Submitted:
29 August 2023
Posted:
30 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Fabrication of composite solid electrolytes
2.2. Fabrication of LFP-supported composite solid electrolytes
2.3. Materials and electrochemical characterization
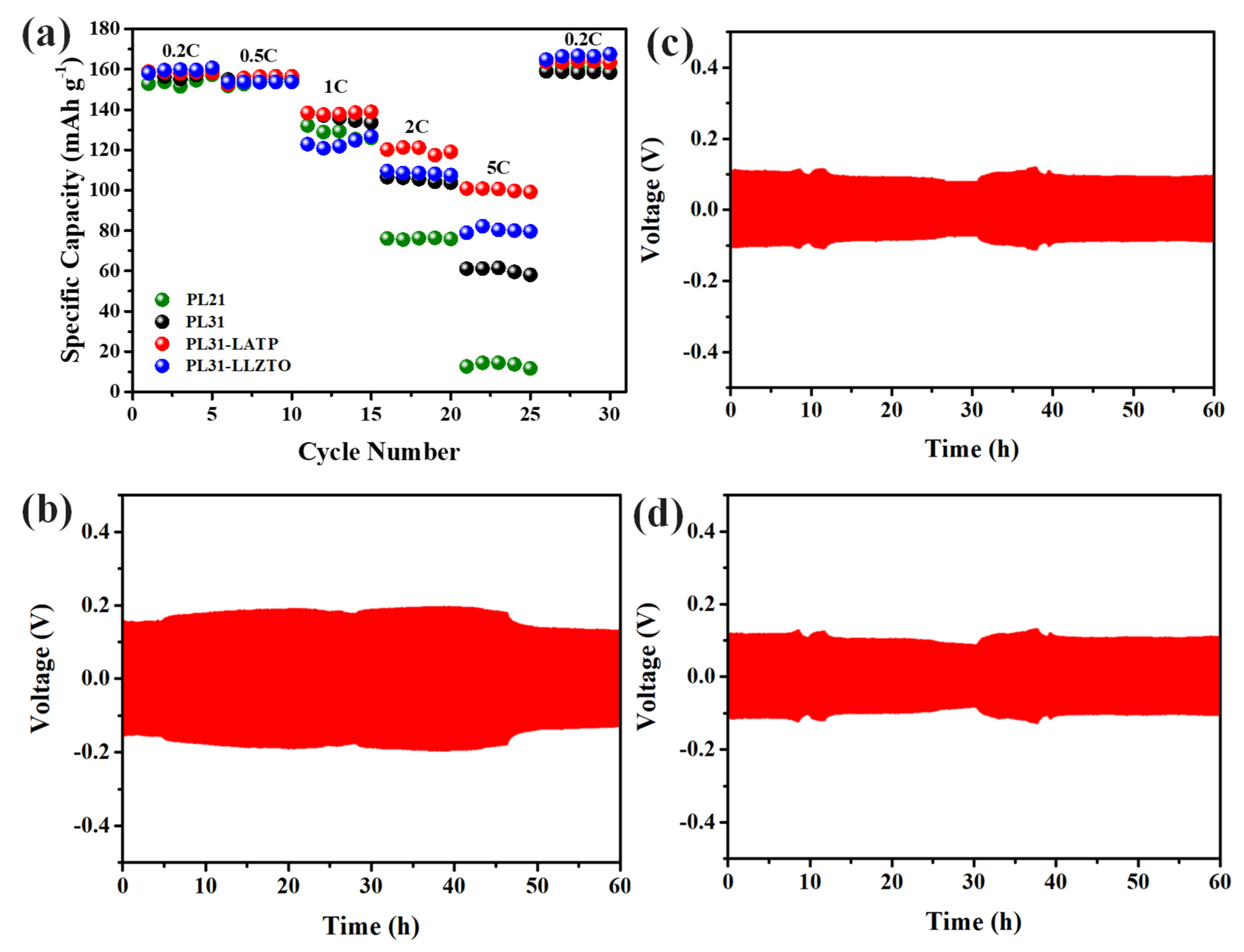
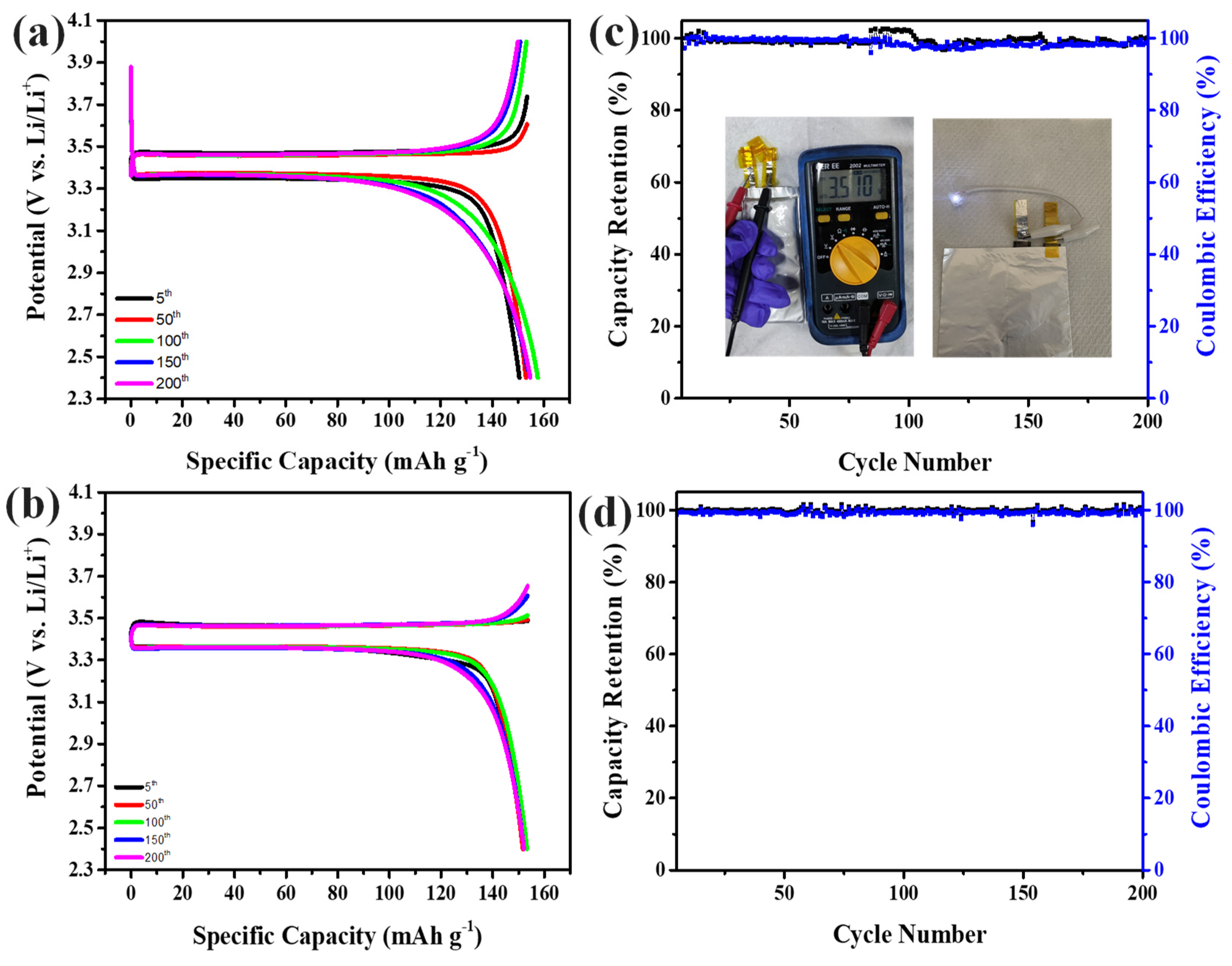
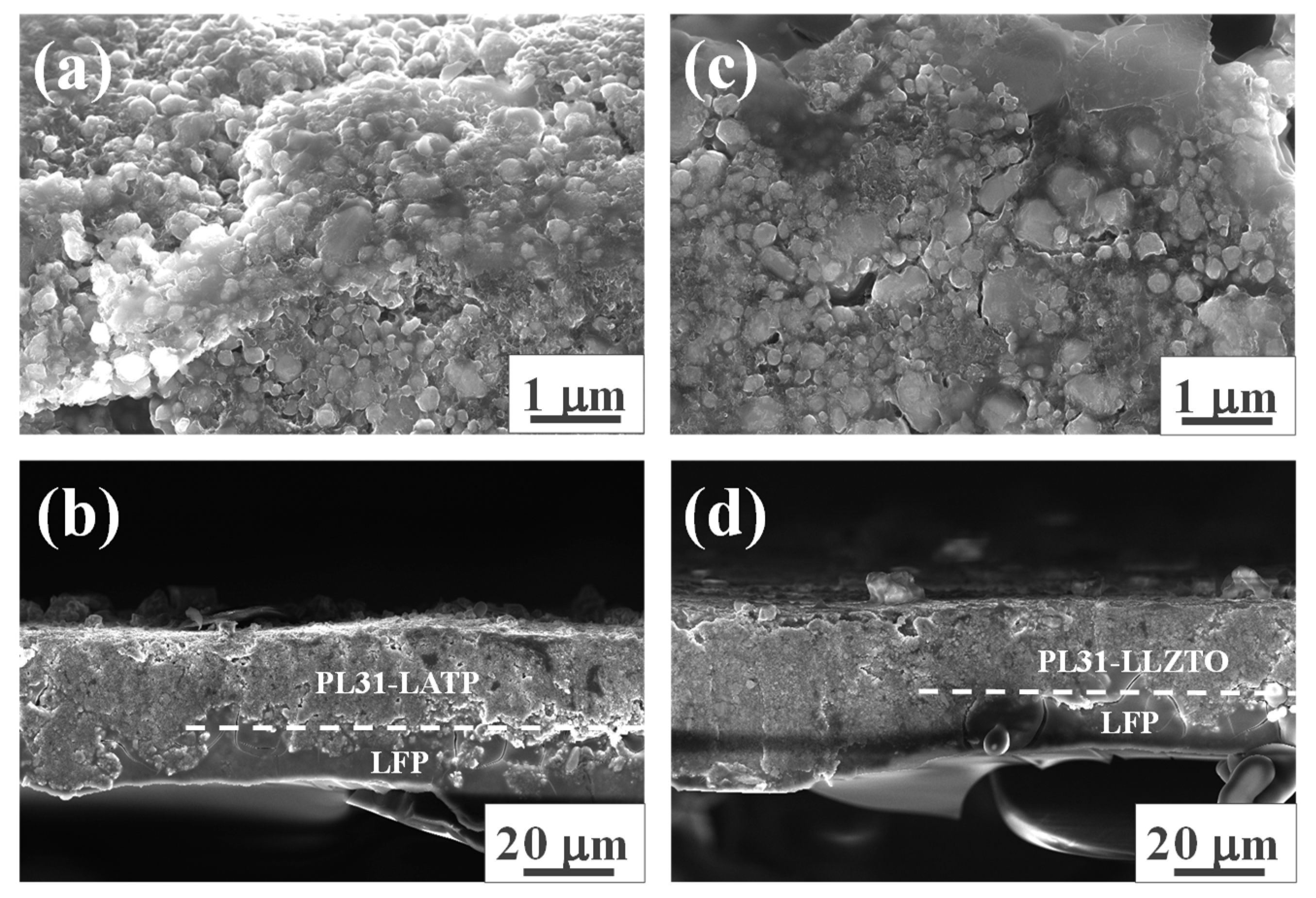
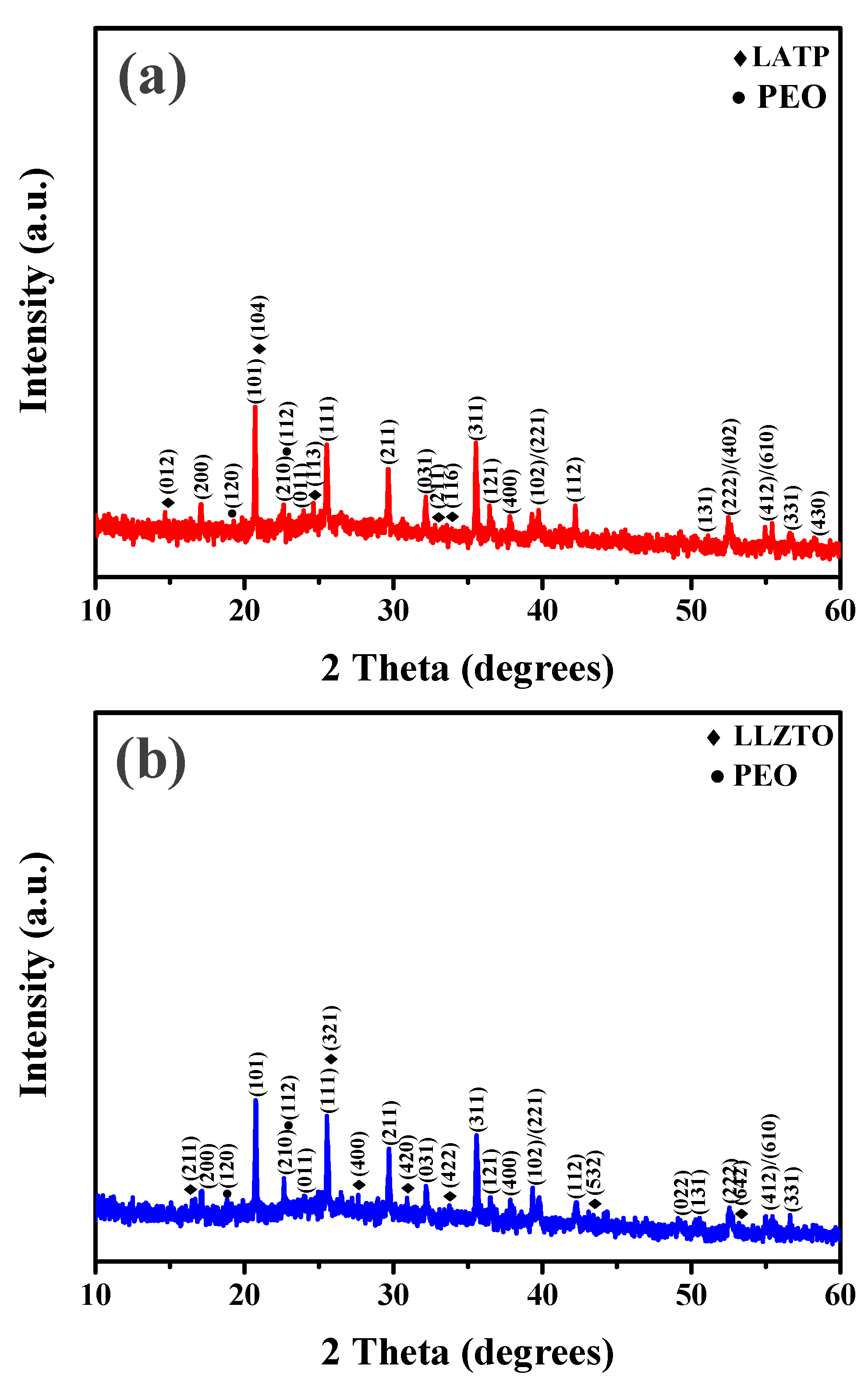
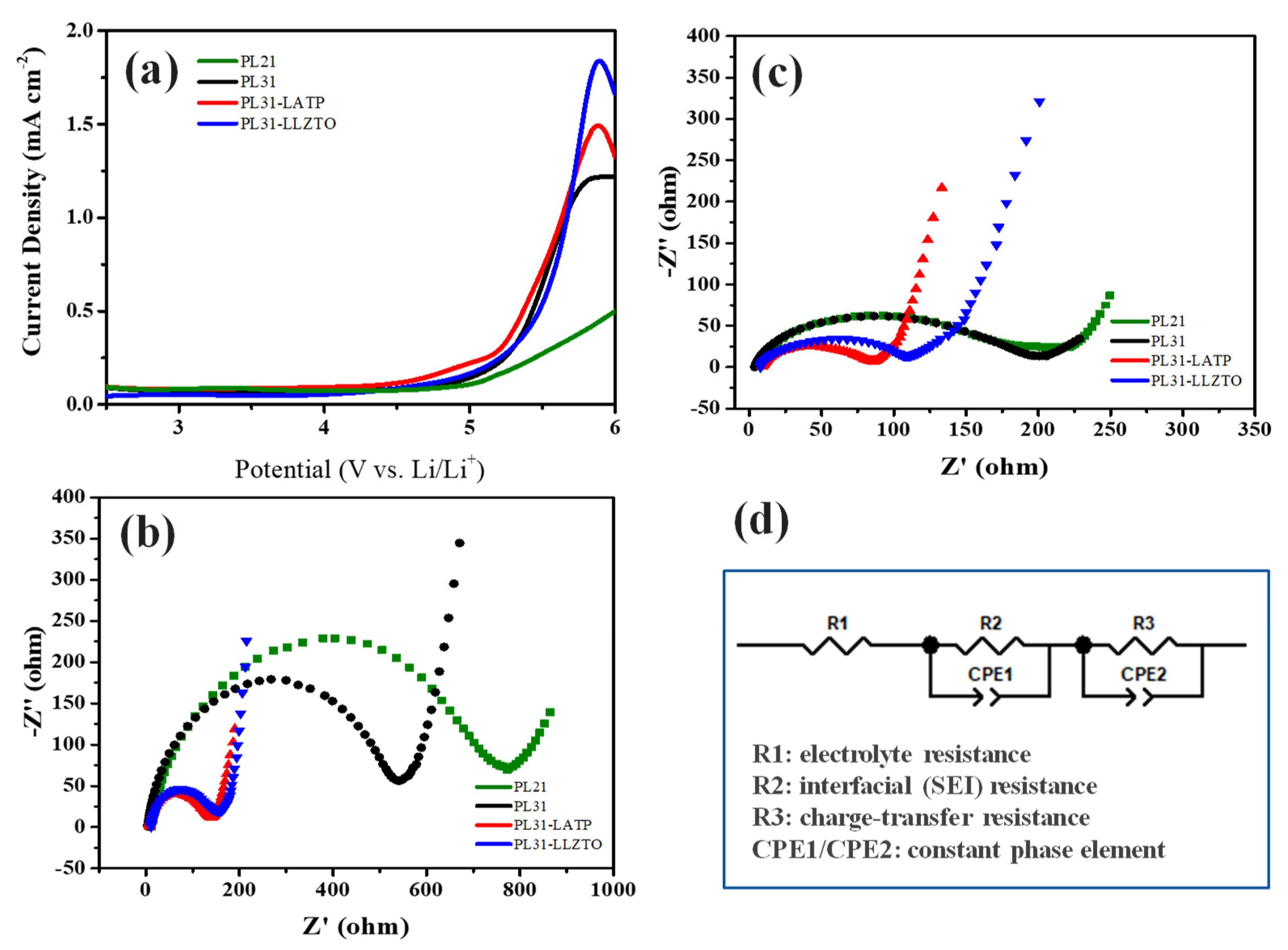
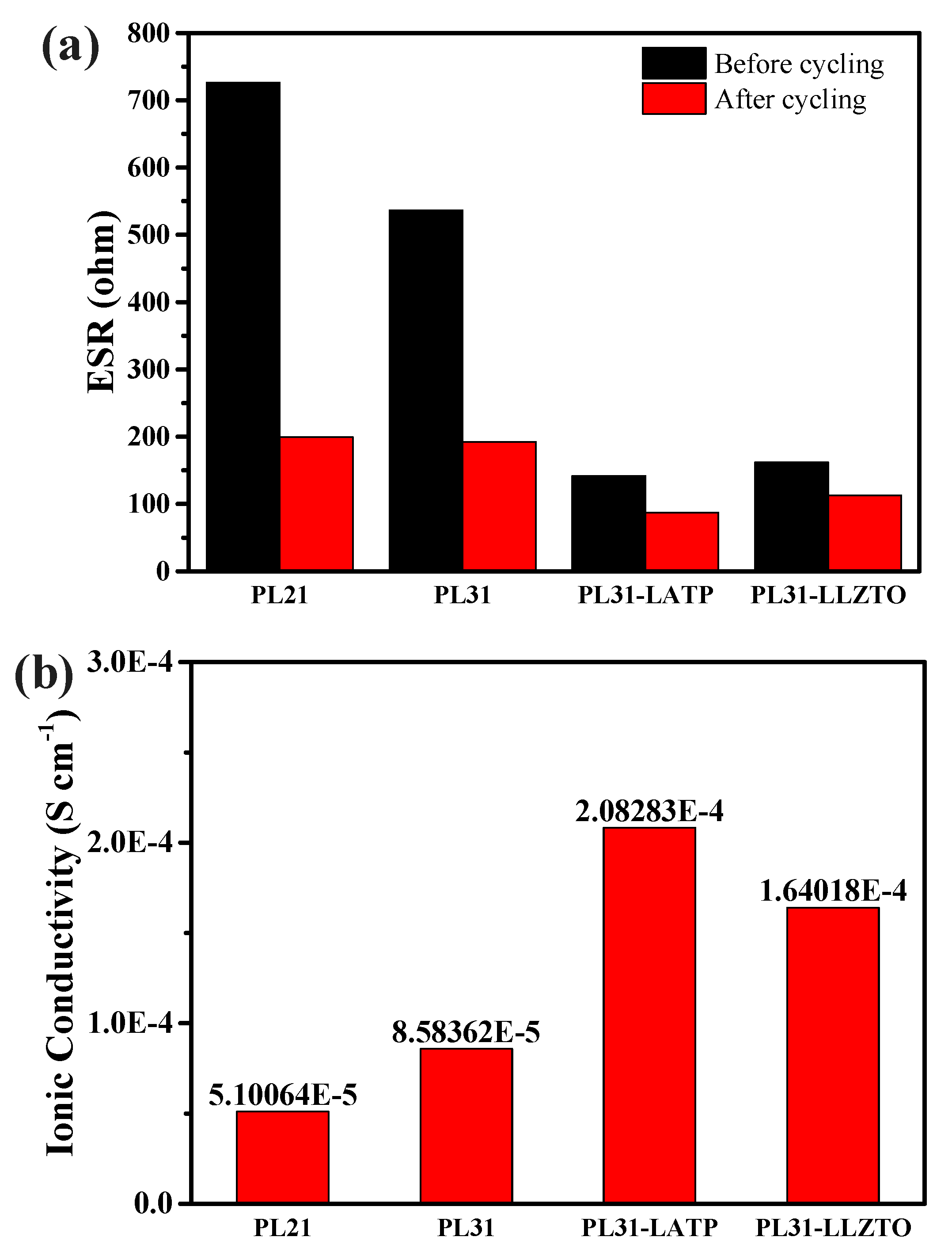
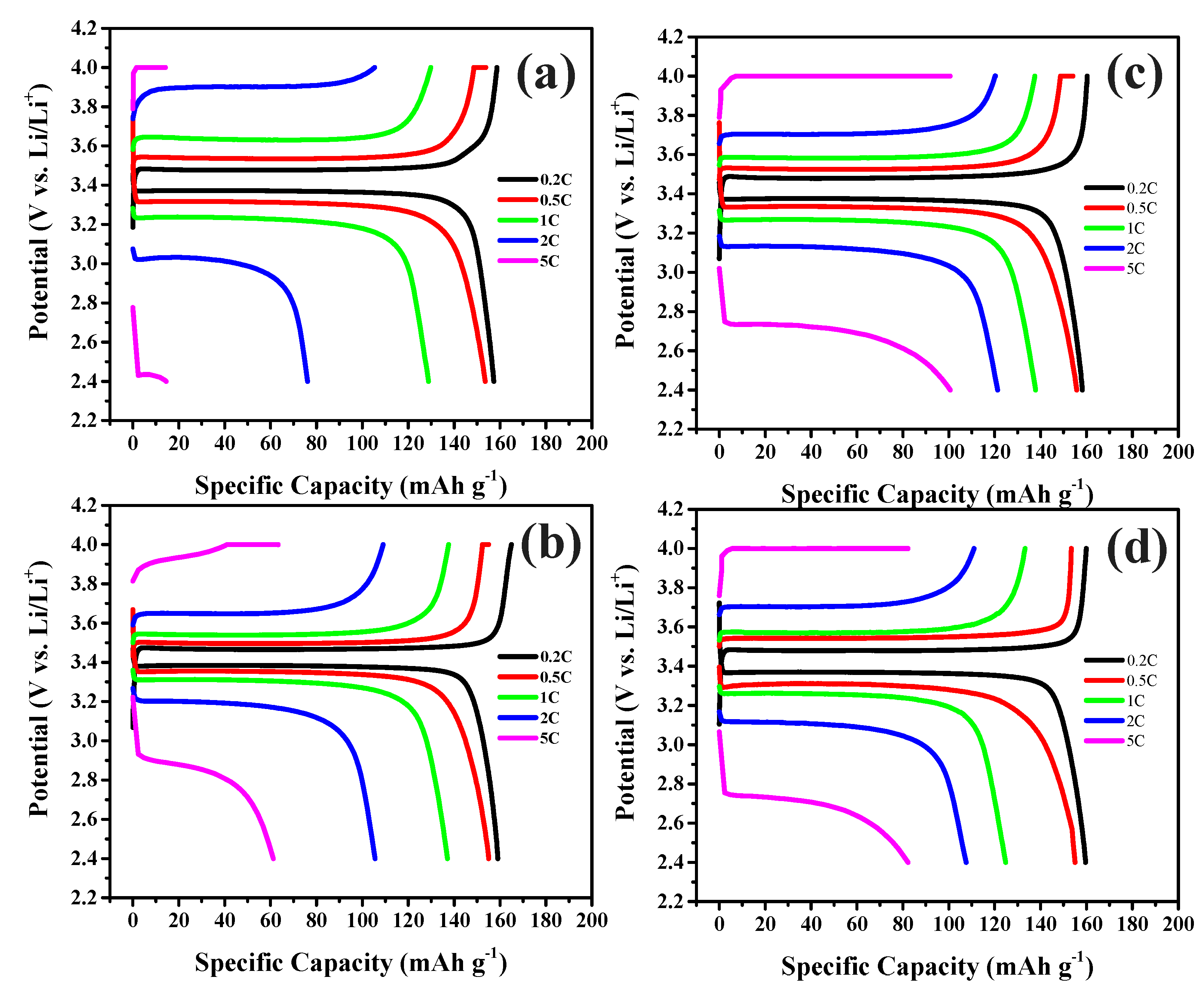
3. Results and discussion
4. Conclusions
CRediT authorship contribution statement
Acknowledgements
Declaration of competing interest
References
- J.M. Tarascon, M. Armand, Issues and challenges facing rechargeable lithium batteries, Nature 414 (2001) 359–367. [CrossRef]
- N. Nitta, F. Wu, J.T. Lee, G. Yushin, Li-ion battery materials: Present and future, Mater Today 18 (2015) 252–264. [CrossRef]
- J. Janek, W.G. Zeier, A solid future for battery development, Nat Energy 1 (2016) 1–4. [CrossRef]
