Submitted:
29 August 2023
Posted:
31 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
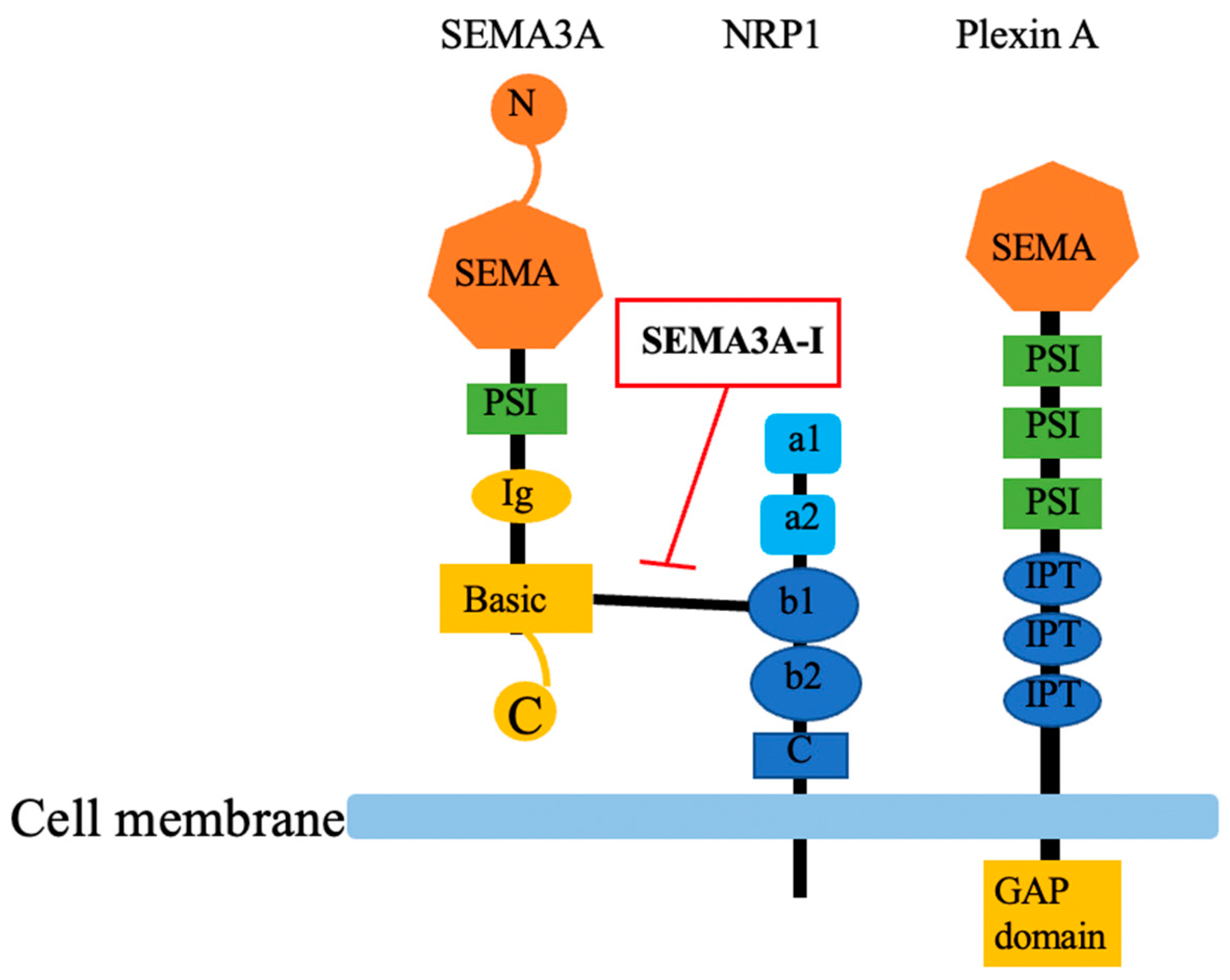
2. SEMA3A and its receptors, and expression in kidneys
3. SEMA3A in kidney development
| Disease | Etiology | Species | Sample | SEMA3A expression |
Ref. |
|---|---|---|---|---|---|
| Proteinuric diseases | MCNS | Human | Urine | Increase | [59] |
| PAN | Rats | Kidney | Increase | [57] | |
| DN | - | Human | Urine | Increase | [60] |
| db/db | Mice | Kidney | Increase | [60] | |
| db/db | Mice | Kidney | Increase | [57] | |
| Streptozotocin | Mice | Kidney | Increase | [61] | |
| AKI | IRI | Mice | Kidney | Increase | [62] |
| LPS | Mice | Kidney | Increase | [45] | |
| contrast | Human | Urine | Increase | [63] | |
| Cardiac operation |
Human | Serum/ Urine | Increase | [43] | |
| CKD | - | Human | Urine | Increase | [64] |
| LN | - | Human | Kidney | Increase | [65] |
| - | Human | Urine | Decrease | [66] |
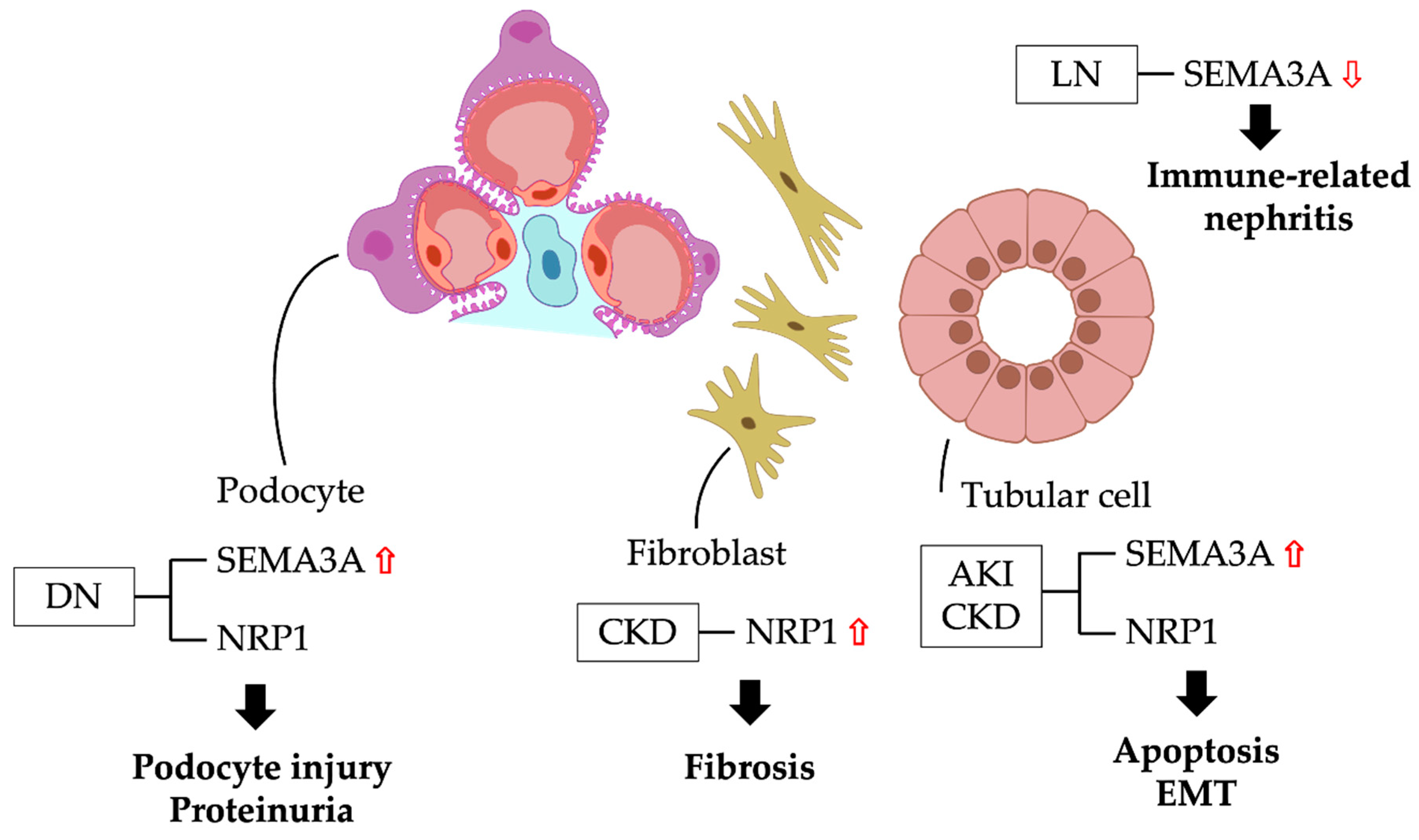
4. SEMA3A and kidney diseases
4.1. Podocytopathy and diabetic nephropathy
4.2. Acute kidney injury
4.3. Chronic kidney disease
4.4. Systemic lupus erythematosus
| Etiology | Model | SEMA3A- targeting |
Targeting Method |
Species | Outcome | Target | Function | Ref |
|---|---|---|---|---|---|---|---|---|
| Podocyte injury | Dox | Down | SEMA3A inhibitor |
Mice | Proteinuria↓ | JNK | Anti-apoptosis | [70] |
| - | - | Up | Recombinant SEMA3A | Mice | Nephrotic proteinuria |
- | Podocytopathy | [44] |
| - | - | Up | Podocyte SEMA3A+ |
Mice | Proteinuria↑ | dysregulation of nephrin, MMP9, αvβ3 integrin | Podocytopathy | [69] |
| DN | STZ | Down | SEMA3A- | Mice | Proteinuria↓ | - | - | [60] |
| STZ | Down | SEMA3A inhibitor |
Mice | Proteinuria↓ Kidney fibrosis↓ Kidney dysfunction↓ |
- | - | [60] | |
| High- glucose |
Down | miR-15b-5p | Podocyte | - | - | Anti-apoptosis Anti-inflammation |
[78] | |
| High- glucose |
Up | KCNQ1OT1 | Podocyte | - | miR-23b-3p | Inflammation Apoptosis |
[79] | |
| High- glucose |
Down | TCF7 silence / SEMA3A- siRNA |
Podocyte | Cytotoxicity↓ | miR-16-5p | - | [80] | |
| Podocyte SEMA3A+ & STZ |
Down | SEMA3A inhibitor (xanthofulvin) |
Mice | Proteinuria↓ Kidney dysfunction↓ |
MICAL1 | - | [42] | |
| AKI | IRI | Down | SEMA3A- / SEMA3A -inhibitor | Mice | Kidney dysfunction↓ Neutrophil infiltration↓ |
- | Anti-apoptosis, Anti-inflammation |
[62] |
| IRI / cisplatin |
Down | Gpr97- | Mice | Kidney dysfunction↓ Neutrophil infiltration↓ |
HuR | Aiti-apoptosis anti-inflammation |
[90] | |
| IRI | Down | LOXblock-I / Curcumin |
Rats | - | - | Anti-apoptosis Anti-inflammation |
[91] | |
| IRI | Down | miR-199a-3p | Mice | Kidney dysfunction↓ | AKT / ERK pathway |
Aiti-apoptosis | [92] | |
| LPS | Down | EGCG | Mice | Kidney dysfunction↓ Neutrophil infiltration↓ |
Rac1/NF-κB p65 / JNK pathway | Anti-apoptosis, Anti-inflammation |
[45] | |
| CKD | UUO | Down | SEMA3A Inhibitor (SM-345431) |
Mice | Kidney fibrosis↓ | JNK | Anti-apoptosis, Anti-EMT |
[64] |
| LN | NZB | Up | Recombinant SEMA3A |
Mice | Proteinuria↓ Immune complex- deposition↓ |
- | - | [104] |
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Q.; Zhu, L. , The Role of Semaphorins in Metabolic Disorders. Int J Mol Sci 2020, 21, (16). [Google Scholar] [CrossRef] [PubMed]
- Alto, L. T.; Terman, J. R. , Semaphorins and their Signaling Mechanisms. Methods Mol Biol 2017, 1493, 1–25. [Google Scholar] [PubMed]
- Kiseleva, E. P.; Rutto, K. V. , Semaphorin 3A in the Immune System: Twenty Years of Study. Biochemistry (Mosc) 2022, 87, 640–657. [Google Scholar] [CrossRef]
