Submitted:
29 August 2023
Posted:
30 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Hand hygiene
1.2. Cold atmospheric plasma for hand disinfection
2. Materials and Methods
2.1. Plasma source and design
2.2. Microbiological testing
2.3. Microbial cultures
2.4. Pre-assays using Petri dishes
2.5. Disinfection assays using steel and silicone disks
3. Results
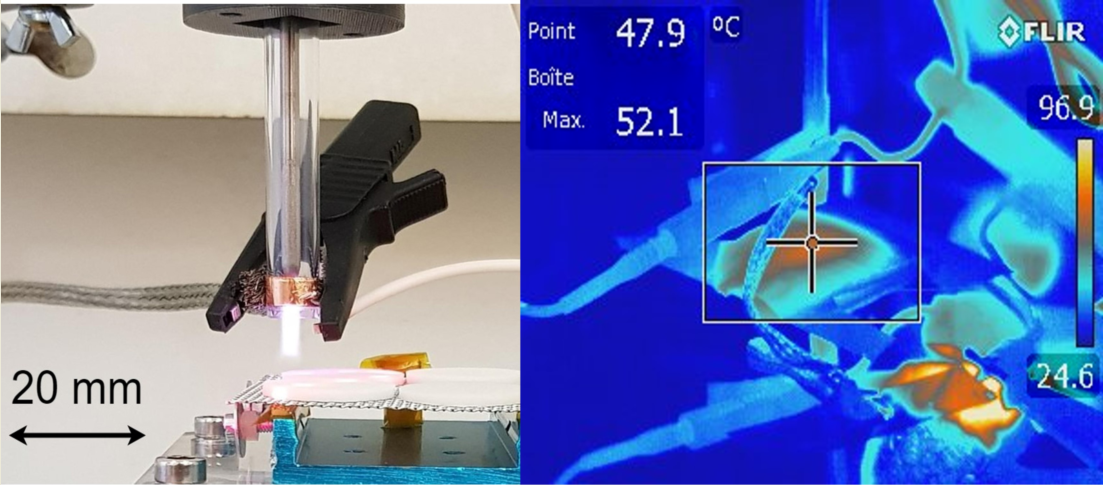
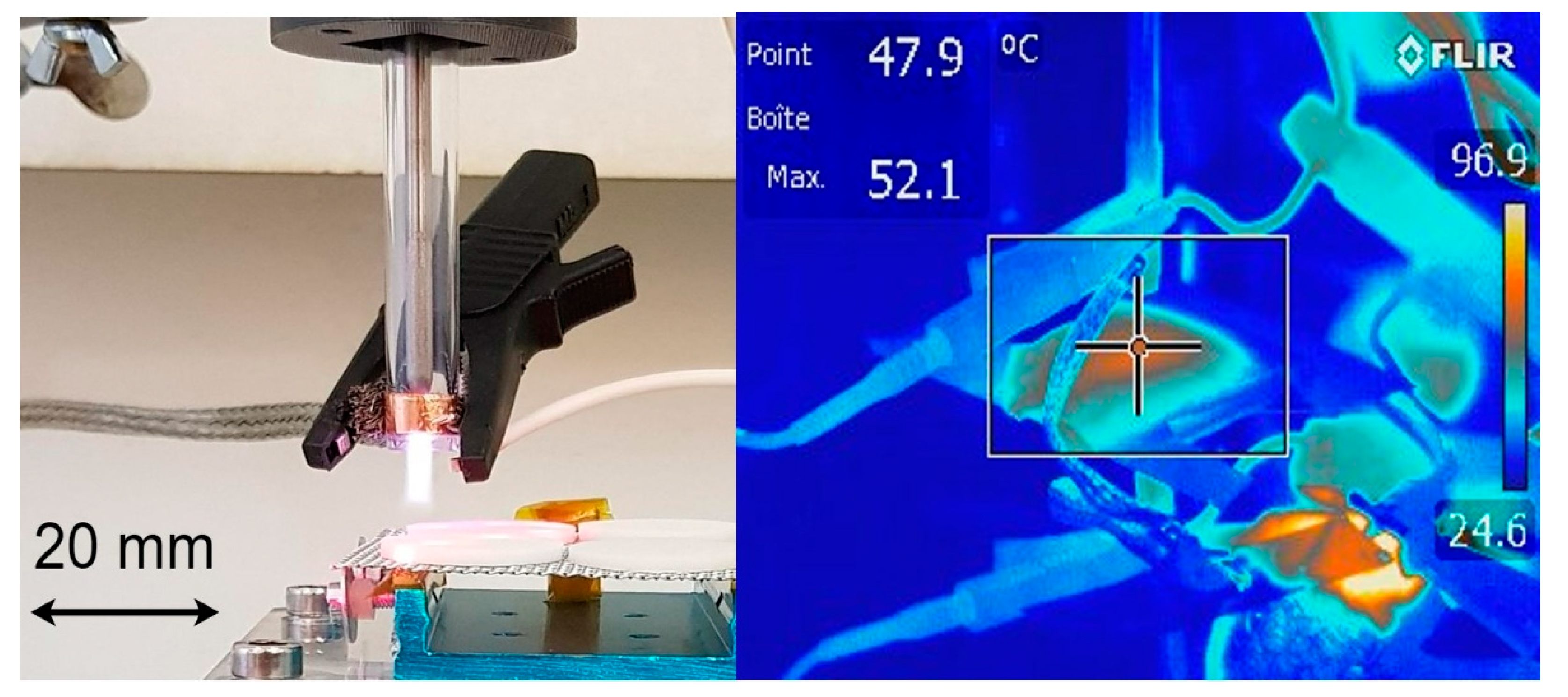
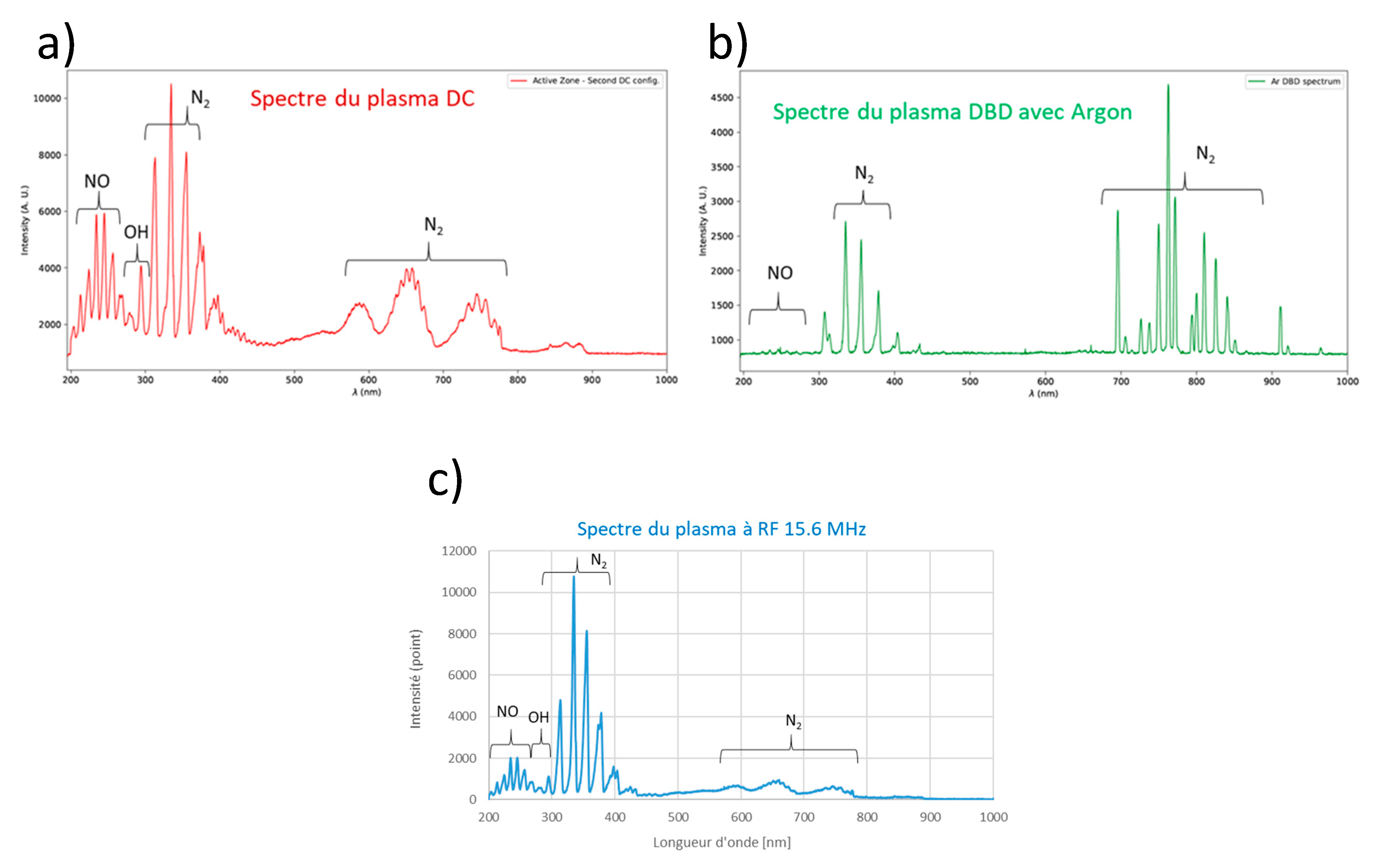
3.1. Plasma source and design
3.2. Pre-assays
3.2.1. The influence of dying methods on culture viability
3.2.2. DC plasma assays
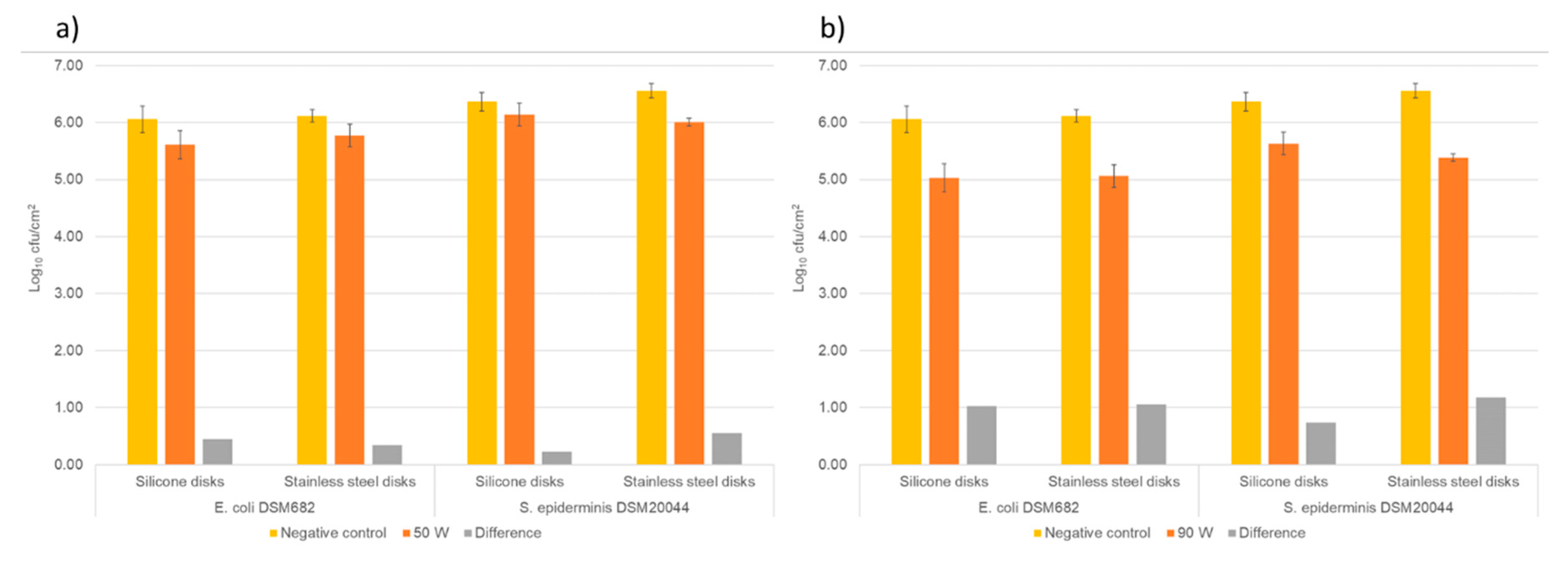
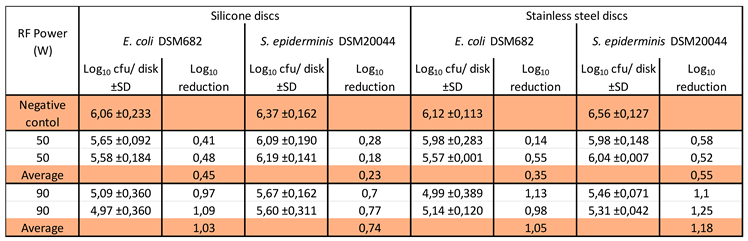
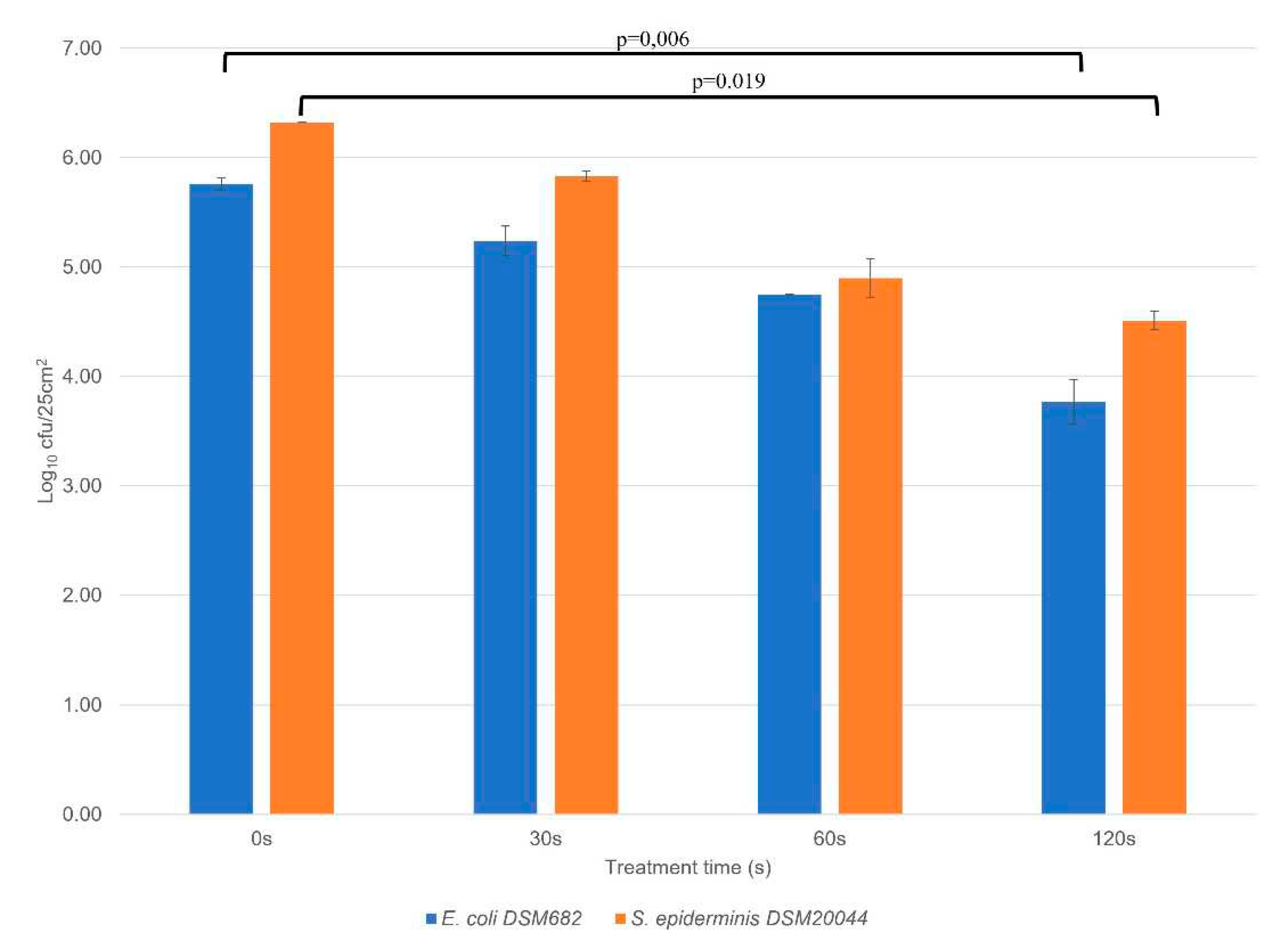
3.3. Disinfection assays using RF AIR CAP
 |
4. Discussion
4.1. RF Plasma mechanism of inactivation
