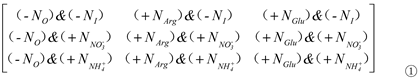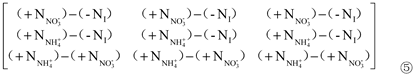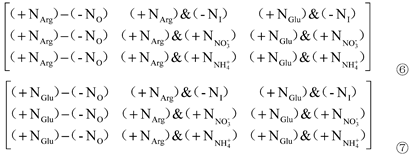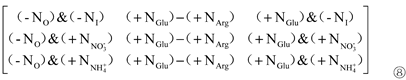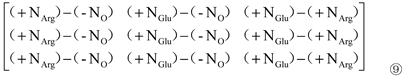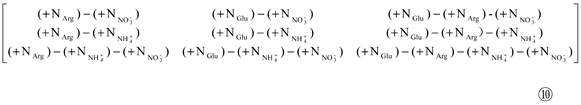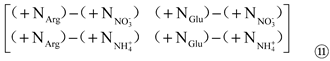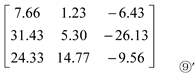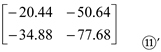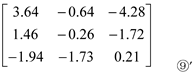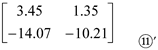1. Introduction
Chromium (Cr), as a naturally occurring heavy metal, is widely distributed in the environmental matrixes through different industrial activities, such as metallurgical and chemical industries (Singh et al., 2013). Due to its high solubility, mobility, and oxidizing potential, Cr has been considered as one of the top 20 hazardous materials to be remediated (Feng et al., 2019). In nature, Cr possesses several oxidation states that ranges from “-2” to “+6”, in which the hexavalent [Cr(VI)] and trivalent chromium [Cr(III)] are the most stable forms of Cr (Singh et al., 2013). Nowadays, Cr pollution is a serious agricultural problem (Fan et al., 2020; Zhang et al., 2022), since it is very crucial to maintain the ecosystem integrity and sustain the agriculture production. Cr(III) is frequently detected in different environmental conditions derived from both natural and anthropogenic sources. Over-accumulation of Cr in photosynthetic organisms has a damaging impact on nitrogen (N) metabolism. For example, Cr exposure disturbed assimilation of nitrate (NO3-) and ammonium (NH4+) through modifying the activities of nitrate reductase (NR), nitrite reductase (NiR), glutamine (GS), and glutamate dehydrogenase (GDH) in Sorghum bicolor and S. lycopersicum (Kumar and Joshi 2008; Martins et al., 2021). In addition, Cr exposure can significantly affect the innate pool of organic and inorganic N in Hordeum vulgare, S. bicolor and Pistia stratoites (Kumar and Joshi 2008; Ganesh et al., 2008; Wyszkowski and Radziemska 2010).
Nitrogen is a crucial macroelement for supporting plant growth and development (Li et al., 2020), wherein NO3- and NH4+ are the most available inorganic N forms. Uptake and subsequent assimilation of NO3- or NH4+ by plants play a critical role in improving the yield and quality of crops (Li et al., 2020). Among the biomolecules derived from assimilation of both inorganic species, amino acids are the major component of plant biomass. Proline (Pro) is the most common biomolecules present in plant cells (Szabados and Savoure 2010). The synthesis of Pro in plants is highly dependent upon two different precursors, i.e., glutamate (Glu) and arginine (Arg) (Kaur and Asthir 2015). Numerous studies have demonstrated that Pro accumulation in plants can maintain osmotic balance, protect subcellular structures, remove reactive oxygen species (ROS), stabilize protein and DNA, and provide N sources and energy in responses to diverse stressful conditions, such as drought, high temperature, salinity, UV radiation, pathogens, and chemical exposure (Pavlíková et al., 2007; Verbruggen and Hermans, 2008; Szabados and Savoure 2010). Therefore, the level of Pro accumulation in plant materials has been suggested as a sensitive indicator to evaluate the overall performance of plants growth in various contaminated sites. It has been reported that the content of amino acids in plants is highly dependent on N nutrition (Näsholm et al., 2009). Our previous work also confirmed that NH4+-fed rice seedlings showed significantly dose-dependent increase of Pro in shoots, while the innate level of Pro in NO3--fed rice seedlings is independent on the dose of NO3- supplied (Li et al., 2022). Additionally, we noticed that accumulation of Pro was observable in rice plants supplied with additional Glu and Arg, whereas the latter demonstrated much higher potential than the former during synthesis of Pro in rice plants (Li et al., 2022).
It is known that unfavorable growth conditions would disturb plant growth and development through different toxic mechanisms, in which disturbing and/or inhibiting acquirement and assimilation of main N nutritional elements is one of the possible mechanisms involved. If so, the original N cycle could be changed in composition and the synthesis rate of amino acids. In fact, literatures are available on Cr-mediation damage on enzymes and metabolites during N assimilation (Sangwan et al., 2014). It is also reported that fertilization of inorganic N (NO3- and NH4+) can influence the bioavailability and toxicity of Cu, Cd and Cr in plants by altering the synthesis of organic N species, e.g., Pro, Glu and Arg (Huo et al., 2020; Chai et al., 2018). Our previous work reported that the innate level of Pro in rice plants is changeable due to the application of different N sources (NO3- and NH4+) under the stress of Cr(VI) pollution (Yu et al., 2017). Rice is one of the world most produced crop and a major energy source in the world. Research on the synthesis of Pro in rice plants from both inorganic (NO3- and NH4+) and organic (Arg and Glu) N sources showed completely different increment pattern (Li et al., 2022). However, no information is available to investigate the indigenous Pro in rice plants fertilized with different nitrogenous chemicals under the Cr(III) stress.