- Raper, J. A.; Kapfhammer, J. P. , The enrichment of a neuronal growth cone collapsing activity from embryonic chick brain. Neuron 1990, 4, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Raible, D.; Raper, J. A. , Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 1993, 75, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.; Koncina, E.; Satkauskas, S.; Cremel, G.; Aunis, D.; Bagnard, D. , The many faces of semaphorins: from development to pathology. Cell Mol Life Sci 2009, 66, 649–666. [Google Scholar] [CrossRef]
- Kim, O.; Tran, P. T.; Gal, M.; Lee, S. J.; Na, S. H.; Hwangbo, C.; Lee, J. H. , RAS-stimulated release of exosomal miR-494-3p promotes the osteolytic bone metastasis of breast cancer cells. Int J Mol Med 2023, 52, (3). [Google Scholar] [CrossRef]
- Kurokawa, S.; Kashimoto, M.; Hagikura, K.; Shimodai-Yamada, S.; Otsuka, N.; Wakamatsu, Y.; Nagashima, K.; Matsumoto, T.; Hao, H.; Okumura, Y. , Intravenous Semaphorin 3A Administration Maintains Cardiac Contractility and Improves Electrical Remodeling in a Mouse Model of Isoproterenol-Induced Heart Failure. Int Heart J 2023, 64, 453–461. [Google Scholar] [CrossRef]
- Qiu, Q.; Yu, X.; Chen, Q.; He, X. , Sema3A inactivates the ERK/JNK signalling pathways to alleviate inflammation and oxidative stress in lipopolysaccharide-stimulated rat endothelial cells and lung tissues. Autoimmunity 2023, 56, 2200908. [Google Scholar] [CrossRef]
- Tian, T.; Chen, L.; Wang, Z.; Zhu, M.; Xu, W.; Wu, B. , Sema3A Drives Alternative Macrophage Activation in the Resolution of Periodontitis via PI3K/AKT/mTOR Signaling. Inflammation 2023, 46, 876–891. [Google Scholar] [CrossRef]
- Ieguchi, K.; Funakoshi, M.; Mishima, T.; Takizawa, K.; Omori, T.; Nakamura, F.; Watanabe, M.; Tsuji, M.; Kiuchi, Y.; Kobayashi, S.; Tsunoda, T.; Maru, Y.; Wada, S. , The Sympathetic Nervous System Contributes to the Establishment of Pre-Metastatic Pulmonary Microenvironments. Int J Mol Sci 2022, 23, (18). [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Nishinaka, A.; Hidaka, Y.; Shimazawa, M.; Thomas, L.; Bakker, R. A.; Hara, H. , Efficacy of an Anti-Semaphorin 3A Neutralizing Antibody in a Male Experimental Retinal Vein Occlusion Mouse Model. Invest Ophthalmol Vis Sci 2022, 63, 14. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, A.; Corredor-Sanchez, M.; Galron, R.; Nahary, L.; Safrin, M.; Bruzel, M.; Moure, A.; Bonet, R.; Perez, Y.; Bujons, J.; Vallejo-Yague, E.; Sacks, H.; Burnet, M.; Alfonso, I.; Messeguer, A.; Benhar, I.; Barzilai, A.; Solomon, A. S. , Inhibition of Sema-3A Promotes Cell Migration, Axonal Growth, and Retinal Ganglion Cell Survival. Transl Vis Sci Technol 2021, 10, 16. [Google Scholar] [CrossRef]
- Kanth, S. M.; Gairhe, S.; Torabi-Parizi, P. , The Role of Semaphorins and Their Receptors in Innate Immune Responses and Clinical Diseases of Acute Inflammation. Front Immunol 2021, 12, 672441. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D. M. O.; Caliva, M.; Schroeder, M.; Carlson, B.; Upadhyayula, P. S.; Milligan, B. D.; Cheshier, S. H.; Weissman, I. L.; Sarkaria, J. N.; Meyer, F. B.; Henley, J. R. , Semaphorin 3A mediated brain tumor stem cell proliferation and invasion in EGFRviii mutant gliomas. BMC Cancer 2020, 20, 1213. [Google Scholar] [CrossRef]
- Karpuz, T.; Araz, M.; Korkmaz, L.; Kilinc, I.; Findik, S.; Karaagac, M.; Eryilmaz, M. K.; Artac, M. , The Prognostic Value of Serum Semaphorin3A and VEGF Levels in Patients with Metastatic Colorectal Cancer. J Gastrointest Cancer 2020, 51, 491–497. [Google Scholar] [CrossRef]