- K. Xu, Electrolytes and interphases in Li-ion batteries and beyond, Chem. Rev. 114 (2014) 11503–11618. [CrossRef]
- C. Chen, Q. Li, Y. Li, Z. Cui, X. Guo, H. Li, Sustainable interfaces between Si anodes and garnet electrolytes for room-temperature solid-state batteries, ACS Appl. Mater. Interfaces 10 (2018) 2185–2190. [CrossRef]
- S.W. Song, K.C. Lee, H.Y. Park, High-performance flexible all-solid-state micro batteries based on solid electrolyte of lithium boron oxynitride, J. Power Sources 328 (2016) 311–317. [CrossRef]
- A. Hammami, N. Raymond, M. Armand, Lithium-ion batteries: Runaway risk of forming toxic compounds, Nature 424 (2003) 635–636. [CrossRef]
- D. Wang, G. Zhong, Y. Li, Z. Gong, M.J. McDonald, J.-X. Mi, R. Fu, Z. Shi, Y. Yang, Enhanced ionic conductivity of Li3.5Si0.5P0.5O4 with addition of lithium borate, Solid State Ionics 283 (2015) 109–114. [CrossRef]
- K. Takada, Progress in solid electrolytes toward realizing solid-state lithium batteries, J. Power Sources 394 (2018) 74–85. [CrossRef]
