Submitted:
28 August 2023
Posted:
30 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals and Dietary Regime
2.2. Behavioural Assessment
2.2.1. Phenotypic Behavioural Assessment
2.2.2. Touchscreen Operant Chambers
2.2.3. Training Stages
2.2.4. Visual Discrimination Task
2.2.5. Motivation Task
2.3. Physiological Assessment
2.3.1. Blood Glucose and Plasma Insulin Measurements
2.3.2. Lipid and Lipoprotein Analysis
2.3.3. Sacrifice and Tissue Collection
2.3.4. White Adipose Tissue and Liver Histology
2.3.5. Immunohistochemistry
2.3.6. Gene Expression Analyses
RNA Isolation
cDNA Synthesis
qPCR
2.3.7. Hippocampal Insulin Levels
2.4. Statistical Analysis
3. Results
3.1. Effects of the Two Dietary Regimes in Healthy Long Evans (LE) Rats on Behaviour and Physiological Outcomes
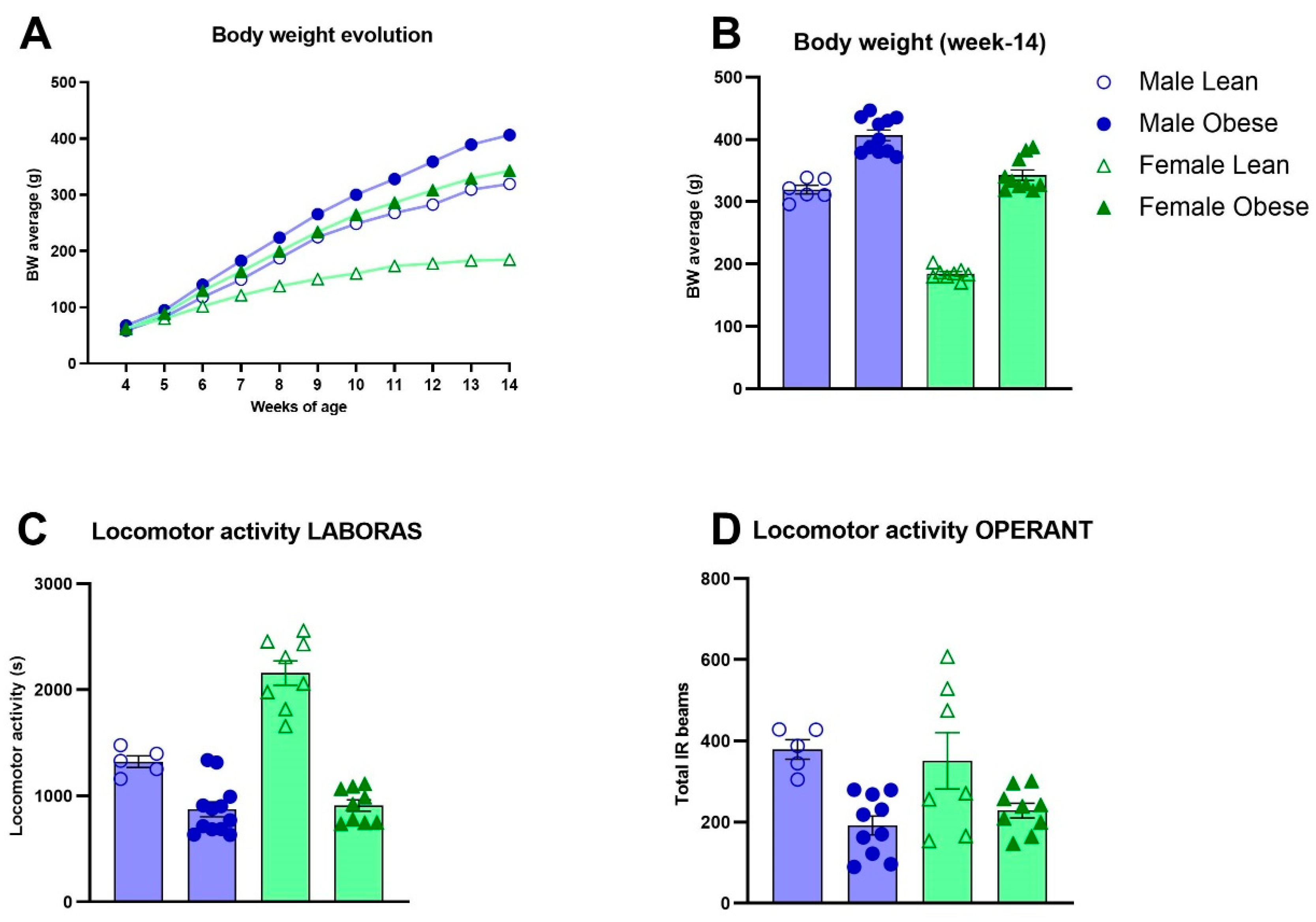
3.2. Body Weight Development, Locomotor Activity and Ad Libitum Food Intake in ZDF Rats
