Submitted:
28 August 2023
Posted:
30 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
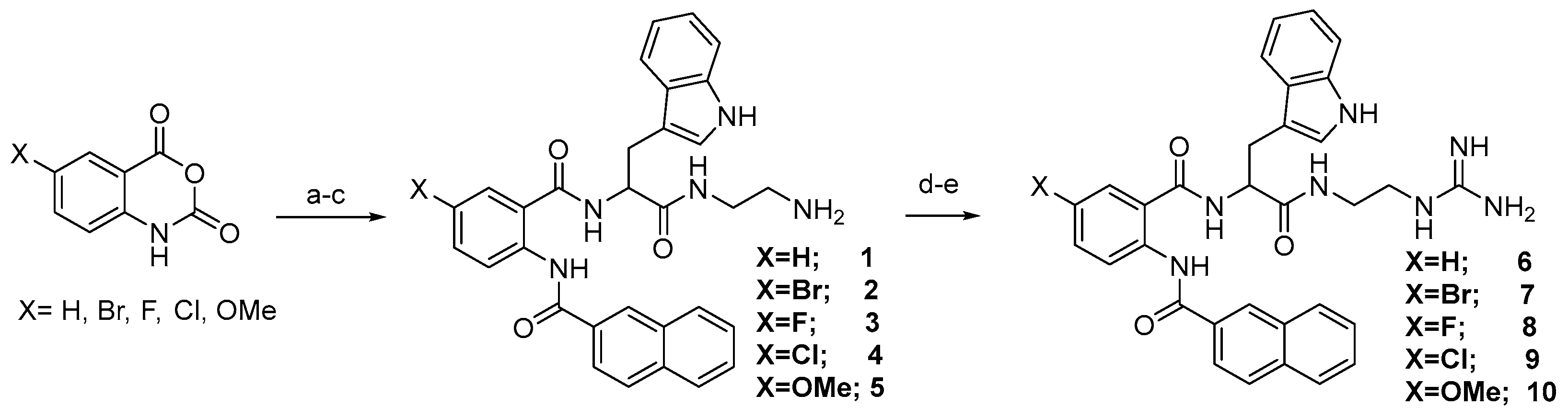
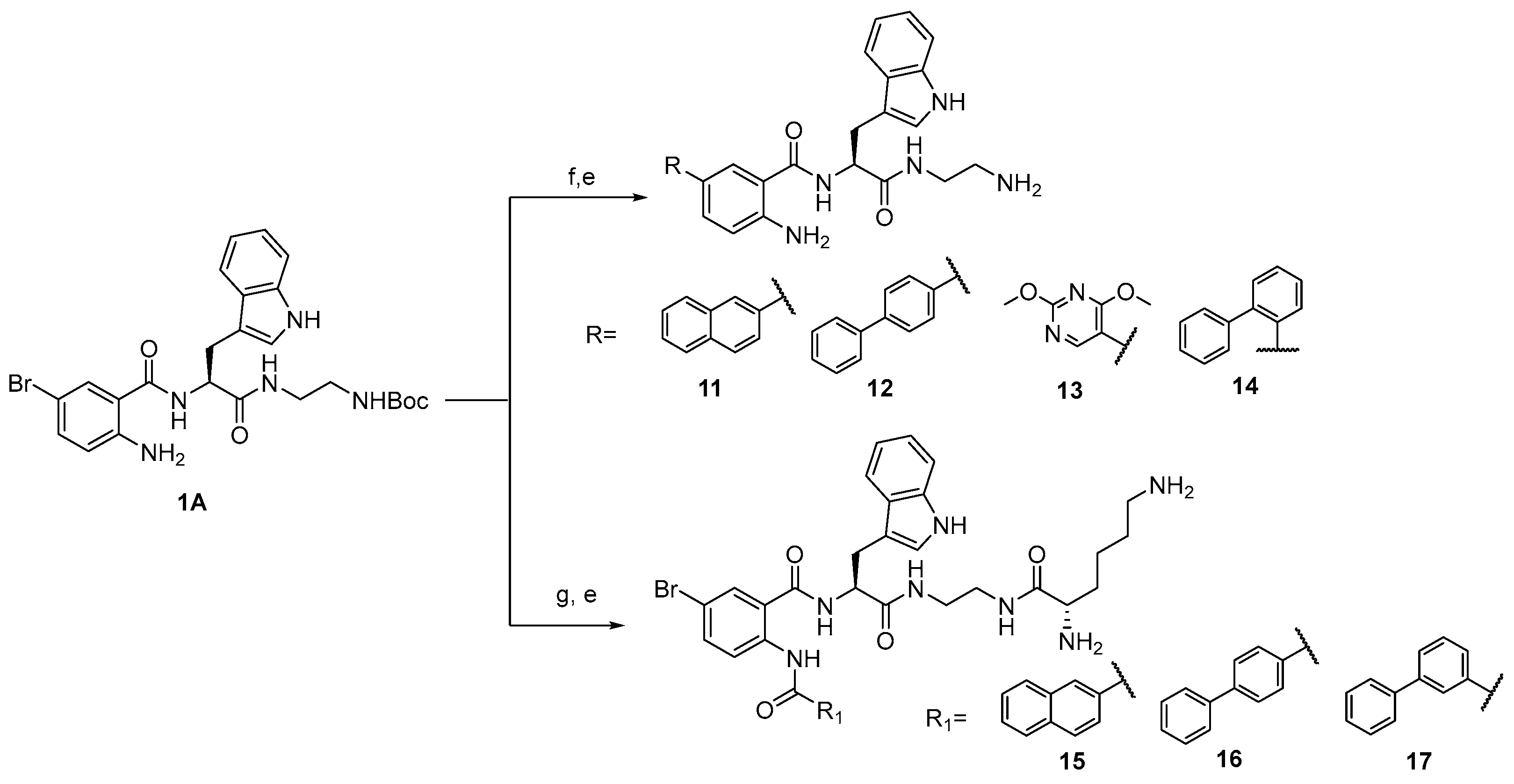
2.1. Chemistry
2.2. Screening of anthranilamide peptidomimetics against MHV-1 and HSV-1
2.3. TEM images
2.4. Cytotoxic activities of active mimetics
3. Materials and Methods
3.1. Chemistry
3.2. Analytical data
3.3. Virus and Cell culture
3.4. Antiviral testing
3.5. Cytotoxicity assay
3.6. Transmission electron microscopy
3.7. Statistical analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javanian, M.; Barary, M.; Ghebrehewet, S.; Koppolu, V.; Vasigala, V.; Ebrahimpour, S. A Brief Review of Influenza Virus Infection. J Med Virol 2021, 93, 4638–4646. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chang, J.; Chen, S.; Wang, L.; Yau, T.O.; Zhao, Q.; Hong, Z.; Ruan, J.; Duan, G.; Gao, S. Genomic Feature Analysis of Betacoronavirus Provides Insights into Sars and Covid-19 Pandemics. Front Microbiol 2021, 12, 614494. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.R.; Navas-Martin, S. Coronavirus Pathogenesis and the Emerging Pathogen Severe Acute Respiratory Syndrome Coronavirus. Microbiol Mol Biol Rev 2005, 69, 635–664. [Google Scholar] [CrossRef] [PubMed]
- MacPhee, P.J.; Dindzans, V.J.; Fung, L.S.; Levy, G.A. Acute and Chronic Changes in the Microcirculation of the Liver in Inbred Strains of Mice Following Infection with Mouse Hepatitis Virus Type 3. Hepatology 1985, 5, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.J.; Weiss, S.R. Pathogenesis of Murine Coronavirus in the Central Nervous System. J Neuroimmune Pharmacol 2010, 5, 336–354. [Google Scholar] [CrossRef]
- Yang, Z.; Du, J.; Chen, G.; Zhao, J.; Yang, X.; Su, L.; Cheng, G.; Tang, H. Coronavirus Mhv-A59 Infects the Lung and Causes Severe Pneumonia in C57bl/6 Mice. Virol Sin 2014, 29, 393–402. [Google Scholar] [CrossRef]