4.2. Plasma integration into a hand dryer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Villa, C.; Russo, E. Hydrogels in Hand Sanitizers. Materials 2021, 14, 1577. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, R.E.; Gutierrez, D.; Peters, C.; Nichols, M.; Boles, B.R. Elucidation of bacteria found in car interiors and strategies to reduce the presence of potential pathogens. Biofouling 2014, 30, 337–346. [Google Scholar] [CrossRef]
- Mathur, P. Hand hygiene: Back to the basics of infection control. Indian J. Med Res. 2011, 134, 611–20. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, C.; Avraam, D.; Cueto-Felgueroso, L.; González, M.C.; Juanes, R. Hand-Hygiene Mitigation Strategies Against Global Disease Spreading through the Air Transportation Network. Risk Anal. 2019, 40, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Price, L.; Gozdzielewska, L.; Alejandre, J.C.; Jorgenson, A.; Stewart, E.; Pittet, D.; Reilly, J. Systematic review on factors influencing the effectiveness of alcohol-based hand rubbing in healthcare. Antimicrob. Resist. Infect. Control. 2022, 11, 1–22. [Google Scholar] [CrossRef]
- Breidablik, H.J.; Johannessen, L.; Andersen, J.R.; Søreide, H.; Kleiven, O.T. Effect of Optimal Alcohol-Based Hand Rub among Nurse Students Compared with Everyday Practice among Random Adults; Can Water-Based Hand Rub Combined with a Hand Dryer Machine Be an Alternative to Remove E. coli Contamination from Hands? Microorganisms 2023, 11, 325. [Google Scholar] [CrossRef]
- Plum, F.; Yüksel, Y.T.; Agner, T.; Nørreslet, L.B. Skin barrier function after repeated short-term application of alcohol-based hand rub following intervention with water immersion or occlusion. Contact Dermat. 2020, 83, 215–219. [Google Scholar] [CrossRef]
- Kivuti-Bitok, L.W.; Chepchirchir, A.; Waithaka, P.; Ngune, I. Dry Taps? A Synthesis of Alternative “Wash” Methods in the Absence of Water and Sanitizers in the Prevention of Coronavirus in Low-Resource Settings. J. Prim. Care Community Heal. 2020, 11. [Google Scholar] [CrossRef]