- Sabag, A. D.; Dias-Polak, D.; Bejar, J.; Sheffer, H.; Bergman, R.; Vadasz, Z. , Altered expression of regulatory molecules in the skin of psoriasis. Immunol Res 2018, 66, 649–654. [Google Scholar] [CrossRef]
- Gao, H.; Ma, X. X.; Guo, Q.; Xie, L. F.; Zhong, Y. C.; Zhang, X. W. , Expression of circulating Semaphorin3A and its association with inflammation and bone destruction in rheumatoid arthritis. Clin Rheumatol 2018, 37, 2073–2080. [Google Scholar] [CrossRef]
- Jacob, S.; Al-Kandari, A.; Alroughani, R.; Al-Temaimi, R. , Assessment of plasma biomarkers for their association with Multiple Sclerosis progression. J Neuroimmunol 2017, 305, 5–8. [Google Scholar] [CrossRef]
- Hira, K.; Ueno, Y.; Tanaka, R.; Miyamoto, N.; Yamashiro, K.; Inaba, T.; Urabe, T.; Okano, H.; Hattori, N. , Astrocyte-Derived Exosomes Treated With a Semaphorin 3A Inhibitor Enhance Stroke Recovery via Prostaglandin D(2) Synthase. Stroke 2018, 49, 2483–2494. [Google Scholar] [CrossRef]
- Matrone, C.; Ferretti, G. , Semaphorin 3A influences neuronal processes that are altered in patients with autism spectrum disorder: Potential diagnostic and therapeutic implications. Neurosci Biobehav Rev 2023, 153, 105338. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, J. W.; Wu, J.; Wu, Z. X.; Li, J.; Ji, H. F.; Liang, N. P.; Zhang, H. J.; Lai, Z. Q.; Dong, Y. F. , Inhibition of semaphorin-3a alleviates lipopolysaccharide-induced vascular injury. Microvasc Res 2022, 142, 104346. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, E. R.; Mahmoud, D. A.; Ebrahim, G. E.; Al Anany, M. G.; Seliem, N.; Hassan, M. M. , Serum metabolomic profiles and semaphorin-3A as biomarkers of diabetic retinopathy progression. Egypt J Immunol 2023, 30, 83–98. [Google Scholar] [CrossRef]
- Valiulyte, I.; Steponaitis, G.; Kardonaite, D.; Tamasauskas, A.; Kazlauskas, A. , A SEMA3 Signaling Pathway-Based Multi-Biomarker for Prediction of Glioma Patient Survival. Int J Mol Sci 2020, 21, (19). [Google Scholar] [CrossRef]
- Wang, P.; Mao, Y. M.; Liu, L. N.; Zhao, C. N.; Li, X. M.; Pan, H. F. , Decreased Expression of Semaphorin 3A and Semaphorin 7A Levels and Its Association with Systemic Lupus Erythematosus. Immunol Invest 2020, 49, (1–2), 69. [Google Scholar] [CrossRef] [PubMed]
- Izycka, N.; Sterzynska, K.; Januchowski, R.; Nowak-Markwitz, E. , Semaphorin 3A (SEMA3A), protocadherin 9 (PCdh9), and S100 calcium binding protein A3 (S100A3) as potential biomarkers of carcinogenesis and chemoresistance of different neoplasms, including ovarian cancer - review of literature. Ginekol Pol 2019, 90, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Qi, L.; Wang, F.; Sun, Z.; Huang, Z.; Xi, Q. , Decreased semaphorin 3A expression is associated with a poor prognosis in patients with epithelial ovarian carcinoma. Int J Mol Med 2015, 35, 1374–1380. [Google Scholar] [CrossRef]
- Luyckx, V. A.; Tonelli, M.; Stanifer, J. W. , The global burden of kidney disease and the sustainable development goals. Bull World Health Organ 2018, 96, 414–422D. [Google Scholar] [CrossRef]
- Collaboration, G. B. D. C. K. D. , Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar]
- The, E.-K. C. G.; Herrington, W. G.; Staplin, N.; Wanner, C.; Green, J. B.; Hauske, S. J.; Emberson, J. R.; Preiss, D.; Judge, P.; Mayne, K. J.; Ng, S. Y. A.; Sammons, E.; Zhu, D.; Hill, M.; Stevens, W.; Wallendszus, K.; Brenner, S.; Cheung, A. K.; Liu, Z. H.; Li, J.; Hooi, L. S.; Liu, W.; Kadowaki, T.; Nangaku, M.; Levin, A.; Cherney, D.; Maggioni, A. P.; Pontremoli, R.; Deo, R.; Goto, S.; Rossello, X.; Tuttle, K. R.; Steubl, D.; Petrini, M.; Massey, D.; Eilbracht, J.; Brueckmann, M.; Landray, M. J.; Baigent, C.; Haynes, R. , Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med 2023, 388, 117–127. [Google Scholar]