- B. Jiang, Y. Wei, J. Wu, H. Cheng, L. Yuan, Z. Li, H. Xu, Y. Huang, Recent progress of asymmetric solid-state electrolytes for lithium/sodium-metal batteries, EnergyChem 3 (2021) 100058. [CrossRef]
- J. Janek, W.A. Zeier, A solid future for battery development, Nat. Energy 1 (2016) 16141. [CrossRef]
- S.Y. Chen, C.T. Hsieh, R.S. Zhang, D. Mohanty, Y. Ashraf Gandomi, I. Ming Hung, Hybrid solid state electrolytes blending NASICON-Type Li1+xAlxTi2–x(PO4)3 with poly(vinylidene fluoride-co-hexafluoropropene) for lithium metal batteries, Electrochim. Acta 427 (2022) 140903. [CrossRef]
- S. Wang, L. Ben, H. Li, L. Chen, Identifying Li+ ion transport properties of aluminum doped lithium titanium phosphate solid electrolyte at wide temperature range, Solid State Ionics (2014). [CrossRef]
- Z. Zhang, Y. Zhao, S. Chen, D. Xie, X. Yao, P. Cui, X. Xu, An advanced construction strategy of all-solid-state lithium batteries with excellent interfacial compatibility and ultralong cycle life, J. Mater. Chem. A 5 (2017) 16984–16993. [CrossRef]