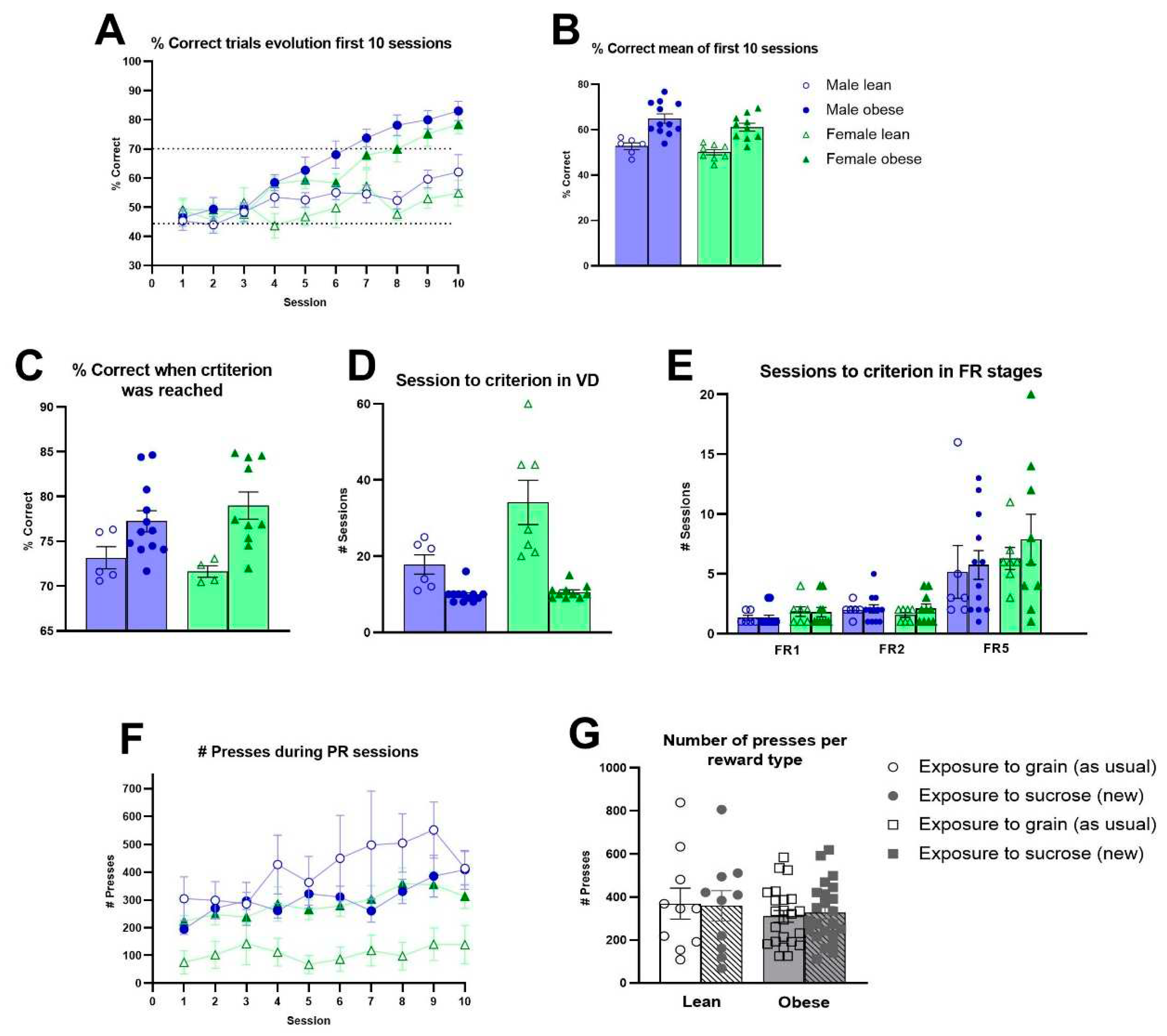
3.3. Cognitive Performance and Level of Motivation in ZDF Rats
3.4. Blood Glucose, Insulin and Lipids in ZDF Rats
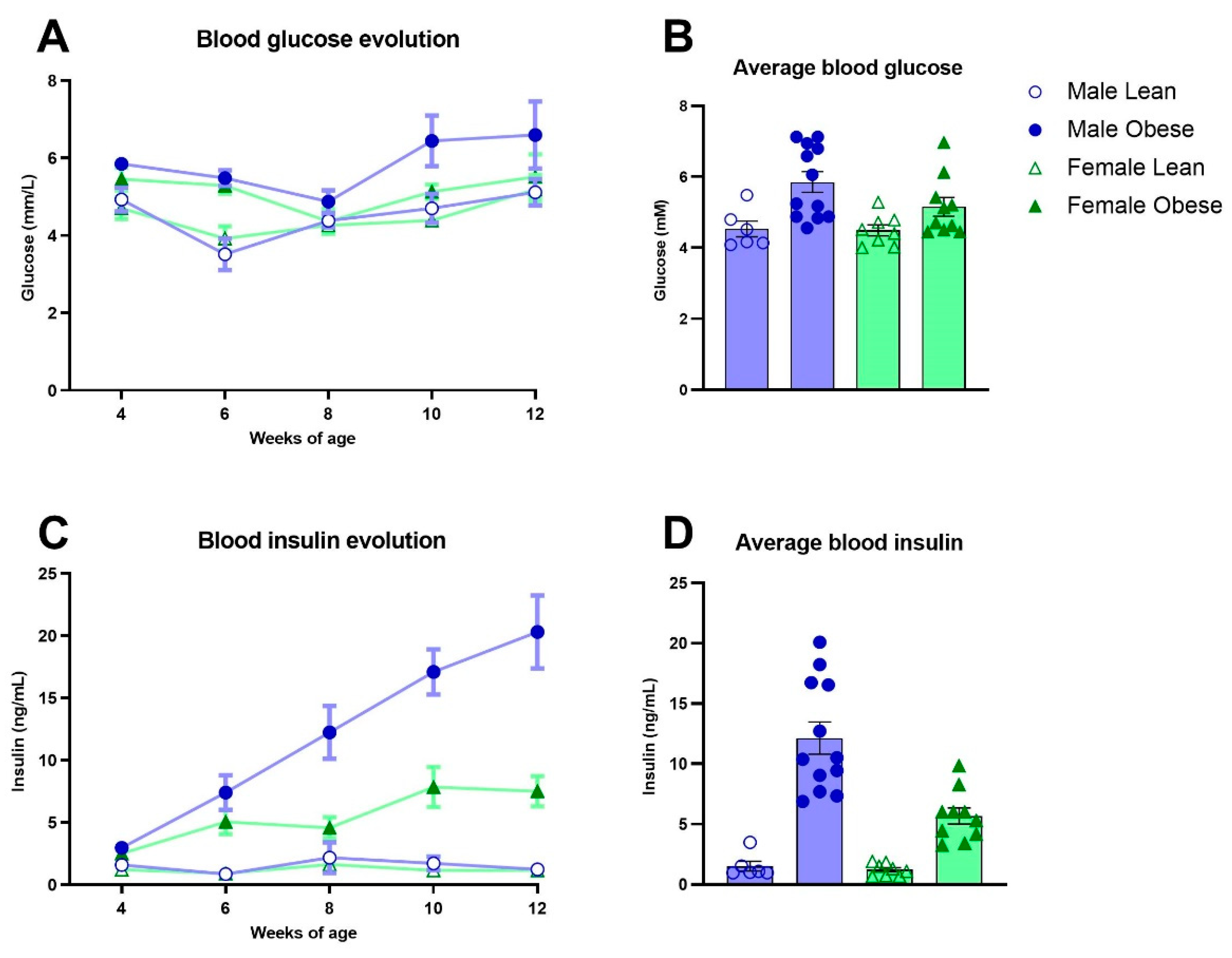
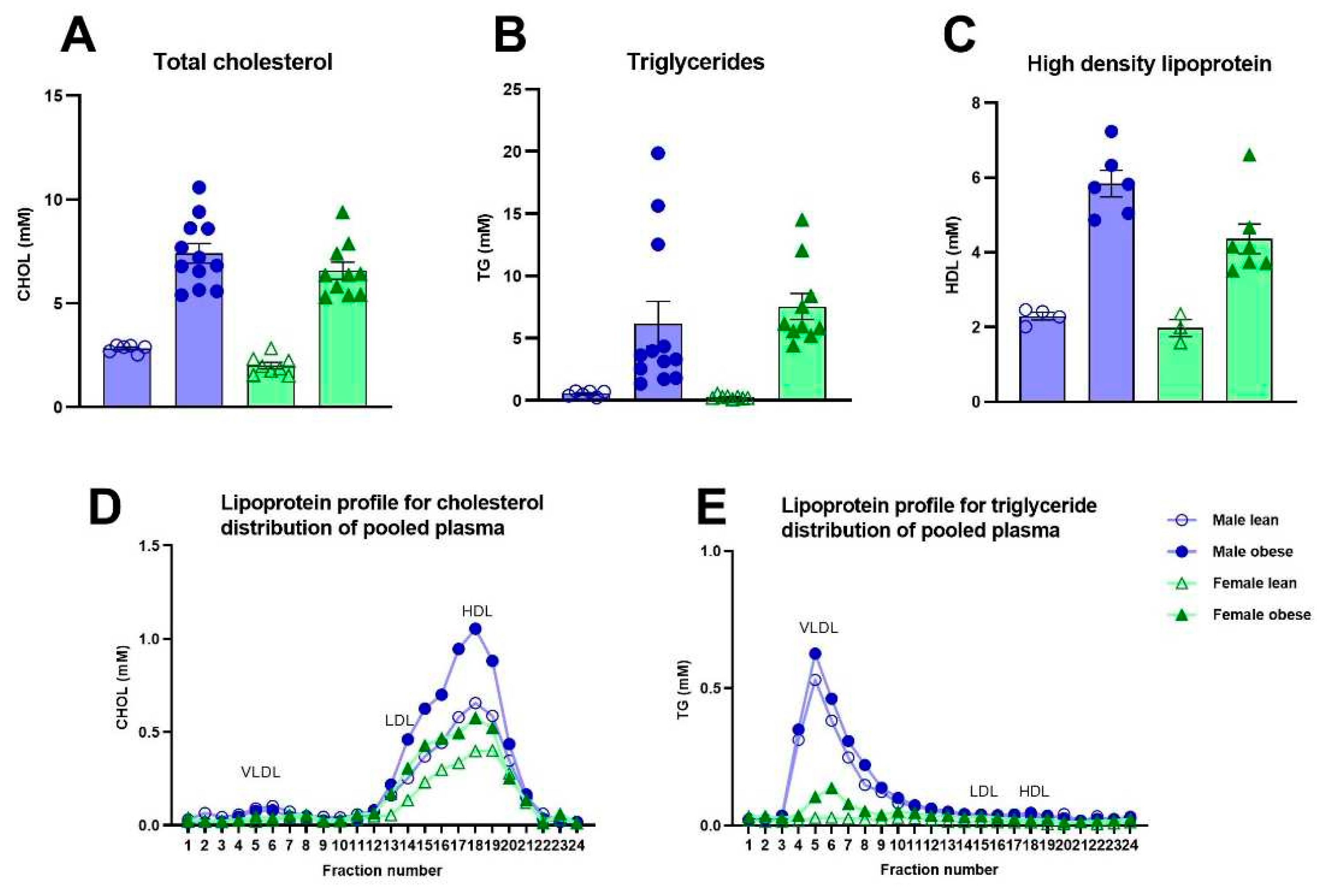
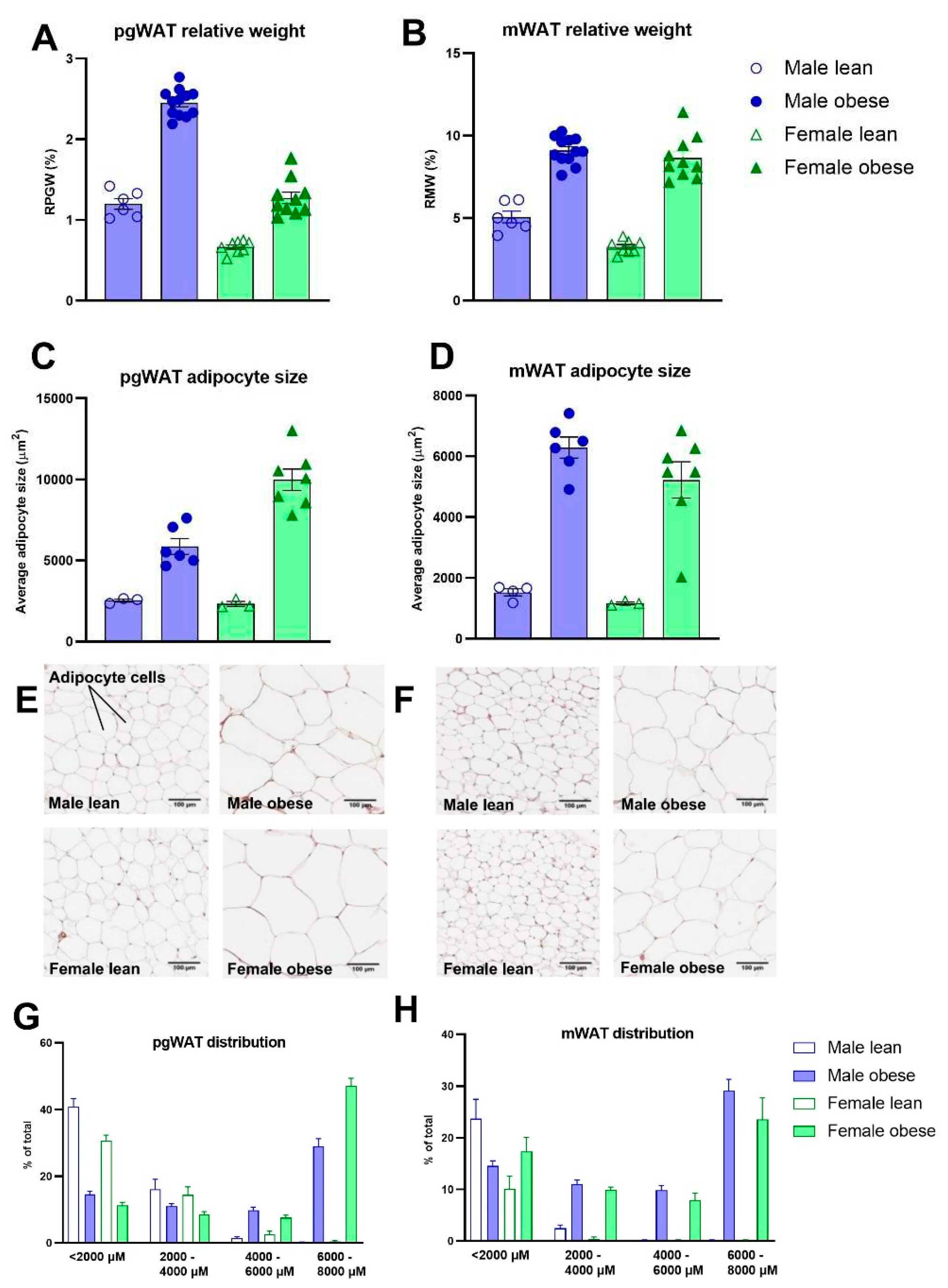
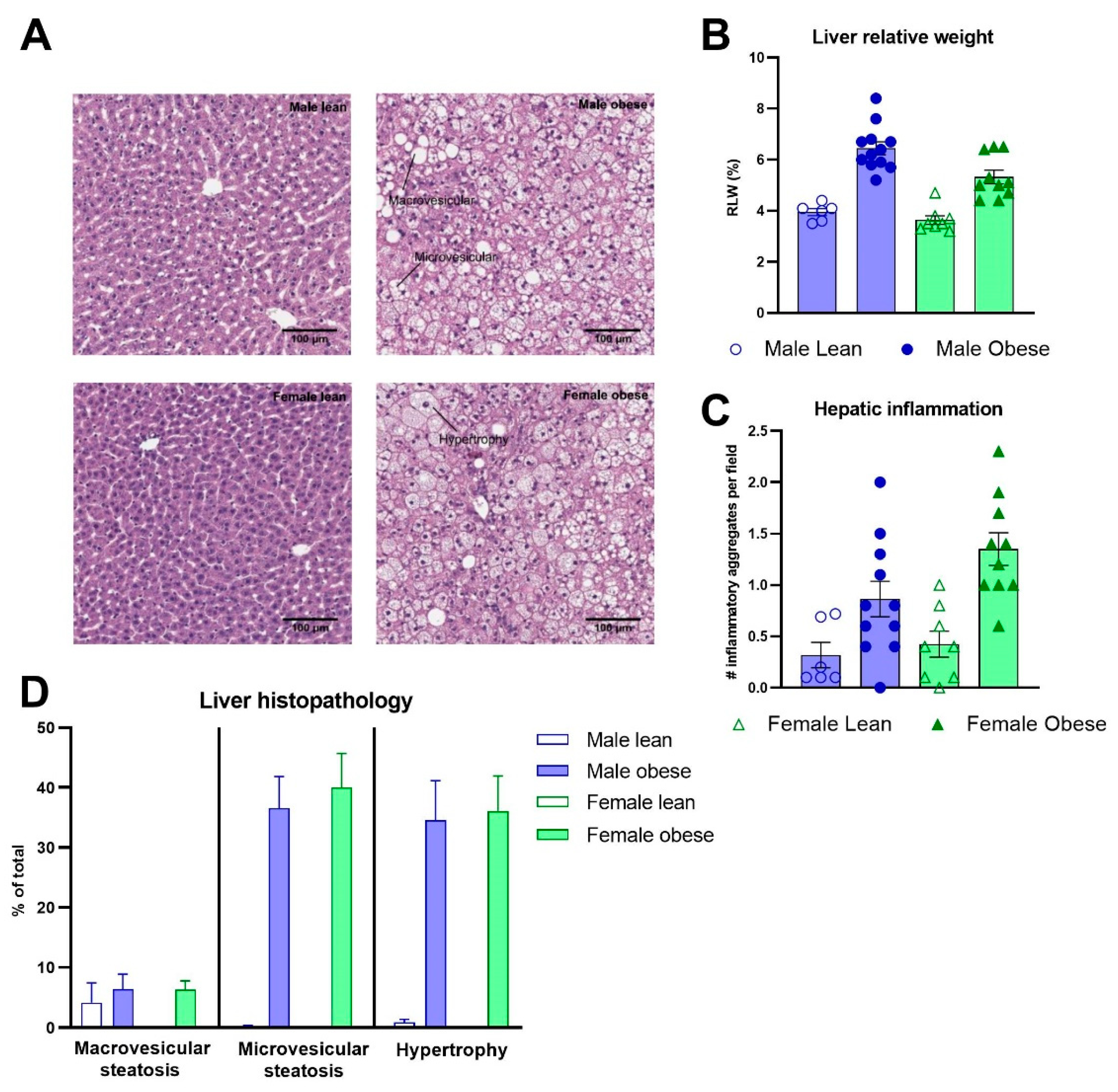
3.5. Histological Analyses of White Adipose Tissues and Liver in ZDF Rats
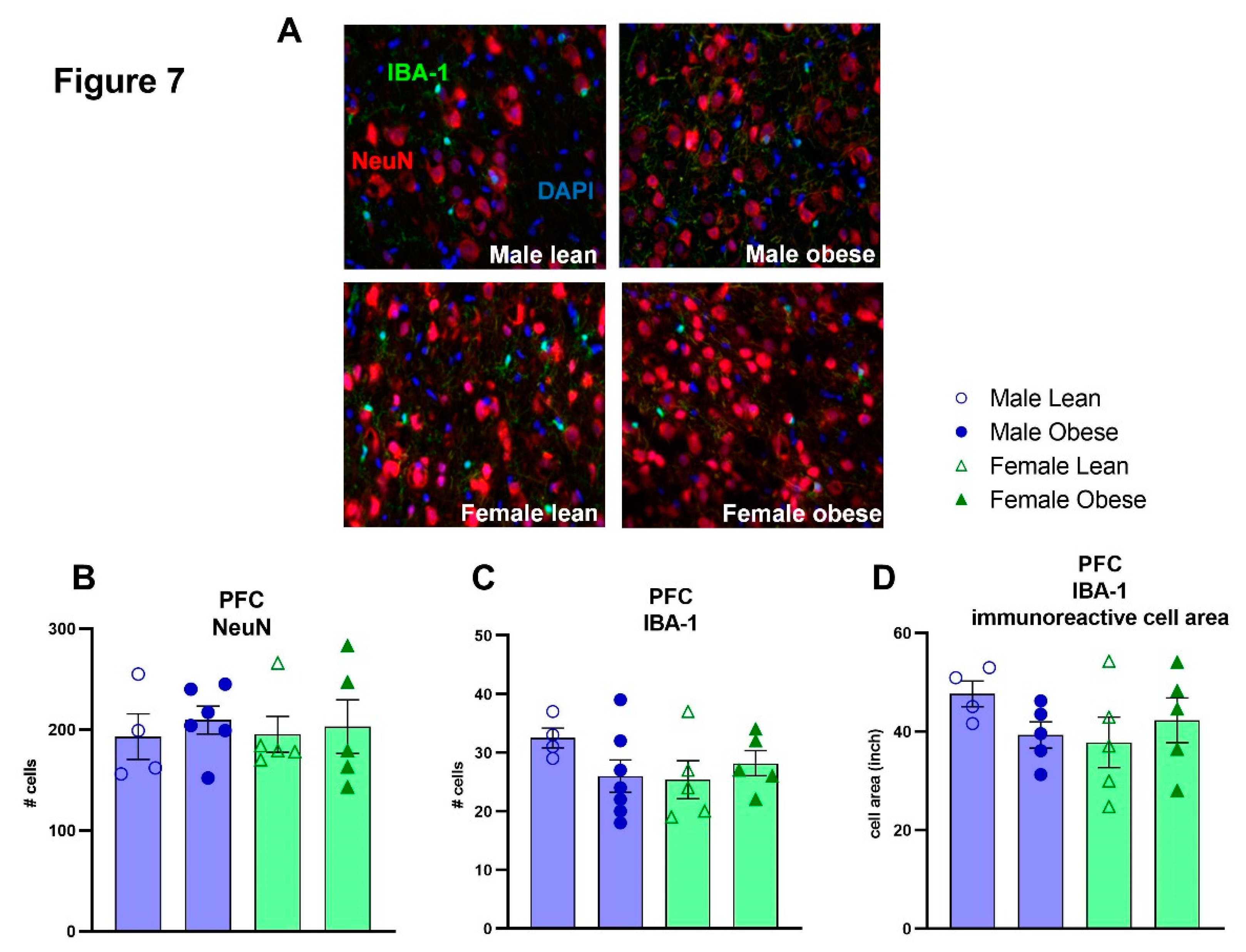
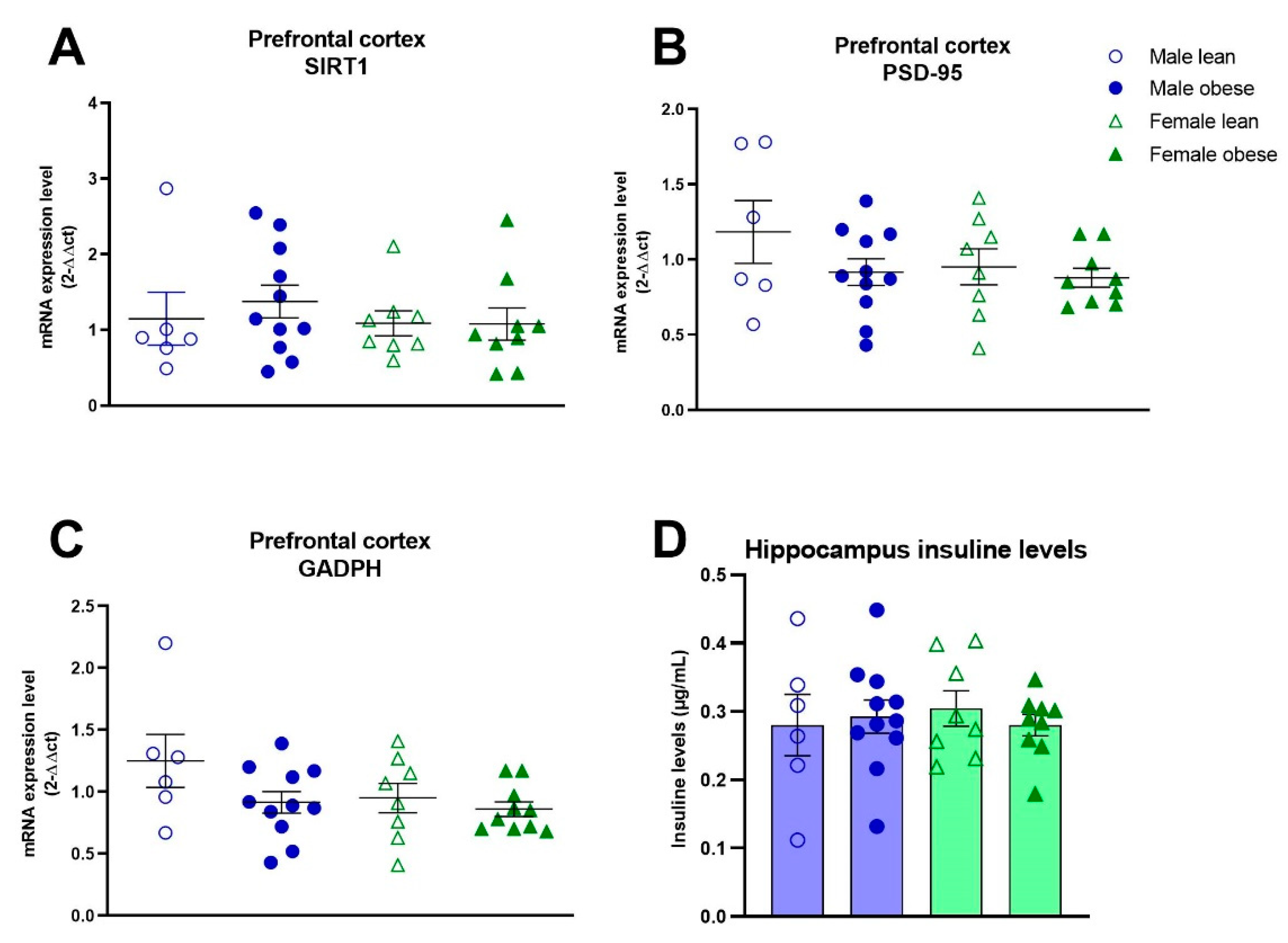
3.6. mPFC NeuN and IBA-1 Positive Cells, mPFC SIRT1 and PSD-95 mRNA Expression, and Hippocampal Insulin Levels in ZDF Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and overweight. Updated , 2021. Available online: https://www.who.int/newsroom/fact-sheets/detail/obesity-and-overweight (accessed on 25 September 2019).
