4.1. Metal pollution in R. mangrove sediment of the Navachiste lagoon complex
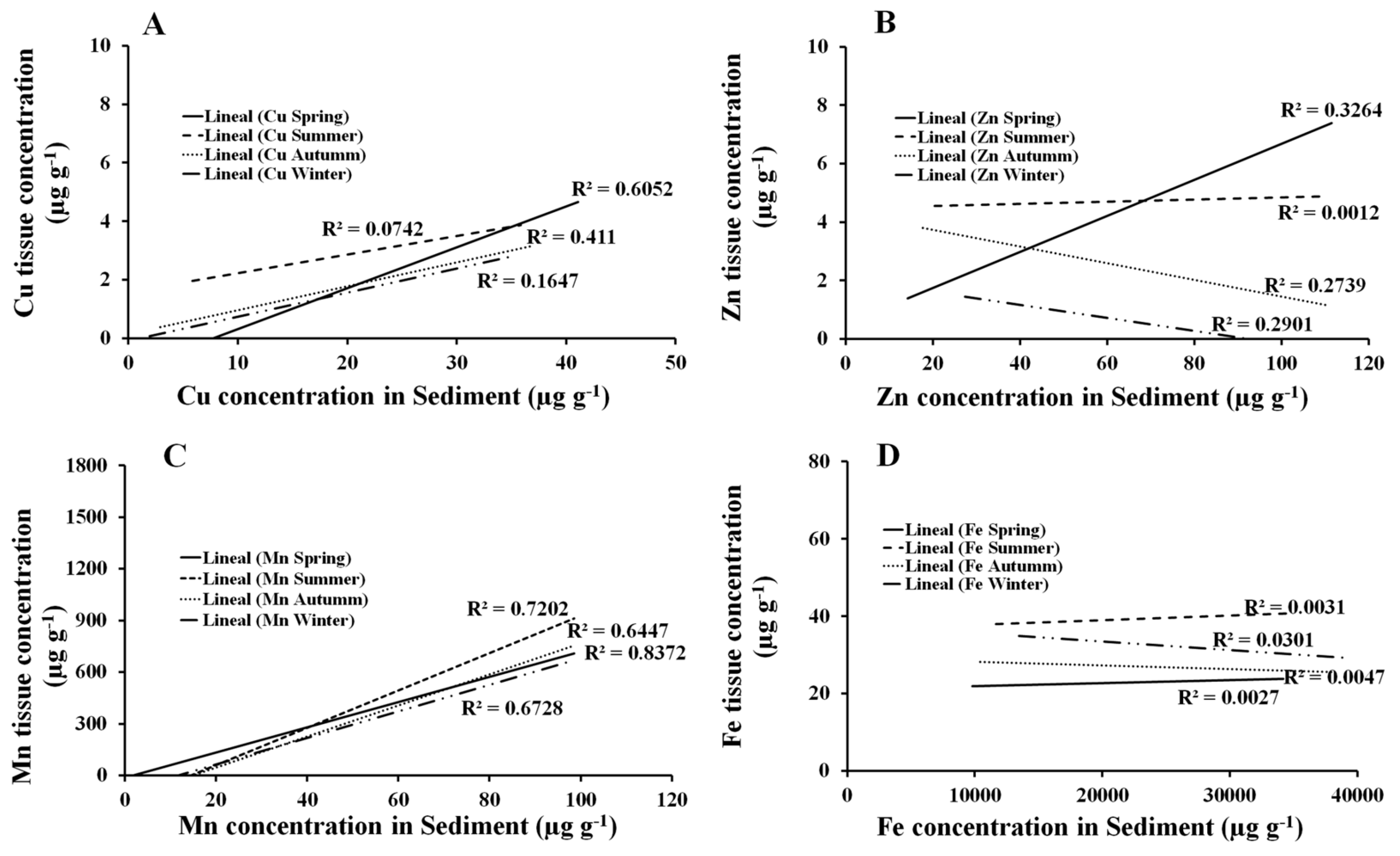
Tissue Mn and Cu concentrations in
R. mangle can be used as bioindicator data to monitor environmental exposure to some metals. Although the mobility/availability of metals within the soil-plant spectrum depends on OM, suspended O
2, texture and pH, the chemical composition of the sediments and mangrove detritus [
20,
21], in this study,
R. mangle demonstrated a significant correlation between the bioavailable contents of Mn and Cu in the sediment and their corresponding contents in its roots and leaves. Other species of this genus, such as
R. mucronata, have already been classified as potential phytoremediators due to their ability to translocate trace metals to their tissues [
22].
The capacity of mangrove species to translocate trace metals to their tissues is influenced by various parameters, including salinity, pH, Electrical conductivity (EC), Total Dissolved Solids (TDS), biochemical and chemical oxygen demand (BOD and COD), which affect the translocation of trace metals from the sediment to the tissues. Additionally, factors such as the type of sediments, life stage, age, morphology of the tree, and biomass also play a role in this process [
5,
22].
