Submitted:
30 August 2023
Posted:
31 August 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Flaxseeds proteins
Albumins of flaxseed
Globulins of flaxseed
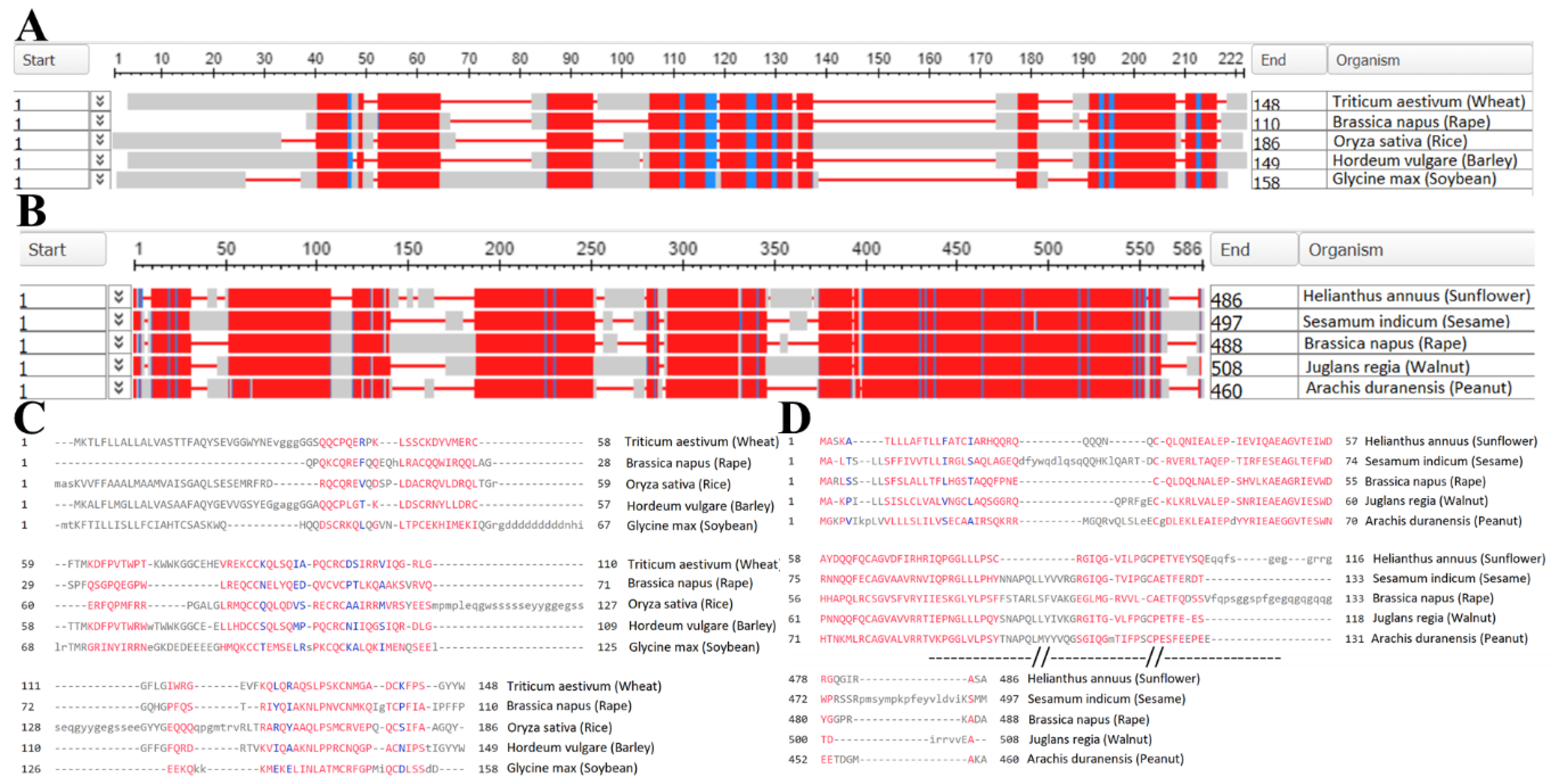
The proteoform level analysis of major proteins from flaxseed
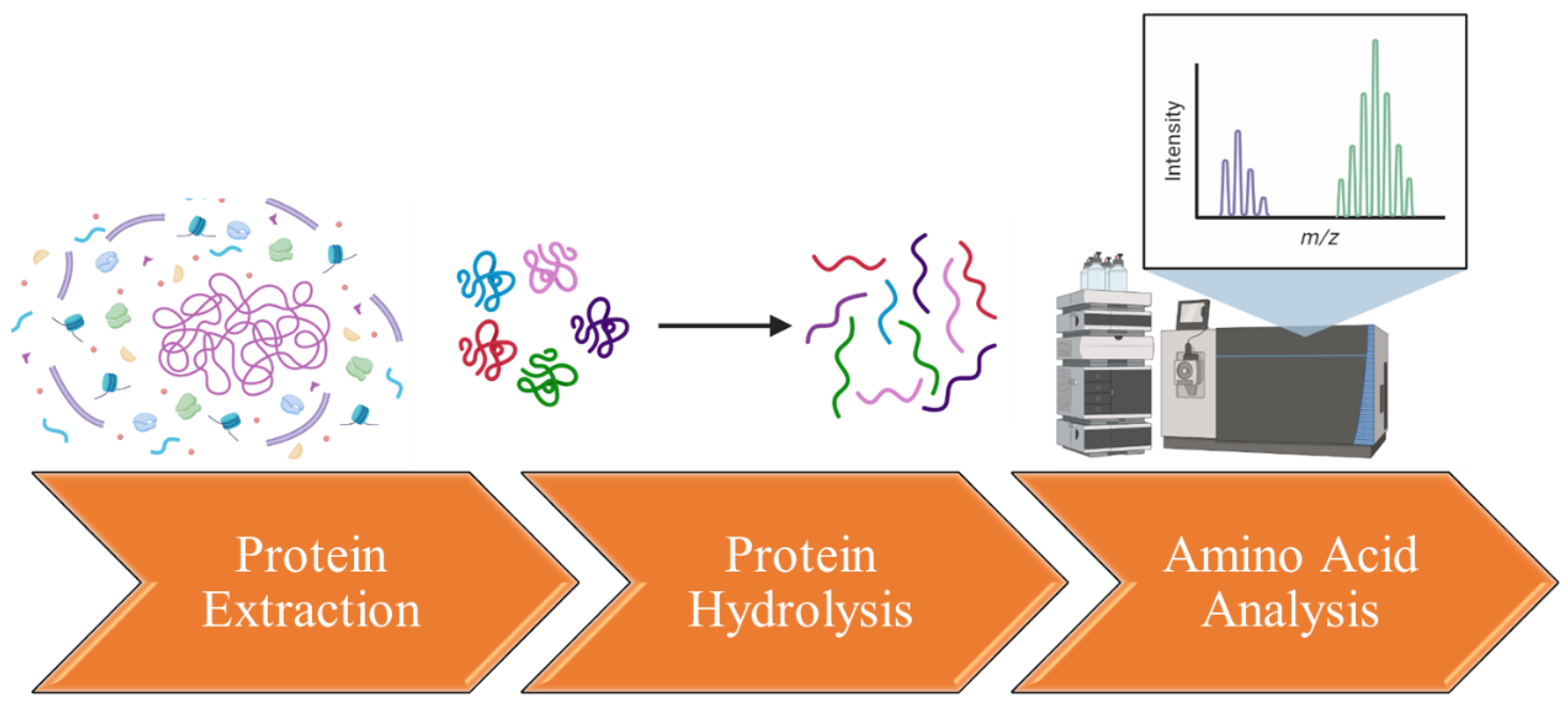
Extraction and characterization of flaxseed`s amino acids
| Amino Acid | Description | Structure | Composition from flaxseed proteins |
| Glutamic Acid | An aliphatic, acidic, conditionally non-essential, crystalline α-amino acid | 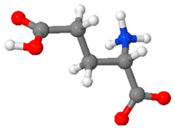 |
19-27 %[114,115,116] |
| Aspartic Acid | An aliphatic, acidic, non-essential, crystalline α-amino acid | 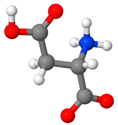 |
8-21 % [117,118,119] |
| Arginine | An aliphatic, non-aromatic, conditionally non-essential basic/cationic amino acid | 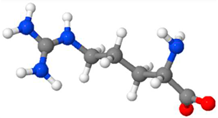 |
8-12 % [120,121,122] |
| Isoleucine | An aliphatic, non-polar, essential, crystalline α-amino acid | 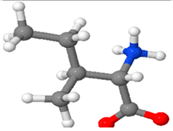 |
4-8 %[118,123,124] |
| Leucine | An aliphatic, non-polar, essential, crystalline α-amino acid | 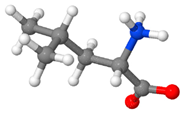 |
4-7 %[116,117,119,121,124] |
Presence of flaxseed proteome in databases
The sequencing and profiling of flaxseed proteins
The combined anti-cancer action of flaxseed proteins
Flaxseed proteome effect on anti-cancer radiotherapy
Further research
Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Merkher, Y.; Weihs, D. Proximity of Metastatic Cells Enhances Their Mechanobiological Invasiveness. Ann. Biomed. Eng. 2017, 45, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Merkher, Y.; Horesh, Y.; Abramov, Z.; Shleifer, G.; Ben-Ishay, O.; Kluger, Y.; Weihs, D. Rapid Cancer Diagnosis and Early Prognosis of Metastatic Risk Based on Mechanical Invasiveness of Sampled Cells. Ann. Biomed. Eng. 2020, 48, 2846–2858. [Google Scholar] [CrossRef]
- Merkher Yulia; Kontareva Elizaveta; Bogdan Elizaveta; Achkasov Konstantin; Grolman Joshua; Leonov Sergey Nanoparticle Cellular Endocytosis as Potential Prognostic Biomarker for Cancer Progression. FEBS Open Bio 2021, 11, 429–430. [CrossRef]
- Merkher, Y.; Kontareva, E.; Melekhova, A.; Leonov, S. Abstract PO-042: Nanoparticles Imaging for Cancer Metastasis Diagnosis. Clin. Cancer Res. 2021, 27, PO–042. [Google Scholar] [CrossRef]
- Weber, G.F. Molecular Mechanisms of Metastasis. Cancer Lett. 2008, 270, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, B.L.; Francis, P.A.; Parker, B.S.; Anderson, R.L. Strategies for the Discovery and Development of Therapies for Metastatic Breast Cancer. Nat. Rev. Drug Discov. 2012, 11, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Barney, L.E.; Jansen, L.; Polio, S.R.; Galarza, S.; Lynch, M.E.; Barney, L.E.; Jansen, L.; Polio, S.R.; Galarza, S.; Lynch, M.E.; et al. The Predictive Link between Matrix and Metastasis. Curr. Opin. Chem. Eng. 2016, 11, 85–93. [Google Scholar] [CrossRef]
- Van Zijl, F.; Krupitza, G.; Mikulits, W. Initial Steps of Metastasis: Cell Invasion and Endothelial Transmigration. Mutat. Res. - Rev. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef]