- Featherstone, A.B.; Brown, A.C.; Chitlapilly Dass, S. Murine Hepatitis Virus, a Biosafety Level 2 Model for Sars-Cov-2, Can Remain Viable on Meat and Meat Packaging Materials for at Least 48 Hours. Microbiol Spectr 2022, 10, e0186222. [Google Scholar] [CrossRef]
- Casanova, L.M.; Jeon, S.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Effects of Air Temperature and Relative Humidity on Coronavirus Survival on Surfaces. Appl Environ Microbiol 2010, 76, 2712–2717. [Google Scholar] [CrossRef]
- Dellanno, C.; Vega, Q.; Boesenberg, D. The Antiviral Action of Common Household Disinfectants and Antiseptics against Murine Hepatitis Virus, a Potential Surrogate for Sars Coronavirus. Am J Infect Control 2009, 37, 649–652. [Google Scholar] [CrossRef]
- Yasir, M.; Kumar Vijay, A.; Willcox, M. Antiviral Effect of Multipurpose Contact Lens Disinfecting Solutions against Coronavirus. Cont Lens Anterior Eye 2021, 101513. [Google Scholar] [CrossRef]
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Smith, E.C.; Case, J.B.; Feng, J.Y.; Jordan, R.; et al. Coronavirus Susceptibility to the Antiviral Remdesivir (Gs-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Burrer, R.; Neuman, B.W.; Ting, J.P.; Stein, D.A.; Moulton, H.M.; Iversen, P.L.; Kuhn, P.; Buchmeier, M.J. Antiviral Effects of Antisense Morpholino Oligomers in Murine Coronavirus Infection Models. Journal of virology 2007, 81, 5637–5648. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.L.; Pruijssers, A.J.; Chappell, J.D.; Gribble, J.; Lu, X.; Andres, E.L.; Bluemling, G.R.; Lockwood, M.A.; Sheahan, T.P.; Sims, A.C.; et al. Small-Molecule Antiviral Β-D-N(4)-Hydroxycytidine Inhibits a Proofreading-Intact Coronavirus with a High Genetic Barrier to Resistance. J Virol 2019, 93. [Google Scholar] [CrossRef]
