Submitted:
29 August 2023
Posted:
30 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
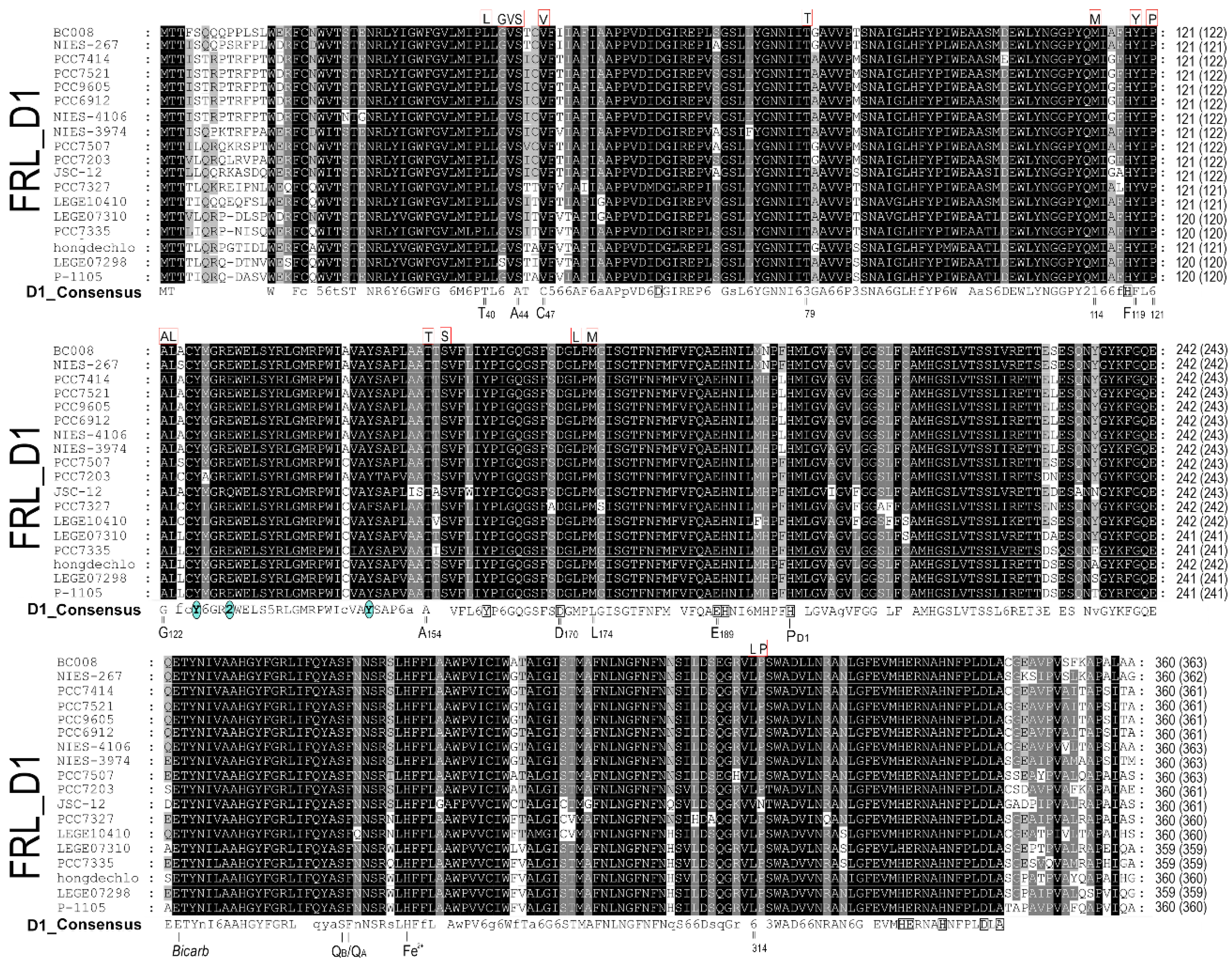
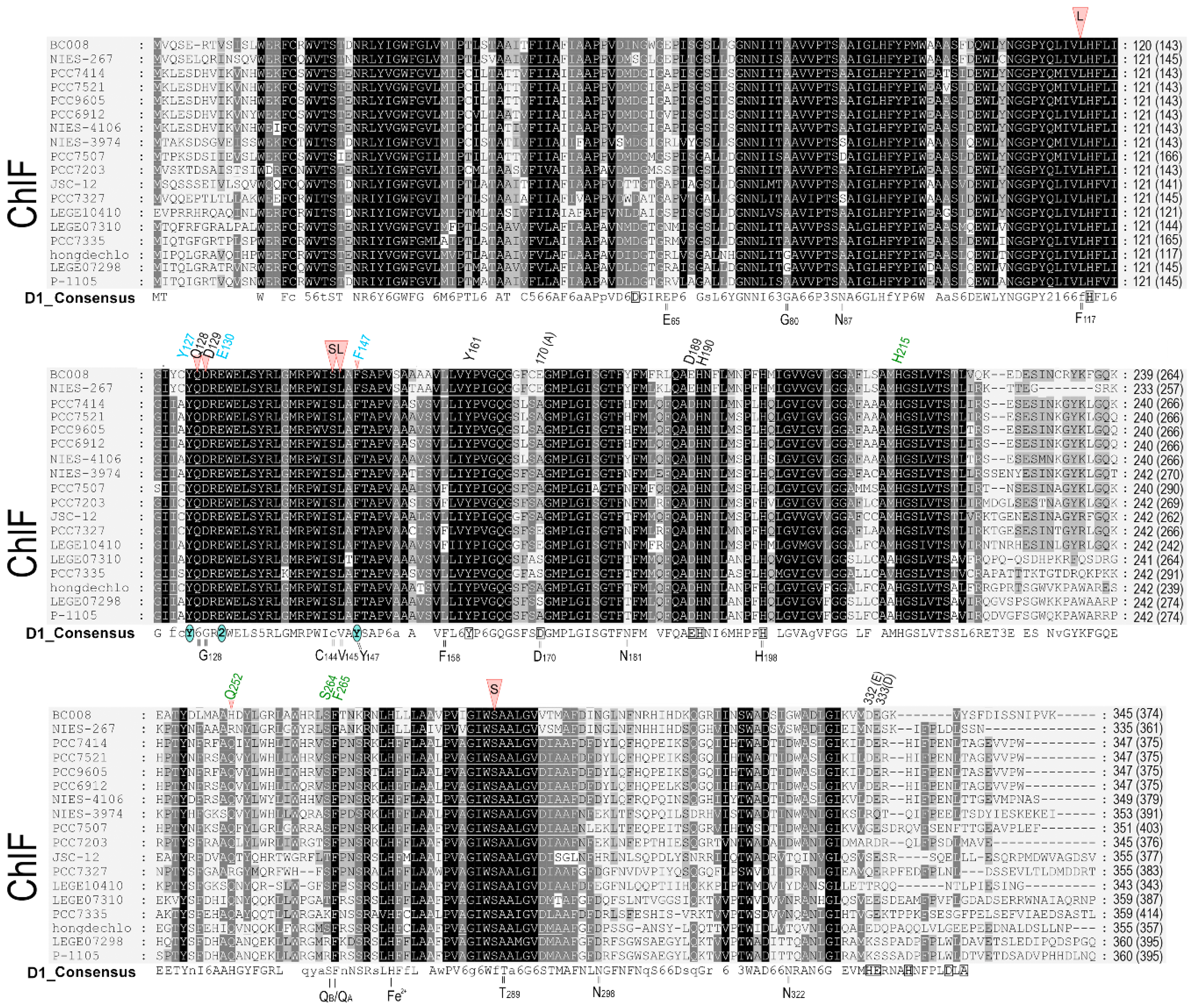
2.1. D1 protein sequence alignment
2.2. The D1 sequence comparison
2.3. Structure modelling and comparison
3. Results
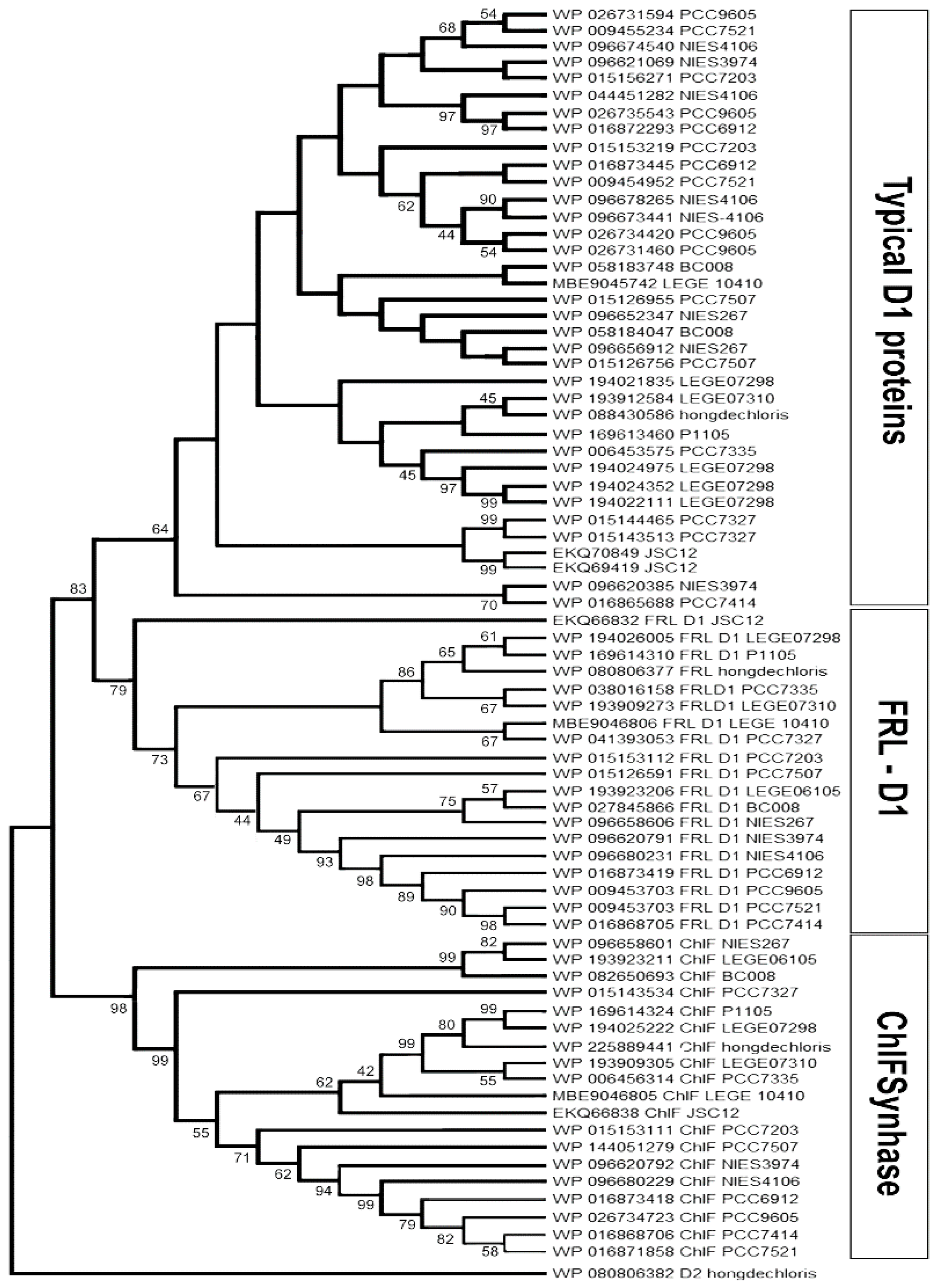
3.1. Alignment and phylogenetic relationship of D1 paralogs in Chl f-producing cyanobacteria
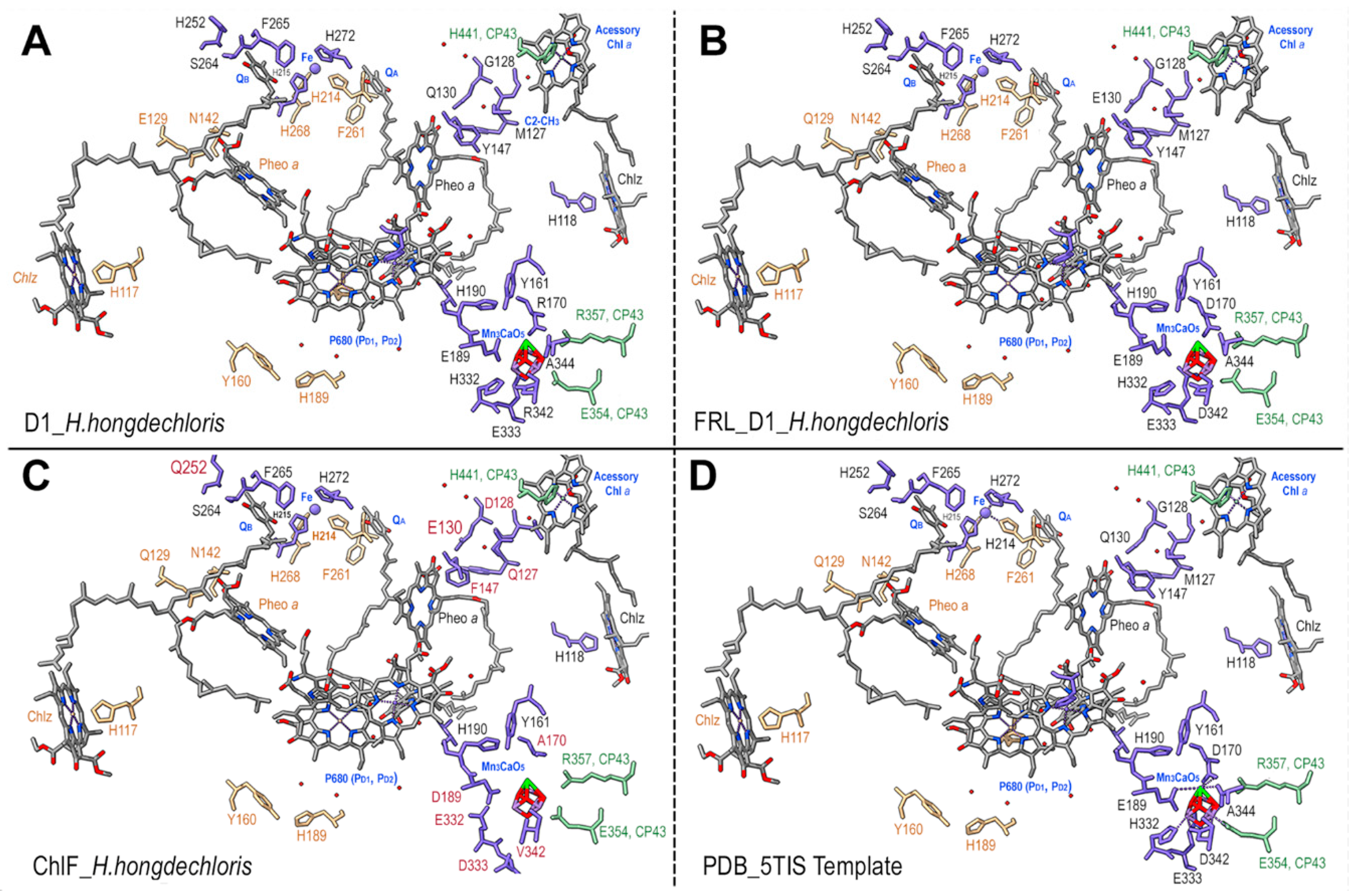
3.2. The modelling of D1 paralogs from H. hongdechloris
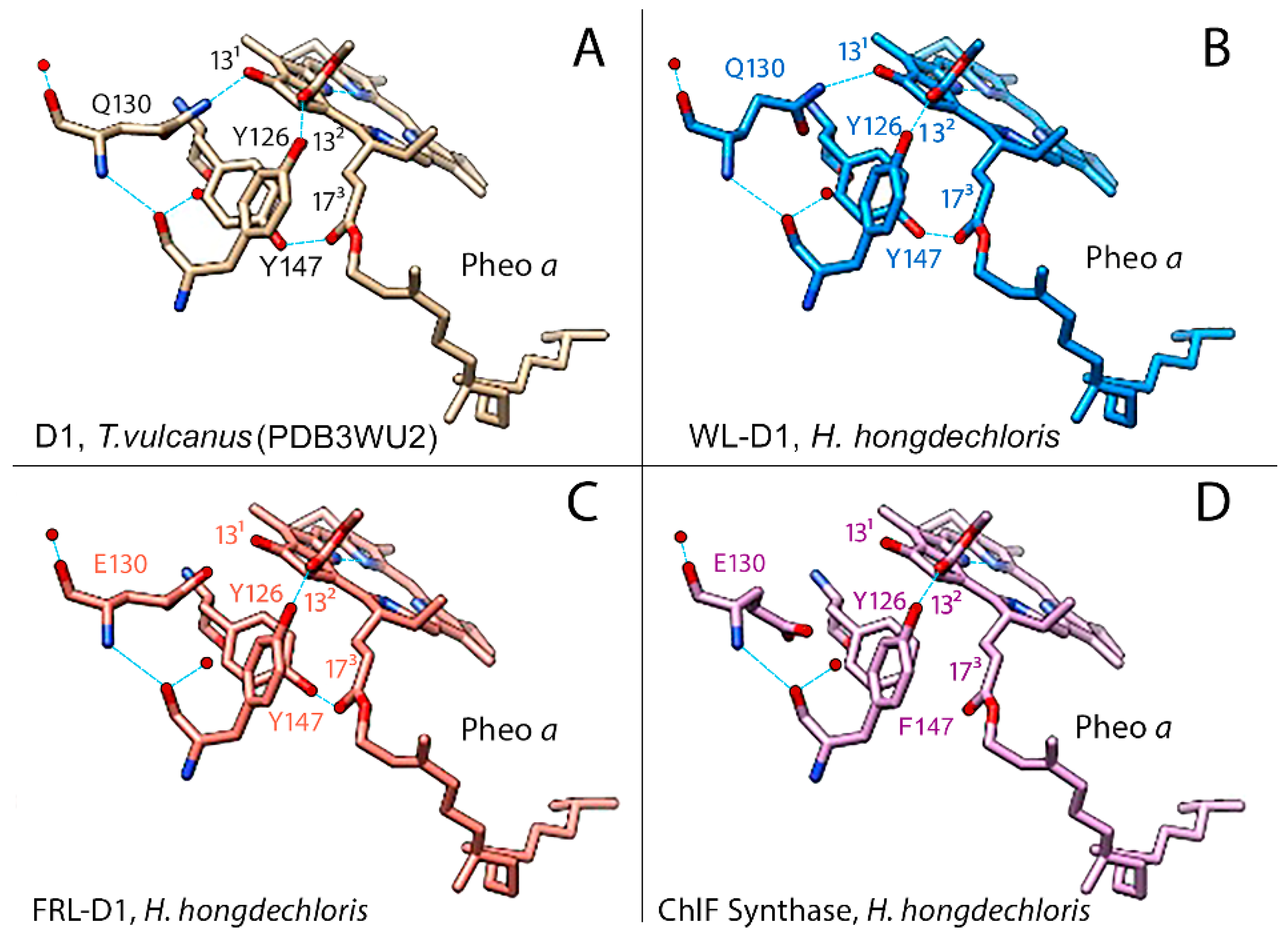
3.3. Pheophytin a binding environment
3.4. QB binding environment
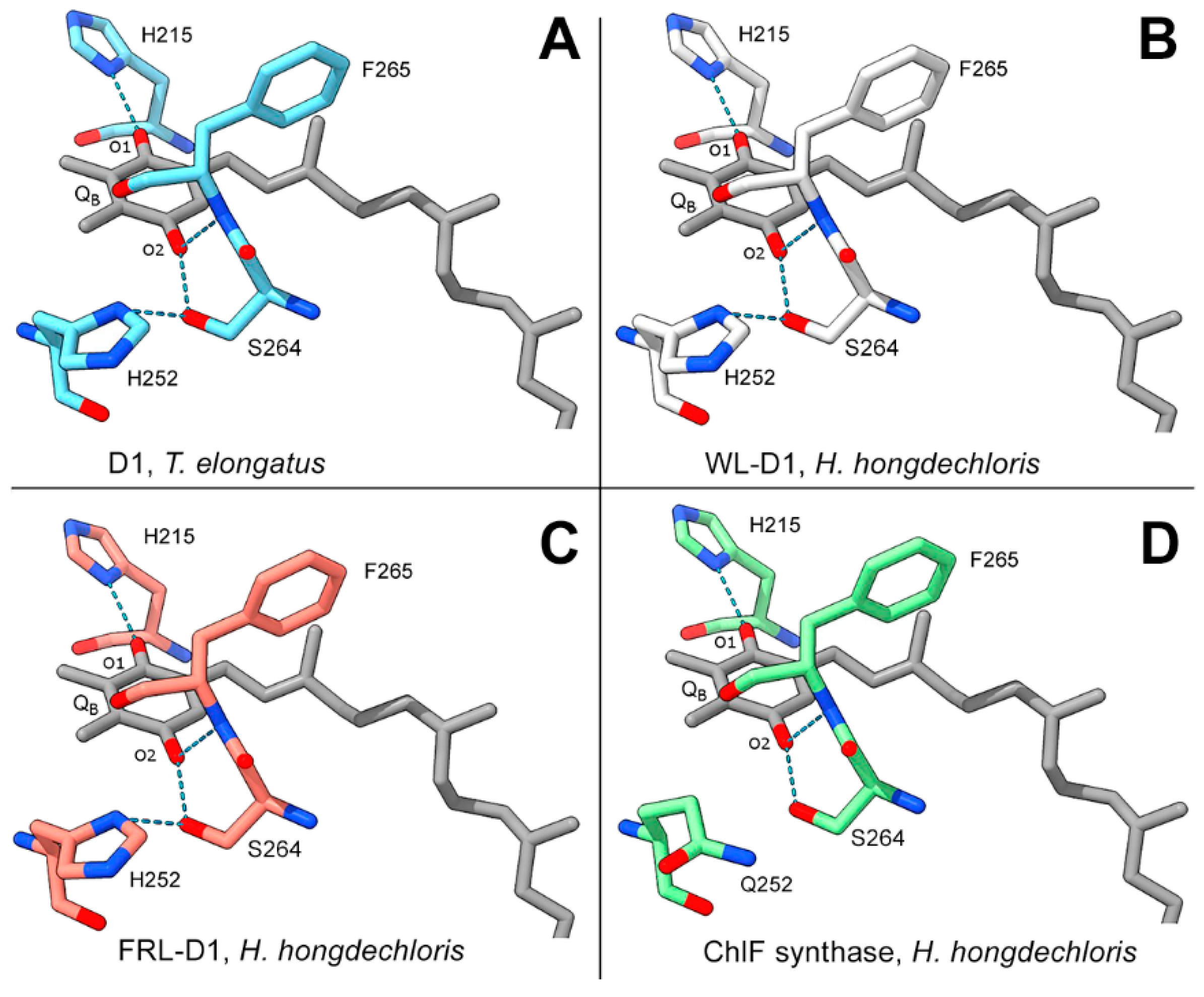
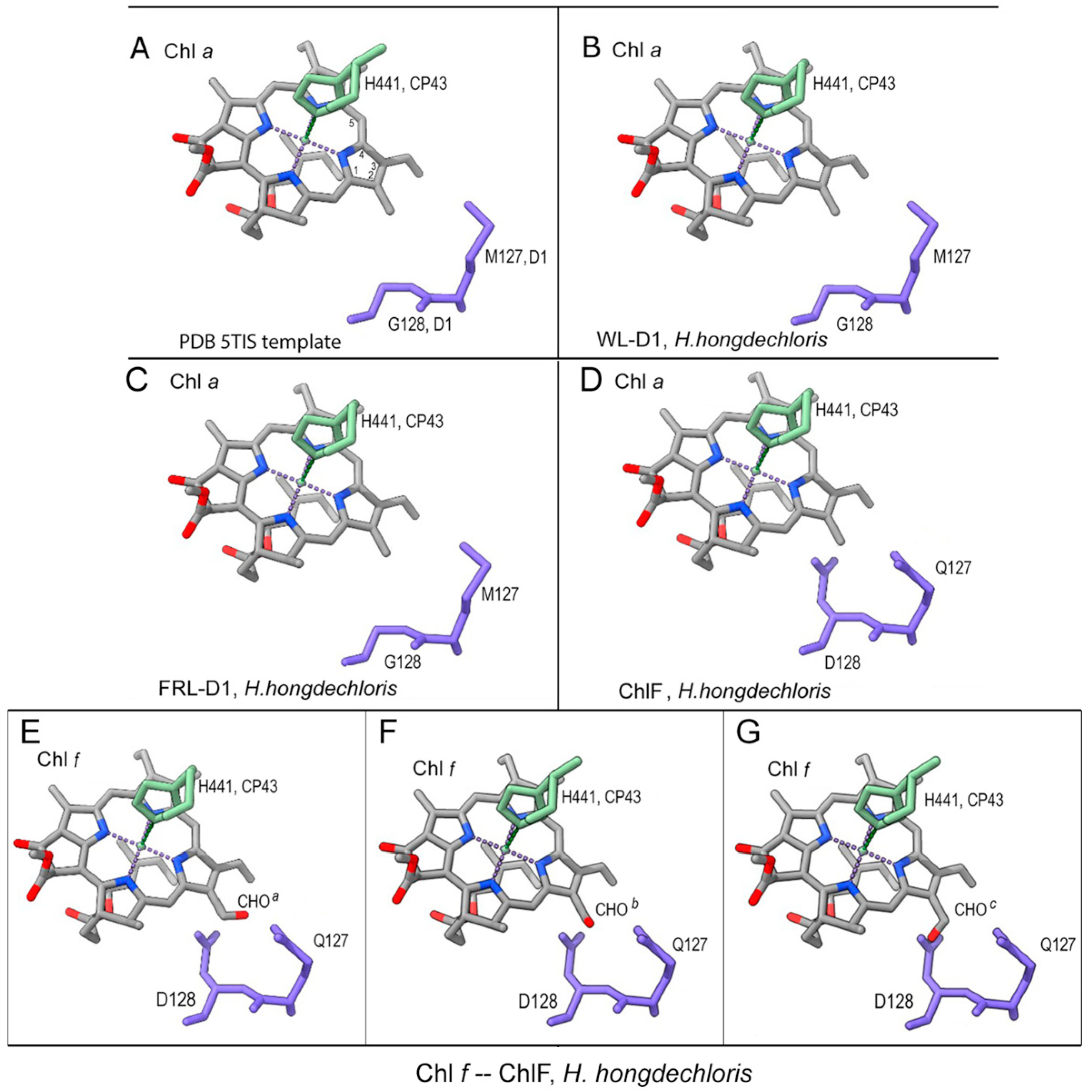
3.5. Putative Chl f-binding region of ChlF-PSII complexes
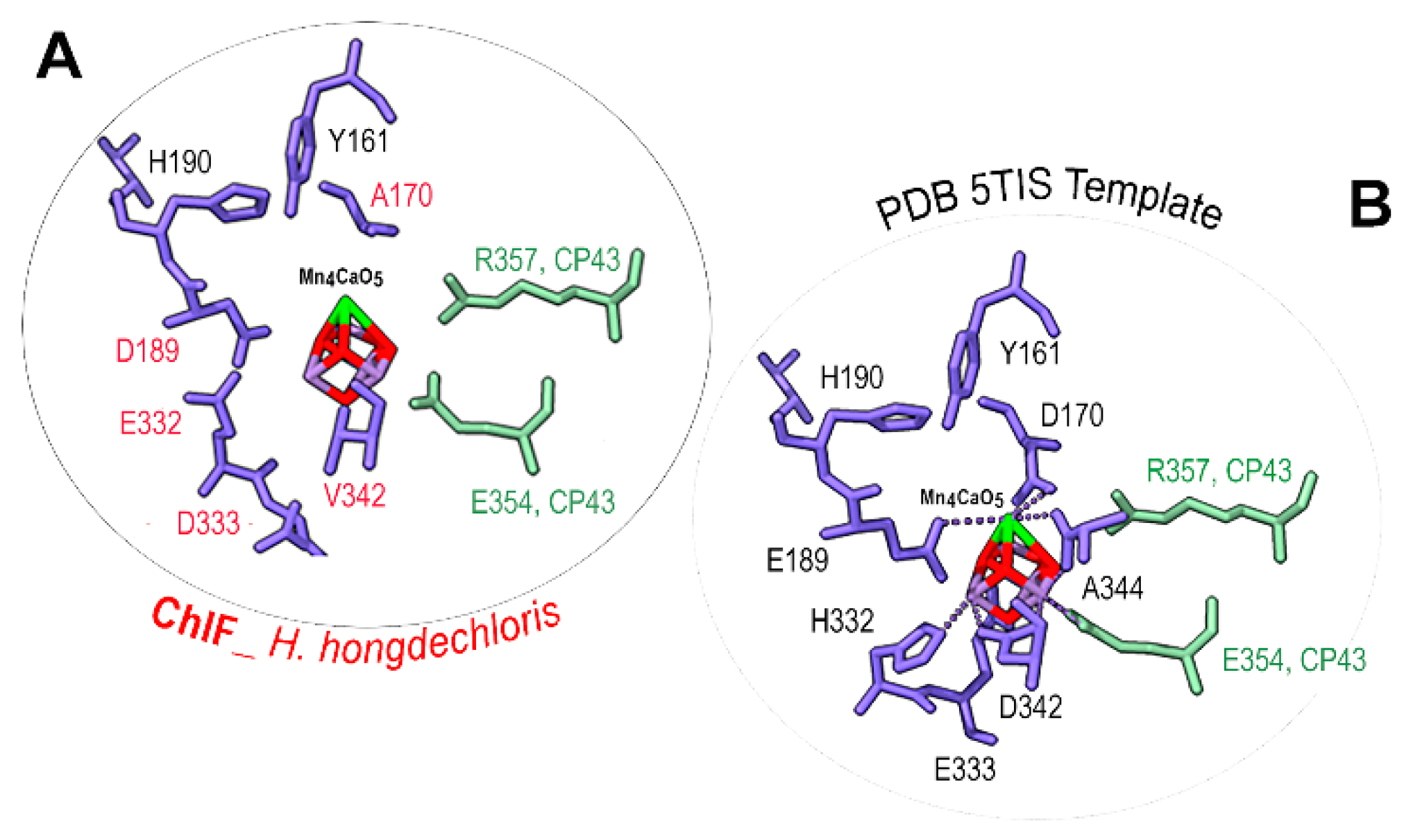
3.6. Mn4CaO5 cluster modelling
4. Discussion
4.1. Proposed uncoupled Mn4CaO5 cluster in PSII-ChlF complexes
4.2. The changed electron transfer pathways in dysfunctional PSII-ChlF complexes
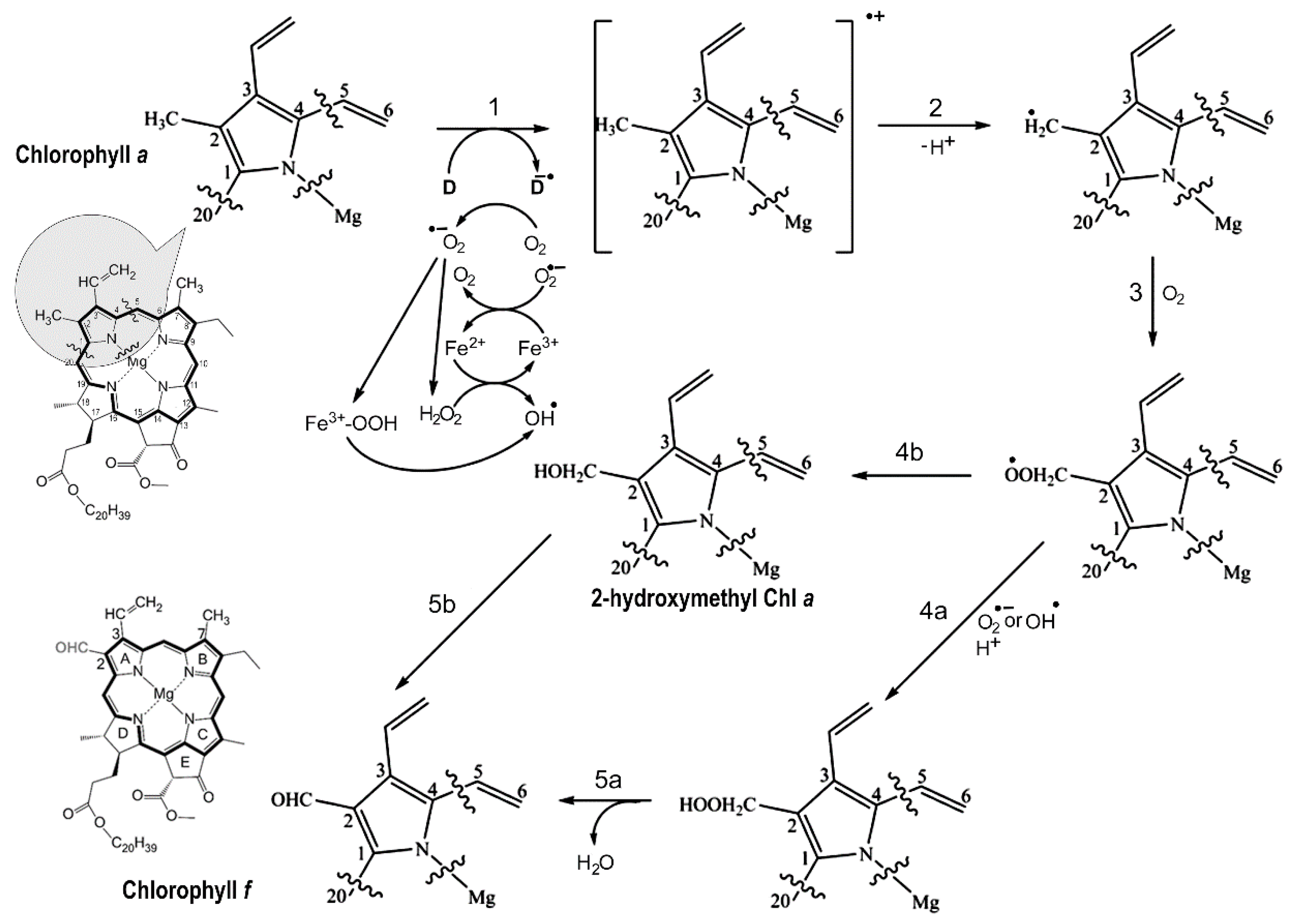
4.3. Reaction mechanism of Chl f biosynthesis pathway
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cardona, T.; Murray, J.W.; Rutherford, A.W. Origin and evolution of water oxidation before the Last Common Ancestor of the Cyanobacteria. Mol Biol Evol 2015, 32, 1310–1328. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.-R. ; Kamiya N Crystal structure of oxygen-evolving photosystem II at a resolution of 19, A. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- Service, R.J.; Yano, J.; McConnell, I.; Hwang, H.J.; Niks, D.; Hille, R.; et al. Participation of glutamate-354 of the CP43 polypeptide in the ligation of manganese and the binding of substrate water in Photosystem II. Biochemistry 2011, 50, 63–81. [Google Scholar] [CrossRef]
- Chu, H.A.; Nguyen, A.P.; Debus, R.J. Amino acid residues that influence the binding of manganese or calcium to photosystem II. 1. The lumenal interhelical domains of the D1 polypeptide. Biochemistry 1995, 34, 5839–5858. [Google Scholar] [CrossRef]
- Diner, B.A. Amino acid residues involved in the coordination assembly of the manganese cluster of photosystem, I.I. Proton-coupled electron transport of the redox-active tyrosines and its relationship to water oxidation. Biochim Biophys Acta Bioenerg 2001, 1503, 147–163. [Google Scholar] [CrossRef]
- Dilbeck, P.L.; Bao, H.; Neveu, C.L.; Burnap, R.L. Perturbing the water cavity surrounding the manganese cluster by mutating the residue D1-valine 185 has a strong effect on the water oxidation mechanism of Photosystem, I.I. Biochemistry 2013, 52, 6824–6833. [Google Scholar] [CrossRef]
- Service, R.J.; Hillier, W.; Debus, R.J. Network of hydrogen bonds near the oxygen-evolving Mn4CaO5 cluster of Photosystem II probed with FTIR difference spectroscopy. Biochemistry 2014, 53, 1001–1017. [Google Scholar] [CrossRef]
- Pokhrel, R.; Debus, R.J.; Brudvig, G.W. Probing the effect of mutations of asparagine 181 in the D1 subunit of Photosystem, I. I. Biochemistry 2015, 54, 1663–1672. [Google Scholar] [CrossRef]
- Nixon, P.J.; Diner, B.A. Aspartate 170 of the photosystem II reaction center polypeptide D1 is involved in the assembly of the oxygen-evolving manganese cluster. Biochemistry 1992, 31, 942–948. [Google Scholar] [CrossRef]
