPB and ICU admission samples. Therefore, we can define lactate as a reliable LCOS predictor, even though interaction curves might suggest an influence of CPB.
ScVO2 is another commonly used marker of tissue hypoxia. This marker has been widely used in pediatric cardiac surgery for a long time, but its use is limited by residual interatrial or intraventricular shunt. We observed a significant association between LCOS and ScVO2 in pre-CPB and post extubation samples in the whole population and in the pre-CPB in the acyanotic CHD. CPB seems to have a different impact on ScVO2 values according to the child’s cardiopathy, decreasing in the acyanotic one and increasing in the cyanotic group. This might be ascribable to an improved perfusion in the cyanotic group.
ERO2 expresses the relationship between DO2 and VO. When CO is significantly reduced (e.g., blood loss, tamponade, anemia or hypoxia), an increase in ERO2 allows VO2 to remain stable until DO2 lowers to the critical cut-off and an anaerobic state is initiated. We found indeed that LCOS was significantly related with this marker only pre-CPB within the whole population and in the acyanotic cohort.
Recently, the Δ(v-a) CO
2 has been proposed as a marker of hypoperfusion.[
14] It correlates with CO in adult critically ill patients, being its increase associated with lower CO and higher morbidity. [
15] Little is known about the utility of ΔPCO
2 in the monitoring of pediatric patients after cardiac surgery. In pediatric patients a relationship between ΔPCO
2 and ScVO
2 has been demonstrated after complete reparation of cardiac defects [
16]. Rhodes et al. recently studied the ability of ΔPCO
2 to predict a poor outcome associated with LCOS in children, concluding that this marker correlates with both cardiac output and oxygen delivery and it may identify patients at risk for important morbidity immediately upon admission in ICU. [
17] Within our study ΔPCO
2 could predict LCOS in the whole population and in the cyanotic CHD group in the pre-CBP phase,it was significantly associated also with LCOS in the post-CPB sample in the acyanotic group. Therefore, it might be an important prognostic tool in this specific groups of patients.
ΔP(v-a)CO
2/ΔC(a-v)O
2 has been demonstrated to be more sensitive than serum lactate levels thanks to the shorter timeframe for signs of hypoperfusion, and more specific as it can detect situations leading to an increase in serum lactates not depending on hypoxia. [
15] Nevertheless, it was not a reliable marker of anaerobic metabolism in our pediatric cardiac population and, therefore, this ratio could not be suggested to predict LCOS in this setting. Our findings are not aligned with the current available literature: Monnet et al. [
7] and Du et al. [
8] demonstrated that this ratio is a marker of anaerobic metabolism, hence apt to predict an adequate response to a DO
2 challenge. Howewer, these studies did not evaluate the ratio as a prognostication marker for LCOS onset.
Patel et al. [
18] consideredΔP(v-a)CO
2/ΔC(a-v)O
2 as a tool to predict the onset of anaerobic metabolism during CPB, Similarly, Mekontso et al [
19] showed its ability to detect anaerobic metabolism in a more reliable way when compared to other indexes (SaO
2, ScVO
2, ERO
2, ΔPCO
2), but none of them investigate its ability to predict and/or detect LCOS. Recently, Dubin et al. [
20] found that ΔP(v-a)CO
2/ΔC(a-v)O
2 is not a reliable marker of anaerobic metabolism in the case of hemodilution. Due to their reduced circulating blood volume and to the need of pre-filling the bypass circuit, pediatric patients are potentially more exposed than adults to hemodilution.
Our results should be interpreted in the light of this last finding. Indeed, the ratio inability to adequately reflect a reduction in blood flow might be due to the fact that in a situation of hemodilution this marker is primarily influenced by hemoglobin rather than by anaerobiosis.
Despite not significant, patients with low CO during CPB presented higher ratio values and, in these patients, an increasing trend can also be observed in the following phases suggesting hypoperfusion during and after CBP. This means that the increase in ratio values during and following CPB can suggest that the patient might be poorly perfused.
Serum lactates are confirmed to be a good marker of hypoperfusion also at the end of CPB and at ICU admission, even though its trend might be amplified by CPB itself.
On the other hand, ScVO2 is a reliable indicator of low perfusion even after extubation.