Therefore, to achieve the objective, we carried out the present study in the following manner: (1) to determine the content of Pro in rice tissues under Cr(III) stress with different inorganic (NO3- and NH4+) and organic (Arg and Glu) N sources alone and in combination; (2) to mathematically evaluate the contribution of different N sources alone and in combination to Pro content in rice plants under Cr(III) stress, based on the mass balance matrix model (MBMM); (3) to predict a suitable combination of different N sources for regulation of Cr(III) stress in rice plants using the content of Pro as a bioindicator. Overall, our study provides a new method to elucidate the contribution of different N sources to Pro accumulation in rice plants under Cr(III) stress.
2. Methods and materials
2.1. Rice seedlings and Cr treatment
Rice (Oryza sativa L. XZX 45) was sown and cultivated as described in our previous work (Li et al., 2020). Briefly, after soaking the seeds with deionized water for 24 h, the seeds were cultivated in sand soils and placed in a growth chamber (temperature: 25 ± 0.5℃ and relative humidity: 60 ± 2%). Rice seedlings were irrigated with a modified ISO8629 nutrient solution during the growth period (16 days) (Feng et al., 2019). Seedlings with similar size were selected for following treatments (Figure 1):
Figure 1.
The design of experimental treatments
(1) ‘Cr(III)+(-NI)’ treatments: Rice seedlings were pre-treated with the nutrition solution without KNO3/NH4Cl (‘-NI’), but with 3 mM Arg (‘+NArg’) or 10 mM Glu (‘+NGlu’) for 12 hours, respectively (Li et al., 2022), and then were exposed to Cr(III) solution at 0, 12.0, 24.0 and 40.0 mg Cr/L for 2 days, respectively (
Figure 1a).
(2) ‘Cr(III)+(+NNO3-)’ treatments: Rice seedlings were pre-treated with the KNO3-containing nutrition solution (‘+ NNO3-’), and with 3 mM Arg (‘+NArg’) or 10 mM Glu (‘+NGlu’) for 12 hours, respectively (Li et al., 2022) [
15], and then were exposed to Cr(III) solution at 0, 12.0, 24.0 and 40.0 mg Cr/L for 2 days, respectively (
Figure 1b).
(3) ‘Cr(III)+(+NNH4+)’ treatments: Rice seedlings were pre-treated with the NH4Cl-containing nutrition solution ‘+NNH4+’, and with 3 mM Arg (‘+NArg’) or 10 mM Glu (‘+NGlu’) for 12 hours, respectively (Li et al., 2022) [
15], and then were exposed to Cr(III) solution at 0, 12.0, 24.0 and 40.0 mg Cr/L for 2 days, respectively (
Figure 1c).
Figure 1.
The design of experimental treatments
(1) ‘Cr(III)+(-NI)’ treatments: Rice seedlings were pre-treated with the nutrition solution without KNO3/NH4Cl (‘-NI’), but with 3 mM Arg (‘+NArg’) or 10 mM Glu (‘+NGlu’) for 12 hours, respectively (Li et al., 2022), and then were exposed to Cr(III) solution at 0, 12.0, 24.0 and 40.0 mg Cr/L for 2 days, respectively (
Figure 1a).
(2) ‘Cr(III)+(+NNO3-)’ treatments: Rice seedlings were pre-treated with the KNO3-containing nutrition solution (‘+ NNO3-’), and with 3 mM Arg (‘+NArg’) or 10 mM Glu (‘+NGlu’) for 12 hours, respectively (Li et al., 2022) [
15], and then were exposed to Cr(III) solution at 0, 12.0, 24.0 and 40.0 mg Cr/L for 2 days, respectively (
Figure 1b).
(3) ‘Cr(III)+(+NNH4+)’ treatments: Rice seedlings were pre-treated with the NH4Cl-containing nutrition solution ‘+NNH4+’, and with 3 mM Arg (‘+NArg’) or 10 mM Glu (‘+NGlu’) for 12 hours, respectively (Li et al., 2022) [
15], and then were exposed to Cr(III) solution at 0, 12.0, 24.0 and 40.0 mg Cr/L for 2 days, respectively (
Figure 1c).
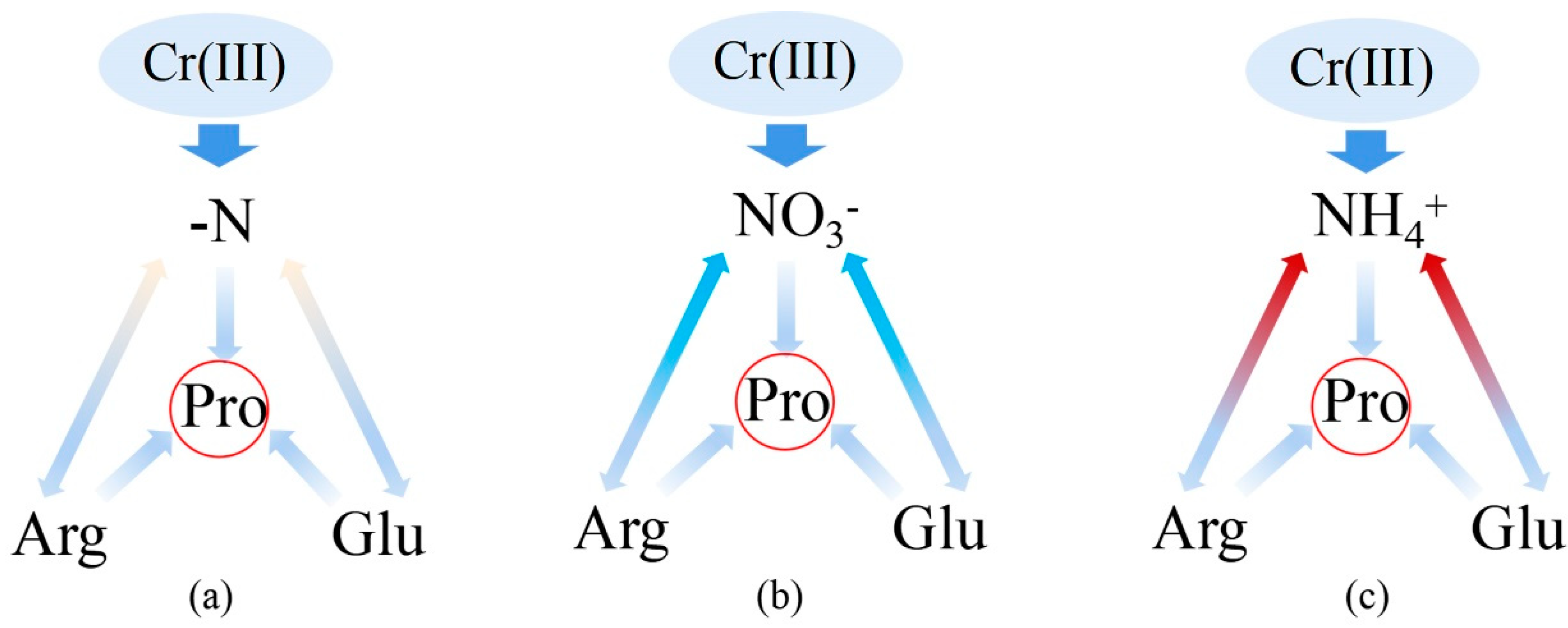