- Kiss, E.; Kós, P.B.; Chen, M.; Vass, I. A unique regulation of the expression of the psbA, psbD, and psbE genes, encoding the D1, D2 and cytochrome b559 subunits of the Photosystem II complex in the chlorophyll d containing cyanobacterium Acaryochloris marina. Biochim Biophys Acta Bioenerg 2012, 1817, 1083–94. [Google Scholar] [CrossRef]
- Murray, J.W. Sequence variation at the oxygen-evolving centre of photosystem II: a new class of ‘rogue’ cyanobacterial D1 proteins. Photosyn Res 2012, 110, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, K.J.; Dunca, E.J.; Eaton-Rye, J.J.; Summerfield, T.C. The diversity and distribution of D1 proteins in cyanobacteria. Photosynth Res 2020, 145, 111–128. [Google Scholar] [CrossRef]
- Mohamed, A.; Eriksson, J.; Osiewacz, H.D.; Jansson, C. Differential expression of the psbA genes in the cyanobacterium Synechocystis 6803. Mol Gen Genet 1993, 238, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Sicora, C.I.; Ho, F.M.; Salminen, T.; Styring, S.; Aro, E.-M. Transcription of a “silent” cyanobacterial psbA gene is induced by microaerobic conditions. Biochim Biophys Acta Bioenerg 2009, 1787, 105–112. [Google Scholar] [CrossRef]
- Zhang, X.; Sherman, L.A. Alternate copies of D1 are used by cyanobacteria under different environmental conditions. Photosyn Res 2012, 114, 133–135. [Google Scholar] [CrossRef]
- Wegener, K.M.; Nagarajan, A.; Pakrasi, H.B. An atypical psbA Gene Encodes a sentinel D1 protein to form a physiologically relevant inactive Photosystem II complex in cyanobacteria. J Biol Chem 2015, 290, 3764–3774. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-Y.; Shen, G.; Canniffe, D.P.; Zhao, C.; Bryant, D.A. Light-dependent chlorophyll f synthase is a highly divergent paralog of PsbA of photosystem II. Science 2016, 353, 886. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Loughlin, P.C.; Willows, R.D.; Chen, M. The C21-formyl group in chlorophyll f originates from molecular oxygen. J Biol Chem 2017, 292, 19279–19289. [Google Scholar] [CrossRef]
- Trinugroho, J.P.; Bečková, M.; Shao, S.; Yu, J.; Zhao, Z.; Murray, J.; et al. Chlorophyll f synthesis by a super-rogue photosystem II complex. Nat Plants 2020, 6, 238–244. [Google Scholar] [CrossRef]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.L.; Neilan, B.A.; Scheer, H. A red-shifted chlorophyll. Science 2010, 329, 1318–1319. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Birch, D.; Willows, R.D. A cyanobacterium that contains chlorophyll f – a red-absorbing photopigment. FEBS Lett 2012, 586, 3249–3254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Li, Z.K.; Yin, Y.C.; Li, Y.; Jia, Y.; Chen, M.; et al. Widespread occurrence and unexpected diversity of red-shifted chlorophyll producing cyanobacteria in humid subtropical forest ecosystems. Environ Microbiol 2019, 21, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Gan, F.; Shen, G.; Bryant, D.A. Occurrence of far-red light photoacclimation (FaRLiP) in diverse cyanobacteria. Life. 2015, 5, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Averina, S.G.; Velichko, N.; Senatskaya, E.; Pinevich, A. Far-red photoadaptations in aquatic cyanobacteria. Hydrobiologia. 2018, 813, 1–17. [Google Scholar] [CrossRef]
- Gómez-Lojero, C.; Leyva-Castillo, L.E.; Herrera-Salgado, P.; Barrero-Rojas, J.; Rios-Castro, E.; Gutiérrez-Cirlos, E.B. Leptolyngbya CCM 4, a cyanobacterium with far-red photoacclimation from Cuatro Ciénegas Basin, México. Photosynthetica. 2018, 56, 342–353. [Google Scholar] [CrossRef]