The complexity of hemodynamic monitoring in patients following cardiac surgery and its need to embrace multiple sets of parameters rather than just following a single biomarker trend has recently been studied by Hong et al [
21] who successfully developed several machines learning models to predict LCOS onset after surgery. In its study he demonstrated that as many as 11 parameters (between biomarkers, echocardiography and clinical indicators) were necessary to successfully predict LCOS onset in a cohort of adults patients undergoing cardiac surgery.
Another recent study [
22] demonstrated the positive correlation between a score composed by 8 items (namely ejection fraction, oliguria, fluid bolus need, capillary refill time, inotrope requirements, arterial lactates and cerebral NIRS) and the length of hospital stay, ICU length of stay, vasoactive-inotropic score, lactate mean and aortic clamp duration in a population of 54 pediatric patients following cardiac surgery.
One single marker cannot detect hemodynamic instability and the evaluation of a critical patient must include a comprehensive approach (clinical course, vital signs, hemodynamic and tissue perfusion monitoring). For instance, after cardiac surgery hyperlactatemia is not exclusively a sign of only anaerobic metabolism, and neither do adequate values of ScVO2 show that resuscitation is being conducted adequately with absolute certainty. The ratio could be a useful parameter for physicians to detect LCOS only if considered together with clinical, laboratory and instrumental data collected during hemodynamic alterations in a critical care setting.
Moreover, the choice of the anticoagulation strategy during CPB or ECMO support could also play a role in defining the severity of hypoperfusion, affecting the development of complications. In the pediatric population, the use of bivalirudin comparedto heparin seems to be able to reduce the risk of bleeding and thrombosis. This could guarantee a better hemodynamic stability, with reduced need of resuscitation therapies and an improved microcirculatory perfusion. Recently, the use of Nafamostat Mesylate (NM), a new regional anticoagulant, has been proposed during ECMO assistance in pediatric and adult patients who had evidence of adverse events due to heparin therapy (resistance, heparin-induced thrombocytopenia, clinically uncontrolled bleeding) [
24].This serin-protease has shown pleiotropic effects like the ability, when administered before myocardial reperfusion in animal experimental models of cardiac infarction, to modulate the inflammatory response and reduce myocardial damage. The suppression of both complement-mediated response and neutrophil activation was associated with an advantage in terms of survival in ECMO patients treated with NM. Considering this evidence, it could be interesting to understand if the choice of the anticoagulation strategy could infactinfluence the onset of LCOS, identified through common markers of tissue perfusion.
This study has some limitations. First, the study was conducted on a small convenient sample of patients (even though comparable to other studies in the field) who presented a large variability for age and underlying cardiac defects.
Second, only patients who underwent open heart surgery with the use of CPB were studied. Third, practice variability with respect to sedative and cathecolamine administration may represent a confounder.
Finally, even if we have taken into account the capacity of 5 markers in predicting LCOS development, this study has not evaluated the capacity of capillary refill time (CRT)in predicting the onset of organ dysfunction.Capillary refill time is defined as “time required for return of color after application of blanching pressure to a distal capillary bed”. Nowadays a lot of literature evidence [
23,
24] suggests that a prolonged CRT (>3 seconds) could be used as a simple, rapid, reproducible and non-invasive parameter with a better ability than hemodynamic macroparameters in identifying microcirculatory hypoperfusion, predicting the severity of hemodynamic instability and the response to resuscitation therapies both in sepsis and cardiogenic shock. Even if it has been demonstrated as effective as conventional markers in adults, CRT presents some critical procedural aspects, for instance the site of measurement, the access to this site in many circumstances (for example surgery) and the position in which we have to perform it. These limitations could be even more exacerbated in the pediatric population. For these reasons and considering that we do not currently have guidelines that standardize its execution, its measurement is not a common clinical practice in ICU.
5. Conclusions
ΔP(v-a)CO2/ΔC(a-v)O2 is not able to predict LCOS onset in pediatric patients undergoing cardiac surgery, despite a rising trend during CPB in patients who then develop LCOS. Besides, rised serum lactate concentrations, reduced ScVO2 and increased ERO2 -measured in the pre-CPB phase- were significantly associated with LCOS onset, thus suggesting them as reliable low perfusion indexes before CPB.