- Blüher, M. Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Cildir, G.; Akıncılar, S.C.; Tergaonkar, V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol. Med. 2013, 19, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Wree, A.; Kahraman, A.; Gerken, G.; Canbay, A. Obesity Affects the Liver – The Link between Adipocytes and Hepatocytes. Digestion 2010, 83, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6-10. Metabolism 2019, 92, 6–10.
- Cildir, G.; Akıncılar, S.C.; Tergaonkar, V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol. Med. 2013, 19, 487–500. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflammation 2008, 5, 37–37. [Google Scholar] [CrossRef]
- Reichelt, A.C.; Morris, M.J.; Westbrook, R.F. Daily access to sucrose impairs aspects of spatial memory tasks reliant on pattern separation and neural proliferation in rats. Learn. Mem. 2016, 23, 386–390. [Google Scholar] [CrossRef]
- Tozuka, Y, Wada E, Wada K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J. 2009, 23, 1920–1934. [CrossRef]
- Barber, T.M.; Kyrou, I.; Randeva, H.S.; Weickert, M.O. Mechanisms of Insulin Resistance at the Crossroad of Obesity with Associated Metabolic Abnormalities and Cognitive Dysfunction. Int. J. Mol. Sci. 2021, 22, 546. [Google Scholar] [CrossRef]
- Kothari, V.; Luo, Y.; Tornabene, T.; O’Neill, A.M.; Greene, M.W.; Geetha, T.; Babu, J.R. High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2017, 1863, 499–508. [Google Scholar] [CrossRef]
- Luo, A.; Xie, Z.; Wang, Y.; Wang, X.; Li, S.; Yan, J.; Zhan, G.; Zhou, Z.; Zhao, Y.; Li, S. Type 2 diabetes mellitus-associated cognitive dysfunction: Advances in potential mechanisms and therapies. Neurosci. Biobehav. Rev. 2022, 137, 104642. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Reagan, L.P. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci. 2015, 16, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Bi, T.; Feng, R.; Zhan, L.; Ren, W.; Lu, X. ZiBuPiYin