Physicochemical parameters, such as moderate salinity, stimulate the lignified exodermis, which delays the entry of metals, while a deficiency of N allows the adsorption of metals in the roots [
23]. In this study, silt, clay, and OM showed correlations with the highest concentrations of metals, similar to those reported by [
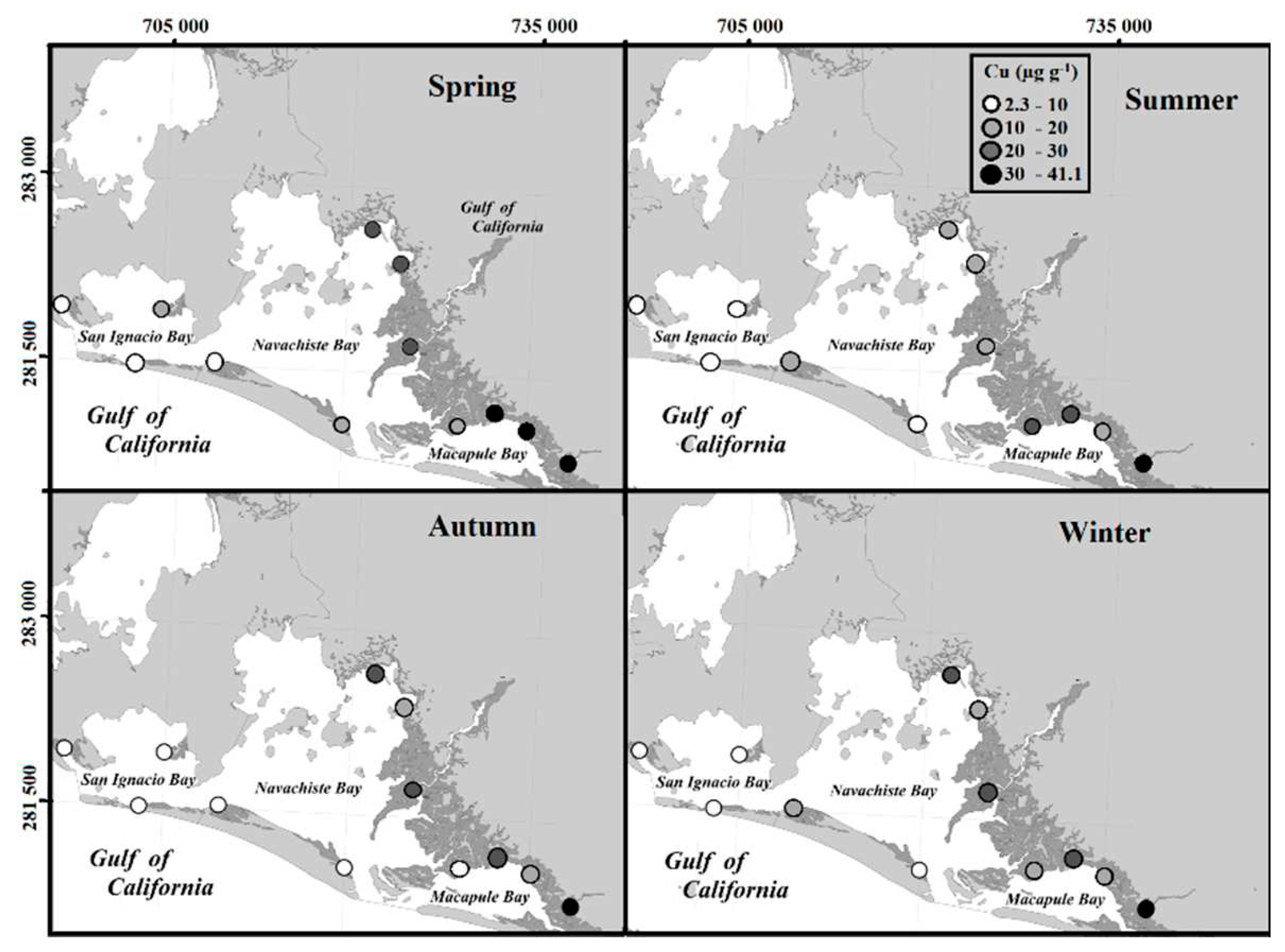
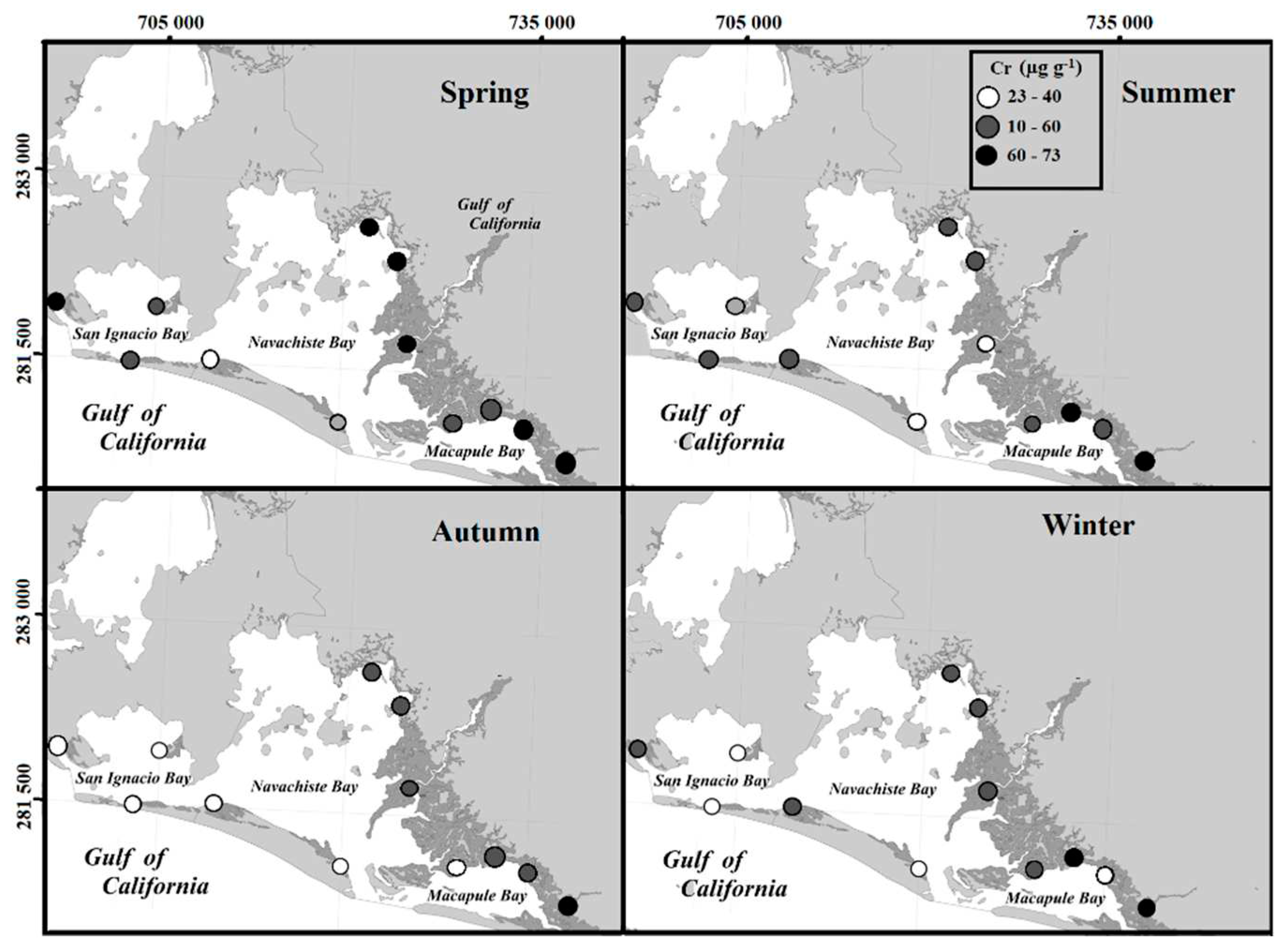
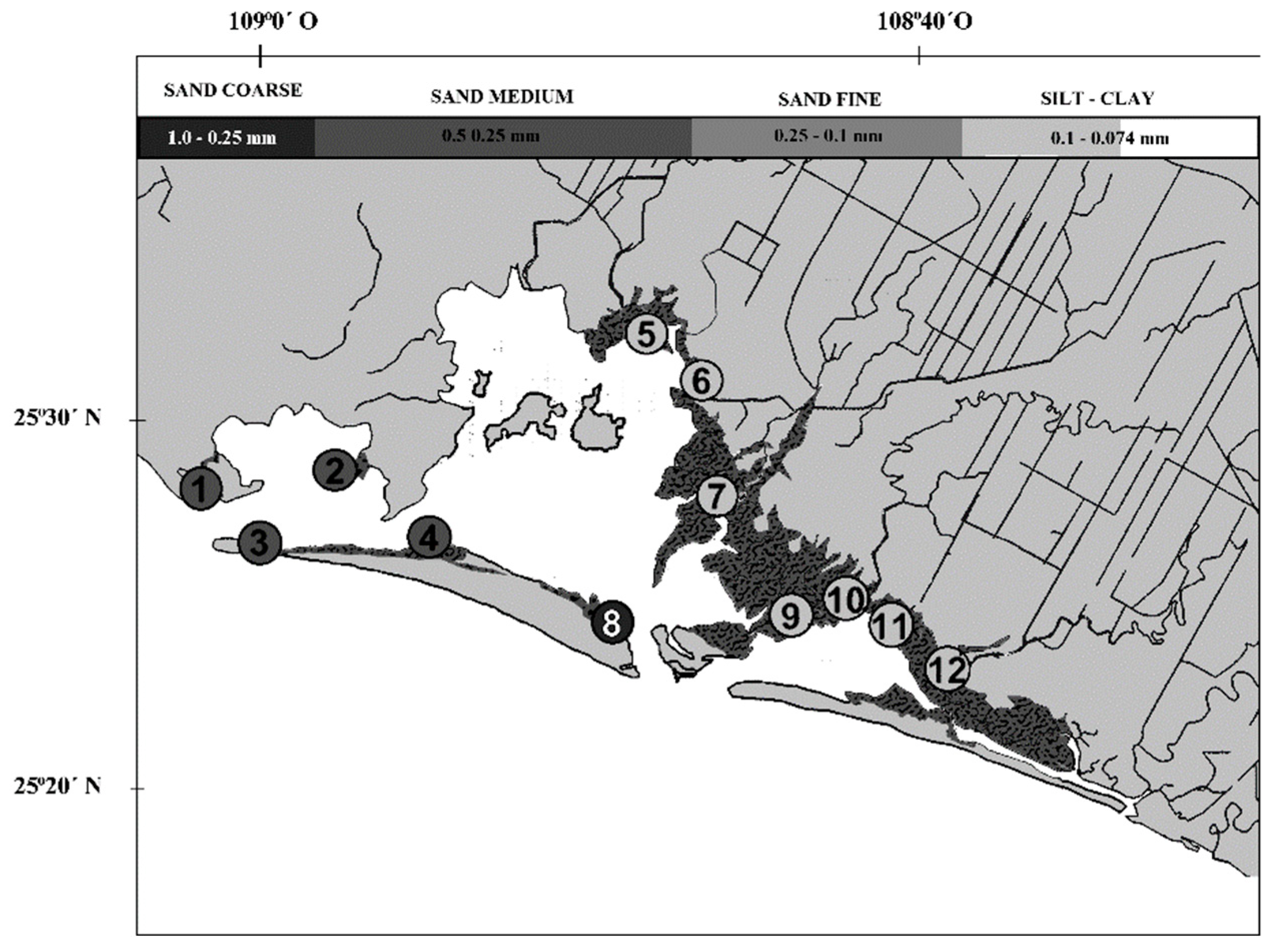
24]. The concentrations of trace metals varied between the collection sites of the NAV, but it is evident that the sample sites surrounding mangrove trees with these textural characteristics presented the highest concentrations of trace metals.
Another aspect that can influence the variation of the concentrations between the sites and the collection station are the speeds of the water circulation patterns that influence the rate of sediment deposit [
25] and, therefore, the distribution of trace metals in the areas of the NAV lagoon complex. Within the NAV lagoon complex, it has been determined that the strongest currents are found at the entrances that are located among the sand islands [