We can conclude that in the evaluation of a cardiac pediatric patient a global approach is always to be preferred, taking into consideration clinical data and invasive and non- invasive monitoring. A comprehensive vision allows for a better outcome prediction compared to single values, despite their accuracy.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: illustrates the correlation between serum lactate levels and LCOS in the whole population.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Ethics committee:ComitatoEticoTerritorialeArea Sud-Ovest Veneto, Verona Hospital and University Trust, Italia, Italyprotocol code 72485 and date of approval on 12 November 2018.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available from the corresponding author on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Whitson BA. Commentary: Low cardiac output syndrome: A definition or a diagnosis code? J Thorac Cardiovasc Surg. 2022 May;163(5):1902-1903. Epub 2020 Sep 14. PMID: 33019969. [CrossRef]
- Algarni KD, Maganti M, Yau TM. Predictors of low cardiac output syndrome after isolated coronary artery bypass surgery: trends over 20 years. Ann Thorac Surg. 2011 Nov;92(5):1678-84. Epub 2011 Sep 21. PMID: 21939957. [CrossRef]
- Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, Tu T; Society for Cardiovascular Angiography and Interventions (SCAI); Heart Failure Society of America (HFSA); Society of Thoracic Surgeons (STS); American Heart Association (AHA), and American College of Cardiology (ACC). 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol. 2015 May 19;65(19):e7-e26. Epub 2015 Apr 7. PMID: 25861963. [CrossRef]
- Parr GV, Blackstone EH, Kirklin JW. Cardiac performance and mortality early after intracardiac surgery in infants and young children. Circulation. 1975 May;51(5):867-74. PMID: 235375. [CrossRef]
- Johnson, W.H., Pediatric Cardiology: The Essential Pocket Guide. Third edition, 2014: John Wiley and Sons.
- Bailey JM, Hoffman TM, Wessel DL, Nelson DP, Atz AM, Chang AC, Kulik TJ, Spray TL, Akbary A, Miller RP, Wernovsky G. A population pharmacokinetic analysis of milrinone in pediatric patients after cardiac surgery. J Pharmacokinet Pharmacodyn. 2004 Feb;31(1):43-59. PMID: 15346851. [CrossRef]
- Monnet X, Julien F, Ait-Hamou N, Lequoy M, Gosset C, Jozwiak M, Persichini R, Anguel N, Richard C, Teboul JL. Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med. 2013 Jun;41(6):1412-20. PMID: 23442986. [CrossRef]
- Du W, Long Y, Wang XT, Liu DW. The Use of the Ratio between the Veno-arterial Carbon Dioxide Difference and the Arterial-venous Oxygen Difference to Guide Resuscitation in Cardiac Surgery Patients with Hyperlactatemia and Normal Central Venous Oxygen Saturation. Chin Med J (Engl). 2015 May 20;128(10):1306-13. PMID: 25963349; PMCID: PMC4830308. [CrossRef]
- Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet. 2005 Mar 5-11;365(9462):871-5. Erratum in: Lancet. 2005 Jul 9-15;366(9480):122. PMID: 15752531. [CrossRef]
- Fink MP. Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit Care Clin. 2001 Jan;17(1):219-37. PMID: 11219231. [CrossRef]
- Svensson S, Svedjeholm R, Ekroth R, Milocco I, Nilsson F, Sabel KG, William-Olsson G. Trauma metabolism and the heart. Uptake of substrates and effects of insulin early after cardiac operations. J Thorac Cardiovasc Surg. 1990 Jun;99(6):1063-73. PMID: 2193199. [CrossRef]
- Rivers EP, Rady MY, Martin GB, Fenn NM, Smithline HA, Alexander ME, Nowak RM. Venous hyperoxia after cardiac arrest. Characterization of a defect in systemic oxygen utilization. Chest. 1992 Dec;102(6):1787-93. PMID: 1446489. [CrossRef]
- Leavy JA, Weil MH, Rackow EC. 'Lactate washout' following circulatory arrest. JAMA. 1988 Aug 5;260(5):662-4. PMID: 3392792. [CrossRef]