Recipe Prevented and Treated Cognitive Decline in ZDF Rats With Diabetes-Associated Cognitive Decline via Microbiota–Gut–Brain Axis Dialogue. Front. Cell Dev. Biol. 2021, 9, 651517. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.K.; Dharavath, R.N.; Chopra, K. Time-response studies on development of cognitive deficits in an experimental model of insulin resistance. Clin. Nutr. 2018, 38, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Grillo, C.A.; Woodruff, J.L.; Macht, V.A.; Reagan, L.P. Insulin resistance and hippocampal dysfunction: Disentangling peripheral and brain causes from consequences. Exp. Neurol. 2019, 318, 71–77. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Gao, F. New insights into insulin: The anti-inflammatory effect and its clinical relevance. World J. Diabetes 2014, 5, 89–96. [Google Scholar] [CrossRef]
- Scherer, T.; Sakamoto, K.; Buettner, C. Brain insulin signalling in metabolic homeostasis and disease. Nat. Rev. Endocrinol. 2021, 17, 468–483. [Google Scholar] [CrossRef]
- Hallschmid, M. Intranasal Insulin for Alzheimer’s Disease. CNS Drugs 2021, 35, 21–37. [Google Scholar] [CrossRef]
- Canteiro, P.B.; Antero, D.C.; Tramontin, N.d.S.; Simon, K.U.; Mendes, C.; Correa, M.E.A.B.; Silveira, P.C.L.; Muller, A.P. Insulin treatment protects the brain against neuroinflammation by reducing cerebral cytokines and modulating mitochondrial function. Brain Res. Bull. 2019, 149, 120–128. [Google Scholar] [CrossRef]
- Aljada A, Ghanim H, Mohanty P, Kapur N, Dandona P. Insulin inhibits the pro-inflammatory transcription factor early growth response gene-1 (egr)-1 expression in mononuclear cells (mnc) and reduces plasma tissue factor (tf) and plasminogen activator inhibitor-1 (pai-1) concentrations. J Clin Endocrinol Metab. 2002, 87, 1419–1422.