26]. The collection sites close to these areas had the lowest concentrations of metals (
Figure 10).
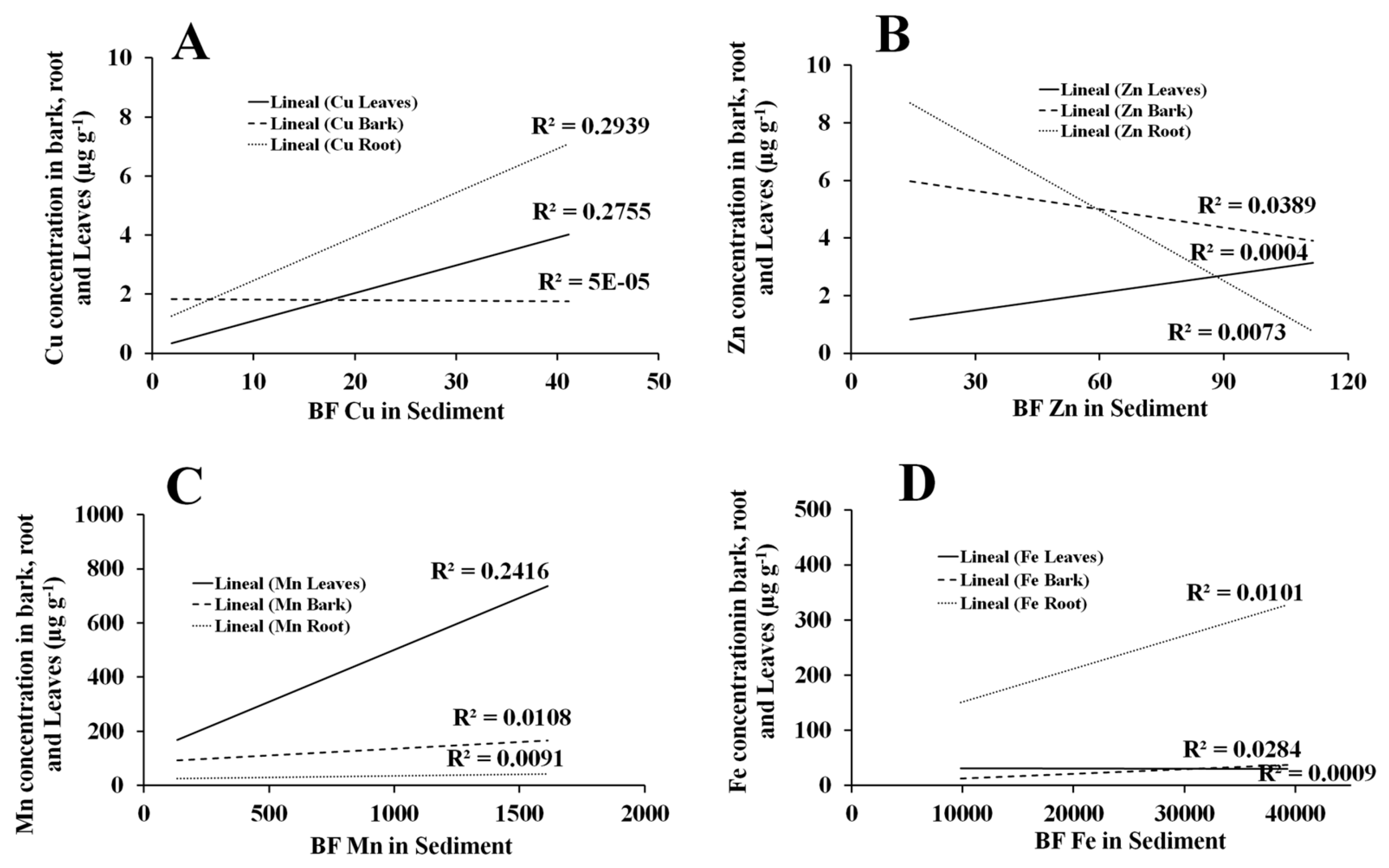
The lack of correlation of the Cu, Zn, Mn, and Fe concentrations between the retention pattern by
R. mangrove and collection sites in the NAV lagoon complex or among seasons of the year may be due to the adsorption pattern of metal(oids) that depends on the characteristics of the species’ roots, the amount and type of iron plaques, and the availability of metal ions in aquatic environments [
27].
Despite the fact that in previous studies the root is reported as a sump for metals such as Co, Cr, Cu, Fe, Mn, Ni, Pb, Zn, and Hg [
6], in this study, this accumulation strategy of metals was not observed in the root but rather in the leaf, which showed the highest correlations of Cu and Mn concentrations throughout most of the year and with the sediment. Unlike other neighboring species, such as
L. racemosa, the bioaccumulation of other metals like Al, Pb, and Zn have been documented [