- Zhou, Q.; Luo, Y.; Zhu, Y.; Chen, Q.; Qiu, J.; Cong, F.; Li, Y.; Zhang, X. Nonsteroidal Anti-Inflammatory Drugs (Nsaids) and Nucleotide Analog Gs-441524 Conjugates with Potent in Vivo Efficacy against Coronaviruses. European Journal of Medicinal Chemistry 2023, 249. [Google Scholar] [CrossRef] [PubMed]
- Tu, E.C.; Hsu, W.L.; Tzen, J.T.C. Strictinin, a Major Ingredient in Yunnan Kucha Tea Possessing Inhibitory Activity on the Infection of Mouse Hepatitis Virus to Mouse L Cells. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Graff Reis, J.; Dai Prá, I.; Michelon, W.; Viancelli, A.; Piedrahita Marquez, D.G.; Schmitz, C.; Maraschin, M.; Moura, S.; Thaís Silva, I.; de Oliveira Costa, G.; et al. Characterization of Planktochlorella Nurekis Extracts and Virucidal Activity against a Coronavirus Model, the Murine Coronavirus 3. International Journal of Environmental Research and Public Health 2022, 19. [Google Scholar] [CrossRef]
- Sainz, B., Jr.; Mossel, E.C.; Gallaher, W.R.; Wimley, W.C.; Peters, C.J.; Wilson, R.B.; Garry, R.F. Inhibition of Severe Acute Respiratory Syndrome-Associated Coronavirus (Sars-Cov) Infectivity by Peptides Analogous to the Viral Spike Protein. Virus Res 2006, 120, 146–155. [Google Scholar] [CrossRef]
- Kim, H.Y.; Eo, E.Y.; Park, H.; Kim, Y.C.; Park, S.; Shin, H.J.; Kim, K. Medicinal Herbal Extracts of Sophorae Radix, Acanthopanacis Cortex, Sanguisorbae Radix and Torilis Fructus Inhibit Coronavirus Replication in Vitro. Antiviral Therapy 2010, 15, 697–709. [Google Scholar] [CrossRef]
- Whitley, R.J.; Roizman, B. Herpes Simplex Virus Infections. The lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS One 2015, 10, e0140765. [Google Scholar] [CrossRef]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and Virulence of Herpes Simplex Virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef] [PubMed]