- Coimbra, T.M.; Janssen, U.; Gröne, H.J.; Ostendorf, T.; Kunter, U.; Schmidt, H.; Brabant, G.; Floege, J. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int. 2000, 57, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Hoshi S, Shu Y, Yoshida F, Inagaki T, Sonoda J, Watanabe T, Nomoto K, Nagata M: Podocyte injury promotes progressive nephropathy in Zucker diabetic fatty rats. Lab Invest. 2002, 82, 25–35. [CrossRef] [PubMed]
- Bussey, T.J.; Padain, T.L.; Skillings, E.A.; Winters, B.D.; Morton, A.J.; Saksida, L.M. The touchscreen cognitive testing method for rodents: How to get the best out of your rat. Learn. Mem. 2008, 15, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Talpos, J.; Steckler, T. Touching on translation. Cell Tissue Res. 2013, 354, 297–308. [Google Scholar] [CrossRef]
- Horner AE, Heath CJ, Hvoslef-Eide M, Kent BA, Kim CH, Nilsson SR, Alsio J, Oomen CA, Holmes A, Saksida LM et al. The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc. 2013, 8, 1961–1984.
- Turner, K.M.; Simpson, C.G.; Burne, T.H.J. BALB/c Mice Can Learn Touchscreen Visual Discrimination and Reversal Tasks Faster than C57BL/6 Mice. Front. Behav. Neurosci. 2017, 11, 16. [Google Scholar] [CrossRef]
- Van de Weerd HA, Bulthuis RJA, Bergman AF, Schlingmann F, Tolboom J, Van Loo PLP, Remie R, Baumans V, Van Zutphen LFM. Validation of a new system for the automatic registration of behaviour in mice and rats. Behavioural Processes 2011, 53, 11–20.
- Cardinal RN and Aitken MRF. Whisker A client—server high-performance multimedia research control system. Behavior Research Methods 2010, 42, 1059–1071. [CrossRef]
- Markou, A.; Salamone, J.D.; Bussey, T.J.; Mar, A.C.; Brunner, D.; Gilmour, G.; Balsam, P. Measuring reinforcement learning and motivation constructs in experimental animals: Relevance to the negative symptoms of schizophrenia. Neurosci. Biobehav. Rev. 2013, 37, 2149–2165. [Google Scholar] [CrossRef]
- Morrison, M.C.; Mulder, P.; Stavro, P.M.; Suárez, M.; Arola-Arnal, A.; van Duyvenvoorde, W.; Kooistra, T.; Wielinga, P.Y.; Kleemann, R. Replacement of Dietary Saturated Fat by PUFA-Rich Pumpkin Seed Oil Attenuates Non-Alcoholic Fatty Liver Disease and Atherosclerosis Development, with Additional Health Effects of Virgin over Refined Oil. PLOS ONE 2015, 10, e0139196. [Google Scholar] [CrossRef]
- Mueller, A.M.; Kleemann, R.; Gart, E.; van Duyvenvoorde, W.; Verschuren, L.; Caspers, M.; Menke, A.; Krömmelbein, N.; Salic, K.; Burmeister, Y.; et al. Cholesterol Accumulation as a Driver of Hepatic Inflammation Under Translational Dietary Conditions Can Be Attenuated by a Multicomponent Medicine. Front. Endocrinol. 2021, 12, 601160. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a General NAFLD Scoring System for Rodent Models and Comparison to Human Liver Pathology. PLOS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed]
- Tengeler AC, Gart E, Wiesmann M, Arnoldussen IAC, van Duyvenvoorde W, Hoogstad M, Dederen PJ et al. Propionic acid and not caproic acid, attenuates nonalcoholic steatohepatitis and improves (cerebro) vascular functions in obese Ldlr−/−.Leiden mice. FASEB J. 2020, 34, 9575–9593. [CrossRef] [PubMed]
- Bélanger, A.; Lavoie, N.; Trudeau, F.; Massicotte, G.; Gagnon, S. Preserved LTP and water maze learning in hyperglycaemic–hyperinsulinemic ZDF rats. Physiol. Behav. 2004, 83, 483–494. [Google Scholar] [CrossRef]
- Jolivalt, C.G.; Aghanoori, M.R.; Navarro-Diaz, M.C.; Han, M.M.; Sanchez, G.; Guernsey, L.; Quach, D.; Johe, K.; Fernyhough, P.; Calcutt, N.A. Enhancement of Mitochondrial Function by the Neurogenic Molecule NSI-189 Accompanies Reversal of Peripheral Neuropathy and Memory Impairment in a Rat Model of Type 2 Diabetes. J. Diabetes Res. 2022, 2022, 8566970. [Google Scholar] [CrossRef]
- Tomassoni D, Martinelli I, Moruzzi M, Micioni Di Bonaventura MV, Cifani C, Amenta F, Tayebati SK. Obesity and Age-Related Changes in the Brain of the Zucker Lepr fa/fa Rats. Nutrients 2020, 12, 1356. [CrossRef]
- Vogel, H.; Kraemer, M.; Rabasa, C.; Askevik, K.; Adan, R.A.; Dickson, S.L. Genetic predisposition to obesity affects behavioural traits including food reward and anxiety-like behaviour in rats. Behav. Brain Res. 2017, 328, 95–104. [Google Scholar] [CrossRef]
- Chander PN, Gealekman O, Brodsky SV, Elitok S, Tojo A, Crabtree M, Gross SS, Goligorsky MS. Nephropathy in Zucker diabetic fat rat is associated with oxidative and nitrosative stress: prevention by chronic therapy with a peroxynitrite scavenger ebselen. 2004; 15(9):2391-403.