- Clark, A.G.; Vignjevic, D.M. Modes of Cancer Cell Invasion and the Role of the Microenvironment. Curr. Opin. Cell Biol. 2015, 36, 13–22. [Google Scholar] [CrossRef]
- Zustiak, S.; Nossal, R.; Sackett, D.L. Multiwell Stiffness Assay for the Study of Cell Responsiveness to Cytotoxic Drugs. Biotechnol. Bioeng. 2014, 111, 396. [Google Scholar] [CrossRef]
- Majcherek, D.; Weresa, M.A.; Ciecierski, C. A Cluster Analysis of Risk Factors for Cancer across EU Countries: Health Policy Recommendations for Prevention. Int. J. Environ. Res. Public Health 2021, 18, 8142. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Yust, M.M.; Pedroche, J.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Production of Ace Inhibitory Peptides by Digestion of Chickpea Legumin with Alcalase. Food Chem. 2003, 81, 363–369. [Google Scholar] [CrossRef]
- Bernacchia, R.; Preti, R.; Vinci, G. Chemical Composition and Health Benefits of Flaxseed. AUSTIN J. Nutr. FOOD Sci. 2022, 2(8), 1–9. [Google Scholar]
- Lowcock, E.C.; Cotterchio, M.; Boucher, B.A. Consumption of Flaxseed, a Rich Source of Lignans, Is Associated with Reduced Breast Cancer Risk. Cancer Causes Control 2013, 24, 813–816. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.E.; Hootman, K.C.; Weaver, A.M.; Thompson, L.U.; Morrison, C.; Hwang, H.; Edge, S.B.; Ambrosone, C.B.; Horvath, P.J.; Kulkarni, S.A. Dietary Intakes of Total and Specific Lignans Are Associated with Clinical Breast Tumor Characteristics. J. Nutr. 2012, 142, 91. [Google Scholar] [CrossRef]
- Freitas, R.D.S.; Campos, M.M. Protective Effects of Omega-3 Fatty Acids in Cancer-Related Complications. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- D’Eliseo, D.; Velotti, F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J. Clin. Med. 2016, 5. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Pruteanu, L.L.; Bailey, D.S.; Grădinaru, A.C.; Jäntschi, L. The Biochemistry and Effectiveness of Antioxidants in Food, Fruits, and Marine Algae. Antioxidants 2023, Vol. 12, Page 860 2023, 12, 860. [Google Scholar] [CrossRef]
- Pal, P.; Hales, K.; Petrik, J.; Hales, D.B. Pro-Apoptotic and Anti-Angiogenic Actions of 2-Methoxyestradiol and Docosahexaenoic Acid, the Biologically Derived Active Compounds from Flaxseed Diet, in Preventing Ovarian Cancer. J. Ovarian Res. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Kajla, P.; Goyal, N.; Bangar, S.P.; Chaudhary, V.; Lorenzo, J.M. Flaxseed Proteins (Linum Usitassimum): Thermal, Functional and Spectroscopic Characterization. Food Anal. Methods 2023, 16, 459–467. [Google Scholar] [CrossRef]
- Mueed, A.; Madjirebaye, P.; Shibli, S.; Deng, Z. Flaxseed Peptides and Cyclolinopeptides: A Critical Review on Proteomic Approaches, Biological Activity, and Future Perspectives. J. Agric. Food Chem. 2022, 70, 14600–14612. [Google Scholar] [CrossRef]
- Sammour, R.H. Proteins of Linseed (Linum Usitatissimum L.), Extraction and Characterization by Electrophoresis. Bot. Bull. Acad. Sin. 1999. [Google Scholar]
- Ayad, A.A. Characterization and Properties of Flaxseed Protein Fractions, 2010.