- Kłysik, K.; Pietraszek, A.; Karewicz, A.; Nowakowska, M. Acyclovir in the Treatment of Herpes Viruses - a Review. Curr Med Chem 2020, 27, 4118–4137. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, H.H.; Snoeck, R.; Andrei, G. Acyclovir Resistance in Herpes Simplex Viruses: Prevalence and Therapeutic Alternatives. Biochem Pharmacol 2022, 206, 115322. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.Y.; Yeh, C.S.; Wang, S.M.; Chen, S.H.; Wang, J.R.; Chen, T.Y.; Tsai, H.P. Acyclovir-Resistant Hsv-1 Isolates among Immunocompromised Patients in Southern Taiwan: Low Prevalence and Novel Mutations. J Med Virol 2023, 95, e28985. [Google Scholar] [CrossRef] [PubMed]
- Urmi, U.L.; Vijay, A.K.; Kuppusamy, R.; Islam, S.; Willcox, M.D.P. A Review of the Antiviral Activity of Cationic Antimicrobial Peptides. Peptides 2023, 166, 171024. [Google Scholar] [CrossRef]
- Chowdhury, A.S.; Reehl, S.M.; Kehn-Hall, K.; Bishop, B.; Webb-Robertson, B.M. Better Understanding and Prediction of Antiviral Peptides through Primary and Secondary Structure Feature Importance. Sci Rep 2020, 10, 19260. [Google Scholar] [CrossRef]
- Mousavi Maleki, M.S.; Sardari, S.; Ghandehari Alavijeh, A.; Madanchi, H. Recent Patents and Fda-Approved Drugs Based on Antiviral Peptides and Other Peptide-Related Antivirals. Int J Pept Res Ther 2023, 29, 5. [Google Scholar] [CrossRef]
- Bradshaw, J. Cationic Antimicrobial Peptides : Issues for Potential Clinical Use. BioDrugs 2003, 17, 233–240. [Google Scholar] [CrossRef]
- Gurwitz, D. Peptide Mimetics: Fast-Forward Look. Drug Dev Res 2017, 78, 231–235. [Google Scholar] [CrossRef]
- Li Petri, G.; Di Martino, S.; De Rosa, M. Peptidomimetics: An Overview of Recent Medicinal Chemistry Efforts toward the Discovery of Novel Small Molecule Inhibitors. J Med Chem 2022, 65, 7438–7475. [Google Scholar] [CrossRef]
- Ding, D.; Xu, S.; da Silva-Júnior, E.F.; Liu, X.; Zhan, P. Medicinal Chemistry Insights into Antiviral Peptidomimetics. Drug Discov Today 2023, 28, 103468. [Google Scholar] [CrossRef] [PubMed]
- Lenci, E.; Trabocchi, A. Peptidomimetic Toolbox for Drug Discovery. Chem Soc Rev 2020, 49, 3262–3277. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Zhang, L.; Weigel, L.F.; Schilz, J.; Graf, D.; Bartenschlager, R.; Hilgenfeld, R.; Klein, C.D. Peptide–Boronic Acid Inhibitors of Flaviviral Proteases: Medicinal Chemistry and Structural Biology. Journal of medicinal chemistry 2017, 60, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Osswald, H.L.; Prato, G. Recent Progress in the Development of Hiv-1 Protease Inhibitors for the Treatment of Hiv/Aids. Journal of medicinal chemistry 2016, 59, 5172–5208. [Google Scholar] [CrossRef]
- Su, B.; Yao, C.; Zhao, Q.-X.; Cai, W.-P.; Wang, M.; Lu, H.-Z.; Mu, T.-T.; Chen, Y.-Y.; Liu, L.; Wang, H. Long-Acting Hiv Fusion Inhibitor Albuvirtide Combined with Ritonavir-Boosted Lopinavir for Hiv-1-Infected Patients after Failing the First-Line Antiretroviral Therapy: 48-Week Randomized, Controlled, Phase 3 Non-Inferiority Talent Study. Journal of Infection 2022, 85, 334–363. [Google Scholar] [CrossRef] [PubMed]
- Awahara, C.; Oku, D.; Furuta, S.; Kobayashi, K.; Teruya, K.; Akaji, K.; Hattori, Y. The Effects of Side-Chain Configurations of a Retro–Inverso-Type Inhibitor on the Human T-Cell Leukemia Virus (Htlv)-1 Protease. Molecules 2022, 27, 1646. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, Å.; Samuelsson, B.; Johansson, P.-O.; Cummings, M.D.; Lenz, O.; Raboisson, P.; Simmen, K.; Vendeville, S.; de Kock, H.; Nilsson, M. Discovery and Development of Simeprevir (Tmc435), a Hcv Ns3/4a Protease Inhibitor. Journal of medicinal chemistry 2014, 57, 1673–1693. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.; Molchanova, N.; Herlan, C.; Fortkort, J.A.; Lin, J.S.; Figgins, E.; Bopp, N.; Ryan, L.K.; Chung, D.; Adcock, R.S.; et al. Potent Antiviral Activity against Hsv-1 and Sars-Cov-2 by Antimicrobial Peptoids. Pharmaceuticals (Basel) 