- D.H. Kim, D.Y. Oh, K.H. Park, Y.E. Choi, Y.J. Nam, H.A. Lee, S.M. Lee, Y.S. Jung, Infiltration of solution-processable solid electrolytes into conventional Li-ion-battery electrodes for all-solid-state Li-ion batteries, Nano Lett. 17 (2017) 3013–3020. [CrossRef]
- R. Koerver, I. Aygün, T. Leichtweiß, C. Dietrich, W. Zhang, J.O. Binder, P. Hartmann, W.G. Zeier, J. Janek, Capacity fade in solid-state batteries: Interphase formation and chemomechanical processes in nickel-rich layered oxide cathodes and lithium thiophosphate solid electrolytes, Chem. Mater. 29 (2017) 5574–5582. [CrossRef]
- B. Zhang, R. Tan, L. Yang, J. Zheng, K. Zhang, S. Mo, Z. Lin, F. Pan, Mechanisms and properties of ion-transport in inorganic solid electrolytes, Energy Storage Mater. 10 (2018) 139–159. [CrossRef]
- Q. Zhou, J. Ma, S. Dong, X. Li, G. Cui, Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries, Adv Mater 31 (2019) 1–21. [CrossRef]
- Y. Yan, J. Ju, S. Dong, Y. Wang, L. Huang, L. Cui, F. Jiang, Q. Wang, Y. Zhang, G. Cui, In situ polymerization permeated three-dimensional Li+-percolated porous oxide ceramic framework boosting All solid-state lithium metal battery, Adv Sci 8 (2021) 1–9. [CrossRef]