- Singh, K.K.; Mridula, D.; Rehal, J.; Barnwal, P. Flaxseed: A Potential Source of Food, Feed and Fiber. 2011, 51, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Saini, C.S. Amino Acid Composition, Nutritional Profiling, Mineral Content and Physicochemical Properties of Protein Isolate from Flaxseeds (Linum Usitatissimum). J. Food Meas. Charact. 2022, 16, 829–839. [Google Scholar] [CrossRef]
- Peng, D.; Ye, J.; Jin, W.; Yang, J.; Geng, F.; Deng, Q. A Review on the Utilization of Flaxseed Protein as Interfacial Stabilizers for Food Applications. JAOCS, J. Am. Oil Chem. Soc. 2022, 99, 723–737. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G. Flaxseed Proteins—a Review. Food Chem. 1993, 48, 109–114. [Google Scholar] [CrossRef]
- Arntfield, S.D. Proteins from Oil-Producing Plants. Proteins Food Process. Second Ed. 2018, 187–221. [Google Scholar] [CrossRef]
- Xie, M.; Liu, D.; Yang, Y. Anti-Cancer Peptides: Classification, Mechanism of Action, Reconstruction and Modification. Open Biol. 2020, 10. [Google Scholar] [CrossRef]
- Fillería, S.G.; Nardo, A.E.; Paulino, M.; Tironi, V. Peptides Derived from the Gastrointestinal Digestion of Amaranth 11S Globulin: Structure and Antioxidant Functionality. Food Chem. Mol. Sci. 2021, 3, 100053. [Google Scholar] [CrossRef]
- Taghizadeh, S.F.; Rezaee, R.; Mehmandoust, M.; Badibostan, H.; Karimi, G. Assessment of in Vitro Bioactivities of Pis v 1 (2S Albumin) and Pis v 2.0101 (11S Globulin) Proteins Derived from Pistachio (Pistacia Vera L.). J. Food Meas. Charact. 2020, 14, 1054–1063. [Google Scholar] [CrossRef]
- Sandoval-Sicairos, E.S.; Milán-Noris, A.K.; Luna-Vital, D.A.; Milán-Carrillo, J.; Montoya-Rodríguez, A. Anti-Inflammatory and Antioxidant Effects of Peptides Released from Germinated Amaranth during in Vitro Simulated Gastrointestinal Digestion. Food Chem. 2021, 343, 128394. [Google Scholar] [CrossRef]
- Martínez, J.H.; Velázquez, F.; Burrieza, H.P.; Martínez, K.D.; Paula Domínguez Rubio, A.; dos Santos Ferreira, C.; del Pilar Buera, M.; Pérez, O.E. Betanin Loaded Nanocarriers Based on Quinoa Seed 11S Globulin. Impact on the Protein Structure and Antioxidant Activity. Food Hydrocoll. 2019, 87, 880–890. [Google Scholar] [CrossRef]
- Luo, X.; Wu, W.; Feng, L.; Treves, H.; Ren, M. Short Peptides Make a Big Difference: The Role of Botany-Derived Amps in Disease Control and Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 11363. [Google Scholar] [CrossRef]
- Paterson, S.; Fernández-Tomé, S.; Hernández-Ledesma, B. Modulatory Effects of a Lunasin-Enriched Soybean Extract on Immune Response and Oxidative Stress-Associated Biomarkers. Biol. Life Sci. Forum 2022, Vol. 12, Page 10 2022, 12, 10. [Google Scholar] [CrossRef]
- Caponio, G.R.; Wang, D.Q.H.; Di Ciaula, A.; De Angelis, M.; Portincasa, P. Regulation of Cholesterol Metabolism by Bioactive Components of Soy Proteins: Novel Translational Evidence. Int. J. Mol. Sci. 2021, 22, 1–18. [Google Scholar] [CrossRef]
- Capraro, J.; De Benedetti, S.; Heinzl, G.C.; Scarafoni, A.; Magni, C. Bioactivities of Pseudocereal Fractionated Seed Proteins and Derived Peptides Relevant for Maintaining Human Well-Being. Int. J. Mol. Sci. 2021, 22, 3543. [Google Scholar] [CrossRef]
- Ateeq, M.; Adeel, M.M.; Kanwal, A.; Qamar, M.T.U.; Saeed, A.; Khaliq, B.; Saeed, Q.; Atiq, M.N.; Bilal, M.; Alharbi, M.; et al. In Silico Analysis and Functional Characterization of Antimicrobial and Insecticidal Vicilin from Moth Bean (Vigna Aconitifolia (Jacq.) Marechal) Seeds. Molecules 2022, 27, 3251. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, Y.Y.; Wu, C.T.; Yu, C.C.; Liao, H.F. Prolamin, a Rice Protein, Augments Anti-Leukaemia Immune Response. J. Cereal Sci. 2010, 51, 189–197. [Google Scholar] [CrossRef]
- Ji, Z.; Mao, J.; Chen, S.; Mao, J. Antioxidant and Anti-Inflammatory Activity of Peptides from Foxtail Millet (Setaria Italica) Prolamins in HaCaT Cells and RAW264.7 Murine Macrophages. Food Biosci. 2020, 36, 100636. [Google Scholar] [CrossRef]
- Jimenez-Pulido, I.J.; Daniel, R.; Perez, J.; Martínez-Villaluenga, C.; De Luis, D.; Martín Diana, A.B. Impact of Protein Content on the Antioxidants, Anti-Inflammatory Properties and Glycemic Index of Wheat and Wheat Bran. Foods 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-de la Paz, S.; Rodriguez-Martin, N.M.; Villanueva, A.; Pedroche, J.; Cruz-Chamorro, I.; Millan, F.; Millan-Linares, M.C. Evaluation of Anti-Inflammatory and Atheroprotective Properties of Wheat Gluten Protein Hydrolysates in Primary Human Monocytes. Foods 2020, 9, 854. [Google Scholar] [CrossRef] [PubMed]
- Aqeel, T.; Gurumallu, S.C.; Bhaskar, A.; Hashimi, S.M.; Lohith, N.C.; Javaraiah, R. Protective Role of Flaxseed Lignan Secoisolariciresinol Diglucoside against Lead-Acetate-Induced Oxidative-Stress-Mediated Nephrotoxicity in Rats. Phytomedicine Plus 2021, 1, 100038. [Google Scholar] [CrossRef]
- Rajesha, J.; Ranga Rao, A.; Madhusudhan, B.; Karunakumar, M. Antibacterial Properties of Secoisolariciresinol Diglucoside Isolated from Indian Flaxseed Cultivars. Curr. Trends Biotechnol. Pharm. 2010, 4, 551–560. [Google Scholar]
- Nguyen, N.P.T.; Cong, T.L.; Tran, T.T.H.; Do, B.N.; Nguyen, S.T.; Vu, B.T.; Nguyen, L.H.T.; Van Ngo, M.; Dinh, H.T.; Huy, H.D.; et al. Lower Plasma Albumin, Higher White Blood Cell Count and High-Sensitivity C-Reactive Protein Are Associated with Femoral Artery Intima-Media Thickness Among Newly Diagnosed Patients with Type 2 Diabetes Mellitus. Int. J. Gen. Med. 2022, 15, 2715–2725. [Google Scholar] [CrossRef]
- Lessomo, F.Y.N.; Fan, Q.; Wang, Z.Q.; Mukuka, C. The Relationship between Leukocyte to Albumin Ratio and Atrial Fibrillation Severity. BMC Cardiovasc. Disord. 2023, 23, 1–8. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G. Pretreatment Serum Albumin as a Predictor of Cancer Survival: A Systematic Review of the Epidemiological Literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef]