- van Beest PA, Lont MC, Holman ND, Loef B, Kuiper MA, Boerma EC. Central venous-arterial pCO₂ difference as a tool in resuscitation of septic patients. Intensive Care Med. 2013 Jun;39(6):1034-9. Epub 2013 Apr 5. PMID: 23559077. [CrossRef]
- Ospina-Tascón GA, Umaña M, Bermúdez W, Bautista-Rincón DF, Hernandez G, Bruhn A, Granados M, Salazar B, Arango-Dávila C, De Backer D. Combination of arterial lactatelevels and venous-arterial CO2 to arterial-venous O 2 content difference ratio as markers of resuscitation in patients with septic shock. Intensive Care Med. 2015 May;41(5):796-805. Epub 2015 Mar 20. PMID: 25792204; PMCID: PMC4414929. [CrossRef]
- Furqan M, Hashmat F, Amanullah M, Khan M, Durani HK, Anwar-ul-Haque. Venoarterial PCO2 difference: a marker of postoperative cardiac output in children with congenital heart disease. J Coll Physicians Surg Pak. 2009 Oct;19(10):640-3. PMID: 19811716. [CrossRef]
- Rhodes LA, Erwin WC, Borasino S, Cleveland DC, Alten JA. Central Venous to Arterial CO2 Difference After Cardiac Surgery in Infants and Neonates. Pediatr Crit Care Med. 2017 Mar;18(3):228-233. PMID: 28121832; PMCID: PMC5336489. [CrossRef]
- Patel R, Solanki A, Patel H, Patel J, Pandya H, Sharma J. Monitoring Microcirculatory Blood Flow during Cardiopulmonary Bypass in Paediatric Cardiac Surgery Patients as a Predictor for Anaerobic Metabolism. J Clin Diagn Res. 2017 Apr;11(4):UC22-UC25. Epub 2017 Apr 1. PMID: 28571240; PMCID: PMC5449886. [CrossRef]
- Mekontso-Dessap A, Castelain V, Anguel N, Bahloul M, Schauvliege F, Richard C, Teboul JL. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med. 2002 Mar;28(3):272-7. Epub 2002 Feb 8. PMID: 11904655. [CrossRef]
- Dubin A, Ferrara G, Kanoore Edul VS, Martins E, Canales HS, Canullán C, Murias G, Pozo MO, Estenssoro E. Venoarterial PCO2-to-arteriovenous oxygen content difference ratio is a poor surrogate for anaerobic metabolism in hemodilution: an experimental study. Ann Intensive Care. 2017 Dec;7(1):65. Epub 2017 Jun 12. PMID: 28608134; PMCID: PMC5468362. [CrossRef]
- Hong L, Xu H, Ge C, Tao H, Shen X, Song X, Guan D, Zhang C. Prediction of low cardiac output syndrome in patients following cardiac surgery using machine learning. Front Med (Lausanne). 2022 Aug 24;9:973147. PMID: 36091676; PMCID: PMC9448978. [CrossRef]
- Aslan N, Yıldızdaş D, Göçen U, Erdem S, Demir F, Yontem A, Horoz ÖÖ, Sertdemir Y. Çocuk yoğun bakım ünitesinde kardiyak cerrahi sonrası hastaların değerlendirilmesinde kullanılan düşük kardiyak debi sendromu skorlaması [Low cardiac output syndrome score to evaluate postoperative cardiac surgery patients in a pediatric intensive care unit]. Turk Kardiyol Dern Ars. 2020 Jul;48(5):504-513. Turkish. PMID: 32633258. [CrossRef]
- Li DH, Sun MW, Zhang JC, Zhang C, Deng L, Jiang H. Isbivalirudin an alternative anticoagulant for extracorporeal membrane oxygenation (ECMO) patients? A systematic review and meta-analysis. Thromb Res. 2022 Feb;210:53-62. Epub 2021 Dec 31. PMID: 35007937. [CrossRef]
- Sanfilippo F, Currò JM, La Via L, Dezio V, Martucci G, Brancati S, Murabito P, Pappalardo F, Astuto M. Use of nafamostatmesilate for anticoagulationduringextracorporeal membrane oxygenation: A systematic review. ArtificialOrgans. 2022;46;2371-2381. [CrossRef]
- La Via L, Sanfilippo F, Continella C, Triolo T, Messina A, Robba C, Astuto M, Hernandez G, Noto A. Agreement betweencapillary refill time measuredat finger and earlobesites in different positions: a pilot prospective study on healthyvolunteers. BMC Anesthesiology. 2023 Jan 18;23(1):30. [CrossRef]
- Merdji H, Curtiaud A, Aheto A, Studer A, Harjola VP, Monnier A, Duarte K, Girerd N, Kibler M, Ait-Oufella H, Helms J, Mebazaa A, Levy B, Kimmoun A, Meziani F. Performance of EarlyCapillary Refill Time Measurement on Outcomes in Cardiogenic Shock: An Observational, ProspectiveMulticentric Study. Am J RespirCrit Care Med. 2022 Nov 15;206(10):1230-1238. PMID: 35849736. [CrossRef]
Figure 1.