- Averina, S.G.; Velichko, N.V.; Pinevich, A.A.; Senatskaya, E.V.; Pinevich, A.V. Non-a chlorophylls in cyanobacteria. Photosynthetica. 2019, 57, 1109–1118. [Google Scholar] [CrossRef]
- Billi, D.; Napoli, A.; Mosca, C.; Fagliarone, C.; de Carolis, R.; Balbi, A.; Scanu, M.; Selinger, V.M.; Antonaru, L.A.; Nürnberg, D.J. Identification of far-red light acclimation in an endolithic Chroococcidiopsis strain and associated genomic features: Implications for oxygenic photosynthesis on exoplanets. Front. Microbiol. 2022, 13, 933404. [Google Scholar] [CrossRef]
- Murray, B.; Ertekin, E.; Dailey, M.; Soulier, N.T.; Shen, G.; Bryant, D.A.; Perez-Fernandez, C.; DiRuggiero, J. Adaptation of cyanobacteria to the endolithic light spectrum in hyper-arid deserts. Microorganisms. 2022, 10, 1198. [Google Scholar] [CrossRef]
- Tros, M.; Bersanini, L.; Shen, G.; Ho, M.-Y.; van Stokkum, I.H.M.; Bryant, D.A.; et al. Harvesting far-red light: Functional integration of chlorophyll f into Photosystem I complexes of Synechococcus sp. PCC 7002. Biochim Biophys Acta Bioenerg 2020, 1861, 148206. [Google Scholar] [CrossRef]
- Nürnberg, D.J.; Morton, J.; Santabarbara, S.; Telfer, A.; Joliot, P.; Antonaru, L.A.; et al. Photochemistry beyond the red limit in chlorophyll f–containing photosystems. Science 2018, 360, 1210–1213. [Google Scholar] [CrossRef]