- Nazha, B.; Moussaly, E.; Zaarour, M.; Weerasinghe, C.; Azab, B. Hypoalbuminemia in Colorectal Cancer Prognosis: Nutritional Marker or Inflammatory Surrogate? World J. Gastrointest. Surg. 2015, 7, 370. [Google Scholar] [CrossRef]
- Van De Wouw, J.; Joles, J.A. Albumin Is an Interface between Blood Plasma and Cell Membrane, and Not Just a Sponge. Clin. Kidney J. 2022, 15, 624–634. [Google Scholar] [CrossRef]
- Cho, H.; Jeon, S.I.; Ahn, C.H.; Shim, M.K.; Kim, K. Emerging Albumin-Binding Anticancer Drugs for Tumor-Targeted Drug Delivery: Current Understandings and Clinical Translation. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Z.; Yu, S.; Chen, S.; Zhou, Y.; Qu, Y.; Xu, P.; Jiang, L.; Yuan, C.; Huang, M. Albumin-Based Drug Carrier Targeting Urokinase Receptor for Cancer Therapy. Int. J. Pharm. 2023, 634. [Google Scholar] [CrossRef] [PubMed]
- Ranaivo, H.R.; Hodge, J.N.; Choi, N.; Wainwright, M.S. Albumin Induces Upregulation of Matrix Metalloproteinase-9 in Astrocytes via MAPK and Reactive Oxygen Species-Dependent Pathways. J. Neuroinflammation 2012, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- von Au, A.; Vasel, M.; Kraft, S.; Sens, C.; Hackl, N.; Marx, A.; Stroebel, P.; Hennenlotter, J.; Todenhöfer, T.; Stenzl, A.; et al. Circulating Fibronectin Controls Tumor Growth. Neoplasia (United States) 2013, 15, 925–938. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, Y.; Wu, A.; Rao, Y.; Huang, Y. Roles of Albumin-Binding Proteins in Cancer Progression and Biomimetic Targeted Drug Delivery. ChemBioChem 2018, 19, 1796–1805. [Google Scholar] [CrossRef]
- Givant-Horwitz, V.; Davidson, B.; Reich, R. Laminin-Induced Signaling in Tumor Cells. Cancer Lett. 2005, 223, 1–10. [Google Scholar] [CrossRef]
- Radovic, R.S.; Maksimovic, R. V.; Brkljacic, M.J.; Varkonji Gasic, I.E.; Savic, P.A. 2S Albumin from Buckwheat (Fagopyrum Esculentum Moench) Seeds. J. Agric. Food Chem. 1999, 47, 1467–1470. [Google Scholar] [CrossRef]
- Souza, P.F.N. The Forgotten 2S Albumin Proteins: Importance, Structure, and Biotechnological Application in Agriculture and Human Health. Int. J. Biol. Macromol. 2020, 164, 4638–4649. [Google Scholar] [CrossRef]
- Bueno-Díaz, C.; Martín-Pedraza, L.; Parrón, J.; Cuesta-Herranz, J.; Cabanillas, B.; Pastor-Vargas, C.; Batanero, E.; Villalba, M. Characterization of Relevant Biomarkers for the Diagnosis of Food Allergies: An Overview of the 2s Albumin Family. Foods 2021, 10. [Google Scholar] [CrossRef]
- Khan, S.; Ali, S.A.; Yasmin, T.; Ahmed, M.; Khan, H. Purification and Characterization of 2S Albumin from Nelumbo Nucifera. Biosci. Biotechnol. Biochem. 2016, 80, 2109–2114. [Google Scholar] [CrossRef]
- Shidal, C.; Inaba, J.I.; Yaddanapudi, K.; Davis, K.R. The Soy-Derived Peptide Lunasin Inhibits Invasive Potential of Melanoma Initiating Cells. Oncotarget 2017, 8, 25525–25541. [Google Scholar] [CrossRef] [PubMed]
- Vuyyuri, S.B.; Shidal, C.; Davis, K.R. Development of the Plant-Derived Peptide Lunasin as an Anticancer Agent. Curr. Opin. Pharmacol. 2018, 41, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Seber, L.E.; Barnett, B.W.; McConnell, E.J.; Hume, S.D.; Cai, J.; Boles, K.; Davis, K.R. Scalable Purification and Characterization of the Anticancer Lunasin Peptide from Soybean. PLoS One 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Dia, V.P.; Gonzalez de Mejia, E. Lunasin Potentiates the Effect of Oxaliplatin Preventing Outgrowth of Colon Cancer Metastasis, Binds to α 5β 1 Integrin and Suppresses FAK/ERK/NF-ΚB Signaling. Cancer Lett. 2011, 313, 167–180. [Google Scholar] [CrossRef]
- Fernández-tomé, S.; Xu, F.; Han, Y.; Hernández-ledesma, B.; Xiao, H. Inhibitory Effects of Peptide Lunasin in Colorectal Cancer Hct-116 Cells and Their Tumorsphere- Derived Subpopulation. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Dia, V.P.; De Mejia, E.G. Lunasin Induces Apoptosis and Modifies the Expression of Genes Associated with Extracellular Matrix and Cell Adhesion in Human Metastatic Colon Cancer Cells. Mol. Nutr. Food Res. 2011, 55, 623–634. [Google Scholar] [CrossRef]
- Jiang, Q.; Pan, Y.; Cheng, Y.; Li, H.; Liu, D.; Li, H. Lunasin Suppresses the Migration and Invasion of Breast Cancer Cells by Inhibiting Matrix Metalloproteinase-2/-9 via the FAK/Akt/ERK and NF-ΚB Signaling Pathways. Oncol. Rep. 2016, 36, 253–262. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Gehlot, P. Inflammation and Cancer: How Friendly Is the Relationship for Cancer Patients? Curr. Opin. Pharmacol. 2009, 9, 351–369. [Google Scholar] [CrossRef]
- Wan, X.; Liu, H.; Sun, Y.; Zhang, J.; Chen, X.; Chen, N. Lunasin: A Promising Polypeptide for the Prevention and Treatment of Cancer. Oncol. Lett. 2017, 13, 3997–4001. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Hsieh, C.C.; de Lumen, B.O. Antioxidant and Anti-Inflammatory Properties of Cancer Preventive Peptide Lunasin in RAW 264.7 Macrophages. Biochem. Biophys. Res. Commun. 2009, 390, 803–808. [Google Scholar] [CrossRef]
- Vuyyuri, S.B.; Shidal, C.; Davis, K.R. Development of the Plant-Derived Peptide Lunasin as an Anticancer Agent. Curr. Opin. Pharmacol. 2018, 41, 27–33. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.J.; Devapatla, B.; Yaddanapudi, K.; Davis, K.R. The Soybean-Derived Peptide Lunasin Inhibits Non-Small Cell Lung Cancer Cell Proliferation by Suppressing Phosphorylation of the Retinoblastoma Protein. Oncotarget 2015, 6, 4649–4662. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Hernández-Ledesma, B.; de Lumen, B.O. Lunasin, a Novel Seed Peptide, Sensitizes Human Breast Cancer MDA-MB-231 Cells to Aspirin-Arrested Cell Cycle and Induced Apoptosis. Chem. Biol. Interact. 2010, 186, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Huang, A.H.C. OCCURRENCE OF LOW MOLECULAR WEIGHT AND HIGH CYSTEINE CONTAINING ALBUMIN STORAGE PROTEINS IN OILSEEDS OF DIVERSE SPECIES. Am. J. Bot. 1981, 68, 44–48. [Google Scholar] [CrossRef]