- Z. Lv, Q. Zhou, S. Zhang, S. Dong , Q. Wang , L. Huang, K. Chen, G. Cui, Cyano-reinforced in-situ polymer electrolyte enabling long-life cycling for high-voltage lithium metal batteries, Energy Storage Mater 37 (2021) 215–223. [CrossRef]
- G. Cui, Reasonable design of high-energy-density solidstate lithium-metal batteries, Matter 2 (2020) 805–815. [CrossRef]
- K. Nie, X. Wang, J. Qiu, Yi Wang, Qi Yang, J. Xu, X. Yu, H. Li, X. Huang, L. Chen, Increasing poly(ethylene oxide) stability to 4.5 V by surface coating of the cathode, ACS Energy Lett 5 (2020) 826–832. [CrossRef]
- J. Zhang, N. Zhao, M. Zhang, Y. Li, P. K. Chu, X. Guo, Z. Di, Xi Wang, H. Li, Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide, Nano Energy 28 (2016) 447–454. [CrossRef]
- L.P. Wang, X.D. Zhang, T.S. Wang, Y.X. Yin, J.L. Shi, C.R. Wang, Y.G. Guo, Ameliorating the interfacial problems of cathode and solid-state electrolytes by interface modification of functional polymers, Adv. Energy Mater. 8 (2018) 1801528. [CrossRef]