- Kurashov, V.; Ho, M.-Y.; Shen, G.; Piedl, K.; Laremore, T.N.; Bryant, D.A.; et al. Energy transfer from chlorophyll f to the trapping center in naturally occurring and engineered Photosystem I complexes. Photosyn Res 2019, 141, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Vella, N.; Chen, M. Characterization of isolated photosystem I from Halomicronema hongdechloris, a chlorophyll f-producing cyanobacterium. Photosynthetica 2018, 56, 306–315. [Google Scholar] [CrossRef]
- Chen, M.; Hernández-Prieto, M.A.; Loughlin, P.C.; Li, Y.; Willows, R.D. Genome and proteome of the chlorophyll f-producing cyanobacterium Halomicronema hongdechloris: adaptative proteomic shifts under different light conditions. BMC Genom 2019, 20, 207. [Google Scholar] [CrossRef]
- Junge, W. Oxygenic photosynthesis: history, status and perspective. Q Rev Biophys 2019, 52, e1. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Y.; Loughlin, P.; Chen, M. Optimization and effects of different culture conditions on growth of Halomicronema hongdechloris – a filamentous cyanobacterium containing chlorophyll f. Front Plant Sci 2014, 5, 67. [Google Scholar] [CrossRef]
- Wang, F.; Chen, M. Chromatic acclimation processes and their relationships with phycobiliprotein complexes. Microorganisms 2022, 10, 1562. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 1992, 8, 275–82. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Torshin, I.Y.; Weber, I.T.; Harrison, R.W. Geometric criteria of hydrogen bonds in proteins and identification of `bifurcated' hydrogen bonds. Protein Eng Des Sel 2002, 15, 359–363. [Google Scholar] [CrossRef]
- McDonald, I.K.; Thornton, J.M. Satisfying hydrogen bonding potential in proteins. J Mol Biol 1994, 238, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.A.; Force, D.A.; Nixon, P.J.; Dole, F.; Diner, B.A.; Britt, R.D. Dual-mode EPR detects the initial intermediate in photo assembly of the photosystem II Mn cluster: The influence of amino acid residue 170 of the D1 polypeptide on Mn coordination. J Am Chem Soc. 2000, 122, 3754–3761. [Google Scholar] [CrossRef]