The symbol ‘-NO’ indicates the N treatment without Arg or Glu supplied; ‘+NArg’ indicates the N treatment with Arg supplied; ‘+NGlu’ indicates the N treatment with Glu supplied; ‘-NI’ indicates the N treatment without KNO3 or NH4Cl supplied; ‘+NNO3-’ indicates the KNO3 treatment’; ‘+NNH4+’ indicates the NH4Cl treatment, respectively. The weight of KNO3 and NH4Cl in the nutrient solution is equal to 39.5 mg N/L. All glass containers were wrapped with aluminum foil in order to minimize water loss and inhibit algae growth. Each treatment was prepared in four biological replicates. All chemicals used were of analytical grade and purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China).
2.2. Measurement of relative growth rate
Rice seedlings were weighed before and at the termination of exposure. The relative growth rate (RGR, %) was calculated based on our previous work (Li et al., 2022).
2.3. Measurement of Pro content in rice seedlings
After 2 days of exposure, rice seedlings were divided into roots and shoots, and then rice tissues were homogenized in a pre-chilled mortar with 3% sulfosalicylic acid (5 mL). The homogenate was transferred to a 10 mL tube for centrifuging (4°C, 11,000 rpm, 15 min). After centrifugation, 2 mL of the supernatant was taken and added with equal quantity i.e. 2.0 mL of glacial acetic acid and 2.5% ninhydrin (glacial acetic acid: 6 mol/L phosphoric acid, 60:40) solution, and was kept in boiling water for 1 h. The remaining procedure was identical to our previous work (Li et al., 2022). The amount of Pro content was estimated using a spectrophotometer at 520 nm against a toluene reference.
2.4. Modeling the “mass balance matrix”
In this study, we developed a “mass balance matrix” model (MBMM), based on the elementary rows (r)/columns (c) transformation to predict the optimal tolerance strategies for rice seedlings grown in different N source conditions under Cr(III) stress using the content of Pro as the dependent variable. Accordingly, the contribution of different N sources to Pro content could be estimated. The elements of all matrixes can be denoted by aij (i, j = 1, 2, 3).
The fundamental matrix is as follows:
The rows (i) and columns (j) of Matrix ① are denoted by ri and cj (i, j = 1, 2, 3), respectively. The ‘&’ indicated the combination of two different N sources. For example, a11refers to the treatment without organic N ‘-NO’ and inorganic N (KNO3/NH4Cl) ‘-NI’; a12 refers to the treatment with organic N (Arg) ‘+NArg’, but without inorganic N (KNO3/NH4Cl) ‘-NI’; a13 refers to the treatment with organic N (Glu) ‘+NGlu’, but without inorganic N (KNO3/NH4Cl) ‘-NI’.
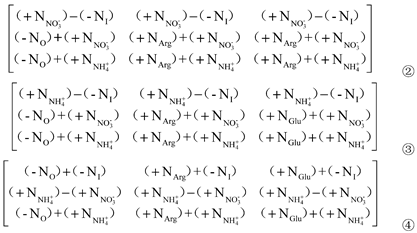
In order to compare the contribution of organic N and inorganic N to Pro content, Matrix ① was performed by the elementary row (r) transformation, i.e., r2-r1, r3-r1, and r3-r2. Therefore, the following three matrices were obtained:
r1 in Matrix ②, r1 in Matrix ③ and r2 in Matrix ④ were all extracted to obtain Matrix ⑤:
Where, r1 is the contribution of inorganic N (KNO3) ‘+NNO3-’ to Pro content; r2 is the contribution of inorganic N (NH4Cl) ‘+NNH4+’ to Pro content; r3 is the difference between the contribution of inorganic N (NH4Cl) ‘+NNH4+’ and inorganic N (KNO3) ‘+NNO3-’ to Pro content.
Additionally, Matrix① was performed by the elementary column (c) transformations, i.e., c2-c1, c3-c1, and c3-c2. Therefore, the following three matrices were obtained:
c1 in Matrix ⑥, c1 in Matrix ⑦ and c2 in Matrix ⑧ were all extracted to obtain Matrix ⑨:
Where, c1 is the contribution of organic N (Arg) ‘+NArg’ to Pro content; c2 is the contribution of organic N (Glu) ‘+NGlu’ to Pro content; c3 is the difference between the contribution of organic N (Glu) ‘+NGlu’ and organic N (Arg) ‘+NArg’ to Pro content.
Next, setting the ‘(-NO) and (-NI)’ in 0, and subtracting Matrix ⑨ from Matrix ⑤to yield Matrix ⑩:
Then, extracting a11, a12, a21, and a22from Matrix ⑩ to form Matrix ⑪:
Wherein, a11 represents the difference between the contribution of ‘Arg and NO3-’ to Pro content; a12represents the difference between the contribution of ‘Glu and NO3-’ to Pro content; a21 represents the difference between the contribution of ‘Arg and NH4+’ to Pro content; a22 represents the difference between the contribution of ‘Glu and NH4+’ to Pro content. In addition, the values of a3j in Matrix ⑤, ai3 in Matrix ⑨, and a11, a12, a21 and a22 in Matrix ⑪ can be use to reflect the contribution of organic (Arg/Glu) and inorganic (KNO3/NH4Cl) N alone to Pro content.
3. Results
3.1. Pro content in rice tissues under ‘Cr(III)+(-NI)’ treatments
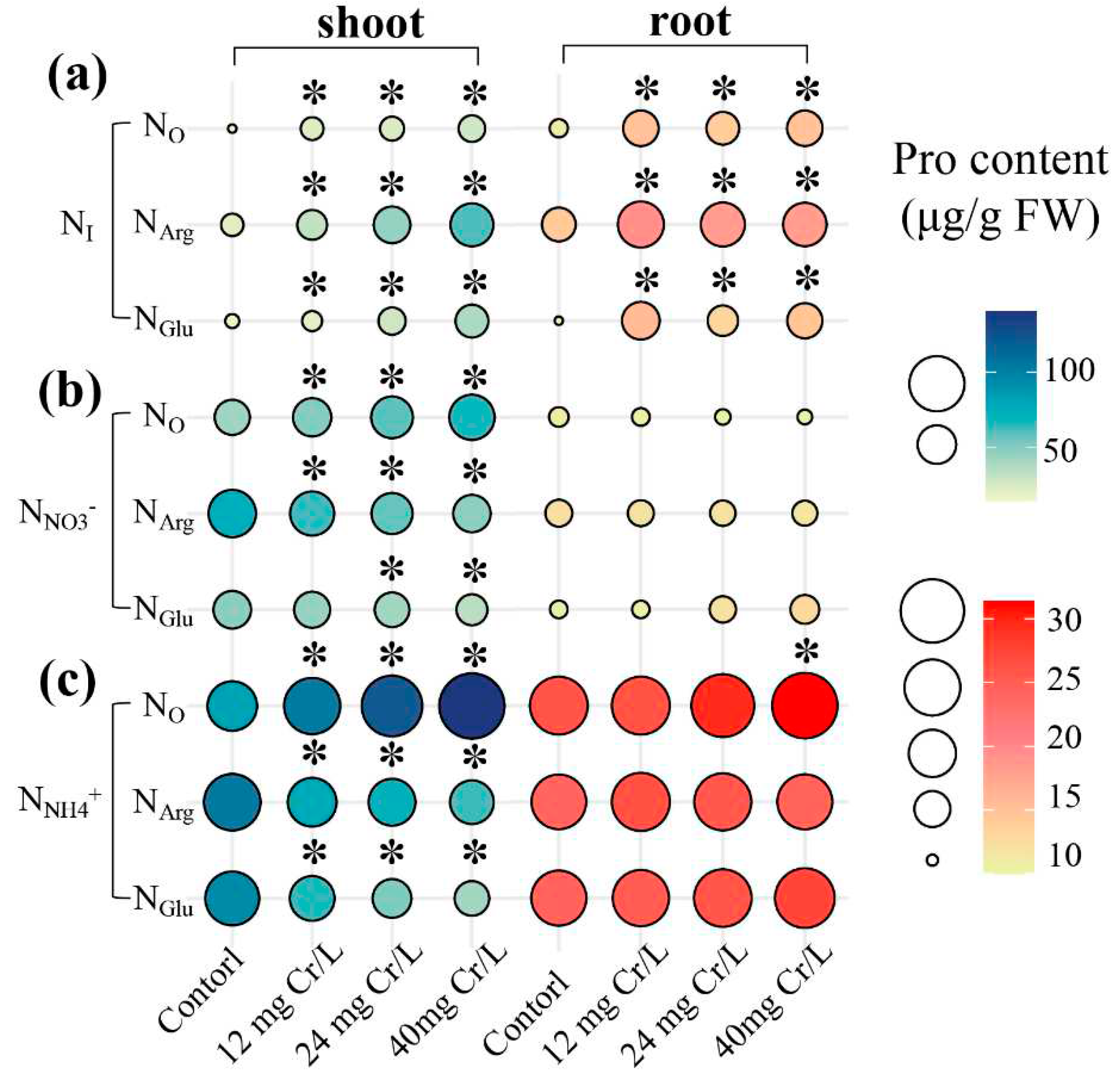
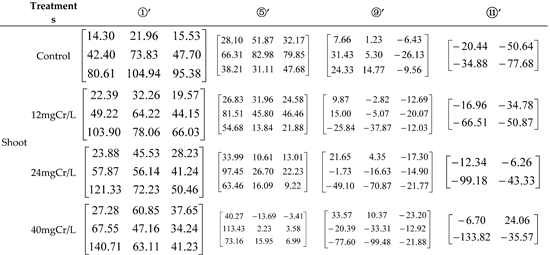
Under ‘Cr(III)+(-NI)’ treatments (Figure 2a), the Pro content in shoots of rice seedlings cultivated with ‘-NO’, ‘+NArg’, ‘+NGlu’ was determined to be “14.30 to 27.28 μg/g FW”, “21.96 to 60.85 μg/g FW”, and “15.53 to 37.65 μg/g FW”, respectively.
Figure 2.
The content of Proline in shoots and roots of rice seedlings under ‘Cr(III)+(-NI)’, ‘Cr(III)+(+NNO3-)’, and ‘Cr(III)+(+NNH4+)’ treatments.
Figure 2.
The content of Proline in shoots and roots of rice seedlings under ‘Cr(III)+(-NI)’, ‘Cr(III)+(+NNO3-)’, and ‘Cr(III)+(+NNH4+)’ treatments.