- P.Y. Sung, M. Lu, C.T. Hsieh, Y. Ashraf Gandomi, S. Gu, W.R. Liu, Sodium super ionic conductor-type hybrid electrolytes for high performance lithium metal batteries, Membranes 13(2) (2023) 201. [CrossRef]
- M. Wang, Y. Xue, K. Zhang, Y. Zhang, Synthesis of FePO4·2H2O nanoplates and their usage for fabricating superior high-rate performance LiFePO4, Electrochim. Acta 56 (2011) 4294–4298. [CrossRef]
- C.T. Hsieh, C.T. Pai, Y.F. Chen, I L. Chen, W.Y. Chen, Preparation of lithium iron phosphate cathode materials with different carbon contents using glucose additive for Li-ion batteries, J. Taiwan Inst. Chem. Eng. 45 (2014) 1501–1508. [CrossRef]
- A.R. Polu, R. Kumar, H.W. Rhe, Magnesium ion conducting solid polymer blend electrolyte based on biodegradable polymers and application in solid-state batteries[J], Ionics 21(1) (2015) 125–132. [CrossRef]
- H. Marceau, C.S. Kim, A. Paolella, S. Ladouceur, M. Lagacé, M. Chaker, A. Vijh, A. Guerfi, C. M. Julien, A. Mauger, M. Armand, P. Hovington, K. Zaghib, In operando