- Madhusudhan, K.T.; Singh, N. Isolation and Characterization of a Small Molecular Weight Protein of Linseed Meal. Phytochemistry 1985, 24, 2507–2509. [Google Scholar] [CrossRef]
- Dev, D.K.; Sienkiewicz, T. Isolation and Subunit Composition of 11 S Globulin of Linseed (Linum Usitatissimum L.). Food / Nahrung 1987, 31, 767–769. [Google Scholar] [CrossRef]
- El-Saadany, A.S.; Hanafy, M.M.; Elkomy, A.E. Flaxseed and Agnus-Castuson Vitex as a Source of Phytoestrogens and Their Impact on Productive Performance, Some Blood Constituents, and Blood Oestradiol Profile of Aged Laying Hens. Ital. J. Anim. Sci. 2022, 21, 821–830. [Google Scholar] [CrossRef]
- Singh, A.; Meena, M.; Kumar, D.; Dubey, A.K.; Hassan, M.I. Structural and Functional Analysis of Various Globulin Proteins from Soy Seed. Crit. Rev. Food Sci. Nutr. 2015, 55, 1491–1502. [Google Scholar] [CrossRef]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine Kinase – Role and Significance in Cancer. Int. J. Med. Sci. 2012, 1, 101–115. [Google Scholar] [CrossRef]
- Bochnak-Niedźwiecka, J.; Szymanowska, U.; Kapusta, I.; Świeca, M. Antioxidant Content and Antioxidant Capacity of the Protein-Rich Powdered Beverages Enriched with Flax Seeds Gum. Antioxidants 2022, 11, 582. [Google Scholar] [CrossRef]
- Yousif, A.N. Effect of Flaxseed on Some Hormonal Profile and Genomic DNA Concentration in Karadi Lambs. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing, November 1 2019; Vol. 388; p. 012035. [Google Scholar]
- Nowak, D.A.; Snyder, D.C.; Brown, A.J.; Demark-Wahnefried, W. The Effect of Flaxseed Supplementation on Hormonal Levels Associated with Polycystic Ovarian Syndrome: A Case Study. Curr. Top. Nutraceutical Res. 2007, 5, 177–181. [Google Scholar] [PubMed]
- Chang, V.C.; Cotterchio, M.; Boucher, B.A.; Jenkins, D.J.A.; Mirea, L.; McCann, S.E.; Thompson, L.U. Effect of Dietary Flaxseed Intake on Circulating Sex Hormone Levels among Postmenopausal Women: A Randomized Controlled Intervention Trial. Nutr. Cancer 2019, 71, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, C.F.; Aluko, R.E. Physicochemical and Functional Properties of 2S, 7S, and 11S Enriched Hemp Seed Protein Fractions. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Kanamori, J.; Masuda, T.; Yagasaki, K.; Kitamura, K.; Mikami, B.; Utsumi, S. Crystal Structure of Soybean 11S Globulin: Glycinin A3B4 Homohexamer. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 7395–7400. [Google Scholar] [CrossRef]
- Quiroga, A. V.; Barrio, D.A.; Añón, M.C. Amaranth Lectin Presents Potential Antitumor Properties. LWT 2015, 60, 478–485. [Google Scholar] [CrossRef]
- Mora-Escobedo, R.; Robles-Ramírez, M. del C.; Ramón-Gallegos, E.; Reza-Alemán, R. Effect of Protein Hydrolysates from Germinated Soybean on Cancerous Cells of the Human Cervix: An In Vitro Study. Plant Foods Hum. Nutr. 2009, 64, 271–278. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Z.; Xie, W.; Peng, C.; Ding, H.; Li, Y.; Feng, S.; Wang, X.; Zhao, C.; Wu, J. 11S Glycinin Up-Regulated NLRP-3-Induced Pyroptosis by Triggering Reactive Oxygen Species in Porcine Intestinal Epithelial Cells. Front. Vet. Sci. 2022, 9, 677. [Google Scholar] [CrossRef]
- Silva-Sánchez, C.; Barba De La Rosa, A.P.; León-Galván, M.F.; De Lumen, B.O.; De León-Rodríguez, A.; González De Mejía, E. Bioactive Peptides in Amaranth (Amaranthus Hypochondriacus) Seed. J. Agric. Food Chem. 2008, 56, 1233–1240. [Google Scholar] [CrossRef]
- Zou, X.G.; Hu, J.N.; Li, J.; Yang, J.Y.; Du, Y.X.; Yu, Y.F.; Deng, Z.Y. ICellular Uptake of [1–9-NαC]-Linusorb B2 and [1–9-NαC]-Linusorb B3 Isolated from Flaxseed, and Their Antitumor Activities in Human Gastric SGC-7901 Cells. J. Funct. Foods 2018, 48, 692–703. [Google Scholar] [CrossRef]
- Zou, X.G.; Hu, J.N.; Wang, M.; Du, Y.X.; Li, J.; Mai, Q.Y.; Deng, Z.Y. [1–9-NαC]-Linusorb B2 and [1–9-NαC]-Linusorb B3 Isolated from Flaxseed Induce G1 Cell Cycle Arrest on SGC-7901 Cells by Modulating the AKT/JNK Signaling Pathway. J. Funct. Foods 2019, 52, 332–339. [Google Scholar] [CrossRef]
- Okinyo-Owiti, D.P.; Dong, Q.; Ling, B.; Jadhav, P.D.; Bauer, R.; Maley, J.M.; Reaney, M.J.T.; Yang, J.; Sammynaiken, R. Evaluating the Cytotoxicity of Flaxseed Orbitides for Potential Cancer Treatment. Toxicol. Reports 2015, 2, 1014–1018. [Google Scholar] [CrossRef]
- Badley, R.A.; Atkinson, D.; Hauser, H.; Oldani, D.; Green, J.P.; Stubbs, J.M. The Structure, Physical and Chemical Properties of the Soy Bean Protein Glycinin. BBA - Protein Struct. 1975, 412, 214–228. [Google Scholar] [CrossRef]
- Gavage, M.; Van Vlierberghe, K.; Van Poucke, C.; De Loose, M.; Gevaert, K.; Dieu, M.; Renard, P.; Arnould, T.; Gillard, N. High-Resolution Mass Spectrometry-Based Selection of Peanut Peptide Biomarkers Considering Food Processing and Market Type Variation. Food Chem. 2020, 304, 125428. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Jin, Y.; Cerny, R.; Ballmer-Weber, B.; Goodman, R.E. Combining 2-DE Immunoblots and Mass Spectrometry to Identify Putative Soybean (Glycine Max) Allergens. Food Chem. Toxicol. 2018, 116, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Magni, C.; Scarafoni, A.; Herndl, A.; Sessa, F.; Prinsi, B.; Espen, L.; Duranti, M. Combined 2D Electrophoretic Approaches for the Study of White Lupin Mature Seed Storage Proteome. Phytochemistry 2007, 68, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Duranti, M.; Horstmann, C.; Gilroy, J.; Croy, R.R.D. The Molecular Basis for N-Glycosylation in the 11S Globulin (Legumin) of Lupin Seed. J. Protein Chem. 1995, 14, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Romero-Rodríguez, M.C.; Maldonado-Alconada, A.M.; Valledor, L.; Jorrin-Novo, J. V. Back to Osborne. Sequential Protein Extraction and Lc-Ms Analysis for the Characterization of the Holm Oak Seed Proteome. Methods Mol. Biol. 2014, 1072, 379–389. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Teixeira, A.R.; Ferreira, R.B. Characterization of Globulins from Common Vetch (Vicia Sativa L.). J. Agric. Food Chem. 2004, 52, 4913–4920. [Google Scholar] [CrossRef]