Interaction plot for P(v-a)CO2/C(a-v)O2 in relation to Low Cardiac Output Syndrome (LCOS) onset (0=no development of LCOS, 1=development of LCOS) in the two subgroups cyanotic (left) and acyanotic (right) at the different sampling times.. Abbreviations: A-pre-CEC: at anesthesia induction (pre-CPB), B-post-CEC: at CPB discontinuation, C-admUTI: upon intensive care unit admission , D-pre-estub: before extubation; E-post-Estub: after extubation.
Figure 1.
Interaction plot for P(v-a)CO2/C(a-v)O2 in relation to Low Cardiac Output Syndrome (LCOS) onset (0=no development of LCOS, 1=development of LCOS) in the two subgroups cyanotic (left) and acyanotic (right) at the different sampling times.. Abbreviations: A-pre-CEC: at anesthesia induction (pre-CPB), B-post-CEC: at CPB discontinuation, C-admUTI: upon intensive care unit admission , D-pre-estub: before extubation; E-post-Estub: after extubation.
Figure 2.
Interaction plot for lactate in relation to Low Cardiac Output Syndrome (LCOS) onset (0=no development of LCOS, 1=development of LCOS) in the two subgroups cyanotic (left) and acyanotic (right) at the different sampling times.Abbreviations: A-pre-CEC: at anesthesia induction (pre-CPB), B-post-CEC: at CPB discontinuation, C-admUTI: upon intensive care unit admission , D-pre-estub: before extubation; E-post-Estub: after extubation.
Figure 2.
Interaction plot for lactate in relation to Low Cardiac Output Syndrome (LCOS) onset (0=no development of LCOS, 1=development of LCOS) in the two subgroups cyanotic (left) and acyanotic (right) at the different sampling times.Abbreviations: A-pre-CEC: at anesthesia induction (pre-CPB), B-post-CEC: at CPB discontinuation, C-admUTI: upon intensive care unit admission , D-pre-estub: before extubation; E-post-Estub: after extubation.
Figure 3.
Interaction plot for ScVO2 in relation to Low Cardiac Output Syndrome (LCOS) onset (0=no development of LCOS, 1=development of LCOS) in the two subgroups cyanotic (left) and acyanotic (right) at the different sampling times.Abbreviations: A-pre-CEC: at anesthesia induction (pre-CPB), B-post-CEC: at CPB discontinuation, C-admUTI: upon intensive care unit admission , D-pre-estub: before extubation; E-post-Estub: after extubation.
Figure 3.
Interaction plot for ScVO2 in relation to Low Cardiac Output Syndrome (LCOS) onset (0=no development of LCOS, 1=development of LCOS) in the two subgroups cyanotic (left) and acyanotic (right) at the different sampling times.Abbreviations: A-pre-CEC: at anesthesia induction (pre-CPB), B-post-CEC: at CPB discontinuation, C-admUTI: upon intensive care unit admission , D-pre-estub: before extubation; E-post-Estub: after extubation.
Figure 4.
Interaction plot for ERO2 in relation to Low Cardiac Output Syndrome (LCOS) onset (0=no development of LCOS, 1=development of LCOS) in the two subgroups cyanotic (left) and acyanotic (right) at the different sampling times. Abbreviations: A-pre-CEC: at anesthesia induction (pre-CPB), B-post-CEC: at CPB discontinuation, C-admUTI: upon intensive care unit admission , D-pre-estub: before extubation; E-post-Estub: after extubation.
Figure 4.
Interaction plot for ERO2 in relation to Low Cardiac Output Syndrome (LCOS) onset (0=no development of LCOS, 1=development of LCOS) in the two subgroups cyanotic (left) and acyanotic (right) at the different sampling times. Abbreviations: A-pre-CEC: at anesthesia induction (pre-CPB), B-post-CEC: at CPB discontinuation, C-admUTI: upon intensive care unit admission , D-pre-estub: before extubation; E-post-Estub: after extubation.