8].
The difference in retention, both in the type and concentration of TM, can be attributed to two situations. The first is related to the location of both species on the coastline, with
R. mangle situated in front of
L. racemosa within the NAV [
3]. In other words, possibly the sediments that are geographically more frontal to the coastline are richer in trace metals. This peculiar information makes it possible to expand future research areas perpendicular to the coast to determine this possible correlation. The second explanation could be given by the physiology of the leaves that depends on the species because concentrations of pentacyclic triterpenes and n-alkanes (such as hentriacontane) in the cuticular waxes influence the retention of trace metals [
8].
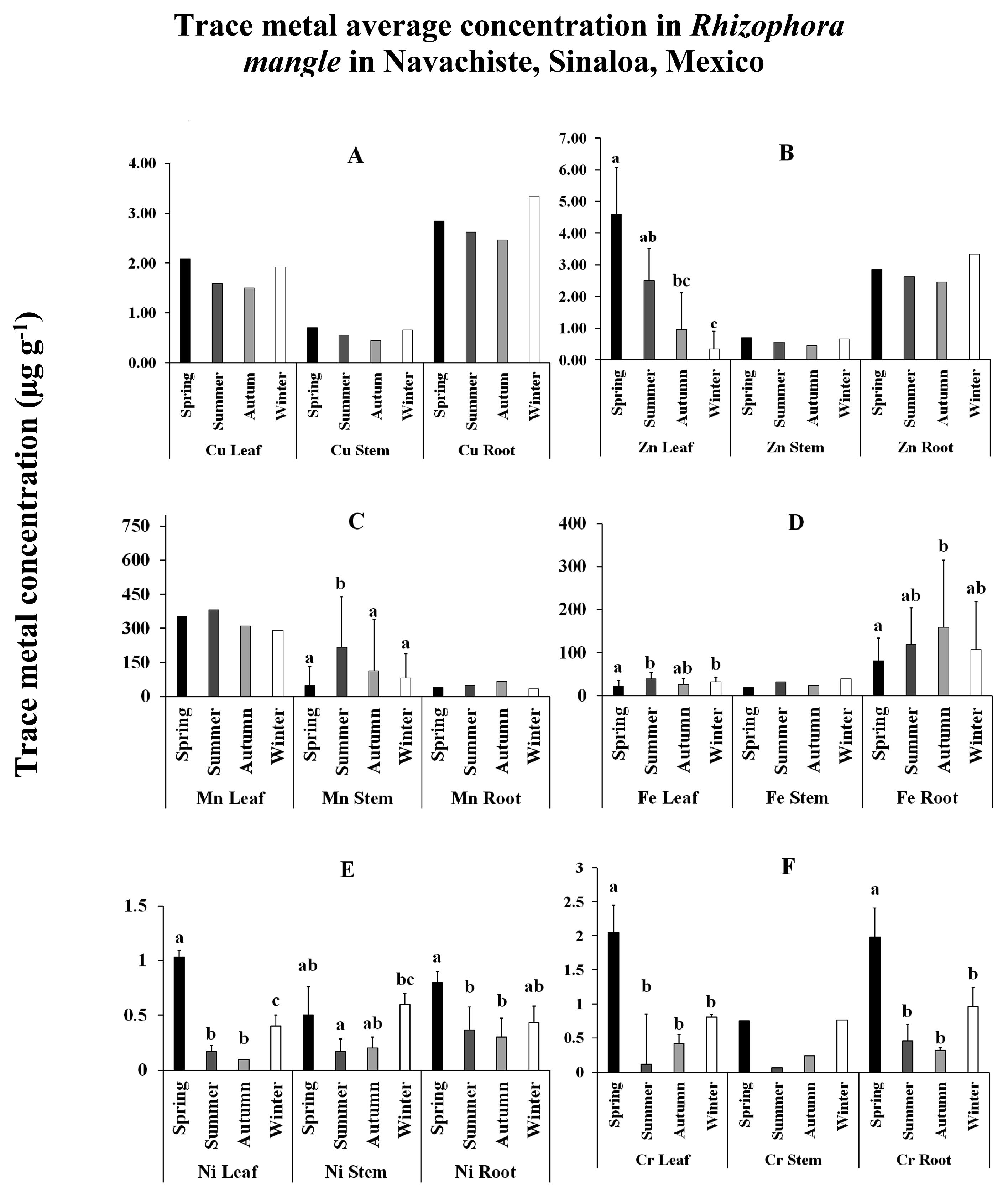
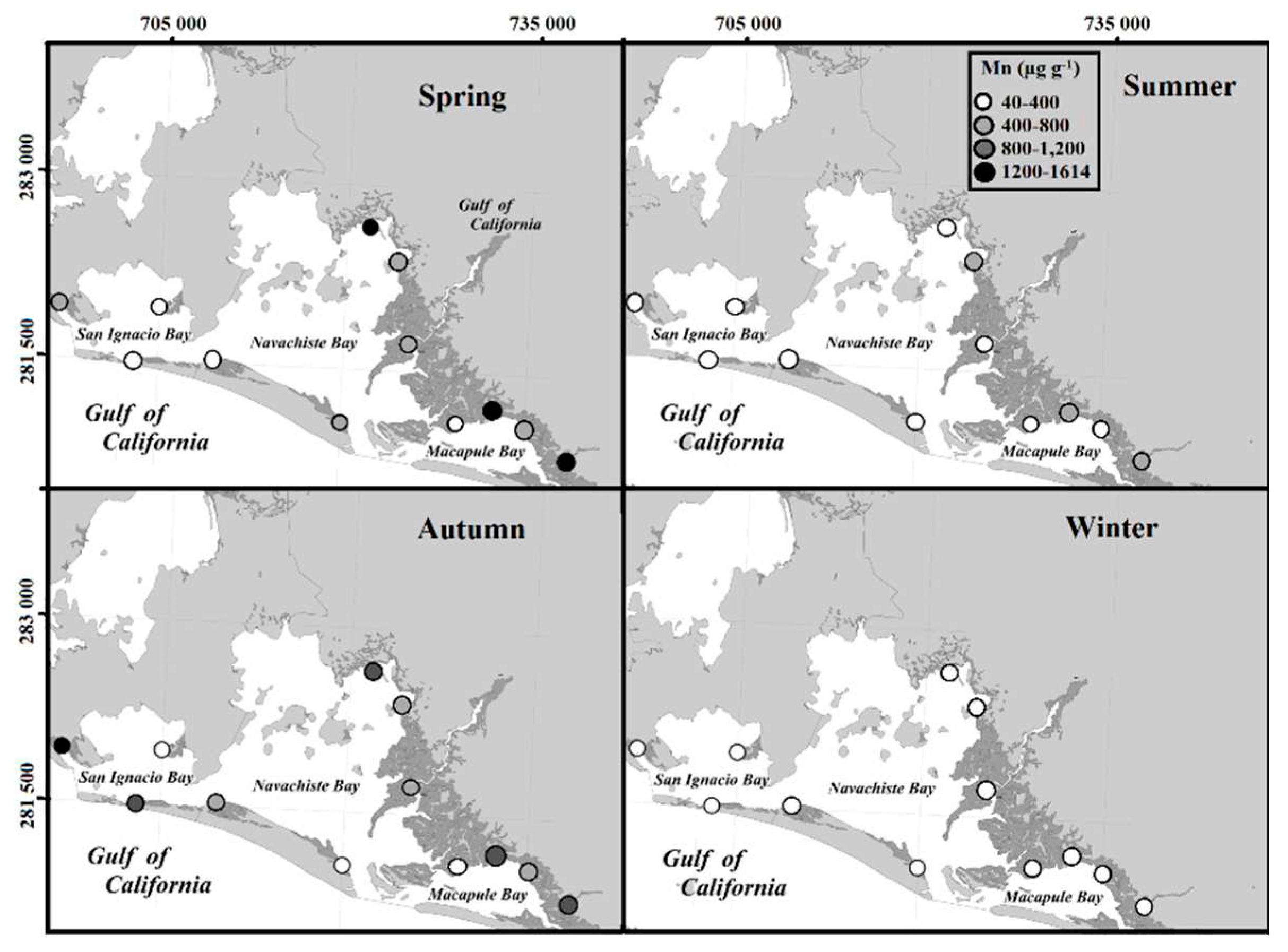
Regarding the total fraction of Mn (563 µg g
-1 y
-1), it was found to be well above the average Mn content in the earth's crust (95 µg g
-1) (Taylor, 1964), which added to its high correlation with Cu, Zn, Cr, and Ni, it could be related to the residues derived from the extensive use of agrochemicals, fertilizers, and pesticides used in agricultural processes (Daulta et al., 2023). For more than 20 years, the use of agrochemicals, such as Maneb
® (SYNGENTA
®), Zineb (VELSIMEX
®), and Cupravit® (BAYER
®), have been reported [
24,
28,
29], and it is highly probable that they are still being used (or their derivatives) in the surrounding agricultural region of Guasave. In this sense, studies should be focused on determining the type of agrochemicals used, as well as the concentration of these residues in the sediments of the channels that flow into the NAV. It has been reported that high Mn levels are associated with the presence of Fe-Mn hydroxides due to the deposit of fine sediments in areas with depressions that favor the deposit of sedimentary material [
30]. This situation is very present in the NAV, as erosion and deposition dominate the coasts and the islands that compose it, forming large extensions of dunes and gentle slopes with a predominance of sand [
31].