- Amponsah, A.; Nayak, B. Evaluation of the Efficiency of Three Extraction Conditions for the Immunochemical Detection of Allergenic Soy Proteins in Different Food Matrices. J. Sci. Food Agric. 2018, 98, 2378–2384. [Google Scholar] [CrossRef]
- Vaintraub, I.A.; Bassüner, R.; Shutov, A.D. The Action of Trypsin and Chymotrypsin on the Reserve Proteins of Some Leguminous Seeds. Food / Nahrung 1976, 20, 763–771. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Adebiyi, A.P.; Doyen, A.; Li, H.; Bazinet, L.; Aluko, R.E. Low Molecular Weight Flaxseed Protein-Derived Arginine-Containing Peptides Reduced Blood Pressure of Spontaneously Hypertensive Rats Faster than Amino Acid Form of Arginine and Native Flaxseed Protein. Food Chem. 2012, 132, 468–475. [Google Scholar] [CrossRef]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-Inhibitory Activity of Enzymatic Protein Hydrolysates from Lupin and Other Legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [CrossRef]
- Ingkaninan, K.; De Best, C.M.; Van Der Heijden, R.; Hofte, A.J.P.; Karabatak, B.; Irth, H.; Tjaden, U.R.; Van Der Greef, J.; Verpoorte, R. High-Performance Liquid Chromatography with on-Line Coupled UV, Mass Spectrometric and Biochemical Detection for Identification of Acetylcholinesterase Inhibitors from Natural Products. J. Chromatogr. A 2000, 872, 61–73. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, S.; Nagarajan, K. Glutamic Acid as Anticancer Agent: An Overview. Saudi Pharm. J. 2013, 21, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, Y.; Li, Y.; Xu, Y.; Song, W. Poly(Glutamic Acid)-Engineered Nanoplatforms for Enhanced Cancer Phototherapy. Curr. Drug Deliv. 2023, 20. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Yamamoto, K.; Sato, Y.; Inoue, S.; Morinaga, T.; Hirano, E. Combination of Aspartic Acid and Glutamic Acid Inhibits Tumor Cell Proliferation. Biomed. Res. 2016, 37, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Shen, M.; Xu, W. Arginine, Glycine, Aspartic Acid Peptide-Modified Paclitaxel and Curcumin Co-Loaded Liposome for the Treatment of Lung Cancer: In Vitro/Vivo Evaluation. Int. J. Nanomedicine 2018, 13, 2561–2569. [Google Scholar] [CrossRef]
- Park, K.G.M.; Hayes, P.D.; Eremin, O.; Sewell, H.; Park, K.G.M.; Garlick, P.J. Stimulation of Lymphocyte Natural Cytotoxicity by L-Arginine. Lancet 1991, 337, 645–646. [Google Scholar] [CrossRef]
- Futaki, S.; Suzuki, T.; Ohashi, W.; Yagami, T.; Tanaka, S.; Ueda, K.; Sugiura, Y. Arginine-Rich Peptides. An Abundant Source of Membrane-Permeable Peptides Having Potential as Carriers for Intracellular Protein Delivery. J. Biol. Chem. 2001, 276, 5836–5840. [Google Scholar] [CrossRef]
- Schmidt, N.; Mishra, A.; Lai, G.H.; Wong, G.C.L. Arginine-Rich Cell-Penetrating Peptides. FEBS Lett. 2010, 584, 1806–1813. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Kang, W.; Ding, B.; Cui, S.; Zhou, L.; Zhang, N.; Luo, H.; Wang, M.; Zhang, F.; et al. High Dose Isoleucine Stabilizes Nuclear PTEN to Suppress the Proliferation of Lung Cancer. Discov. Oncol. 2023, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Li, L.; Yu, X.; Deng, Q.; Xiang, Q.; Zhu, Y. Comparative Composition Structure and Selected Techno-Functional Elucidation of Flaxseed Protein Fractions. Foods 2022, 11. [Google Scholar] [CrossRef]
- Mueed, A.; Shibli, S.; Korma, S.A.; Madjirebaye, P.; Esatbeyoglu, T.; Deng, Z. Flaxseed Bioactive Compounds: Chemical Composition, Functional Properties, Food Applications and Health Benefits-Related Gut Microbes. Foods 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.W.Y.; Lei, B.; Li-Chan, E.C.Y. Isolation and Structural Characterization of the Major Protein Fraction from NorMan Flaxseed (Linum Usitatissimum L.). Food Chem. 2005, 90, 271–279. [Google Scholar] [CrossRef]
- Bhatty, R.S.; Cherdkiatgumchai, P. Compositional Analysis of Laboratory-Prepared and Commercial Samples of Linseed Meal and of Hull Isolated from Flax. J. Am. Oil Chem. Soc. 1990, 67, 79–84. [Google Scholar] [CrossRef]
- Lee, K.H.; Qi, G.H.; Sim, J.S. Metabolizable Energy and Amino Acid Availability of Full-Fat Seeds, Meals, and Oils of Flax and Canola. Poult. Sci. 1995, 74, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Porokhovinova, E.A.; Shelenga, T. V.; Kerv, Y.A.; Khoreva, V.I.; Konarev, A. V.; Yakusheva, T. V.; Pavlov, A. V.; Slobodkina, A.A.; Brutch, N.B. Features of Profiles of Biologically Active Compounds of Primary and Secondary Metabolism of Lines from VIR Flax Genetic Collection, Contrasting in Size and Color of Seeds. Plants 2022, 11, 750. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.Y.; Gui, B.; Arnison, P.G.; Wang, Y.; Reaney, M.J.T. Flaxseed (Linum Usitatissimum L.) Bioactive Compounds and Peptide Nomenclature: Areview. Trends Food Sci. Technol. 2014, 38, 5–20. [Google Scholar] [CrossRef]
- Kolodziejczyk, P.; Ozimek, L.; Kozłowska, J. The Application of Flax and Hemp Seeds in Food, Animal Feed and Cosmetics Production. In Handbook of Natural Fibres; Woodhead Publishing, 2012; pp. 329–366. [Google Scholar]
- Wu, S.; Wang, X.; Qi, W.; Guo, Q. Bioactive Protein/Peptides of Flaxseed: A Review. Trends Food Sci. Technol. 2019, 92, 184–193. [Google Scholar] [CrossRef]
- Pirmohammadi, A.; Khalaji, S.; Yari, M. Effects of Linseed Expansion on Its Dietary Molecular Structures, and on Broiler Chicks Digestive Enzymes Activity, Serum Metabolites, and Ileal Morphology. J. Appl. Poult. Res. 2019, 28, 997–1012. [Google Scholar] [CrossRef]
- Ahmed Abdullah Bakhashwain, A.A.B. Evaluation of Different Flax Cultivars in Their Oil, Fatty Acids Protein AndAmino Acids and Correlations. J. King Abdulaziz Univ. - Meteorol. Environ. Arid L. Agric. Sci. 2017, 27, 51–58. [Google Scholar] [CrossRef]