Figure 5.
Interaction plot for ∆P(v – a)CO2 in relation to Low Cardiac Output Syndrome (LCOS) onset (0=no development of LCOS, 1=development of LCOS) in the two subgroups cyanotic (left) and acyanotic (right) at the different sampling times.. Abbreviations: A-pre-CEC: at anesthesia induction (pre-CPB), B-post-CEC: at CPB discontinuation, C-admUTI: upon intensive care unit admission , D-pre-estub: before extubation; E-post-Estub: after extubation.
Figure 5.
Interaction plot for ∆P(v – a)CO2 in relation to Low Cardiac Output Syndrome (LCOS) onset (0=no development of LCOS, 1=development of LCOS) in the two subgroups cyanotic (left) and acyanotic (right) at the different sampling times.. Abbreviations: A-pre-CEC: at anesthesia induction (pre-CPB), B-post-CEC: at CPB discontinuation, C-admUTI: upon intensive care unit admission , D-pre-estub: before extubation; E-post-Estub: after extubation.
Table 1.
Principal cardiac malformations.. Abbreviations: AS: aortic stenosis; ASD: atrial septum defect; AVSD: atrioventricular septum defect; COA: coartation; d-TGV: transposition of great arteries; PDA patent ductus arteriosus; TAPVR: Total anomalous pulmonary venous return; TOF Tetralogy of Fallot.
Table 1.
Principal cardiac malformations.. Abbreviations: AS: aortic stenosis; ASD: atrial septum defect; AVSD: atrioventricular septum defect; COA: coartation; d-TGV: transposition of great arteries; PDA patent ductus arteriosus; TAPVR: Total anomalous pulmonary venous return; TOF Tetralogy of Fallot.
| Pulmonary flow |
Acyanotic |
Cyanotic |
| Augmented |
Left to right shunt:
VSD, PDA, ASD, AVSD |
Commistion lesions:
d-TGA, TAPVR |
| Normal |
Obstructive lesions
AS, PS, COA, Cardiomyopathies |
- |
| Reduced |
- |
Pulmonary flow obstruction with septal defect:
TOF, tricuspidal atresia,
Ebstein malformation |
Table 2.
Patients’ Demographic Data.
Table 2.
Patients’ Demographic Data.
| Sex |
Male |
56,1 % (n=55) |
|
| Female |
43,9% (n=43) |
Age
(med±SD) |
32,0 ±48,0 months |
Male |
35,9 ±51,1 months |
| Female |
27,0 ±34,7 months |
Height
(med ±SD) |
82,9±35,4cm |
Male |
85,0±37,7cm |
| Female |
78,0±32,1cm |
Weight
(med ±SD) |
12,7±13,8 kg |
Male |
13,9 ±14,9 kg |
| Female |
11,0 ±12,1 kg |
Table 3.
Incidence of the different congenital heart defects and related incidence of Low Cardiac Output Syndrome (LCOS).
Table 3.
Incidence of the different congenital heart defects and related incidence of Low Cardiac Output Syndrome (LCOS).
| Population (n=98) |
LCOS |
| Acyanotic, n (%): 63 (62%) |
20 (31.7 %) |
| Cyanotic, n (%): 35 (38%) |
26 (74.2%) |
Table 4.
P(v-a)CO2/C(a-v)O2 values (mmHg/ml) at the different sampling times correlated with presence/absence of Low Cardiac Output Syndrome (LCOS). Abbreviations: LCOS (Low cardiac output syndrome), CPB (Cardiopulmonary bypass), ICU (Intensive Care Unit), SD (standard deviation), CHD (congenital heart defect).
Table 4.