- Heerspink, H. J. L.; Stefansson, B. V.; Correa-Rotter, R.; Chertow, G. M.; Greene, T.; Hou, F. F.; Mann, J. F. E.; McMurray, J. J. V.; Lindberg, M.; Rossing, P.; Sjostrom, C. D.; Toto, R. D.; Langkilde, A. M.; Wheeler, D. C.; Committees, D.-C. T. ; Investigators, Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Prattichizzo, F.; de Candia, P.; Ceriello, A. , Diabetes and kidney disease: emphasis on treatment with SGLT-2 inhibitors and GLP-1 receptor agonists. Metabolism 2021, 120, 154799. [Google Scholar] [CrossRef] [PubMed]
- Nangaku, M.; Takama, H.; Ichikawa, T.; Mukai, K.; Kojima, M.; Suzuki, Y.; Watada, H.; Wada, T.; Ueki, K.; Narita, I.; Kashihara, N.; Kadowaki, T.; Hase, H.; Akizawa, T. , Randomized, double-blind, placebo-controlled phase 3 study of bardoxolone methyl in patients with diabetic kidney disease: design and baseline characteristics of the AYAME study. Nephrol Dial Transplant 2023, 38, 1204–1216. [Google Scholar] [CrossRef] [PubMed]
- Leehey, D. J.; Carlson, K.; Reda, D. J.; Craig, I.; Clise, C.; Conner, T. A.; Agarwal, R.; Kaufman, J. S.; Anderson, R. J.; Lammie, D.; Huminik, J.; Polzin, L.; McBurney, C.; Huang, G. D.; Emanuele, N. V. , Pentoxifylline in diabetic kidney disease (VA PTXRx): protocol for a pragmatic randomised controlled trial. BMJ Open 2021, 11, e053019. [Google Scholar] [CrossRef]
- Chertow, G. M.; Pergola, P. E.; Chen, F.; Kirby, B. J.; Sundy, J. S.; Patel, U. D.; Investigators, G.-U.-. . Effects of Selonsertib in Patients with Diabetic Kidney Disease. J Am Soc Nephrol 2019, 30, 1980–1990. [Google Scholar] [CrossRef]
- Tuttle, K. R.; Brosius, F. C., 3rd; Adler, S. G.; Kretzler, M.; Mehta, R. L.; Tumlin, J. A.; Tanaka, Y.; Haneda, M.; Liu, J.; Silk, M. E.; Cardillo, T. E.; Duffin, K. L.; Haas, J. V.; Macias, W. L.; Nunes, F. P.; Janes, J. M. , JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial. Nephrol Dial Transplant 2018, 33, 1950–1959. [Google Scholar] [CrossRef]
- Lin, C. W.; Mostafa, N. M.; D, L. A.; J, J. B.; Klein, C. E.; Awni, W. M. , Relationship Between Atrasentan Concentrations and Urinary Albumin to Creatinine Ratio in Western and Japanese Patients With Diabetic Nephropathy. Clin Ther 2018, 40, 242–251. [Google Scholar] [CrossRef]
- Toledano, S.; Nir-Zvi, I.; Engelman, R.; Kessler, O.; Neufeld, G. , Class-3 Semaphorins and Their Receptors: Potent Multifunctional Modulators of Tumor Progression. Int J Mol Sci 2019, 20, (3). [Google Scholar] [CrossRef]
- Tamagnone, L.; Artigiani, S.; Chen, H.; He, Z.; Ming, G. I.; Song, H.; Chedotal, A.; Winberg, M. L.; Goodman, C. S.; Poo, M.; Tessier-Lavigne, M.; Comoglio, P. M. , Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 1999, 99, 71–80. [Google Scholar] [CrossRef]
- Janssen, B. J.; Malinauskas, T.; Weir, G. A.; Cader, M. Z.; Siebold, C.; Jones, E. Y. , Neuropilins lock secreted semaphorins onto plexins in a ternary signaling complex. Nat Struct Mol Biol 2012, 19, 1293–1299. [Google Scholar] [CrossRef]
- Lu, D.; Shang, G.; He, X.; Bai, X. C.; Zhang, X. , Architecture of the Sema3A/PlexinA4/Neuropilin tripartite complex. Nat Commun 2021, 12, 3172. [Google Scholar] [CrossRef]
- Aggarwal, P. K.; Veron, D.; Thomas, D. B.; Siegel, D.; Moeckel, G.; Kashgarian, M.; Tufro, A. , Semaphorin3a promotes advanced diabetic nephropathy. Diabetes 2015, 64, 1743–1759. [Google Scholar] [CrossRef]
- Jayakumar, C.; Ranganathan, P.; Devarajan, P.; Krawczeski, C. D.; Looney, S.; Ramesh, G. , Semaphorin 3A is a new early diagnostic biomarker of experimental and pediatric acute kidney injury. PLoS One 2013, 8, e58446. [Google Scholar] [CrossRef] [PubMed]
- Tapia, R.; Guan, F.; Gershin, I.; Teichman, J.; Villegas, G.; Tufro, A. , Semaphorin3a disrupts podocyte foot processes causing acute proteinuria. Kidney Int 2008, 73, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Gan, H.; Zeng, Y.; Zhao, H.; Tang, R.; Xia, Y. , Inhibition of semaphorin-3a suppresses lipopolysaccharide-induced acute kidney injury. J Mol Med (Berl) 2018, 96, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Tsuji, K.; Inoue-Torii, A.; Fukushima, K.; Kitamura, S.; Wada, J. , Semaphorin3A-Inhibitor Ameliorates Doxorubicin-Induced Podocyte Injury. Int J Mol Sci 2020, 21, (11). [Google Scholar] [CrossRef]
- Tufro, A. , Semaphorin3a signaling, podocyte shape, and glomerular disease. Pediatr Nephrol 2014, 29, 751–755. [Google Scholar] [CrossRef]
- Reidy, K. J.; Villegas, G.; Teichman, J.; Veron, D.; Shen, W.; Jimenez, J.; Thomas, D.; Tufro, A. , Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development. Development 2009, 136, 3979–3989. [Google Scholar] [CrossRef]
- Guan, F.; Villegas, G.; Teichman, J.; Mundel, P.; Tufro, A. , Autocrine class 3 semaphorin system regulates slit diaphragm proteins and podocyte survival. Kidney Int 2006, 69, 1564–1569. [Google Scholar] [CrossRef]
- Wu, H.; Kirita, Y.; Donnelly, E. L.; Humphreys, B. D. , Advantages of Single-Nucleus over Single-Cell RNA Sequencing of Adult Kidney: Rare Cell Types and Novel Cell States Revealed in Fibrosis. J Am Soc Nephrol 2019, 30, 23–32. [Google Scholar] [CrossRef]
- Kolodkin, A. L.; Levengood, D. V.; Rowe, E. G.; Tai, Y. T.; Giger, R. J.; Ginty, D. D. , Neuropilin is a semaphorin III receptor. Cell 1997, 90, 753–762. [Google Scholar] [CrossRef]
- Omoto, M.; Yoshida, S.; Miyashita, H.; Kawakita, T.; Yoshida, K.; Kishino, A.; Kimura, T.; Shibata, S.; Tsubota, K.; Okano, H.; Shimmura, S. , The semaphorin 3A inhibitor SM-345431 accelerates peripheral nerve regeneration and sensitivity in a murine corneal transplantation model. PLoS One 2012, 7, e47716. [Google Scholar] [CrossRef] [PubMed]
- Ivakhnitskaia, E.; Chin, M. R.; Siegel, D.; Guaiquil, V. H. , Vinaxanthone inhibits Semaphorin3A induced axonal growth cone collapse in embryonic neurons but fails to block its growth promoting effects on adult neurons. Sci Rep 2021, 11, 13019. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, R.; Yamazoe, K.; Yoshida, S.; Hatou, S.; Inagaki, E.; Okano, H.; Tsubota, K.; Shimmura, S. , The Semaphorin 3A inhibitor SM-345431 preserves corneal nerve and epithelial integrity in a murine dry eye model. Sci Rep 2017, 7, 15584. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, K.; Stichel, J.; Bellmann-Sickert, K.; Baumann, L.; Bierer, D.; Riedl, B.; Beck-Sickinger, A. G. , Pinpointing the interaction site between semaphorin-3A and its inhibitory peptide. J Pept Sci 2023, 29, e3460. [Google Scholar] [CrossRef]
- Tran, T. S.; Kolodkin, A. L.; Bharadwaj, R. , Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol 2007, 23, 263–292. [Google Scholar] [CrossRef]
- Reidy, K.; Tufro, A. , Semaphorins in kidney development and disease: modulators of ureteric bud branching, vascular morphogenesis, and podocyte-endothelial crosstalk. Pediatr Nephrol 2011, 26, 1407–1412. [Google Scholar] [CrossRef]