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, R.; Yasir, M.; Yu, T.T.; Voli, F.; Vittorio, O.; Miller, M.J.; Lewis, P.; Black, D.S.; Willcox, M.; Kumar, N. Tuning the Anthranilamide Peptidomimetic Design to Selectively Target Planktonic Bacteria and Biofilm. Antibiotics 2023, 12, 585. [Google Scholar] [CrossRef]
- Kuppusamy, R.; Yasir, M.; Yee, E.; Willcox, M.; Black, D.S.; Kumar, N. Guanidine Functionalized Anthranilamides as Effective Antibacterials with Biofilm Disruption Activity. Organic & Biomolecular Chemistry 2018, 16, 5871–5888. [Google Scholar]
- Chianese, A.; Zannella, C.; Monti, A.; De Filippis, A.; Doti, N.; Franci, G.; Galdiero, M. The Broad-Spectrum Antiviral Potential of the Amphibian Peptide Ar-23. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Zannella, C.; Chianese, A.; Palomba, L.; Marcocci, M.E.; Bellavita, R.; Merlino, F.; Grieco, P.; Folliero, V.; De Filippis, A.; Mangoni, M.; et al. Broad-Spectrum Antiviral Activity of the Amphibian Antimicrobial Peptide Temporin L and Its Analogs. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Tooze, S.A.; Tooze, J.; Warren, G. Site of Addition of N-Acetyl-Galactosamine to the E1 Glycoprotein of Mouse Hepatitis Virus-A59. J Cell Biol 1988, 106, 1475–1487. [Google Scholar] [CrossRef]
- Perrier, A.; Bonnin, A.; Desmarets, L.; Danneels, A.; Goffard, A.; Rouille, Y.; Dubuisson, J.; Belouzard, S. The C-Terminal Domain of the Mers Coronavirus M Protein Contains a Trans-Golgi Network Localization Signal. J Biol Chem 2019, 294, 14406–14421. [Google Scholar] [CrossRef]
- McBride, C.E.; Li, J.; Machamer, C.E. The Cytoplasmic Tail of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein Contains a Novel Endoplasmic Reticulum Retrieval Signal That Binds Copi and Promotes Interaction with Membrane Protein. J Virol 2007, 81, 2418–2428. [Google Scholar] [CrossRef]
- Ahmad, I.; Wilson, D.W. Hsv-1 Cytoplasmic Envelopment and Egress. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Ivanova, P.T.; Myers, D.S.; Milne, S.B.; McClaren, J.L.; Thomas, P.G.; Brown, H.A. Lipid Composition of the Viral Envelope of Three Strains of Influenza Virus Not All Viruses Are Created Equal. ACS infectious diseases 2015, 1, 435–442. [Google Scholar] [CrossRef]
- Chazal, N.; Gerlier, D. Virus Entry, Assembly, Budding, and Membrane Rafts. Microbiology and molecular biology reviews 2003, 67, 226–237. [Google Scholar] [CrossRef]
- Kirui, J.; Abidine, Y.; Lenman, A.; Islam, K.; Gwon, Y.-D.; Lasswitz, L.; Evander, M.; Bally, M.; Gerold, G. The Phosphatidylserine Receptor Tim-1 Enhances Authentic Chikungunya Virus Cell Entry. Cells 2021, 10, 1828. [Google Scholar] [CrossRef] [PubMed]
- Tate, P.M.; Mastrodomenico, V.; Cunha, C.; McClure, J.; Barron, A.E.; Diamond, G.; Mounce, B.C.; Kirshenbaum, K. Peptidomimetic Oligomers Targeting Membrane Phosphatidylserine Exhibit Broad Antiviral Activity. ACS Infect Dis 2023. [Google Scholar] [CrossRef]
- Lorin, C.; Saidi, H.; Belaid, A.; Zairi, A.; Baleux, F.; Hocini, H.; Bélec, L.; Hani, K.; Tangy, F. The Antimicrobial Peptide Dermaseptin S4 Inhibits Hiv-1 Infectivity in Vitro. Virology 2005, 334, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Savoia, D.; Donalisio, M.; Civra, A.; Salvadori, S.; Guerrini, R. In Vitro Activity of Dermaseptin S1 Derivatives against Genital Pathogens. Apmis 2010, 118, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, C.F.; Liao, Y.D. Fetal Bovine Serum Albumin Inhibits Antimicrobial Peptide Activity and Binds Drug Only in Complex with A1-Antitrypsin. Sci Rep 2021, 11, 1267. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhang, Q.; Hong, W.; Xie, Y.; Liu, Y.; Li, W.; Wu, Y.; Cao, Z. A Scorpion Defensin Bmkdfsin4 Inhibits Hepatitis B Virus Replication in Vitro. Toxins (Basel) 2016, 8. [Google Scholar] [CrossRef]