P(v-a)CO2/C(a-v)O2 values (mmHg/ml) at the different sampling times correlated with presence/absence of Low Cardiac Output Syndrome (LCOS). Abbreviations: LCOS (Low cardiac output syndrome), CPB (Cardiopulmonary bypass), ICU (Intensive Care Unit), SD (standard deviation), CHD (congenital heart defect).
| |
|
Whole population |
|
Cyanotic
CHD |
|
Acyanotic
CHD |
|
| Sampling times |
LCOS |
Mean value
(SD)
mmHg/ml |
P-value |
Mean value
(SD)
mmHg/ml |
P-value |
Mean value
(SD)
mmHg/ml |
P-value |
| Pre-CPB |
Yes |
1,55 (1,00) |
0,023 |
1,41 (0,85) |
0,131 |
1,74 (1,18) |
0,286 |
| |
No |
2,05 (1,05) |
|
2,10 (1,22) |
|
2,06 (1,02) |
|
| Post-CPB |
Yes |
2,44 (2,94) |
0,5 |
2,40 (3,79) |
0,377 |
2,49 (1,33) |
0,167 |
| |
No |
1,87 (1,26) |
|
1,58 (0,91) |
|
1,92 (1,30) |
|
| ICU adm. |
Yes |
2,21 (1,67) |
0,256 |
2,47 (2,06) |
0,160 |
1,84 (0,82) |
0,990 |
| |
No |
1,95 (1,64) |
|
1,37 (0,94) |
|
2,09 (1,75) |
|
| Pre-ext. |
Yes |
2,61 (4,41) |
0,770 |
3,09 (5,86) |
- |
2,02 (1,24) |
0,958 |
| |
No |
2,04 (1,53) |
|
0,45 (-) |
|
2,11 (1,53) |
|
| Post-ext. |
Yes |
2,04 (1,74) |
0,239 |
2,22 (1,99) |
0,99 |
1,76 (1,32) |
0,586 |
| |
No |
1,52 (1,41) |
|
2,25 (2,22) |
|
1,47 (1,39) |
|
Table 6.
ScVO2 values (mmol/l) at the different sampling times correlated with presence/absence of Low Cardiac Output Syndrome (LCOS). Abbreviations: LCOS (Low cardiac output syndrome), CPB (Cardiopulmonary bypass), ICU (Intensive Care Unit), SD (standard deviation), CHD (congenital heart defect).
Table 6.
ScVO2 values (mmol/l) at the different sampling times correlated with presence/absence of Low Cardiac Output Syndrome (LCOS). Abbreviations: LCOS (Low cardiac output syndrome), CPB (Cardiopulmonary bypass), ICU (Intensive Care Unit), SD (standard deviation), CHD (congenital heart defect).
| |
|
Whole population |
|
Cyanotic CHD |
|
Acyanotic CHD |
|
| Samplingtimes |
LCOS |
Mean value (SD) mmol/l |
P-value |
Mean value (SD) mmol/l |
P-value |
Mean value (SD) mmol/l |
P-value |
| Pre-CPB |
Yes |
61,83 (14,73) |
0,000061 |
58,41 (14,63) |
0,792 |
66,28 (13,98) |
0,002 |
| |
No |
74,06 (14,73) |
|
59,48 (13,99) |
|
77,03(9,82) |
|
| Post-CPB |
Yes |
67,89 (12,44) |
0,197 |
65,86 (12,95) |
0,919 |
70,53 (11,55) |
0,652 |
| |
No |
70,59 (14,39) |
|
63,29 (22,26) |
|
71,38 (12,57) |
|
| ICUadm. |
Yes |
65,36 (15,78) |
0,224 |
63,25 (17,63) |
0,345 |
68,41 (12,52) |
0,309 |
| |
No |
69,01 (13,79) |
|
57,04 (15,49) |
|
71,62 (11,82) |
|
| Pre-ext. |
Yes |
67,04 (15,36) |
0,301 |
63,52 (17,62) |
0,689 |
72,45 (9,25) |
0,940 |
| |
No |
70,86 (14,00) |
|
72,20 (23,50) |
|
70,77 (13,77) |
|
| Post-ext. |
Yes |
62,17 (15,85) |
0,05 |
57,74 (16,78) |
0,848 |
69,18 (11,73) |
0,654 |
| |
No |
69,61 (12,54) |
|
61,07 (16,67) |
|
70,41 (12,12) |
|
Table 7.
ERO2 values (%) at the different sampling times correlated with presence/absence of Low Cardiac Output Syndrome (LCOS). Abbreviations: LCOS (Low cardiac output syndrome), CPB (Cardiopulmonary bypass), ICU (Intensive Care Unit), SD (standard deviation), CHD (congenital heart defect).
Table 7.