- Moura, I.B.; Ewin, D.; Wilcox, M.H. From the hospital toilet to the ward: A pilot study on microbe dispersal to multiple hospital surfaces following hand drying using a jet air dryer versus paper towels. Infect. Control. Hosp. Epidemiology 2021, 43, 241–244. [Google Scholar] [CrossRef]
- Menashi, W.P. 1968, Treatment of surfaces, USA, US3383163 A.
- Laroussi, M.; Bekeschus, S.; Keidar, M.; Bogaerts, A.; Fridman, A.; Lu, X.P.; Ostrikov, K.; Hori, M.; Stapelmann, K.; Miller, V.; Reuter, S.; Laux, C.; Mesbah, A.; Walsh, J.; Jiang, C.; Mededovic Thagard, S.; Tanaka, H.; Liu, D.W.; Yan, D.; Yusupov, M. Low-temperature plasma for biology, hygiene, and medicine: Perspective and roadmap. IEEE Transactions on Radiation and Plasma Medical Sciences arXiv:2108.03158, 2022. [CrossRef]
- Fridman, A. Plasma Biology and Plasma Medicine. In: Fridman A (ed.) Plasma Chemistry Cambridge: Cambridge University Press, 2009, 848-914. [CrossRef]
- Busco, G.; Robert, E.; Chettouh-Hammas, N.; Pouvesle, J.-M.; Grillon, C. The emerging potential of cold atmospheric plasma in skin biology. Free. Radic. Biol. Med. 2020, 161, 290–304. [Google Scholar] [CrossRef]
- Osman, I.; Ponukumati, A.; Vargas, M.; Bhakta, D.; Ozoglu, B.; Bailey, C. Plasma-Activated Vapor for Sanitization of Hands. Plasma Med. 2016, 6, 235–245. [Google Scholar] [CrossRef]
- Deng, X.L.; Nikiforov, A.Y.; Vanraes, P.; Leys, C. Direct current plasma jet at atmospheric pressure operating in nitrogen and air. J. Appl. Phys. 2013, 113. [Google Scholar] [CrossRef]
- Kassir, A.M.; Sonnard, J.; Roulin, L.; Baudin, M.; Courret, G.; Brück, W.M. Fast Prototyping for Atmospheric Plasma Sources Integration into Air Hand Dryers. International Journal of Chemical and Molecular Engineering 2022,16(12),2022. publications.waset.org/10012844/pdf.