- Subba, P.; Narayana Kotimoole, C.; Prasad, T.S.K. Plant Proteome Databases and Bioinformatic Tools: An Expert Review and Comparative Insights. Omi. A J. Integr. Biol. 2019, 23, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Barvkar, V.T.; Pardeshi, V.C.; Kale, S.M.; Kadoo, N.Y.; Giri, A.P.; Gupta, V.S. Proteome Profiling of Flax (Linum Usitatissimum) Seed: Characterization of Functional Metabolic Pathways Operating during Seed Development. J. Proteome Res. 2012, 11, 6264–6276. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.; Raina, P.; Shinde, K.; Mansara, P.; Karandikar, M.; Kaul-Ghanekar, R. Flax Seed Oil Reduced Tumor Growth, Modulated Immune Responses and Decreased HPV E6 and E7 Oncoprotein Expression in a Murine Model of Ectopic Cervical Cancer. Prostaglandins Other Lipid Mediat. 2019, 143, 106332. [Google Scholar] [CrossRef]
- Sung, N.Y.; Jeong, D.; Shim, Y.Y.; Ratan, Z.A.; Jang, Y.J.; Reaney, M.J.T.; Lee, S.; Lee, B.H.; Kim, J.H.; Yi, Y.S.; et al. The Anti-Cancer E_ect of Linusorb B3 from Flaxseed Oil through the Promotion of Apoptosis, Inhibition of Actin Polymerization, and Suppression of Src Activity in Glioblastoma Cells. Molecules 2020, 25, 5881. [Google Scholar] [CrossRef]
- Lee, J.; Cho, K. Flaxseed Sprouts Induce Apoptosis and Inhibit Growth in MCF-7 and MDA-MB-231 Human Breast Cancer Cells. Vitr. Cell. Dev. Biol. - Anim. 2012, 48, 244–250. [Google Scholar] [CrossRef]
- De Silva, S.F.; Alcorn, J. Flaxseed Lignans as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets. Pharmaceuticals 2019, 12. [Google Scholar] [CrossRef]
- de Mey, S.; Dufait, I.; De Ridder, M. Radioresistance of Human Cancers: Clinical Implications of Genetic Expression Signatures. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Fukui, R.; Saga, R.; Matsuya, Y.; Tomita, K.; Kuwahara, Y.; Ohuchi, K.; Sato, T.; Okumura, K.; Date, H.; Fukumoto, M.; et al. Tumor Radioresistance Caused by Radiation-Induced Changes of Stem-like Cell Content and Sub-Lethal Damage Repair Capability. Sci. Reports 2022 121 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Engel, A.L.; Lorenz, N.I.; Klann, K.; Münch, C.; Depner, C.; Steinbach, J.P.; Ronellenfitsch, M.W.; Luger, A.L. Serine-Dependent Redox Homeostasis Regulates Glioblastoma Cell Survival. Br. J. Cancer 2020, 122, 1391–1398. [Google Scholar] [CrossRef]
- Tang, L.; Wei, F.; Wu, Y.; He, Y.; Shi, L.; Xiong, F.; Gong, Z.; Guo, C.; Li, X.; Deng, H.; et al. Role of Metabolism in Cancer Cell Radioresistance and Radiosensitization Methods. J. Exp. Clin. Cancer Res. 2018 371 2018, 37, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Okunieff, P.; Swarts, S.; Keng, P.; Sun, W.; Wang, W.; Kim, J.; Yang, S.; Zhang, H.; Liu, C.; Williams, J.P.; et al. ANTIOXIDANTS REDUCE CONSEQUENCES OF RADIATION EXPOSURE. Adv. Exp. Med. Biol. 2008, 614, 165. [Google Scholar] [CrossRef]
- Di Maggio, F.M.; Minafra, L.; Forte, G.I.; Cammarata, F.P.; Lio, D.; Messa, C.; Gilardi, M.C.; Bravatà, V. Portrait of Inflammatory Response to Ionizing Radiation Treatment. J. Inflamm. (United Kingdom) 2015, 12, 1–11. [Google Scholar] [CrossRef]
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.J.; Mosleh-Shirazi, M.A.; Tavassoli, A.R.; Taheri, M.; Mehdizadeh, A.R.; Namazi, S.A.S.; Jamali, A.; Ghalandari, R.; Bonyadi, S.; Haghani, M.; et al. Increased Radioresistance to Lethal Doses of Gamma Rays in Mice and Rats after Exposure to Microwave Radiation Emitted by a GSM Mobile Phone Simulator. Dose. Response. 2012, 11, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Taibi, A.; Thompson, L.; Comelli, E. Flaxseed Alters Gut Microbiota-Mammary Gland MicroRNA Relationships Differently Than Its Oil and Lignan Components. Curr. Dev. Nutr. 2022, 6, 342. [Google Scholar] [CrossRef]
- Christofidou-Solomidou, M.; Pietrofesa, R.; Arguiri, E.; McAlexander, M.A.; Witwer, K.W. Dietary Flaxseed Modulates the MiRNA Profile in Irradiated and Non-Irradiated Murine Lungs: A Novel Mechanism of Tissue Radioprotection by Flaxseed. Cancer Biol. Ther. 2014, 15, 930–937. [Google Scholar] [CrossRef]
- Lee, J.C.; Krochak, R.; Blouin, A.; Kanterakis, S.; Chatterjee, S.; Arguiri, E.; Vachani, A.; Solomides, C.C.; Cengel, K.A.; Christofidou-Solomidou, M. Dietary Flaxseed Prevents Radiation-Induced Oxidative Lung Damage, Inflammation and Fibrosis in a Mouse Model of Thoracic Radiation Injury. Cancer Biol. Ther. 2009, 8, 47–53. [Google Scholar] [CrossRef]
- Christofidou-Solomidou, M.; Tyagi, S.; Tan, K.S.; Hagan, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Heitjan, D.F.; Solomides, C.C.; Cengel, K.A. Dietary Flaxseed Administered Post Thoracic Radiation Treatment Improves Survival and Mitigates Radiation-Induced Pneumonopathy in Mice. BMC Cancer 2011, 11, 1–15. [Google Scholar] [CrossRef]
- Yau, A.; Lee, J.; Chen, Y. Nanomaterials for Protein Delivery in Anticancer Applications. Pharmaceutics 2021, 13, 1–23. [Google Scholar] [CrossRef]
- Lila, A.S.A.; Ishida, T. Liposomal Delivery Systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Corsaro, E.; Molfino, A. Awareness of Cancer-Related Malnutrition and Its Management: Analysis of the Results From a Survey Conducted Among Medical Oncologists. Front. Oncol. 2021, 11, 1669. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H. Nutritional Issues in Patients with Cancer. Intest. Res. 2019, 17, 455–462. [Google Scholar] [CrossRef] [PubMed]
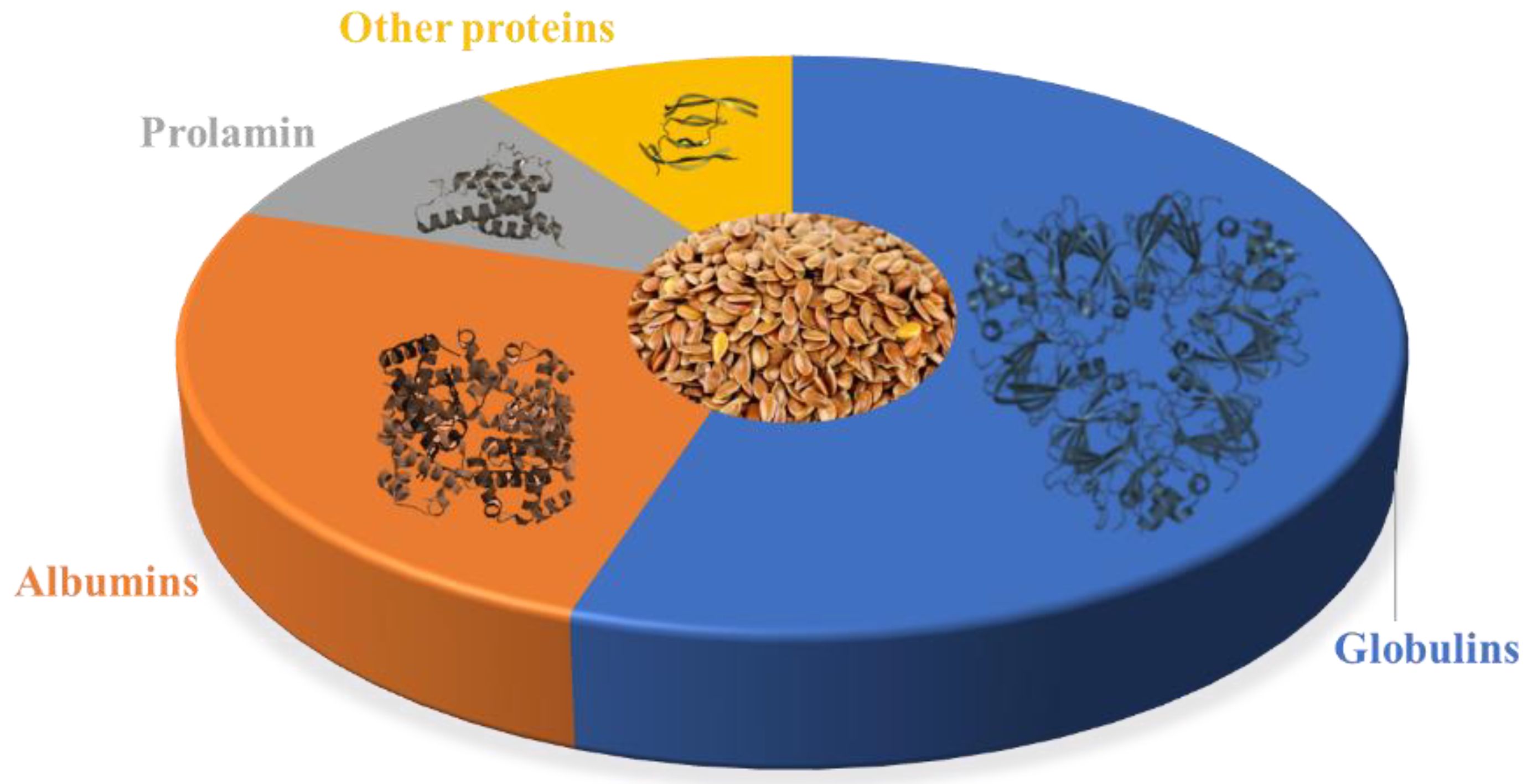
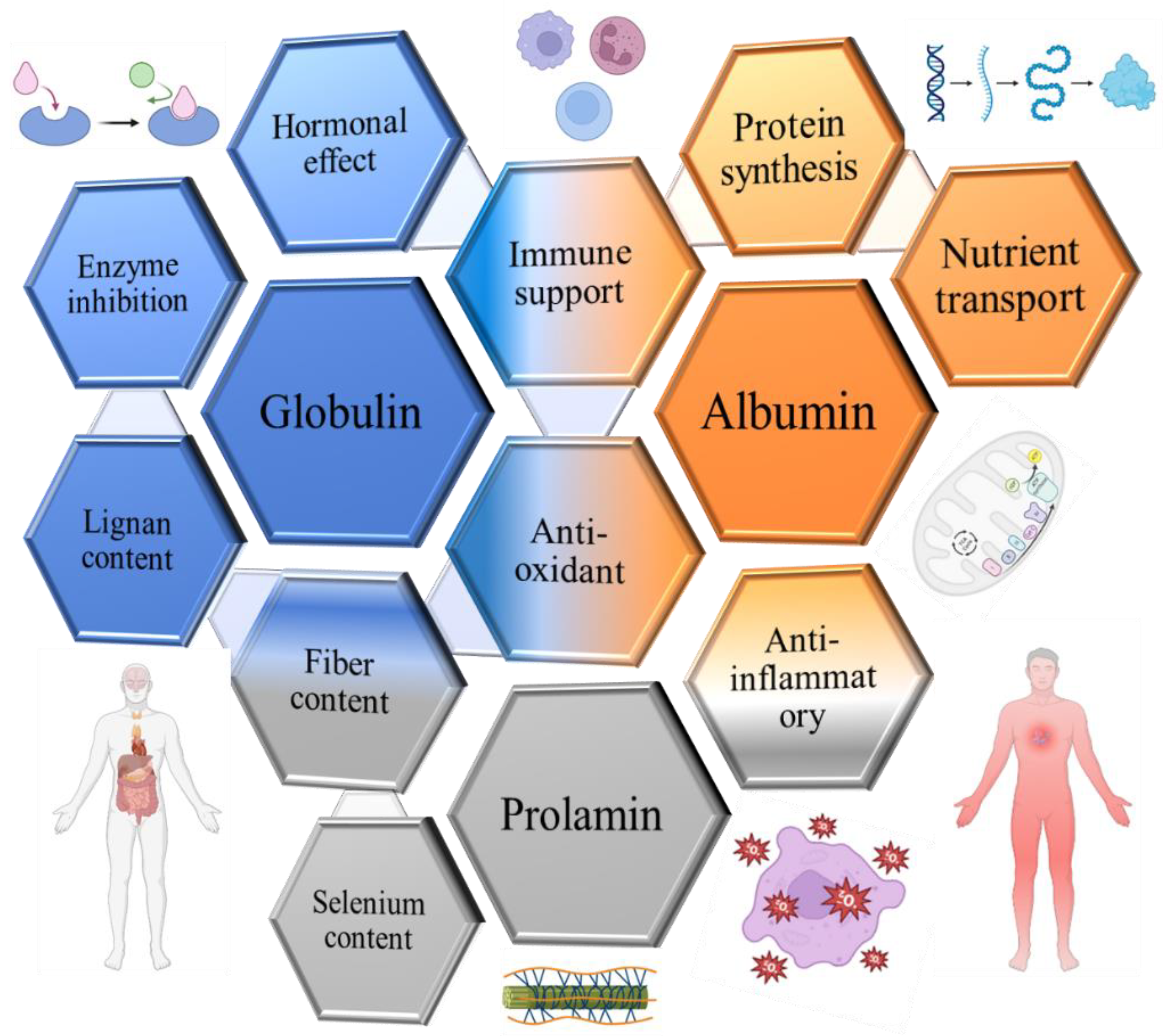
| Proteom Component | Structure | Percentage of Total Protein | Potential Health Benefits |
| 11S Globulin | 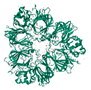 |
43-48% | Contains anti-cancer peptides [31,32,33]; may have anti-inflammatory and antioxidant effects [34,35] |
| 2S Аlbumin | 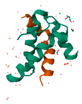 |
16-18% | Contains peptides with potential anti-cancer and anti-inflammatory effects [33,36,37]; may have cholesterol-lowering effects [38] |
| 7S Vicilin-Like Globulin | 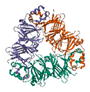 |