While the Pro content in roots of rice seedlings cultivated with ‘-NO’, ‘+NArg’, ‘+NGlu’ was “10.59 to 14.82 μg/g FW”, “14.23 to 19.35 μg/g FW”, and “9.95 to 15.64 μg/g FW”, respectively.
3.2. Pro content in rice tissues under ‘Cr(III)+(+NNO3-)’ treatments
Under ‘Cr(III)+(+NNO3-)’ treatments (Figure 2b), the Pro content in shoots of rice seedlings cultivated with ‘-NO’, ‘+NArg’, ‘+NGlu’ was determined to be “42.40 to 67.55 μg/g FW”, “47.16 to 73.83 μg/g FW”, and “34.24 to 47.70 μg/g FW”, respectively.
While the Pro content in roots of rice seedlings cultivated with ‘-NO’, ‘+NArg’, ‘+NGlu’ was “10.21 to 10.78 μg/g FW”, “11.78 to 12.24 μg/g FW”, and “10.50 to 12.72 μg/g FW”, respectively.
3.3. Pro content in rice tissues under ‘Cr(III)+(+NNH4+)’ treatments
Under ‘Cr(III)+(+NNH4+)’ treatment (Figure 2c), the Pro content in shoots of rice seedlings cultivated with ‘-NO’, ‘+NArg’, ‘+NGlu’ was determined to be “80.61 to 140.71 μg/g FW”, “63.11 to 104.94 μg/g FW”, and “41.23 to 95.38 μg/g FW”, respectively.
While the Pro content in roots of rice seedlings cultivated with ‘-NO’, ‘+NArg’, ‘+NGlu’ was “26.12 to 31.39 μg/g FW”, “24.18 to 26.34 μg/g FW”, and “24.39 to 27.37 μg/g FW”, respectively.
3.4. The contribution of organic and inorganic N application to Pro content in rice seedlings
Herein, we take the treatment with 0.0 mg Cr/L application as an example to predicate the contribution of organic and inorganic N alone and in combination to Pro content in rice seedlings (Table 1).
Table 1.
The ranking of contribution of organic and inorganic N to Pro content in rice seedlings.
Table 1.
The ranking of contribution of organic and inorganic N to Pro content in rice seedlings.
| Treatments |
Organic and inorganic N treatments alone |
Organic and inorganic N treatments in combination |
| Shoot |
Root |
Shoot |
Root |
| Control |
NNH4+>NNO3->NArg>NGlu
|
NArg>NNH4+>NGlu>NNO3-
|
NNH4++NArg>NNH4++NGlu>NNO3-+NArg>NNO3-+NGlu
|
NNH4+NArg≈NNH4+NGlu>NNO3+NArg≈NNO3+NGlu
|
| 12 mg Cr/L |
NNH4+>NNO3->NArg>NGlu
|
NArg>NNH4+>NGlu>NNO3-
|
NNH4++NArg>NNH4++NGlu>NNO3-+NArg>NNO3-+NGlu
|
NNH4+NArg≈NNH4+NGlu>NNO3+NArg≈NNO3+NGlu
|
| 24 mg Cr/L |
NNH4+>NNO3->NArg>NGlu
|
NArg>NNH4+>NGlu>NNO3-
|
NNH4++NArg>NNO3-+NArg>NNH4++NGlu>NNO3-+NGlu
|
NNH4+NArg≈NNH4+NGlu>NNO3+NArg≈NNO3+NGlu
|
| 40 mg Cr/L |
NNH4+>NNO3->NArg>NGlu
|
NArg>NNH4+>NGlu>NNO3-
|
NNH4++NArg>NNO3-+NArg>NNH4++NGlu>NNO3-+NGlu
|
NNH4+NArg≈NNH4+NGlu>NNO3+NArg≈NNO3+NGlu
|
| In summary |
NNH4+>NNO3->NArg>NGlu
|
NArg>NNH4>NGlu>NNO3
|
0 and 12 mg Cr/L treatment:NNH4++NArg>NNH4++NGlu>NNO3-+NArg>NNO3-+NGlu24 and 40 mg Cr/L treatment:NNH4++NArg>NNO3-+NArg>NNH4++NGlu>NNO3-+NGlu
|
NNH4+NArg≈NNH4+NGlu>NNO3+NArg≈NNO3+NGlu
|
3.4.1. Pro content in shoots of Cr(III)-treated rice seedlings
Using the measured data of Pro content in rice shoots, Matrix ①’ was formulated as follows:
As shown in matrix ①’, the contribution of ‘NI + NO’ application to Pro content was in the order: ‘NNH4++NArg’ > ‘NNH4++NGlu’ > ‘NNO3-+NArg’ > ‘NNO3-+NGlu’.
In order to compare the contribution of inorganic N (NNH4+/NNO3-) application to Pro content, the result was shown in Matrix ⑤’:
According to the calculation presented in the third row (r3) of Matrix ⑤’, the NNH4+ application showed a higher contribution to Pro content than the NNO3- application.
Then, Matrix ⑨’ can be obtained as follows:
Based on the results presented in the third column (c3) of matrix ⑨’, the contribution of NArg application to Pro content is higher than that of NGlu application.
The contribution of each individual organic/inorganic N to Pro content was calculated, based on Matrix ⑪ and the result is shown as follows:
It is obvious that the contribution of inorganic/organic N application to Pro content is: ‘NNO3-’ > ‘NArg’, ‘NNO3-’ > ‘NGlu’, ‘NNH4+’ > ‘NArg’, and ‘NNH4+’ > ‘NGlu’. Accordingly, based on the values of a3j in Matrix ⑤’, ai3 in Matrix ⑨’, and a11, a12, a21 and a22 in Matrix ⑪’, the contribution of inorganic/organic N application to Pro content is: ‘NNH4+’ > ‘NNO3-’ > ‘NArg’ > ‘NGlu’.