- Johnson, J.E.; Webb, S.M.; Thomas, K.; Ono, S.; Kirschvink, J.L.; Fischer, W.W. Manganese-oxidizing photosynthesis before the rise of cyanobacteria. Proc Natl Acad Sci U S A. 2013, 110, 11238–43. [Google Scholar] [CrossRef] [PubMed]
- Young, I.D.; Ibrahim, M.; Chatterjee, R.; Gul, S.; Fuller, F.D.; Koroidov, S.; et al. Structure of photosystem II and substrate binding at room temperature. Nature 2016, 540, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, F.; Guergova-Kuras, M.; Nixon, P.J.; Diner, B.A.; Lavergne, J. Kinetics pathways of charge recombination in photosystem, I. I. Biochemistry 2002, 41, 8518–8527. [Google Scholar] [CrossRef]
- Dorlet, P.; Xiong, L.; Sayre, R.T.; Un, S. High field EPR study of the pheophytin anion radical in wild type and D1-E130 mutants of Photosystem II in Chlamydomonas reinhardtii. J Biol Chem 2001, 276, 22313–22316. [Google Scholar] [CrossRef]
- Lupı́nková, L.; Metz, J.G.; Diner, B.A.; Vass, I.; Komenda, J. Histidine residue 252 of the Photosystem II D1 polypeptide is involved in a light-induced cross-linking of the polypeptide with the α subunit of cytochrome b-559: study of a site-directed mutant of Synechocystis PCC 6803. Biochim Biophys Acta Bioenerg 2002, 1554, 192–201. [Google Scholar] [CrossRef]
- Petrouleas, V.; Crofts, A. The iron -quinone acceptor complex In: Wydrzynski TJ, Satoh K editors Photosystem II: the light-driven water:plastoquinone oxidoreductase. Dordrecht: Springer. P177-206, 2005.
- Chu, H.A.; Nguyen, A.P.; Debus, R.J. Amino acid residues that influence the binding of manganese or calcium to photosystem II. 2. The carboxy-terminal domain of the D1 polypeptide. Biochemistry. 1995, 34, 5859–82. [Google Scholar] [CrossRef]
- Gisriel, C.J.; Shen, G.; Ho, M.Y.; Kurashov, V.; Flesher, D.A.; Wang, J.; Armstrong, W.H.; Golbeck, J.H.; Gunner, M.R.; Vinyard, D.J.; Debus, R.J.; Brudvig, G.W.; Bryant, D.A. Structure of a monomeric photosystem II core complex from a cyanobacterium acclimated to far-red light reveals the functions of chlorophylls d and f. J Biol Chem. 2022, 298, 101424. [Google Scholar] [CrossRef]
- Gisriel, C.J.; Cardona, T.; Bryant, D.A.; Brudvig, G.W. Molecular Evolution of Far-Red Light-Acclimated Photosystem II. Microorganisms. 2022, 10, 1270. [Google Scholar] [CrossRef]
- Gisriel, C.J.; Shen, G.; Flesher, D.A.; Kurashov, V.; Golbeck, J.H.; Brudvig, G.W.; Amin, M.; Bryant, D.A. Structure of a dimeric photosystem II complex from a cyanobacterium acclimated to far-red light. J Biol Chem. 2023, 299, 102815. [Google Scholar] [CrossRef] [PubMed]
- Renger, G.; Renger, T. Photosystem II: The machinery of photosynthetic water splitting. Photosyn Res 2008, 98, 53–80. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Kodama, N.; Sun, X.Y.; Ozawa, S.; Takahashi, Y. Requirement for Asn298 on D1 protein for oxygen evolution: analyses by exhaustive amino acid substitution in the green alga Chlamydomonas reinhardtii. Plant Cell Physiol. 2014, 55, 1266–75. [Google Scholar] [CrossRef] [PubMed]
- Faller, P.; Rutherford, A.W.; Debus, R.J. Tyrosine D oxidation at cryogenic temperature in photosystem, I.I. Biochemistry 2002, 41, 12914–12920. [Google Scholar] [CrossRef]
- Cser, K.; Vass, I. Radiative and non-radiative charge recombination pathways in Photosystem II studied by thermoluminescence and chlorophyll fluorescence in the cyanobacterium Synechocystis 6803. Biochim Biophys Acta. 2007, 1767, 233–43. [Google Scholar] [CrossRef]
- Shen, G.; Canniffe, D.P.; Ho, M.-Y.; Kurashov, V.; van der Est, A.; Golbeck, J.H.; et al. Characterization of chlorophyll f synthase heterologously produced in Synechococcus sp. PCC 7002. Photosyn. Res 2019, 140, 77–92. [Google Scholar] [CrossRef]
- Hakala, M.; Tuominen, I.; Keränen, M.; Tyystjärvi, T.; Tyystjärvi, E. Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem, I.I. Biochim Biophys Acta Bioenerg 2005, 1706, 68–80. [Google Scholar] [CrossRef]
- Wood, P.M. The potential diagram for oxygen at pH 7. Biochemical Journal 1988, 253, 287–289. [Google Scholar] [CrossRef]
- Vass, I. Molecular mechanisms of photodamage in the Photosystem II complex. Biochim Biophys Acta Bioenerg 2012, 1817, 209–217. [Google Scholar] [CrossRef]
- Julliard, M.; Galadi, A.; Chanon, M. Oxidation of methyl aromatic compounds by redox photosensitization. J Photochem Photobiol A: Chemistry 1990, 54, 79–90. [Google Scholar] [CrossRef]
- Kiener, A. Enzymatic oxidation of methyl groups on aromatic heterocycles: a versatile method for the preparation of heteroaromatic carboxylic acids. Angew Chem Int Ed Engl 1992, 31, 774–775. [Google Scholar] [CrossRef]
- Bienvenu, C.; Wagner, J.R.; Cadet, J. Photosensitized oxidation of 5-methyl-2‘-deoxycytidine by 2-methyl-1,4-naphthoquinone: characterization of 5-(hydroperoxymethyl)-2‘-deoxycytidine and stable methyl group oxidation products. J Am Chem Soc 1996, 118, 11406–11411. [Google Scholar] [CrossRef]
- Lendzian, F.; Mobius, K.; Lubitz, W. The pheophytin a anion radical. 14N and 1H endor and triple resonance in liquid solution. Chem Phys Lett. 1982, 90, 375–381. [Google Scholar] [CrossRef]
- Pospíšil, P.; Arató, A.; Krieger-Liszkay, A.; Rutherford, A.W. Hydroxyl radical generation by Photosystem, I.I. Biochemistry 2004, 43, 6783–6792. [Google Scholar] [CrossRef] [PubMed]
- Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J Exp Bot 2005, 56, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P. Production of reactive oxygen species by photosystem, I.I. Biochim Biophys Acta Bioenerg 2009, 1787, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Knaff, D.B. The effect of pH on the midpoint oxidation-reduction potentials of components associated with plant photosystem II. FEBS Lett 1975, 60, 331–335. [Google Scholar] [CrossRef]
- Klimov, V.V. Pheophytin as the primary electron acceptor in photosystem II reaction centres. Photosynthetica 1981, 15, 592–609. [Google Scholar]
- Tanaka, A.; Ito, H.; Tanaka, R.; Tanaka, N.K.; Yoshida, K.; Okada, K. Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc Natl Acad Sci U.S.A. 1998, 95, 12719–12723. [Google Scholar] [CrossRef]
- Abe, T.; Tanaka, S.; Ogawa, A.; Tamura, M.; Sato, K.; Itoh, S. Copper-catalyzed selective oxygenation of methyl and benzyl substituents in pyridine with O2. Adv Synth Catal 2017, 46, 348–350. [Google Scholar] [CrossRef]
- Ye, R.; Cao, Y.; Xi, X.; Liu, L.; Chen, T. Metal- and radical-free aerobic oxidation of heteroaromatic methanes: an efficient synthesis of heteroaromatic aldehydes. Org Biomol Chem 2019, 17, 4220–4224. [Google Scholar] [CrossRef]
- Chen, G.X.; Kazimir, J.; Cheniae, G.M. Photoinhibition of hydroxylamine-extracted photosystem II membranes: studies of the mechanism. Biochemistry 1992, 31, 11072–11083. [Google Scholar] [CrossRef]
- Eckert, H.J.; Geiken, B.; Bernarding, J.; Napiwotzki, A.; Eichler, H.J.; Renger, G. Two sites of photoinhibition of the electron transfer in oxygen evolving and Tris-treated PS II membrane fragments from spinach. Photosyn Res 1991, 27, 97–108. [Google Scholar] [CrossRef]
- Blubaugh, D.J.; Atamian, M.; Babcock, G.T.; Golbeck, J.H.; Cheniae, G.M. Photoinhibition of hydroxylamine-extracted photosystem II membranes: identification of the sites of photodamage. Biochemistry 1991, 30, 7586–7597. [Google Scholar] [CrossRef]
- Tyystjärvi, E. Photoinhibition of Photosystem II and photodamage of the oxygen evolving manganese cluster. Coord Chem Rev 2008, 252, 361–376. [Google Scholar] [CrossRef]
- Taguchi, F.; Yamamoto, Y.; Satoh, K. Recognition of the structure around the site of cleavage by the carboxyl-terminal processing protease for D1 precursor protein of the Photosystem II reaction center. J Biol Chem 1995, 270, 10711–10716. [Google Scholar] [CrossRef] [PubMed]
- Conjeaud, H.; Mathis, P. The effect of pH on the reduction kinetics of P-680 in tris-treated chloroplasts. Biochim Biophys Acta Bioenerg 1980, 590, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Styring, S.; Sjöholm, J.; Mamedov, F. Two tyrosines that changed the world: Interfacing the oxidizing power of photochemistry to water splitting in photosystem, I.I. Biochim Biophys Acta Bioenerg 2012, 1817, 76–87. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