- Tufro, A.; Teichman, J.; Woda, C.; Villegas, G. , Semaphorin3a inhibits ureteric bud branching morphogenesis. Mech Dev 2008, 125, (5–6), 558. [Google Scholar] [CrossRef]
- Inoue-Torii, A.; Kitamura, S.; Wada, J.; Tsuji, K.; Makino, H. , The level of urinary semaphorin3A is associated with disease activity in patients with minimal change nephrotic syndrome. Int J Nephrol Renovasc Dis 2017, 10, 167–174. [Google Scholar] [CrossRef]
- Mohamed, R.; Ranganathan, P.; Jayakumar, C.; Nauta, F. L.; Gansevoort, R. T.; Weintraub, N. L.; Brands, M.; Ramesh, G. , Urinary semaphorin 3A correlates with diabetic proteinuria and mediates diabetic nephropathy and associated inflammation in mice. J Mol Med (Berl) 2014, 92, 1245–1256. [Google Scholar] [CrossRef]
- Veron, D.; Bertuccio, C. A.; Marlier, A.; Reidy, K.; Garcia, A. M.; Jimenez, J.; Velazquez, H.; Kashgarian, M.; Moeckel, G. W.; Tufro, A. , Podocyte vascular endothelial growth factor (Vegf(1)(6)(4)) overexpression causes severe nodular glomerulosclerosis in a mouse model of type 1 diabetes. Diabetologia 2011, 54, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Jayakumar, C.; Mohamed, R.; Weintraub, N. L.; Ramesh, G. , Semaphorin 3A inactivation suppresses ischemia-reperfusion-induced inflammation and acute kidney injury. Am J Physiol Renal Physiol 2014, 307, F183–F194. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Li, Z.; Wei, D.; Chen, H.; Yang, C.; Wu, D.; Wang, Y.; Zhang, J. , Urinary semaphorin 3A as an early biomarker to predict contrast-induced acute kidney injury in patients undergoing percutaneous coronary intervention. Braz J Med Biol Res 2018, 51, e6487. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Tsuji, K.; Fukushima, K.; Takahashi, K.; Kitamura, S.; Wada, J. , Semaporin3A inhibitor ameliorates renal fibrosis through the regulation of JNK signaling. Am J Physiol Renal Physiol 2021, 321, F740–F756. [Google Scholar] [CrossRef] [PubMed]
- Vadasz, Z.; Ben-Izhak, O.; Bejar, J.; Sabo, E.; Kessel, A.; Storch, S.; Toubi, E. , The involvement of immune semaphorins and neuropilin-1 in lupus nephritis. Lupus 2011, 20, 1466–1473. [Google Scholar] [CrossRef]
- Vadasz, Z.; Toubi, E. , Semaphorin 3A - a marker for disease activity and a potential putative disease-modifying treatment in systemic lupus erythematosus. Lupus 2012, 21, 1266–1270. [Google Scholar] [CrossRef]
- Koehler, S.; Miner, J. H.; Staruschenko, A. , Call for papers: podocyte physiology and pathophysiology. Am J Physiol Renal Physiol 2023, 324, F505–F510. [Google Scholar] [CrossRef]
- Tufro, A. , Podocyte Shape Regulation by Semaphorin 3A and MICAL-1. Methods Mol Biol 2017, 1493, 393–399. [Google Scholar]
- Reidy, K. J.; Aggarwal, P. K.; Jimenez, J. J.; Thomas, D. B.; Veron, D.; Tufro, A. , Excess podocyte semaphorin-3A leads to glomerular disease involving plexinA1-nephrin interaction. Am J Pathol 2013, 183, 1156–1168. [Google Scholar] [CrossRef]
- Kumagai, K.; Hosotani, N.; Kikuchi, K.; Kimura, T.; Saji, I. , Xanthofulvin, a novel semaphorin inhibitor produced by a strain of Penicillium. J Antibiot (Tokyo) 2003, 56, 610–616. [Google Scholar] [CrossRef]
- Samsu, N. , Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed Res Int 2021, 2021, 1497449. [Google Scholar] [CrossRef] [PubMed]
- Valencia, W. M.; Florez, H. , How to prevent the microvascular complications of type 2 diabetes beyond glucose control. BMJ 2017, 356, i6505. [Google Scholar] [CrossRef] [PubMed]
- Burrows, N. R.; Hora, I.; Geiss, L. S.; Gregg, E. W.; Albright, A. , Incidence of End-Stage Renal Disease Attributed to Diabetes Among Persons with Diagnosed Diabetes - United States and Puerto Rico, 2000-2014. MMWR Morb Mortal Wkly Rep 2017, 66, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Lane, P. H.; Steffes, M. W.; Mauer, S. M. , Renal histologic changes in diabetes mellitus. Semin Nephrol 1990, 10, 254–259. [Google Scholar]
- Maezawa, Y.; Takemoto, M.; Yokote, K. , Cell biology of diabetic nephropathy: Roles of endothelial cells, tubulointerstitial cells and podocytes. J Diabetes Investig 2015, 6, 3–15. [Google Scholar] [CrossRef]
- Cao, Z.; Cooper, M. E. , Pathogenesis of diabetic nephropathy. J Diabetes Investig 2011, 2, 243–247. [Google Scholar] [CrossRef]
- Kumar Pasupulati, A.; Chitra, P. S.; Reddy, G. B. , Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomol Concepts 2016, 7, (5–6), 293. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, C.; Zhang, D.; Chu, X.; Zhang, Y.; Li, J. , miR-15b-5p ameliorated high glucose-induced podocyte injury through repressing apoptosis, oxidative stress, and inflammatory responses by targeting Sema3A. J Cell Physiol 2019, 234, 20869–20878. [Google Scholar] [CrossRef]
- Fei, B.; Zhou, H.; He, Z.; Wang, S. , KCNQ1OT1 inhibition alleviates high glucose-induced podocyte injury by adsorbing miR-23b-3p and regulating Sema3A. Clin Exp Nephrol 2022, 26, 385–397. [Google Scholar] [CrossRef]
- Jiang, Z.; Qian, L.; Yang, R.; Wu, Y.; Guo, Y.; Chen, T. , LncRNA TCF7 contributes to high glucose-induced damage in human podocytes by up-regulating SEMA3A via sponging miR-16-5p. J Diabetes Investig 2023, 14, 193–204. [Google Scholar] [CrossRef]
- Singbartl, K.; Kellum, J. A. , AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int 2012, 81, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Jang, H. R.; Rabb, H. , Immune cells in experimental acute kidney injury. Nat Rev Nephrol 2015, 11, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J. A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H. J. , Acute kidney injury. Nat Rev Dis Primers 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, L.; Matuszkiewicz-Rowinska, J.; Jayakumar, C.; Oldakowska-Jedynak, U.; Looney, S.; Galas, M.; Dutkiewicz, M.; Krawczyk, M.; Ramesh, G. , Netrin-1 and semaphorin 3A predict the development of acute kidney injury in liver transplant patients. PLoS One 2014, 9, e107898. [Google Scholar] [CrossRef]
- Parikh, C. R.; Mishra, J.; Thiessen-Philbrook, H.; Dursun, B.; Ma, Q.; Kelly, C.; Dent, C.; Devarajan, P.; Edelstein, C. L. , Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int 2006, 70, 199–203. [Google Scholar] [CrossRef]
- Doi, K.; Negishi, K.; Ishizu, T.; Katagiri, D.; Fujita, T.; Matsubara, T.; Yahagi, N.; Sugaya, T.; Noiri, E. , Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med 2011, 39, 2464–2469. [Google Scholar] [CrossRef]
- Bennett, M.; Dent, C. L.; Ma, Q.; Dastrala, S.; Grenier, F.; Workman, R.; Syed, H.; Ali, S.; Barasch, J.; Devarajan, P. , Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 2008, 3, 665–673. [Google Scholar] [CrossRef]
- Tian, M.; Zhao, S. , [The early diagnostic value of urinary Sema3A for ICU adult patients with acute kidney injury]. Zhonghua Yi Xue Za Zhi 2015, 95, 1457–1462. [Google Scholar]
- Doi, K.; Noiri, E.; Nangaku, M.; Yahagi, N.; Jayakumar, C.; Ramesh, G. , Repulsive guidance cue semaphorin 3A in urine predicts the progression of acute kidney injury in adult patients from a mixed intensive care unit. Nephrol Dial Transplant 2014, 29, 73–80. [Google Scholar] [CrossRef]