11-13% | May have antioxidant and anti-inflammatory effects [35,39]; contains peptides with potential anti-cancer effects [40] |
| Prolamin |  |
4-6% | Contains peptides with potential anti-cancer effects [41,42] |
| Glutelin-Like Protein |  |
2-3% | May have antioxidant and anti-inflammatory effects [43,44] |
| Other Proteins | 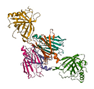 |
10-15% | May include lignans, which have antioxidant and anti-inflammatory and thus potential anti-cancer effects [45,46] |
| Action | Description | Evidence |
| Inhibits cancer proliferation and induce apoptosis | Lunasin is critical for cellular internalization and activity against cancer-stem cells through interaction with the αvβ3 integrin via the FAK/ERK/NF-κB signaling pathway [64,65] | Lunasin inhibited the proliferation and the tumorsphere-forming capacity of HCT-116 cells. Lunasin induced apoptosis through activation of caspase-3 and cleavage of PARP, and could modulate cell cycle progress through the cyclin-dependent kinase inhibitor p21 [66,67] |
| Inhibits cancer cell invasion and migration | Lunasin can interact with cellular receptors, such as integrins and EGFR, and disrupt the activity of key signaling proteins, such as matrix metalloproteinase-2/-9 via the FAK/Akt/ERK and NF-κB signaling pathways that promote cancer cell invasion and spread [68] | Lunasin caused an increase in the expression of the inhibitor of kappa B alpha (IκB-α), a decrease in nuclear p50 NF-κB and a reduction in the migration of HCT-116 and KM12L4 colon cancer cells [65]. It was demonstrated that Lunasin effectively inhibited the migration and invasion activity and expression of matrix metalloproteinase (MMP)-2/-9 in MDA-MB-231 and MCF-7 breast cancer cell lines [68] |
| Reduces inflammation and oxidation | Lunacin has anti-inflammatory properties that may help to reduce inflammation, which is known to contribute to cancer development and progression [69,70] | The anti-inflammatory effects were demonstrated in mouse macrophage cells (LPS-stimulated RAW 264.7), by reducing the production of certain inflammatory markers (ROS, TNF-α and IL-6) [71]. |
| Reduces cancer cells colonization | Lunasin reduced activating phosphorylation of the intracellular kinases FAK and AKT as well as reduced histone acetylation of lysine residues in H3 and H4 histones [62] | Mice receiving Lunasin treatment had significantly reduced pulmonary colonization after injection of highly metastatic B16-F10 melanoma cells compared to mice in the control group [62,72] |
| Arrest cell cycle | Lunasin arrested the cell cycle at the G2/M and G1/S phase in various cancer types. Lunasin altered the expression of the G1 specific cyclin-dependent kinase complex components, increased levels of p27Kip1, reduced levels of phosphorylated Akt [67,73] | NSCLC H661 cells showed that Lunasin inhibited cell cycle progression at the G1/S phase [73]. Lunasin increased twice the amount of colon cancer cells KM12L4 undergoing apoptosis by arresting G2/M phase [67]. Lunasin treatment of MDA-MB-231 breast cancer cells resulted in a notable increase of RB1 level, which lead to arrest of G1 phase [74] |
| Mechanism of Action | Description | Evidence |
| Inhibits cancer cell growth and proliferation | 11S-globulin peptides in the more active fraction (MPI-h(V)) also presented antiproliferative activity [87]. Acid fraction of glycinin, composed of low molecular weight peptides is able to inhibit cancer cells growth [88]. | Studies have shown that 11S globulin inhibits the proliferation of UMR106 rat osteosarcoma-derived cells [87] and inhibited the growth of HeLa cells in a dose-dependent manner [88]. |
| Antioxidant effect | Small (~1kDa) peptides generated from 11S globulin demonstrated peroxyl radical scavenging activity [32] | The peptides were able to inhibit the formation of hydroxyl radicals by reaction of H2O2 and Co+2 in human adenocarcinoma cell line, Caco-2 [32] |
| Induces cancer cell death (apoptosis) | 11S globulin (glycinin) has been shown to induce cell death, or apoptosis, in cancer epithelial cells [89]. Flaxseed orbitides have been shown to be involved in apoptosis process [23]. | Glutelin extracts digested with trypsin, showed the induction of apoptosis against HeLa cells [90]. Human gastric SGC-7901 cell apoptosis was induced by flaxseed orbitides by a mitochondrial- and death receptor-mediated intrinsic and extrinsic pathways [91] |
| Arresting cell cycle | Cyclolinopeptides/linusorbs are capable to arrest cell cycle and thus reduce metastasis spreading. | flaxseed LOs arresting the cell cycle in SGC-7901 cells at the G1 phase by downregulation of CDK2/4 and overexpression of p21WAF1/CIP1 and p27KIP1 genes [92] |
| Cytotoxic effect on cancer cells | Cyclolinopeptides/linusorbs (LOs), which are cyclic peptides with 8–10 amino acid residues have strong cytotoxic effect over cancer cells [23]. 11S globulin was shown to have the cytotoxic effect at high concentration on a variety of human cancer cell lines [33]. | LOs from flaxseed globulin are cytotoxic to human melanoma cells A375 and breast cancer cells Sk-Br-3 and MCF7 at high concentration [93] |
|
Proteins Plants |
11S Globulin | 2S Albumin | 7S Vicilin-like protein | Prolamin |
| Linum Usitatissimum (Flax) | 0 | 12 | 0 | 0 |
| Triticum Aestivum (Wheat) | 3397 | 3944 | 22 | 2925 |
| Brassica Napus (Rape) | 136 | 2252 | 57 | 998 |
| Oryza Sativa (Rice) | 73 | 1920 | 20 | 215 |
| Hordeum Vulgare (Barley) | 286 | 539 | 2 | 284 |
| Glycine Max (Soybean) | 19 | 574 | 70 | 62 |
| Arachis Hypogaea (Peanut) | 15 | 342 | 13 | 29 |
| Avena Sativa (Oat) | 182 | 210 | 0 | 174 |
| Helianthus Annuus (Sunflower) | 34 | 181 | 2 | 8 |
| Sesamum Indicum (Sesame) | 9 | 105 | 13 | 4 |
| Juglans Regia (Walnut) | 8 | 102 | 4 | 3 |
| Carya Illinoinensis (Pecan) | 4 | 92 | 5 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