3.4.2. Pro content in roots of Cr(III)-treated rice seedlings
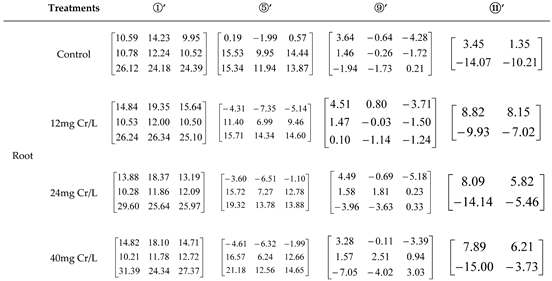
Using the measured data of Pro content in rice roots, Matrix ①’ was formulated as follows:
It can be seen from Matrix ①’ that the contribution of ‘NI + NO’ application to Pro content is: ‘NNH4++NArg’ ≈ ‘NNH4++NGlu’ > ‘NNO3-+NArg’ ≈ ‘NNO3-+NGlu’.
To obtain matrix⑤’:
According to the third row of Matrix ⑤’, the contribution of inorganic N sources to Pro content is: ‘NNH4+’ > ‘NNO3-’.
Then, to obtain Matrix ⑨’:
Based on the third column of Matrix ⑨’, the contribution of organic N sources to Pro content is: ‘NArg’ > ‘NGlu’.
Then, generating a new Matrix ⑪’:
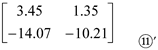
It can be seen from Matrix ⑪’ that the contribution of inorganic or organic N sources to Pro content is ‘NArg’ > ‘NNO3-’, ‘NGlu’ > ‘NNO3-’, ‘NArg’ > ‘NNH4+’, and ‘NNH4+’ > ‘NGlu’. Similarly, based on the values of a3j in Matrix ⑤’, ai3 in Matrix ⑨’, and a11, a12, a21 and a22 in Matrix ⑪’, the contribution of inorganic or organic N sources to Pro content is ‘NArg’ > ‘NNH4+’ > ‘NGlu’ > ‘NNO3-’. Moreover, we also calculated the contribution of inorganic and organic N sources alone and in combination to Pro content in both roots and shoots of rice seedlings under 12, 24, and 40 mg Cr/L treatments (Table 2&3). Apparently, with the application of N sources alone, ‘NNH4+’ showed the largest contribution to Pro content in rice shoots under different concentrations of Cr(III), followed by ‘NNO3-’, ‘NArg’, and ‘NGlu’ application. While, ‘NArg’ application displayed the largest contribution to the Pro content in rice roots under different concentrations of Cr(III), followed by ‘NNH4+’, ‘NGlu’, and ‘NNO3-’ application. Regarding the application of N sources in combination, ‘NNH4++NArg’ application contributed the largest to Pro content in both roots and shoots of rice seedlings under different concentrations of Cr(III), while ‘NNO3-+NGlu’ application contributed the least to Pro content in both roots and shoots of Cr(III)-treated rice seedlings. These results suggested that the contribution of different N sources to Pro content in Cr(III)-treated rice seedling is different.
Table 2.
The contribution of inorganic and organic N alone and in combination to Pro content in shoot of rice seedlings under 12, 24, and 40 mg Cr/L treatments.
Table 2.
The contribution of inorganic and organic N alone and in combination to Pro content in shoot of rice seedlings under 12, 24, and 40 mg Cr/L treatments.
Table 3.
The contribution of inorganic and organic N alone and in combination to Pro content in root of rice seedlings under 12, 24, and 40 mg Cr/L treatments.
Table 3.
The contribution of inorganic and organic N alone and in combination to Pro content in root of rice seedlings under 12, 24, and 40 mg Cr/L treatments.
4. Discussion
The toxic effects of Cr in plants are already reported, such as delaying seed germination, inhibiting root growth, reducing plant height, changing the antioxidative enzyme activities, nutrient elements uptake, and amino acid content (Shanker et al., 2005). In recent years, many strategies have been proposed to curtail negative influence imposed by Cr pollution, wherein plant growth regulators (PGRs) are thought to be the most practical and cost-effective method used (Choudhary et al., 2011). However, often, great difficulties arise in the selection of PGRs for evaluating the efficiency of these PGRs used in the field trail (Lin et al., 2021). In this study, we investigated the effect of different nitrogenous compounds as substrates for synthesizing Pro in Cr(III)-treated rice seedlings by the MBMM. The innate level of Pro in plants is highly dependent on the plant’s growth conditions (Szabados and Savoure 2010). Previous study also demonstrated that the aerial part is the major site of Pro synthesis in rice plants (Stein et al., 2011). Herein, we observed that the accumulation of Pro in shoots of rice seedlings was dependent on the doses of Cr(III) exposure, which was highly regulated by the different N fertilizations. Accordingly, the accumulation of Pro in rice shoots due to application of different N sources was discussed.