- Xiao, C.; Chen, X.; Xie, Q.; Li, G.; Xiao, H.; Song, J.; Han, H. Virus Identification in Electron Microscopy Images by Residual Mixed Attention Network. Comput Methods Programs Biomed 2021, 198, 105766. [Google Scholar] [CrossRef]
- Zannella, C.; Chianese, A.; Annunziata, G.; Ambrosino, A.; De Filippis, A.; Tenore, G.C.; Novellino, E.; Stornaiuolo, M.; Galdiero, M. Antiherpetic Activity of Taurisolo(®), a Grape Pomace Polyphenolic Extract. Microorganisms 2023, 11. [Google Scholar] [CrossRef]
- Liu, P.; Zhong, L.; Xiao, J.; Hu, Y.; Liu, T.; Ren, Z.; Wang, Y.; Zheng, K. Ethanol Extract from Artemisia Argyi Leaves Inhibits Hsv-1 Infection by Destroying the Viral Envelope. Virol J 2023, 20, 8. [Google Scholar] [CrossRef]
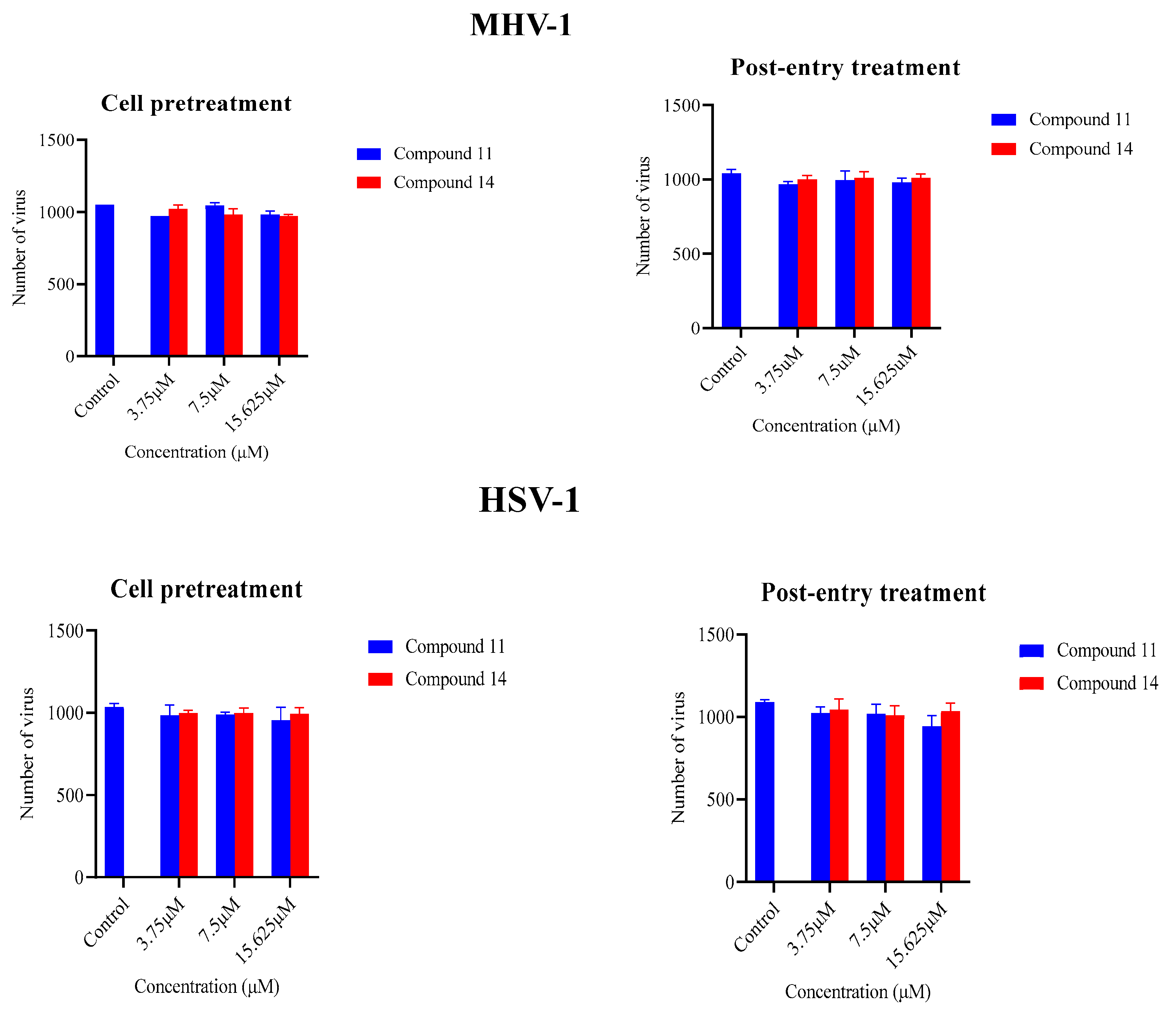
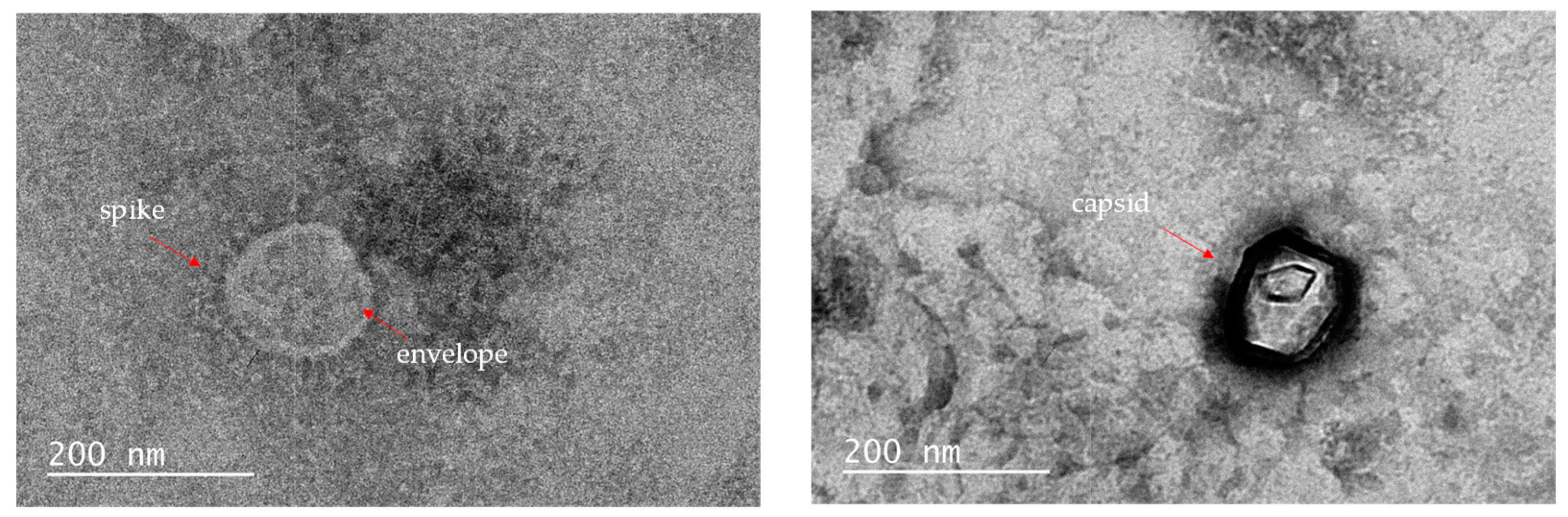
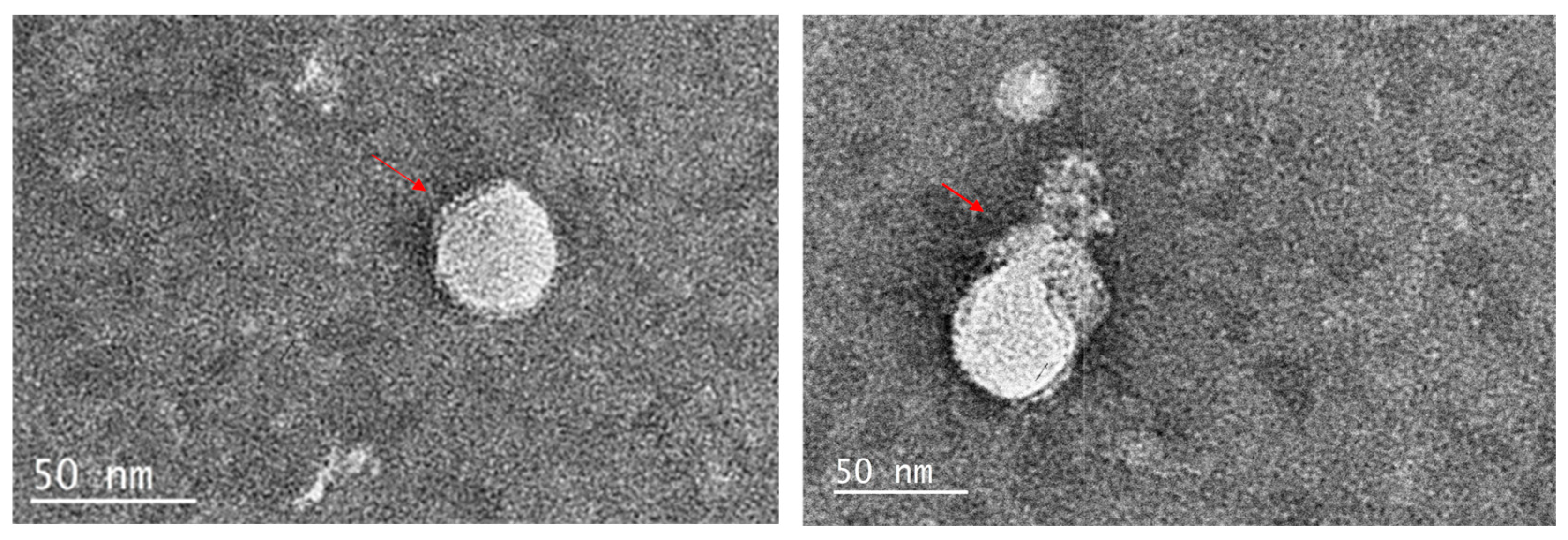
| Compound Number |
MHV-1 IC50 (µM) |
HSV-1 IC50 (µM) |
|---|---|---|
| 1 | >62.5 | >62.5 |
| 2 | >62.5 | >62.5 |
| 3 | >62.5 | >62.5 |
| 4 | >62.5 | >62.5 |
| 5 | >62.5 | >62.5 |
| 6 | >62.5 | >62.5 |
| 7 | >62.5 | 32.7 |
| 8 | >62.5 | >62.5 |
| 9 | >62.5 | 14.8 |
| 10 | >62.5 | >62.5 |
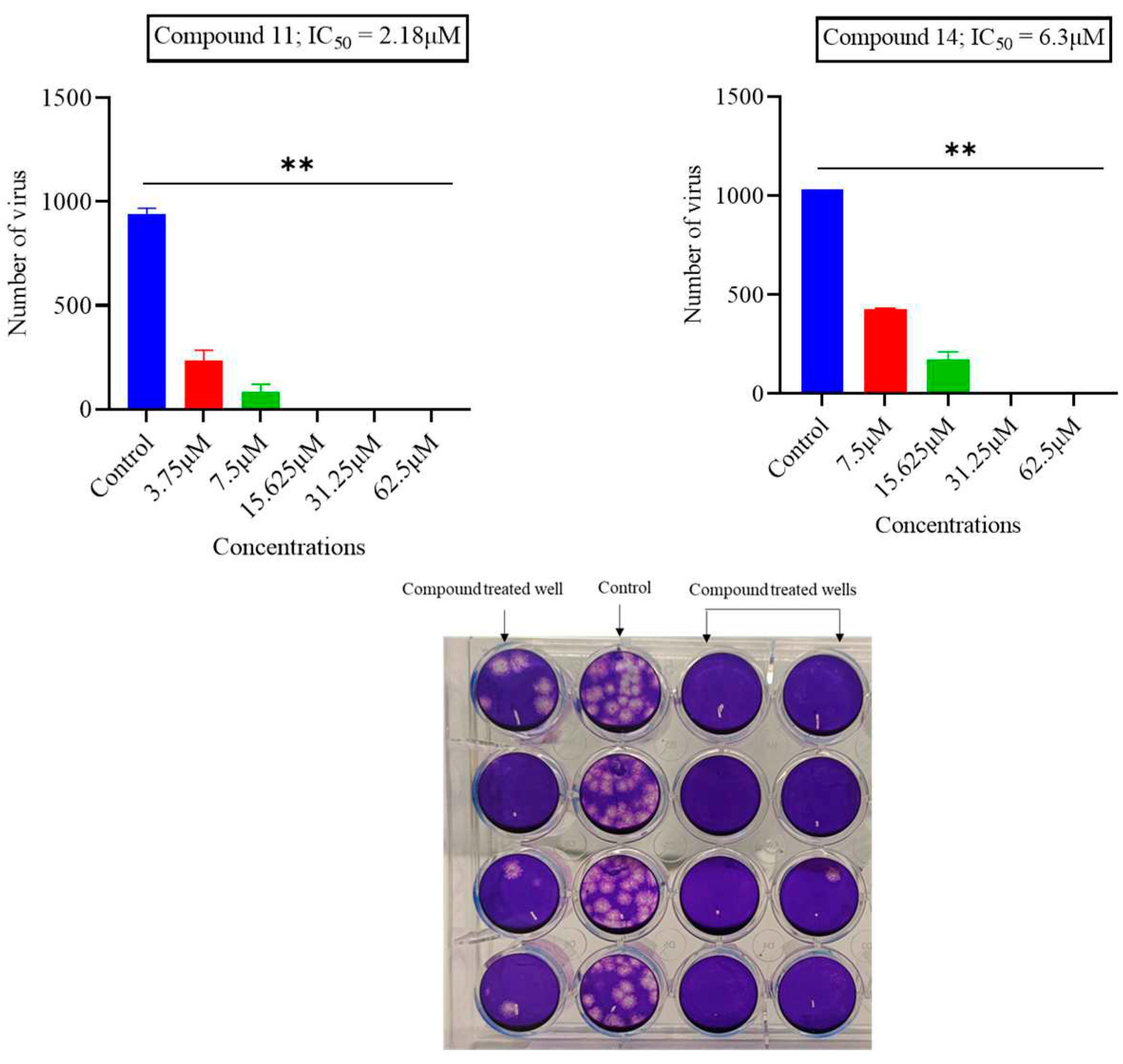
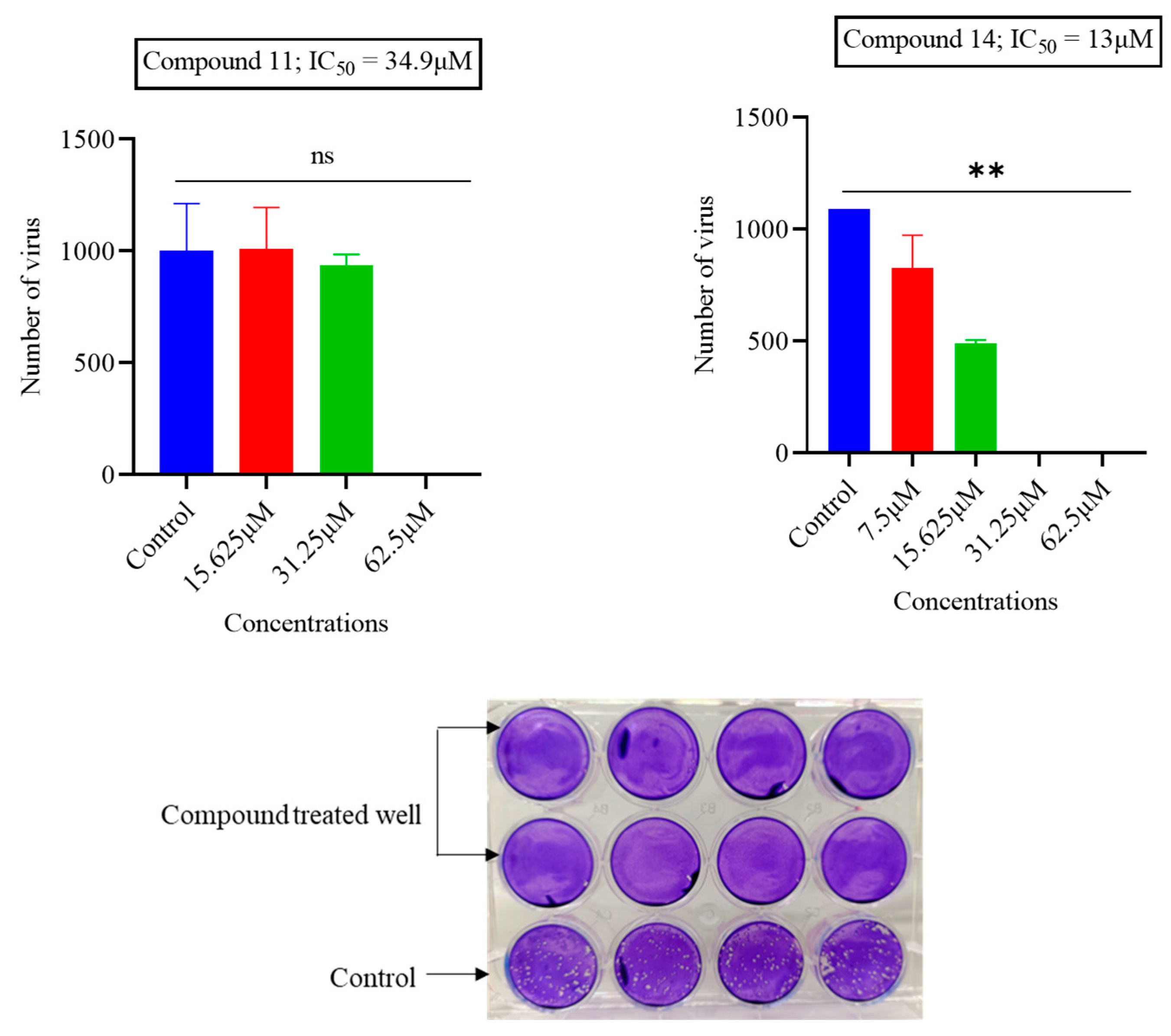
| 11 | 2.38 | 34.9 |
| 12 | >62.5 | >62.5 |
| 13 | >62.5 | >62.5 |
| 14 | 6.3 | 13 |
| 15 | 11.09 | >62.5 |
| 16 | 22.5 | >62.5 |
| 17 | 11.8 | >62.5 |
| CompoundNumber | MHV-1 (A9 cells) | HSV-1 (Vero cells) | ||||
|---|---|---|---|---|---|---|
| IC50 (µM) | CC50 (µM) | Therapeutic index | IC50 (µM) | CC50 (µM) | Therapeutic index | |
| 7 | >62.5 | - | - | 32.76 | 127 | 3.87 |
| 9 | >62.5 | - | - | 14.88 | 45.02 | 3.03 |
| 11 | 2.18 | 20.52 | 9.41 | 34.9 | 16.69 | 0.48 |
| 14 | 6.3 | 10.20 | 1.6 | 13 | 15.18 | 1.16 |
| 15 | 11.09 | 62.68 | 5.65 | >62.5 | - | - |
| 16 | 22.56 | 81.64 | 3.62 | >62.5 | - | - |
| 17 | 11.79 | 114.5 | 9.71 | >62.5 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