- Slavkovsky, R.; Kohlerova, R.; Tkacova, V.; Jiroutova, A.; Tahmazoglu, B.; Velebny, V.; Rezačová, M.; Sobotka, L.; Kanta, J. Zucker diabetic fatty rat: A new model of impaired cutaneous wound repair with type II diabetes mellitus and obesity. Wound Repair Regen. 2011, 19, 515–525. [Google Scholar] [CrossRef]
- Rasmussen, E.B.; Huskinson, S.L. Effects of rimonabant on behavior maintained by progressive ratio schedules of sucrose reinforcement in obese Zucker (fa/fa) rats. Behav. Pharmacol. 2008, 19, 735–742. [Google Scholar] [CrossRef]
- Kullmann, S.; Kleinridders, A.; Small, D.M.; Fritsche, A.; Häring, H.-U.; Preissl, H.; Heni, M. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol. 2020, 8, 524–534. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Schenk, S.; Imamura, T.; Babendure, J.L.; Sonoda, N.; Bae, E.J.; Oh, D.Y.; Lu, M.; Milne, J.C.; Westphal, C.; et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E419–E428. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Gong, Z. The Beneficial Roles of SIRT1 in Neuroinflammation-Related Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 6782872. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Luo, A.; Sun, R.; Tang, X.; Zhao, Y.; Zhang, J.; Zhou, B.; Zheng, H.; Yu, H.; Li, S. Resveratrol Mitigates Hippocampal Tau Acetylation and Cognitive Deficit by Activation SIRT1 in Aged Rats following Anesthesia and Surgery. Oxidative Med. Cell. Longev. 2020, 2020, 4635163. [Google Scholar] [CrossRef] [PubMed]
- Corpas, R.; Revilla, S.; Ursulet, S.; Castro-Freire, M.; Kaliman, P.; Petegnief, V.; Giménez-Llort, L.; Sarkis, C.; Pallàs, M.; Sanfeliu, C. SIRT1 Overexpression in Mouse Hippocampus Induces Cognitive Enhancement Through Proteostatic and Neurotrophic Mechanisms. Mol. Neurobiol. 2017, 54, 5604–5619. [Google Scholar] [CrossRef]
- Nam, S.M.; Na Kim, Y.; Yoo, D.Y.; Yi, S.S.; Choi, J.H.; Hwang, I.K.; Seong, J.K.; Yoon, Y.S. Hypothyroidism affects astrocyte and microglial morphology in type 2 diabetes. Neural Regen. Res. 2013, 8, 2458–2467. [Google Scholar] [CrossRef]
- Hwang, I.K.; Choi, J.H.; Nam, S.M.; Park, O.K.; Yoo, D.Y.; Kim, W.; Yi, S.S.; Won, M.-H.; Seong, J.K.; Yoon, Y.S. Activation of microglia and induction of pro-inflammatory cytokines in the hippocampus of type 2 diabetic rats. Neurol. Res. 2014, 36, 824–832. [Google Scholar] [CrossRef]
- Martinelli, I.; Tomassoni, D.; Roy, P.; Amenta, F.; Tayebati, S.K. Altered Brain Cholinergic and Synaptic Markers in Obese Zucker Rats. Cells 2021, 10, 2528. [Google Scholar] [CrossRef]
- Martin B, Ji S, Maudsley S, and Mattson MP. “Control” laboratory rodents are metabolically morbid: Why it matters. PNAS 2010, 107, 6127–6133. [CrossRef]
- Noble, E.E.; Olson, C.A.; Davis, E.; Tsan, L.; Chen, Y.-W.; Schade, R.; Liu, C.; Suarez, A.; Jones, R.B.; de La Serre, C.; et al. Gut microbial taxa elevated by dietary sugar disrupt memory function. Transl. Psychiatry 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef]
| Learning stage | Criteria | Description |
|---|---|---|
| Habituation | Complete one session | The rat was provided with 25 pellets at variable time intervals. |
| Autoshaping | All 25 pellets eaten | In order to learn the association between the stimulus and the reward; the rat received a reward regardless of whether it touched the stimulus and would receive the reward immediately when it touched the image. |
| Must touch | Two sessions 25 correct responses and all 25 pellets eaten | The rat had to touch the stimulus to receive a reward, otherwise no reward was given. |
| Punish incorrect | At least 70% correct responses for two sessions (Nr correct/Nr correct+Nr incorrect)*100 | The rat receives negative feedback (house light on) when it touches the screen in the wrong location. Then, no pellet is given. |
| Moving punish incorrect | At least 70% correct responses for two sessions (Nr correct/Nr correct+Nr incorrect)*100 | Similar to the punish incorrect, but the stimulus changes position from left to right in a pseudorandom order. |
| Visual discrimination | At least 10 sessions & average 70% correct responses across three consecutive sessions (Nr correct/Nrcorrect+Nr incorrect)*100 | Similar to moving punish incorrect, however the circular stimulus is replaced by two striped images. The designated correct stimulus (counterbalanced between rats) changes position in a pseudorandom order. |
| Fixed Ratio 1 | 50 correct responses | The rat had to press once to receive a reward. |
| Fixed Ratio 2 | 100 correct responses | The rat had to press twice to receive a reward. |
| Fixed Ratio 5 | 250 correct responses | The rat had to press five times to receive the reward. |
| Progressive Ratio | Complete 10 (ZDF rats) or 20 sessions (LE rats) | On each subsequent trial the reward response requirement increased on a linear basis (+4). |
| Name | Sequece 5’ -> 3’ | Tm | GC% |
|---|---|---|---|
| YWHAZ | fw: GACAAGAAAGGAATTGTGGACCAGT rv: GGGCCAGACCCAGTCTGATG |
61.44 62.26 |
44.00 65.00 |
| GAPDH | fw: CCACCAACTGCTTAGCCCCC rv: TGGTCATGAGCCCTTCCACG |
63.12 62.18 |
65.00 60.00 |
| SIRT1 | fw: AGATACCTTGGAGCAGGTTGCAG rv: AGATGCTGTTGCAAAGGAACCATGA |
62.51 63.48 |
52.17 44.00 |
| β-actin | fw: CGTGAAAAGATGACCCAGATCA rv: AGAGGCATACAGGGACAACAC |
58.40 59.72 |
45.45 52.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