Generally, plant growth hig hly depends on the forms of N present in plant growth media, the amount of N available, and the plant species (Cambui et al., 2011; Carlisle et al., 2012). Herein, we noticed that the responses of Pro content were different between organic N-fed and inorganic N-fed seedlings under Cr(III) exposure, indicating that the contribution of these N compounds to Pro content is different (Li et al., 2022). Notably, under ‘Cr(III)+(-NI)’ treatments, relatively higher Pro content was observed in ‘+NArg’-fed rice seedlings than ‘-No’-fed and ‘+NGlu’-fed rice seedlings, suggesting that fertilization of Arg is more effective than Glu on Pro synthesis in rice plant under Cr(III) stress. Regarding to ‘Cr(III)+(+NNO3-)’ and ‘Cr(III)+(+NNH4+)’ treatments, the Pro content in ‘-No’ and ‘+NArg’-fed rice seedlings significantly higher than that of ‘+NGlu’-fed seedlings. Moreover, fertilization of NO3- and NH4+ increased the Pro content in ‘-No’-fed rice seedlings, but reduced the Pro content in ‘+NArg’ and ‘+NGlu’-fed seedlings, suggesting that the utilization and conversion of these inorganic and organic N sources into Pro synthesis in Cr(III)-treated rice seedlings is different. Apparently, the content of Pro in both roots and shoots of rice seedlings under ‘Cr(III)+(+NNH4+)’ treatments was significantly higher (‘NNH4+’ > ‘NNO3-’ > ‘NArg’ > ‘NGlu’) than other treatments, suggesting that ‘NH4+’ is the preferred N source to contribute Pro synthesis in rice plants under Cr(III) stress. Therefore, Cr(III) exposure did not change the rice N preference because rice is an NH4+-like crop, the uptake rate and assimilation rate of NH4+ by rice plants is more rapidly than other N sources (Miller and Cramer 2005; Gaur et al., 2012; Thornton and Robinson 2015). Moreover, NH4+ is mainly assimilated in rice roots (Li et al., 2020), while NO3− is chiefly assimilated in rice shoots (Cao et al., 2018).
In addition, we found that the content of Pro increased in rice shoots under Cr(III) treatments with ‘+NNO3-’ or ‘+NNH4+’ supplied. However, the content of Pro decreased in rice shoots under “Cr(III)+(+NNO3-)” and “Cr(III)+(+NNH4+)” treatments with ‘+NArg’ or ‘+NGlu’ supplied. The MBMM also suggested that the relatively higher content of Pro in rice shoots treated with N source alone was higher than that of treated with N source in combination. This may be due to the (1) application of organic N sources influences the uptake and metabolism of inorganic N in rice seedlings; (2) application of organic N sources promotes the catabolism of Pro in rice seedlings. That is to say, the limiting steps of Pro metabolism in plants are different under Cr(III) stress with different N source supplied (Coque and Gallais 2006; Iqbal et al., 2015). In order to maintain the homeostasis of the internal environment, plants should rapidly metabolize the Pro to maintain the normal N levels against Cr(III) stress (Nanjo et al., 2003). In the future, more physiological, biochemical and molecular studies should be carried out to reveal the effects of different N sources on Pro synthesis under Cr(III) stress.
5. Conclusions
Present study provides a theoretical estimation for the contribution of organic and inorganic N alone and in combination on the Pro synthesis in rice seedlings under Cr(III) stress. Apparently, the content of Pro in rice seedlings depended on the doses of Cr(III) exposure and different N sources. ‘NNH4+’ application possesses the largest contribution to the content of Pro in rice tissues under Cr(III) stress, while ‘NArg’ application possesses the largest contribution to the content of Pro in rice roots under Cr(III) stress. ‘NNH4++NArg’ application contributed the largest to the content of Pro in both root and shoot of rice seedlings under Cr(III) stress. In contrast, ‘NNO3-+NGlu’ application contributed the least to the content of Pro in Cr(III)-treated rice seedlings. In conclusion, plants can regulate the content of Pro in plant tissues to cope with the potential threat induced by Cr(III) exposure under different nutritional N sources.
Author Contributions
Conceptualization, Methodology, Supervision, Writing-reviewing and Editing, and Funding acquisition, Xiao-Zhang Yu; Investigation, Data analysis, Visualization, and Software, Cheng-Zhi Li; Writing original draft preparation, Visualization, Yu-Xi Feng; Investigation and Software Peng Tian. All of the authors contributed to the final review of the manuscript.
Funding
This work is financially supported by the Natural Science Foundation of Guangxi (No. 2018GXNSFDA281024).
Ethics approval and consent to participate
The use of plant materials in the paper fully complied with our institutional guidelines and legislation.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.sd
Competing interests
The authors declare no conflicts of interest
References
- Cambui: C. A., Svennerstam, H., Gruffman, L., Nordin, A., Ganeteg, U., Näsholm, T., 2011. Patterns of plant biomass partitioning depend on nitrogen source. PLoS One 6, e19211. [CrossRef]
- Cao, X. C., Zhong, C., Zhu, C. Q., Zhu, L. F., Zhang, J. H., Wu, L. H., Jin, Q. Y., 2018. Ammonium uptake and metabolism alleviate PEG-induced water stress in rice seedlings. Plant Physiol. Bioch. 132, 128-137. [CrossRef]
- Carlisle, E., Myers, S., Raboy, V., Bloom, A., 2012. The effects of inorganic nitrogen form and CO2 concentration on wheat yield and nutrient accumulation and distribution. Front. Plant Sci. 3, 195. [CrossRef]
- Chai, M., Li, R., Shen, X., Tam, N. F. Y., Zan, Q., Li, R., 2018. Does ammonium nitrogen affect accumulation, subcellular distribution and chemical forms of cadmium in Kandelia obovata?. Ecotox. Environ. Safe. 162, 430-437. [CrossRef]