- Qian, M.-Y.; Ren, C.-S.; Wang, D.-Z.; Fan, Q.-Q.; Nie, Q.-Y.; Wen, X.-Q.; Zhang, J.-L. Investigations on an Atmospheric Dielectric Barrier Discharge Plasma Jet With a Concentric Wire-Mesh Cylinder Electrode Configuration. IEEE Trans. Plasma Sci. 2011, 40, 1134–1141. [Google Scholar] [CrossRef]
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 47–60. [Google Scholar] [CrossRef]
- Chen, Z.; Garcia Jr, G.; Arumugaswami, V.; Wirz, R.E. Cold atmospheric plasma for SARS-CoV-2 inactivation. Physics of Fluids 2020, 32(11), 111702. [Google Scholar] [CrossRef]
- Anonymous (2019). EN 13697:2015+A1:2019. Chemical disinfectants and antiseptics, Quantitative non-porous surface test for the evaluation of bactericidal and/or fungicidal activity of chemical disinfectants used in food, industrial, domestic and institutional areas (phase 2/step 2)” Available at: https://connect.snv.ch/en/ (accessed 04 August 2021).
- Nachman, M.; Franklin, S. Artificial Skin Model simulating dry and moist in vivo human skin friction and deformation behaviour. Tribol. Int. 2016, 97, 431–439. [Google Scholar] [CrossRef]
- Franklin, S.E.; Baranowska, J.; Furgala, J. Friction of natural human, procine and synthetic skin. Proceedings 5th International Conference on Mechanics of Biomaterials and Tissues. Sitges, Spain, 8-12 Dec. 2013.
- Chen, Z.; Wirz, R. Cold Atmospheric Plasma for COVID-19. Preprints.org 2020, 2020040126. [Google Scholar] [CrossRef]
- Suva, Valeurs limites d’exposition aux postes de travail, 1903.f, www.suva.ch/1903.f, February 2021.
- Defrin, R.; Shachal-Shiffer, M.; Hadgadg, M.; Peretz, C. Quantitative Somatosensory Testing of Warm and Heat-Pain Thresholds: The Effect of Body Region and Testing Method. Clin. J. Pain 2006, 22, 130–136. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Huang, Y.; Liao, B.; Cheng, L.; Ren, B. Reactive Oxygen Species in Pathogen Clearance: The Killing Mechanisms, the Adaption Response, and the Side Effects. Front. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, R.; Tang, Z.; Liu, W.; He, C.; Xia, D. Reactive Nitrogen Species Mediated Inactivation of Pathogenic Microorganisms during UVA Photolysis of Nitrite at Surface Water Levels. Environ. Sci. Technol. 2022, 56, 12542–12552. [Google Scholar] [CrossRef]
- Waskow, A.; Betschart, J.; Butscher, D.; Oberbossel, G.; Klöti, D.; Büttner-Mainik, A.; Adamcik, J.; von Rohr, P.R.; Schuppler, M. Characterization of Efficiency and Mechanisms of Cold Atmospheric Pressure Plasma Decontamination of Seeds for Sprout Production. Front. Microbiol. 2018, 9, 3164. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, T.; Zou, L.; Wang, X.; Zhang, Y. Molecular dynamics simulations of membrane properties affected by plasma ROS based on the GROMOS force field. Biophys. Chem. 2019, 253, 106214. [Google Scholar] [CrossRef]
- Yahaya, A.G.; Kristof, J.; Blajan, M.; Mustafa, F.; Shimizu, K. Effect of Plasma Discharge on Epidermal Layer Structure in Pig Skin. Plasma Med. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





