Submitted:
28 August 2023
Posted:
31 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Raw materials
2.2. Organomineral fertilizers (OMF) production
2.3. Physical characterization
2.4. Fertilizer P solubilization dynamics
2.5. Sampling and analyses
2.6. Statistical analysis
3. Results and Discussion
3.1. Physical-chemical characterization
3.2. Leaching Column Experiment
3.2.1. Leachate pH values
3.2.2. Fertilizer P solubilization dynamics
3.2.3. