1. Introduction
Brain metastases are the most common intracranial neoplasms in adults. Newly diagnosed brain metastasis incidence is 3 to 10 times higher than that of primary brain tumors [
1]. In the last decades, metastases to the central nervous system have gained increasing clinical interest due to the development of different therapeutic alternatives such as surgical resection, whole-brain radiotherapy, radiosurgery and targeted systemic therapies that have significantly improved survivals of these patients [
1,
2].
Specifically, given their greater frequency, breast and lung cancer are the most common tumors to metastasize to the brain [
1,
2], with the former approximately developing in 10-30% of the tumor population [
1,
3]. Intracerebral metastasis may represent the first manifestation of a systemic disease or, more commonly, present itself metachronously, with patients experiencing headaches, nausea, seizures, defects in speech, behaviour, coordination and neurocognition, lowering their quality of life. Various risk factors have been identified and associated with the risk of brain metastatic breast cancer (BC), with one of the most recent systematic review including younger age, estrogen receptor (ER)-negative status, HER2-positive status, higher tumour stage, higher histologic grade, large tumour size, and high Ki67 labelling index as independent risk factors [
4,
5]. Brain metastases have been long considered a late event in the progression of the disease occurring even after a decade after the primary cancer diagnosis, preceded by lung, liver and bone metastasis, albeit it’s not that uncommon to identify direct BC to brain metastasis course of the disease (≅ 12%) [
4]. Some studies have tried to assess the microenvironment of brain metastasis from BC, lot of them projecting to a prognostic significance regarding the presence of necrosis, gliosis, immune infiltrate and haemorrhage [
6].
The majority of patients affected by brain metastases from BC develops multiple intracerebral metastases, while a solitary mass occurs only in 14% of cases [
1]; furthermore, BC frequently exhibits leptomeningeal spread via hematogenous route, direct extension and/or extension along nerves and lymphatic vessels [
1], and the pia madre or the arachnoid represent the most commonly affected meningeal layers [
1].
Since, as far as we are aware, there are currently no morphological clues capable of making a pathologist suspect a possible mammary origin of a metastatic lesion to the brain in the absence of adequate clinical information, the histologic diagnosis of brain metastasis from BC is mainly based on the immunohistochemical expression of mammary gland markers such as GATA-3, ERs, progesterone receptors (PgRs) and HER-2 [
7].
The present retrospective study investigated the presence of potential recurrent histologic features on a series of 30 cases of brain metastases from breast carcinoma, in order to collect some useful morphological features capable to suggest to the pathologist a potential mammary origin of the neoplasm even in the absence of clinical information.
2. Materials and Methods
The present research was in accordance with the Declaration of Helsinki and obtained the approval of the local ethics committee, Catania 1 (CE 165/2015/PO). Histologic specimens of all brain metastases from BCs, surgically excised at the Neurosurgery Unit of the “Policlinico “G. Rodolico -San Marco” University hospital between 2018 and 2023, were retrospectively collected. Surgical samples were fixed in 10% neutral buffered formalin, embedded in paraffin, cut to 4-5 micron and stained with hematoxylin and eosin; the corresponding clinical (age, gender and anatomic site of the metastatic tumor) and immunohistochemical data were retrieved from the original pathological reports. Hematoxylin and eosin-stained sections were separately evaluated by two pathologists (J.F. and G.B.) with no awareness of the clinical data of the patients. We collected the following histologic features: (i) solid growth pattern; (ii) presence of comedonecrosis; (iii) glandular differentiation. Their presence was graded by semi-quantitative optical analysis according to a four-tiered system (0% of the tumor = absent; 1–10% of the tumor = focal; 11–50% of the tumor = heterogeneous; >50% of the tumor = diffuse), as previously described [8-10].
3. Results
The clinico-pathologic and immunohistochemical features of the cases from our series are summarized in
Table 1.
3.1. Clinico-Pathologic Features
The study included 30 female patients (median age: 51,6 years; age range: 34-75). One patient had a further recurrence after one year from the first brain metastasis diagnosis. Intracerebral metastases were found in 27 patients, while 3 patients had cerebellar involvement. Of those 27 patients with intraparenchymal metastasis, 6 had a temporal localization, 3 parietal, 3 frontal, 2 tentorial, 1 peri-trigonal, 1 pterional and 11 had no specific site reported on the original report.
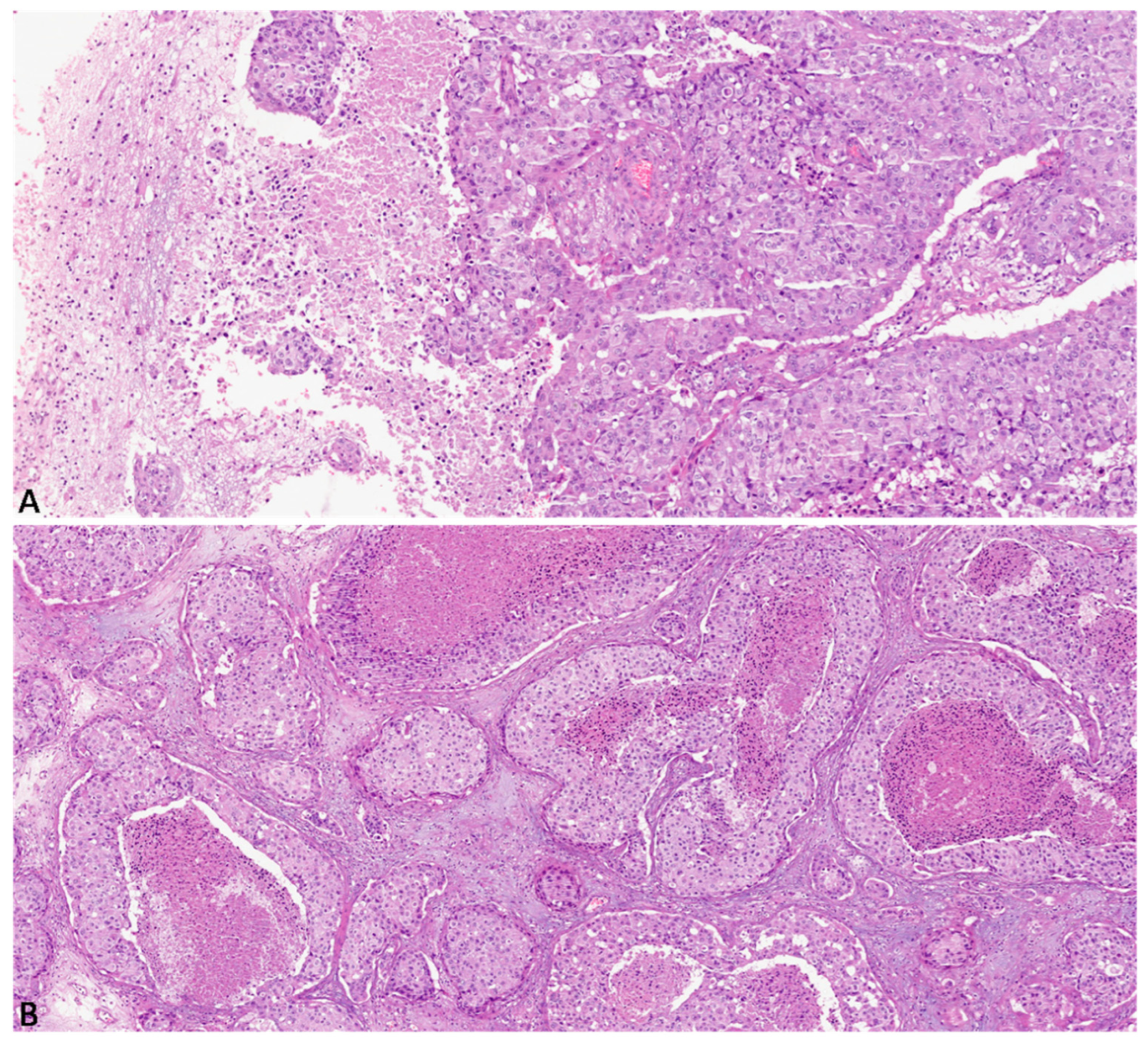
Histologically, 14 cases (47%) exhibited diffuse solid growth pattern with no glandular differentiation (
Figure 1A), 9 cases (30%) had a heterogeneous solid growth pattern, 8 of which also showed a focal glandular differentiation; the remaining 7 cases (23%) showed focal solid growth pattern. Diffuse comedonecrosis was found in 7 cases (23%) (
Figure 1B), while heterogeneous and focal comedonecrosis was found in 11 (37%) and 9 (30%) cases, respectively. 3 cases (10%) showed no necrosis.
3.2. Immunohistochemical features
Among the whole cohort of 30 metastatic breast cancer to brain, 8 cases (27%) showed a triple negative/basal-like phenotype, 3 (10%) were luminal-A, 1 (3%) was luminal-B and 5 tumors (17%) were HER-2-enriched. In 13 cases (43%) the immunophenotype was not available in the original pathology report.
4. Discussion
Brain metastases from BC are an often devastating complication that can significantly impact patients’ quality of life [1-3]. BC is one of the most common types of cancer in women, and it has a high potential to spread to other organs, including the brain [11-13]. Several symptoms, including headaches, seizures, confusion, and difficulty with movement and coordination may be caused by brain metastases [14-16].
The incidence of brain metastases from BC has been increasing in recent years, likely due to improvements in cancer treatments that have led to longer survival times [14-18]. However, the prognosis for patients with brain metastases still remains poor, with a median survival time of only a few months.
Treatment options for brain metastases from BC may include surgery, radiation therapy, chemotherapy, and targeted therapy [17-20]. Surgery may be used to remove a single metastasis or to relieve pressure on the brain caused by mass effect and peritumoral edema. Radiation therapy can be used to shrink tumors and relieve symptoms, while chemotherapy and targeted therapy may be used to slow the growth of cancer cells and improve overall survival [17-20]. Early diagnosis and aggressive treatment are crucial for improving outcomes for patients with brain metastases from BC. Regular monitoring and imaging tests are mandatory to detect brain metastases early. Additionally, ongoing research is needed to develop new and more effective treatments for brain metastases from BC.
While it is relatively straightforward for a pathologist to distinguish between a metastatic carcinoma to the brain and a primary central nervous system neoplasm, it may be more challenging to hypothesize the potential origin of the tumor on the basis of morphology alone and above all in the absence of accurate anamnestic information from other clinicians. Therefore, immunohistochemistry (IHC) is a mandatory ancillary method for the identification of the origin of a metastatic carcinoma to the cerebral parenchyma, allowing that a purely morphological diagnosis of "adenocarcinoma" or "squamous cell carcinoma" is not rendered [
21]. However, although IHC is currently a widely accessible and widespread method in almost all Pathology units, the frequent lack of anamnestic information about the patient's previous neoplasms means that pathologists must use a wide immunohistochemical panel, working blindly and trying to cover all the potential sites of origin of the tumor, with an often important waste of resources and time.
The present research aimed to identify some purely morphological features of metastatic carcinomas to brain, which could potentially be useful to suggest to the pathologist a potential mammary origin of the neoplasm. In more detail, our results demonstrated that the majority of metastatic BCs from our cohort histologically exhibited an almost “pure” solid growth pattern with at least focal comedonecrosis, producing an overall morphology closely reminiscent of mammary high-grade ductal carcinoma in situ.
In our series, as expected, most cases exhibited an immunoprofile consistent with the triple negative/ basal-like and HER-2-enriched subtypes. The possibility of differences in receptor status and/or genomic profiling between brain metastases from BC and primary tumor has been reported in literature [
5]. In particular, the loss of ER, PgR and HER-2 expression has been described by Duchnoswka et al. [
22] and it has been found in about 20% of patients by Thomson et al. [
23]; Schrijver et al. [
24] showed that ER conversion rates were significantly higher in brain that in liver BC metastases, while PgR conversion ones were much more negligible in central nervous system than in bone metastases. Some clinicians also reported that the loss of hormone receptors was closely associated with poorer outcome [
5]. HER2 mRNA levels were found to be increased up to five times in brain metastasis from BC tissues compared to those of primary tumors [
5]. With regards to the genomic sequencing (GS) data, it has been reported that
RB1, ZFHX3, HER2, ATR, FAT1, ARID1A, ATM, CHEK2, TP53, BRCA1, CDH1, PTEN, COL6A3, FLT3, MLH1, BRCA2, MAP3K1, IGFN1, KMT2D, MET, PIK3CA and
KMT2C are the most frequent mutated genes in BC metastases to the central nervous system [
14]. Several authors investigated the GS differences between primary and metastatic BC to the brain [
5]; in this regard, the overexpression of
FGFR4 and
FLT1, combined with the downregulation of ESR1, has been reported [
5]. Brain metastases and primary lesions showed similar rates of mutations of
RB1, PIK3CA, LH1, RB1 and
KIT, while
TP53 resulted more frequently mutated in the former [
5]. Based on this studies, it is possible to deduce that, as brain metastases from BC and primary tumors may exhibit different immunophenotypes and GS data, clinical oncologists should take into account the most appropriate and “personalized” therapeutic approach for these patients. For this purpose, the potential use of drugs targeting mutated genes in the BCBM, including abemaciclib, entrectinib and GDC-0084, was evaluated by a phase II clinical trial (NCT03994796).
Some studies have tried to histologically detail the microenvironment of brain metastases from BC in order to provide prognostic guidance and, perhaps, reveal new molecular targets for future therapeutic options [
6]. Sambade et al. reported four histopathological biomarkers found within the breast cancer brain metastases microenvironment, namely gliosis, immune infiltrate, hemorrhage, and necrosis, and assessed their associations with breast cancer subtype along with their prognostic significance. The study demonstrated that gliosis and immune infiltrate correlated with a better prognosis, while the presence of necrosis was a poor prognostic finding; in more detail, it was shown that gliosis correlated with a better prognosis in triple negative subtype, while the immune infiltrate conferred better prognosis to HER-2- enriched subtype. Specifically, the immune composition of tumor infiltrating lymphocytes (TILs), macrophages, programmed cell death protein -1 and -2 receptors (PDL-1 and -2), and glial fibrillary acid protein (GFAP) have been assessed and it was found that the expression of PDL-1 on tumor infiltrating lymphocytes positively correlated with the overall survival [
6]. When compared to a model that used purely clinical variables to assess the prognosis and treatment decisions, the histological biomarkers provided a higher predictive value [
6]. Therefore, including these histopathological features in pathology reports of brain metastasis from breast cancer cases could positively impact on prognostic information.
In the last years some authors evaluated the radiological extent of tumor necrosis in different types of metastatic tumors to the central nervous system and correlated it to the outcome of patients surgically treated by craniotomy [
25]. They found that brain metastases from lung cancer (neuroendocrine and squamous cell subtypes in particular) exhibited more extensive necrosis than those from other primary tumors including breast, genitourinary and gastrointestinal malignancies [
25]. Furthermore, highly-necrotic brain metastases showed poorer outcome after craniotomy than those harboring less necrosis [
25]. With respect to our study, these authors mainly focused on the radiological extent of necrosis in brain metastases from lung cancer and did not mention the histological pattern of necrosis, while our results highlight more the fact that comedonecrosis may be a morphological features suggestive of the mammary origin of a metastatic carcinoma to the brain.
5. Conclusions
Although we are aware that the above-mentioned morphological parameters are not strictly specific to a mammary origin, we believe that they may have an important diagnostic utility in leading pathologists to suspect a possible breast primary tumor and to include GATA-3, ERs, PgRs and HER-2 to the immunohistochemical panel when dealing with a brain metastasis in their daily diagnostic practice [26-30]. Although to the best of our knowledge this is the first research that aims to select purely morphological features capable to predict the mammary origin of a brain metastasis, in our opinion its limitations include the relatively low number of cases from our cohort and the impossibility to compare the morphology of brain metastases of breast origin with those from other organs, such as the lung, ovary etc. Accordingly, the future perspective of this study is to expand our cohort of cases, by also including brain metastases from non-mammary carcinomas.
Author Contributions
Conceptualization, J.F. and G.B.; validation, M.Z., A.P. and Maurizio Zizzo; investigation, G.A., L.S., G.M.V., L.P. and G.M; resources, R.A., F.C., G.M.V.B. and S.P.; data curation, X.X.; writing—original draft preparation, J.F. and G.B.; writing—review and editing, G.B. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The present research was conducted in accordance with the Declaration of Helsinki and obtained the approval of the local ethics committee, Catania 1 (CE 165/2015/PO).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data presented in this research are available from the corresponding author upon reasonable request.
Acknowledgments
The study was partially supported by the Italian Ministry of Health—Ricerca Corrente Annual Program 2024.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weil, R. J.; Palmieri, D. C.; Bronder, J. L.; Stark, A. M.; Steeg, P. S. Breast Cancer Metastasis to the Central Nervous System. Am. J. Pathol. 2005, 167 (4), 913–920. [CrossRef]
- Lassman, A. B.; DeAngelis, L. M. Brain Metastases. Neurol. Clin. 2003, 21 (1), 1–23. [CrossRef]
- Gil-Gil, M. J.; Martinez-Garcia, M.; Sierra, A.; Conesa, G.; Del Barco, S.; González-Jimenez, S.; Villà, S. Breast Cancer Brain Metastases: A Review of the Literature and a Current Multidisciplinary Management Guideline. Clin. Transl. Oncol. 2014, 16, 436–446. [CrossRef]
- Koniali, L.; Hadjisavvas, A.; Constantinidou, A.; Christodoulou, K.; Christou, Y.; Demetriou, C.; Panayides, A. S.; Pitris, C.; Pattichis, C. S.; Zamba-Papanicolaou, E.; Kyriacou, K. Risk Factors for Breast Cancer Brain Metastases: A Systematic Review. Oncotarget 2020, 11, 650. [CrossRef]
- Watase, C.; Shiino, S.; Shimoi, T.; Noguchi, E.; Kaneda, T.; Yamamoto, Y.; Yonemori, K.; Takayama, S.; Suto, A. Breast Cancer Brain Metastasis—Overview of Disease State, Treatment Options and Future Perspectives. Cancers (Basel). 2021, 13, 1–24. [CrossRef]
- Sambade, M. J.; Prince, G.; Deal, A. M.; Trembath, D.; McKee, M.; Garrett, A.; Keith, K.; Ramirez, J.; Midkiff, B.; Blackwell, K.; Sammons, S.; Leone, J. P.; Brufsky, A.; Morikawa, A.; Brogi, E.; Seidman, A.; Ewend, M.; Carey, L. A.; Moschos, S. J.; Hamilton, R. L.; Vincent, B.; Anders, C. Examination and Prognostic Implications of the Unique Microenvironment of Breast Cancer Brain Metastases. Breast Cancer Res. Treat. 2019, 176, 321. [CrossRef]
- Ding, Q.; Huo, L.; Peng, Y.; Yoon, E.C.; Li, Z.; Sahin, A.A. Immunohistochemical Markers for Distinguishing Metastatic Breast Carcinoma from Other Common Malignancies: Update and Revisit. Semin Diagn Pathol. 2022, 39, 313-321. [CrossRef]
- Broggi, G.; Lo Giudice, A.; Di Mauro, M.; Asmundo, M.G.; Pricoco, E.; Piombino, E.; Caltabiano, R.; Morgia, G.; Russo, G.I. SRSF-1 and microvessel density immunohistochemical analysis by semi-automated tissue microarray in prostate cancer patients with diabetes (DIAMOND study). Prostate. 2021, 81, 882-892. [CrossRef]
- Piombino, E.; Broggi, G.; Barbareschi, M.; Castorina, S.; Parenti, R.; Bartoloni, G.; Salvatorelli, L.; Magro, G. Wilms' Tumor 1 (WT1): A Novel Immunomarker of Dermatofibrosarcoma Protuberans-An Immunohistochemical Study on a Series of 114 Cases of Bland-Looking Mesenchymal Spindle Cell Lesions of the Dermis/Subcutaneous Tissues. Cancers (Basel). 2021, 13, 252. [CrossRef]
- Broggi, G.; Musumeci, G.; Puzzo, L.; Russo, A.; Reibaldi, M.; Ragusa, M.; Longo, A.; Caltabiano, R. Immunohistochemical Expression of ABCB5 as a Potential Prognostic Factor in Uveal Melanoma. Appl. Sci. 2019, 9, 1316. [CrossRef]
- Broggi, G.; Filetti, V.; Ieni, A.; Rapisarda, V.; Ledda, C.; Vitale, E.; Varricchio, S.; Russo, D.; Lombardo, C.; Tuccari, G.; Caltabiano, R.; Loreto, C. MacroH2A1 Immunoexpression in Breast Cancer. Front Oncol. 2020, 10, 1519. [CrossRef]
- Magro, G.; Salvatorelli, L.; Puzzo, L.; Piombino, E.; Bartoloni, G.; Broggi, G.; Vecchio, G.M. Practical approach to diagnosis of bland-looking spindle cell lesions of the breast. Pathologica. 2019, 111, 344-360. [CrossRef]
- Cammarata, F.P.; Forte, G.I.; Broggi, G.; Bravatà, V.; Minafra, L.; Pisciotta, P.; Calvaruso, M.; Tringali, R.; Tomasello, B.; Torrisi, F.; et al. Molecular Investigation on a Triple Negative Breast Cancer Xenograft Model Exposed to Proton Beams. Int. J. Mol. Sci. 2020, 21, 6337. [CrossRef]
- Morgan, A.J.; Giannoudis, A.; Palmieri, C. The genomic landscape of breast cancer brain metastases: a systematic review. Lancet Oncol. 2021, 22, e7-e17. [CrossRef]
- Kuksis, M.; Gao, Y.; Tran, W.; Hoey, C.; Kiss, A.; Komorowski, A.S.; Dhaliwal, A.J.; Sahgal, A.; Das, S.; Chan, K.K.; Jerzak, K.J. The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol. 2021, 23, 894-904. [CrossRef]
- Martin, A.M.; Cagney, D.N.; Catalano, P.J.; Warren, L.E.; Bellon, J.R.; Punglia, R.S.; Claus, E.B.; Lee, E.Q.; Wen, P.Y.; Haas-Kogan, D.A.; Alexander, B.M.; Lin, N.U.; Aizer, A.A. Brain Metastases in Newly Diagnosed Breast Cancer: A Population-Based Study. JAMA Oncol. 2017, 3, 1069-1077. [CrossRef]
- Bailleux, C.; Eberst, L.; Bachelot, T. Treatment strategies for breast cancer brain metastases. Br J Cancer. 2021, 124, 142-155. [CrossRef]
- Corti, C.; Antonarelli, G.; Criscitiello, C.; Lin, N.U.; Carey, L.A.; Cortés, J.; Poortmans, P.; Curigliano, G. Targeting brain metastases in breast cancer. Cancer Treat Rev. 2022, 103, 102324. [CrossRef]
- Shah, N.; Mohammad, A.S.; Saralkar, P.; Sprowls, S.A.; Vickers, S.D.; John, D.; Tallman, R.M.; Lucke-Wold, B.P.; Jarrell, K.E.; Pinti, M.; Nolan, R.L.; Lockman, P.R. Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol Res. 2018, 132, 47-68. [CrossRef]
- Wang, Y.; Ye, F.; Liang, Y.; Yang, Q. Breast cancer brain metastasis: insight into molecular mechanisms and therapeutic strategies. Br J Cancer. 2021, 125, 1056-1067. [CrossRef]
- Anderson, G.G.; Weiss, L.M. Determining tissue of origin for metastatic cancers: meta-analysis and literature review of immunohistochemistry performance. Appl Immunohistochem Mol Morphol. 2010, 18, 3-8. [CrossRef]
- Duchnowska, R.; Dziadziuszko, R.; Trojanowski, T.; Mandat, T.; Och, W.; Czartoryska-Arłukowicz, B.; Radecka, B.; Olszewski, W.; Szubstarski, F.; Kozłowski, W.; et al. Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res. 2012, 14, R119. [CrossRef]
- Thomson, A.H.; McGrane, J.; Mathew, J.; Palmer, J.; Hilton, D.A.; Purvis, G.; Jenkins, R. Changing molecular profile of brain metastases compared with matched breast primary cancers and impact on clinical outcomes. Br. J. Cancer. 2016, 114, 793–800. [CrossRef]
- Schrijver, W.A.M.E.; Suijkerbuijk, K.P.M.; Van Gils, C.H.; Van Der Wall, E.; Moelans, C.B.; Van Diest, P.J. Receptor Conversion in Distant Breast Cancer Metastases: A Systematic Review and Meta-analysis. Jnci. J. Natl. Cancer Inst. 2018, 110, 568–580. [CrossRef]
- Yoo, J.; Cha, Y.J.; Park, H.H.; Park, M.; Joo, B.; Suh, S.H.; Ahn, S.J. The Extent of Necrosis in Brain Metastases May Predict Subtypes of Primary Cancer and Overall Survival in Patients Receiving Craniotomy. Cancers 2022, 14, 1694. [CrossRef]
- Braxton, D.R.; Cohen, C.; Siddiqui, M.T. Utility of GATA3 immunohistochemistry for diagnosis of metastatic breast carcinoma in cytology specimens. Diagn Cytopathol. 2015, 43, 271-7. [CrossRef]
- Yang, Y.; Lu, S.; Zeng, W.; Xie, S.; Xiao, S. GATA3 expression in clinically useful groups of breast carcinoma: a comparison with GCDFP15 and mammaglobin for identifying paired primary and metastatic tumors. Ann Diagn Pathol. 2017, 26, 1-5. [CrossRef]
- Sangoi, A.R.; Shrestha, B.; Yang, G.; Mego, O.; Beck, A.H. The Novel Marker GATA3 is Significantly More Sensitive Than Traditional Markers Mammaglobin and GCDFP15 for Identifying Breast Cancer in Surgical and Cytology Specimens of Metastatic and Matched Primary Tumors. Appl Immunohistochem Mol Morphol. 2016, 24, 229-37. [CrossRef]
- Deftereos, G.; Sanguino Ramirez, A.M.; Silverman, J.F.; Krishnamurti, U. GATA3 immunohistochemistry expression in histologic subtypes of primary breast carcinoma and metastatic breast carcinoma cytology. Am J Surg Pathol. 2015, 39, 1282-9. [CrossRef]
- Liu, H. Application of immunohistochemistry in breast pathology: a review and update. Arch Pathol Lab Med. 2014, 138, 1629-42. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).