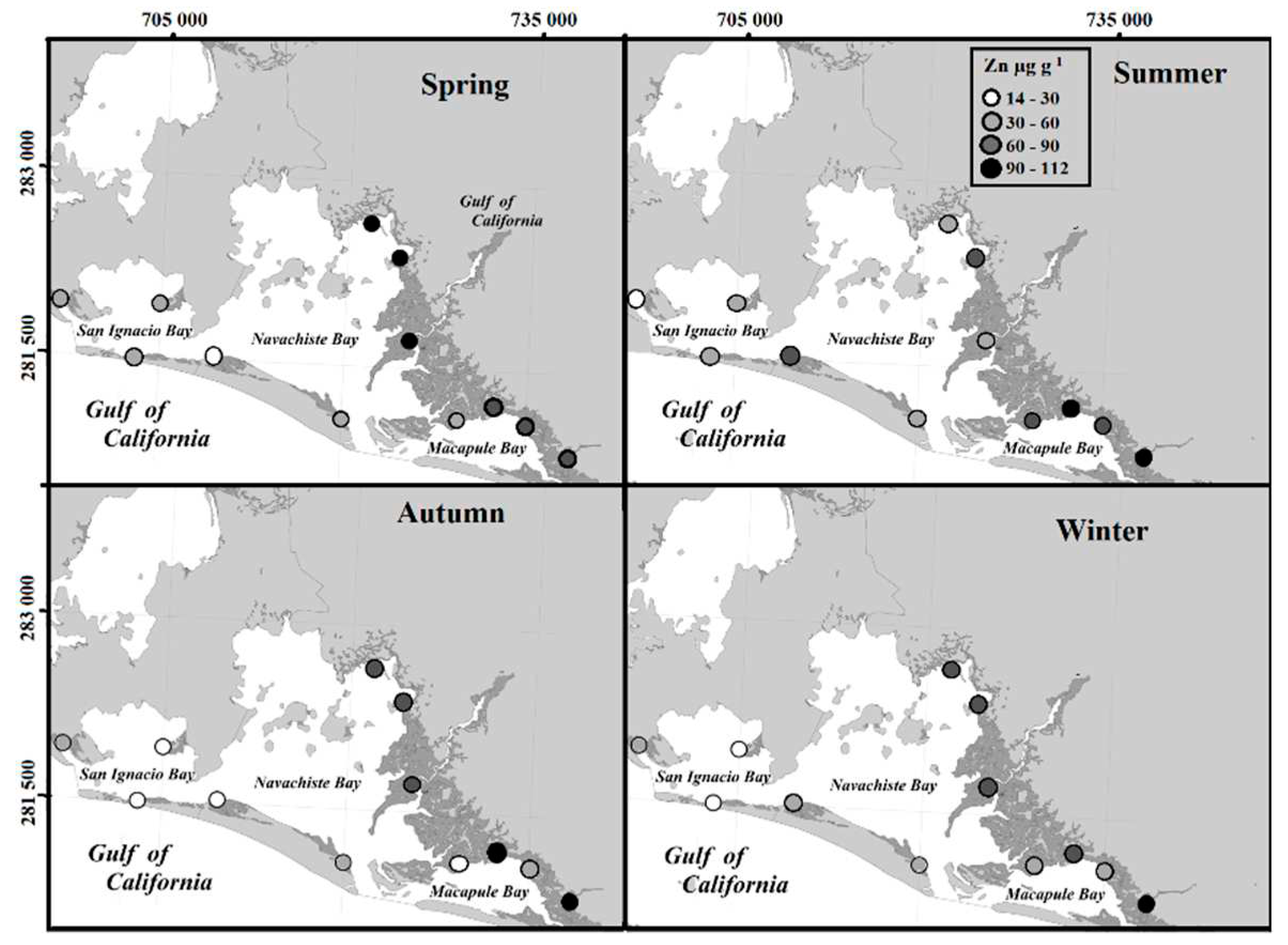
The highest values of Zinc (112 µg g
-1) were determined to be above the average value of the earth's crust of 70 µg g
-1 [
32], at various sites within the NAV (
Figure 4). According to the EF, the Zn content in the MAB and NAB collection sites was classified as enriched throughout the four seasons of the year, agreeing with the crop cycles of the adjacent agricultural valley and the torrential rains that occur occasionally. This enrichment may be due to metallic residues from chemical fertilizers used in the Guasave valley, for the cultivation of vegetables and grains that increase the concentration of heavy metals in agricultural soil [
33]. The increase in concentration adds to the suspended particulate matter, sediments, and water that end up in coastal areas [
34]. This area includes the NAV impacted by the Guasave Valley [
29], which is the most important agricultural area in Mexico, with more than 400 thousand hectares of intensive cultivation.
The Cr variation during autumn and winter in sediments was closely associated with the concentrations of Cu and Ni, and due to the same anthropogenic origin and the natural erosive contribution of the rocky material associated with the silt fraction of the sediment. Even though in the nearby coastal lagoons, such as Ohuira and Topolobampo, whose Cr concentration has been reported to be lower (29.9 ± 11.9 ppm) [
35], it is clear that in the NAV lagoon complex, the contribution of erosive material is greater, managing to form larger areas of dunes and sand islands that make up this coastal lagoon [
31]. Another potential source of Cr is through residues of the food used for shrimp in aquaculture, which provides 23 % of the supply accumulated by shrimp [
36], and considering that there is a feed conversion rate of 1.79 regarding commercial product [
37], it is likely that 6 % of the total 21 % of the uneaten product discharges through the drains towards the lagoon complex.