scanning electron microscopy and ultraviolet-visible spectroscopy studies of lithium/sulfur cells using all solid-state polymer electrolyte[J], J. Power Sources 319 (2016) 247–254. [CrossRef]
- Y. Liang, Z. Lin, Y. Qiu, X. Zhang, Fabrication and characterization of LATP/PAN composite fiber-based lithium-ion battery separators, Electrochim. Acta 56 (2011) 6474–6480. [CrossRef]
- J. Kumar, P. Kichambare, A.K. Rai, R. Bhattacharya, S. Rodrigues, G.A. Subramanyam, A high performance ceramic-polymer separator for lithium batteries, J. Power Sources 301 (2016) 194–198. [CrossRef]
- S.A Yoon, N.R. Oh, A.R. Yoo, H.G. Lee, H.C. Lee, Preparation and characterization of Ta-substituted Li7La3Zr2-xO12 garnet solid electrolyte by sol-gel processing, J. Korean Ceram. Soc. 54 (2017) 278–284. [CrossRef]
- H. Xie, C. Li, W.H. Kan, M. Avdeev, C. Zhu, Z. Zhao, X. Chu, D. Mu, F. Wu, Consolidating the grain boundary of the garnet electrolyte LLZTO with Li3BO3 for high-performance LiNi0.8Co0.1Mn0.1O2/LiFePO4 hybrid solid batteries, J. Mater. Chem. A 7 (2019) 20633.