- Choudhary, S. P., Kanwar, M., Bhardwaj, R., Gupta, B. D., Gupta, R. K., 2011. Epibrassinolide ameliorates Cr (VI) stress via influencing the levels of indole-3-acetic acid, abscisic acid, polyamines and antioxidant system of radish seedlings. Chemosphere 84, 592-600. [CrossRef]
- Coque, M., Gallais, A., 2006. Genomic regions involved in response to grain yield selection at high and low nitrogen fertilization in maize. Theor. Appl. Genet. 112, 1205-1220. [CrossRef]
- Fan, W. J., Feng, Y. X., Li, Y. H., Lin, Y. J., Yu, X. Z., 2020. Unraveling genes promoting ROS metabolism in subcellular organelles of Oryza sativa in response to trivalent and hexavalent chromium. Sci. Total Environ. 744, 140951. [CrossRef]
- Feng, Y. X., Yu, X. Z., Mo, C. H., Lu, C. J., 2019. Regulation network of sucrose metabolism in response to trivalent and hexavalent chromium in Oryza sativa. J. Agr. Food Chem. 67, 9738-9748. [CrossRef]
- Ganesh, K. S., Baskaran, L., Rajasekaran, S., Sumathi, K., Chidambaram, A. L. A., Sundaramoorthy, P., 2008. Chromium stress induced alterations in biochemical and enzyme metabolism in aquatic and terrestrial plants. Colloid. Surfaces B, 63, 159-163. [CrossRef]
- Gaur, V. S., Singh, U. S., Gupta, A. K., Kumar, A., 2012. Understanding the differential nitrogen sensing mechanism in rice genotypes through expression analysis of high and low affinity ammonium transporter genes. Mol. Biol. Rep. 39, 2233-2241. [CrossRef]
- Huo, K., Shangguan, X., Xia, Y., Shen, Z., Chen, C., 2020. Excess copper inhibits the growth of rice seedlings by decreasing uptake of nitrate. Ecotox. Environ. Safe. 190, 110105. [CrossRef]
- Iqbal, N., Umar, S., Khan, N. A., 2015. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 178, 84-91. [CrossRef]
- Kaur, G., Asthir, B. J. B. P., 2015. Proline: a key player in plant abiotic stress tolerance. Biol. Plantarum 59, 609-619. [CrossRef]
- Kumar, S., Joshi, U. N., 2008. Nitrogen metabolism as affected by hexavalent chromium in sorghum (Sorghum bicolor L.). Environ. Exp. Bot. 64, 135-144. [CrossRef]
- Li, C. Z., Yang, L., Lin, Y. J., Zhang, H., Rad, S., Yu, X. Z., 2020. Assimilation of exogenous cyanide cross talk in Oryza sativa L. to the key nodes in nitrogen metabolism. Ecotoxicology 29, 1552-1564. [CrossRef]
- Li, Y. H., Tian, P., Li, C. Z., Yu, X. Z., 2022. Elucidating comportment of the glutamate and ornithine pathway on proline accumulation in rice under different nitrogenous nutrition. Int. J. Environ. Sci. Technol. 19, 2993-3000. [CrossRef]
- Lin Y.J., Feng Y.X., Li Y.H., Yu G., Yu X.Z. 2021. Fuzzy synthetic evaluation of the impact of plant growth regulators on the root phenotype traits of rice seedlings under thiocyanate stress. Plant Physiol. Biochem. 158, 182-189.
- Martins, M., Lopes, J., Sousa, B., Soares, C., Valente, I. M., Rodrigues, J. A., Fidalgo, F., Teixeira, J., 2021. Cr (VI)-induced oxidative damage impairs ammonia assimilation into organic forms in Solanum lycopersicum L. Plant Stress 2, 100034. [CrossRef]
- Miller, A. J., Cramer, M. D., 2005. Root nitrogen acquisition and assimilation. Plant Soil 274, 1-36. [CrossRef]
- Nanjo, T., Fujita, M., Seki, M., Kato, T., Tabata, S., Shinozaki, K., 2003. Toxicity of free proline revealed in an Arabidopsis T-DNA-tagged mutant deficient in proline dehydrogenase. Plant Cell Physiol. 44, 541-548. [CrossRef]
- Näsholm, T., Kielland, K., Ganeteg, U., 2009. Uptake of organic nitrogen by plants. New Phytol., 182, 31-48. [CrossRef]
- Pavlíková, D., Pavlík, M., Staszková, L., Tlustos, P., Száková, J., Balík, J., 2007. The effect of potentially toxic elements and sewage sludge on the activity of regulatory enzyme glutamate kinase. Plant Soil Environ. 53, 201.
- Sangwan, P., Kumar, V., Joshi, U. N., 2014. Effect of chromium (VI) toxicity on enzymes of nitrogen metabolism in clusterbean (Cyamopsis tetragonoloba L.). Enzym. Res. 2014,784036. [CrossRef]
- Shanker, A. K., Cervantes, C., Loza-Tavera, H., Avudainayagam, S., 2005. Chromium toxicity in plants. Environ. Int. 31, 739-753. [CrossRef]
- Singh, H. P., Mahajan, P., Kaur, S., Batish, D. R., Kohli, R. K., 2013. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 11, 229-254. [CrossRef]
- Stein, H., Honig, A., Miller, G., Erster, O., Eilenberg, H., Csonka, L. N., Sazbados, L., Koncz, C., Zilberstein, A., 2011. Elevation of free proline and proline-rich protein levels by simultaneous manipulations of proline biosynthesis and degradation in plants. Plant Sci. 181, 140-150. [CrossRef]
- Szabados, L., Savouré, A., 2010. Proline: a multifunctional amino acid. Trends Plant Sci. 15, 89-97. [CrossRef]
- Thornton, B., Robinson, D., 2005. Uptake and assimilation of nitrogen from solutions containing multiple N sources. Plant Cell Environ. 28, 813-821. [CrossRef]
- Verbruggen, N., Hermans, C., 2008 Proline accumulation in plants: a review. Amino Acids 35, 753-759. [CrossRef]
- Wyszkowski, M., Radziemska, M., 2010. Effects of chromium (III and VI) on spring barley and maize biomass yield and content of nitrogenous compounds. J. Toxicol. Environ. Health. A. 73, 1274-1282. [CrossRef]
- Yu, X. Z., Lin, Y. J., Fan, W. J., Lu, M. R., 2017. The role of exogenous proline in amelioration of lipid peroxidation in rice seedlings exposed to Cr (VI). Internat. Biodeter. Biodegr. 123, 106-112. [CrossRef]
- Zhang, Q., Feng, Y. X., Lin, Y. J., Yu, X. Z., 2022. Mathematical quantification of interactive complexity of transcription factors involved in proline-mediated regulative strategies in Oryza sativa under chromium stress. Plant Physiol. Biochem. 182, 36-44. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).