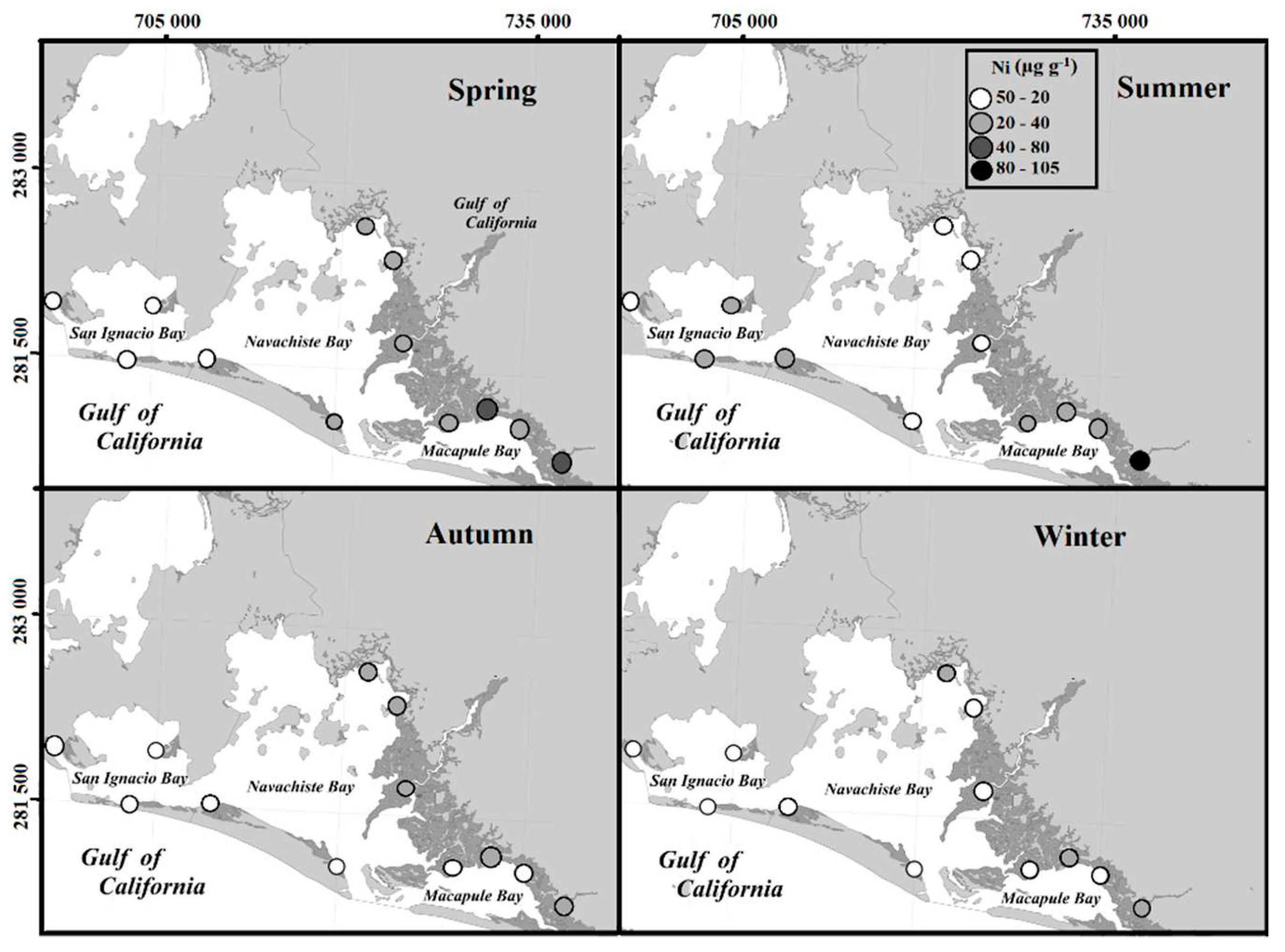
The annual average concentration of Ni (23 µg g
-1), mainly associated with Zn, Cu, Cr, and the silt fraction of the sediment, presented the highest concentrations in spring. At the beginning of this season, intensive irrigation occurs in the agricultural valley of Guasave, slowing down with the beginning of the rains in the summer. This surplus of water carries agricultural residues, including traces of metals such as Ni. However, anthropogenic contamination induces a greater bioavailability of Ni in soils and even greater in soils with high geochemical contribution [
38]. This is observed throughout the year, in which Ni values remain below 40 µg g
-1, which is less than the concentration in the earth's crust (75 µg g
-1) [
32]. This can be attributed to the fact that in the areas surrounding the NAV, secondary activities do not present such an important development as is the case with primary activities like agriculture, livestock, and aquaculture.
The highest levels of Cu concentration were closely related to the discharge drains from agricultural areas, as previously described [
39]. Cu concentrations were also closely related to discharges from shrimp aquaculture. Regarding shrimp farms, 67% of Cu accumulation by shrimp comes mainly from food ingestion [
36]. That is, considering the feed conversion rate [
37], it is probable that 17.8 % of Cu that was not consumed in the aquaculture feed of more than 290 thousand hectares of ponds, is discharged to the lagoon complex, increasing the concentrations of Cu. In the same way, the concentration of Cu in the areas receiving agricultural and aquaculture discharges was related to t he time of the year, silt and clay content found in the adjacent sediments of the mangrove trees. It has previously been determined how the concentration of Cu varies notably from the conditions of the pedosphere and in areas with greater impact from anthropogenic sources such as industrial plants, large urban areas, and high-seas ports, where the concentration values are higher [
24].
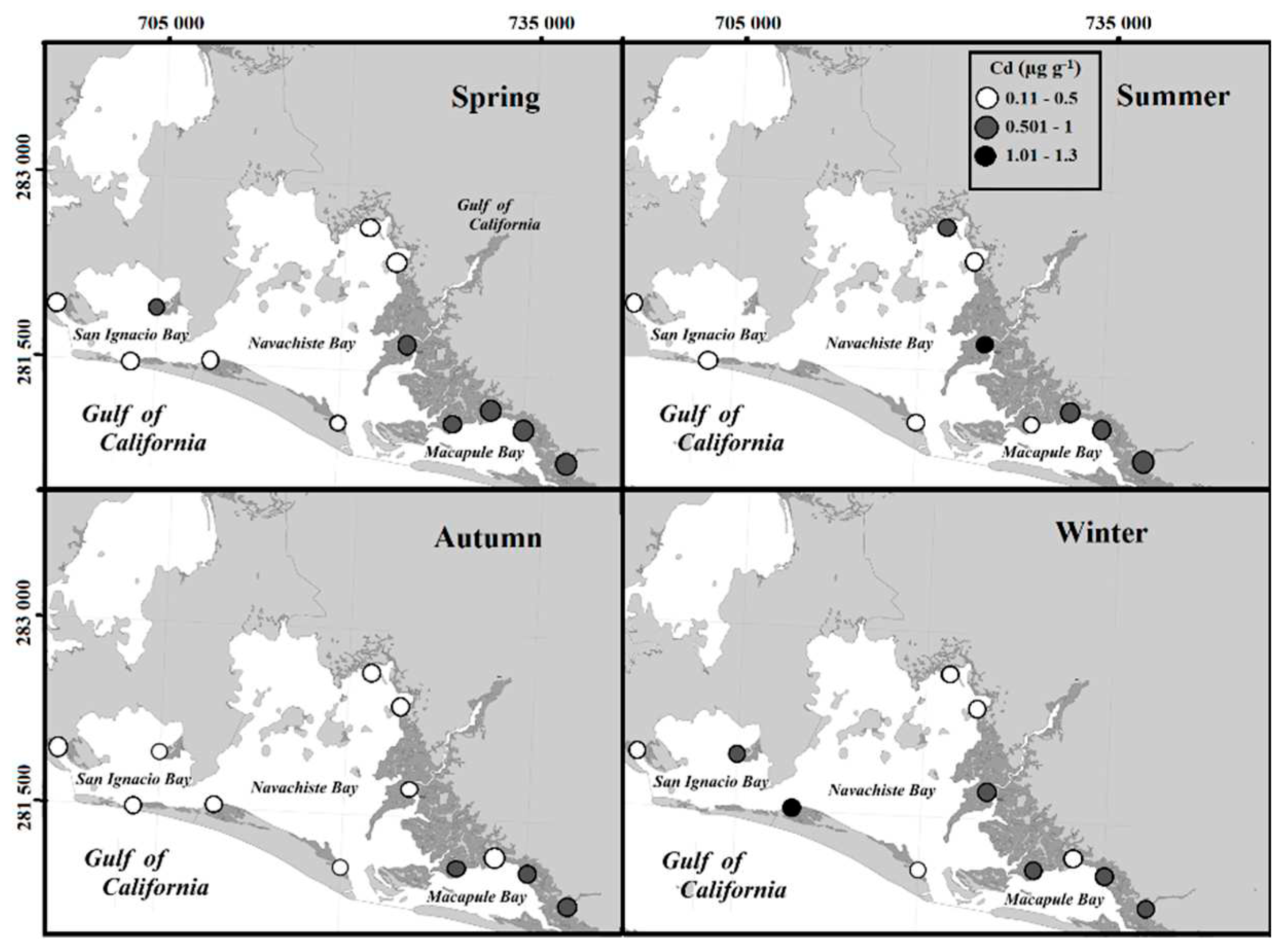
The variation of Cd concentrations in the leaf, stem, and root of
R. mangle, even with concentrations present in the sediments during the year, is associated with sediment components potentially reactive to diagenetic processes, such as OM, oxides, and carbonates near the surface [
40]. Another source of Cd may come from the use of phosphate fertilizers based on phosphate rocks that are used in agriculture [
41]. Another important factor is the 400,000 plus hectares of intensive cultivation in the Guasave Valley, which is likely to be the most important source of Cd. This can also be supported by the high correlation of Cd with those of Zn (r = 0.92) and Fe (r= 0.91) during summer through winter when one of the two irrigation stages occurs in the Guasave Valley and the contributions of waste to the NAV could increase.