- Fang, W.; Wang, Z.; Li, Q.; Wang, X.; Zhang, Y.; Sun, Y.; Tang, W.; Ma, C.; Sun, J.; Li, N.; Yi, F. , Gpr97 Exacerbates AKI by Mediating Sema3A Signaling. J Am Soc Nephrol 2018, 29, 1475–1489. [Google Scholar] [CrossRef]
- Kar, F.; Hacioglu, C.; Senturk, H.; Donmez, D. B.; Kanbak, G.; Uslu, S. , Curcumin and LOXblock-1 ameliorate ischemia-reperfusion induced inflammation and acute kidney injury by suppressing the semaphorin-plexin pathway. Life Sci 2020, 256, 118016. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Pei, L.; Lin, F.; Yin, H.; Li, X.; He, W.; Liu, N.; Gou, X. , Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J Cell Physiol 2019, 234, 23736–23749. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R. R. , Targeting the progression of chronic kidney disease. Nat Rev Nephrol 2020, 16, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Viazzi, F.; Ramesh, G.; Jayakumar, C.; Leoncini, G.; Garneri, D.; Pontremoli, R. , Increased urine semaphorin-3A is associated with renal damage in hypertensive patients with chronic kidney disease: a nested case-control study. J Nephrol 2015, 28, 315–320. [Google Scholar] [CrossRef]
- Iwano, M.; Plieth, D.; Danoff, T. M.; Xue, C.; Okada, H.; Neilson, E. G. , Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 2002, 110, 341–350. [Google Scholar] [CrossRef]
- Takagawa, S.; Nakamura, F.; Kumagai, K.; Nagashima, Y.; Goshima, Y.; Saito, T. , Decreased semaphorin3A expression correlates with disease activity and histological features of rheumatoid arthritis. BMC Musculoskelet Disord 2013, 14, 40. [Google Scholar] [CrossRef]
- Rimar, D.; Nov, Y.; Rosner, I.; Slobodin, G.; Rozenbaum, M.; Halasz, K.; Haj, T.; Jiries, N.; Kaly, L.; Boulman, N.; Vadasz, Z. , Semaphorin 3A: an immunoregulator in systemic sclerosis. Rheumatol Int 2015, 35, 1625–1630. [Google Scholar] [CrossRef]
- Kiriakidou, M.; Ching, C. L. , Systemic Lupus Erythematosus. Ann Intern Med 2020, 172, ITC81–ITC96. [Google Scholar] [CrossRef]
- Kessel, A.; Haj, T.; Peri, R.; Snir, A.; Melamed, D.; Sabo, E.; Toubi, E. , Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev 2012, 11, 670–677. [Google Scholar] [CrossRef]
- Luft, F. C. , Semaphorin-3A is a repulsive but attractive renal guidance cue to therapy. J Mol Med (Berl) 2014, 92, 1225–1227. [Google Scholar] [CrossRef]
- Summers, S. A.; Hoi, A.; Steinmetz, O. M.; O’Sullivan, K. M.; Ooi, J. D.; Odobasic, D.; Akira, S.; Kitching, A. R.; Holdsworth, S. R. , TLR9 and TLR4 are required for the development of autoimmunity and lupus nephritis in pristane nephropathy. J Autoimmun 2010, 35, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Capolunghi, F.; Rosado, M. M.; Cascioli, S.; Girolami, E.; Bordasco, S.; Vivarelli, M.; Ruggiero, B.; Cortis, E.; Insalaco, A.; Fanto, N.; Gallo, G.; Nucera, E.; Loiarro, M.; Sette, C.; De Santis, R.; Carsetti, R.; Ruggiero, V. , Pharmacological inhibition of TLR9 activation blocks autoantibody production in human B cells from SLE patients. Rheumatology (Oxford) 2010, 49, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Doron, R.; Merav, L.; Nasrin, E.; Adi, S. D.; Elias, T.; Gleb, S.; Itzhak, R.; Michael, R.; Zahava, V. , Low Urine Secretion of Semaphorin3A in Lupus Patients with Proteinuria. Inflammation 2022, 45, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Bejar, J.; Kessler, O.; Sabag, A. D.; Sabo, E.; Itzhak, O. B.; Neufeld, G.; Vadasz, Z. , Semaphorin3A: A Potential Therapeutic Tool for Lupus Nephritis. Front Immunol 2018, 9, 634. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