ERO2 values (%) at the different sampling times correlated with presence/absence of Low Cardiac Output Syndrome (LCOS). Abbreviations: LCOS (Low cardiac output syndrome), CPB (Cardiopulmonary bypass), ICU (Intensive Care Unit), SD (standard deviation), CHD (congenital heart defect).
| |
|
Whole population |
|
Cyanotic CHD |
|
Acyanotic CHD |
|
| Samplingtimes |
LCOS |
Mean value (SD)% |
P-value |
Mean value (SD)% |
P-value |
Mean value (SD)% |
P-value |
| Pre-CPB |
Yes |
32,1 (14,4) |
0,027 |
30,4 (15,4) |
0,938 |
43,2 (12,5) |
0,013 |
| |
No |
27,6 (12,1) |
|
31,4 (13,1) |
|
26,9 (11,8) |
|
| Post-CPB |
Yes |
29,3 (15,1) |
0,874 |
25,6 (13,2) |
0,542 |
33,8 (16,4) |
0,403 |
| |
No |
30,1 (14,7) |
|
31,7 (21,2) |
|
30,3 (13,6) |
|
| ICUadm. |
Yes |
32,6 (17,2) |
0,580 |
30,6 (17,4) |
0,375 |
35,6 (17,0) |
0,223 |
| |
No |
32,3 (18,4) |
|
36,9 (15,9) |
|
31,5 (18,8) |
|
| Pre-ext. |
Yes |
34,4 (22,7) |
0,921 |
34,6 (23,5) |
0,309 |
34,2 (22,5) |
0,769 |
| |
No |
34,2 (22,2) |
|
18,8 (16,8) |
|
37,7 (25,1) |
|
| Post-ext. |
Yes |
37,8 (23,6) |
0,549 |
37,4 (23,3) |
0,340 |
38,4 (24,9) |
0,902 |
| |
No |
36,2 (23,0) |
|
26,6 (9,1) |
|
38,9 (25,7) |
|
Table 8.
Delta P(v-a)CO2 values (mmHg) at the different sampling times correlated with presence/absence of Low Cardiac Output Syndrome (LCOS). Abbreviations: LCOS (Low cardiac output syndrome), CPB (Cardiopulmonary bypass), ICU (Intensive Care Unit), SD (standard deviation), CHD (congenital heart defect).
Table 8.
Delta P(v-a)CO2 values (mmHg) at the different sampling times correlated with presence/absence of Low Cardiac Output Syndrome (LCOS). Abbreviations: LCOS (Low cardiac output syndrome), CPB (Cardiopulmonary bypass), ICU (Intensive Care Unit), SD (standard deviation), CHD (congenital heart defect).
| |
|
Whole population |
|
Cyanotic CHD |
|
Acyanotic CHD |
|
| Samplingtimes |
LCOS |
Mean value (SD) mmHg |
P-value |
Mean value (SD) mmHg |
P-value |
Mean value (SD) mmHg |
P-value |
| Pre-CPB |
Yes |
6,49(4,71) |
0,034 |
5,39 (3,67) |
0,018 |
7,89 (5,57) |
0,734 |
| |
No |
8,27 (3,92) |
|
9,56 (4,50) |
|
7,97 (3,71) |
|
| Post-CPB |
Yes |
8,25 (5,11) |
0,837 |
6,24 (3,94) |
0,512 |
12,24 (6,93) |
0,013 |
| |
No |
8,26 (5,21) |
|
8,00 (5,22) |
|
7,80 (3,83) |
|
| ICUadm. |
Yes |
10,29 (5,85) |
0,303 |
11,13 (6,46) |
0,650 |
9,22 (4,95) |
0,990 |
| |
No |
8,92 (5,66) |
|
10,13 (7,81) |
|
8,55 (4,99) |
|
| Pre-ext. |
Yes |
8,43 (6,98) |
0,512 |
8,94 (8,02) |
- |
7,75 (5,58) |
0,646 |
| |
No |
7,00 (5,05) |
|
2,00 (-) |
|
6,93 (5,13) |
|
| Post-ext. |
Yes |
6,85 (5,86) |
0,734 |
7,58 (6,79) |
0,99 |
7,89 (5,57) |
0,734 |
| |
No |
6,96 (5,71) |
|
6,50 (4,95) |
|
7,97 (3,71) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).