- K. Prasanna, T. Subburaj, W.J. Lee, C.W. Lee, Polyethylene separator: Stretched and coated with porous nickel oxide nanoparticles for enhancement of its efficiency in Li-ion batteries, Electrochim. Acta 137 (2014) 273–279. [CrossRef]
- X. Fan, L. Chen, X. Ji, T. Deng, S. Hou, J. Chen, J. Zheng, F. Wang, J. Jiang, K. Xu, C. Wang, Highly fluorinated interphases enable high-voltage Li-metal batteries, Chem 4 (2018) 174–185. [CrossRef]
- G.M.A. Girard, M. Hilder, D. Nucciarone, K. Whitbread, S. Zavorine, M. Moser, M. Forsyth, D.R. MacFarlane, P.C. Howlett, Role of Li concentration and the SEI layer in enabling high performance Li metal electrodes using a phosphonium bis(fluorosulfonyl)imide ionic liquid, J. Phys. Chem. C 121 (2017) 21087–21095. [CrossRef]
- C.T. Hsieh, C.T. Pai, Y.F. Chen, I.L. Chen, W.Y. Chen, Preparation of lithium iron phosphate cathode materials with different carbon contents using glucose additive for Li-ion batteries, J. Taiwan Inst. Chem. Eng. 45 (2014) 1501–1508. [CrossRef]
- C.T. Hsieh, C.T. Pai, Y.F. Chen, P.Y. Yu, R.S. Juang, Electrochemical performance of lithium iron phosphate cathodes at various temperatures, Electrochim. Acta 115 (2014) 96–102. [CrossRef]
- Y.H. Nien, J.R. Carey, J.S. Chen, Physical and electrochemical properties of LiFePO4/C composite cathode prepared from various polymer-containing precursors, J. Power Sources 193 (2009) 822. [CrossRef]
- H.C. Wu, H.C. Wu, E. Lee, N.L. Wu, High-temperature carbon-coated aluminum current collector for enhanced power performance of LiFePO4 electrode of Li-ion batteries, Electrochem. Commun. 12 (2010) 488. [CrossRef]
- J. Zhang, N. Zhao, M. Zhang, Y. Li, P.K. Chu, X. Guo, Z. Di, X. Wang, H. Li, Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide, Nano Energy 28 (2016) 447–454. [CrossRef]
- W. Wang, E. Yi, A.J. Fici, R.M. Laine, J. Kieffer, Lithium ion conducting poly(ethylene oxide)-based solid electrolytes contain-ing active or passive ceramic nanoparticles, J. Phys. Chem. C 121 (2017) 2563–2573. [CrossRef]
- G. Xu, F. Li, Z. Tao, X. Wei, Y. Liu, X. Li, Z. Ren, G. Shen, G. Han, Monodispersed LiFePO4@C core–shell nanostructures for a high power Li-ion battery cathode, J. Power Sources. 246 (2014) 696–702. [CrossRef]
- S. Yu, R.D. Schmidt, R. Garcia-Mendez, E. Herbert, N.J. Dudney, J.B. Wolfenstine, J. Sakamoto, D.J. Siegel, Elastic properties of the solid electrolyte Li7La3Zr2O12 (LLZO), Chem. Mater. 28 (2015) 197. [CrossRef]
- G. Yan, J. Malzbender, S. Fu, J.P. Gross, S. Yu, R.-A. Eichel, R. Schwaiger, Fracture behavior of solid electrolyte LATP material based on micro-pillar splitting method, J. Eur. Ceram. Soc. 41 (2021) 5240–5247. [CrossRef]
- H. Huo, Y. Chen, J. Luo, X. Yang, X. Guo, X. Sun, Rational design of hierarchical “ceramic-in-polymer” and “polymer-in-ceramic” electrolytes for dendrite-free solid-state batteries, Adv. Energy Mater. 9 (2019) 1804004. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