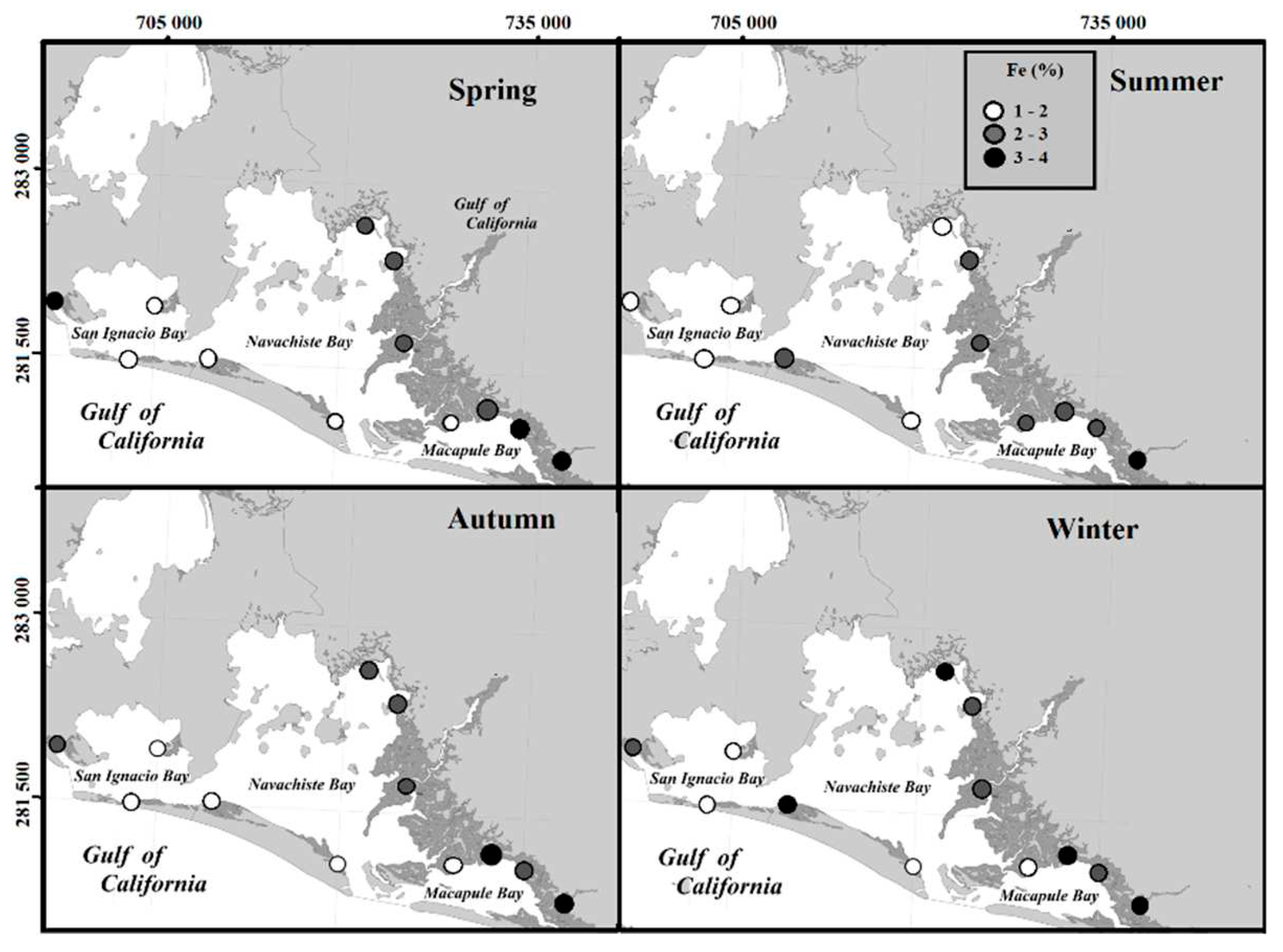
The correlation of Fe concentration in sediment with OM content in sediments during the spring and in silt in autumn and winter occurs in alkaline systems where the presence of organic matter and other oxidizable compounds in the sediment prevent constant oxidation due to the interaction with nanoparticles that favor aggregation and sedimentation [
42].
For this reason, when there is a greater sedimentation and interaction, the BioF of Fe was found to be higher in summer, specifically regarding concentrations equivalent to the total Fe content in the sediment. The Fe concentrations in the roots were also correlated with the Fe concentrations in the summer. That is, the bioavailability of Fe in the sediment was reduced due to the lower interaction and/or its accumulation through the exclusion/regulation mechanism called "iron plaques" present in the roots of
R. mangrove [
7]. Other aspects that influence the formation of Fe plaques are the remobilization and introduction of Fe available in Fe-rich sediments, different water conditions, and wetland types, and acidic pH [
43,
44], which are potentially correlated with the formation of Fe plaques [
6].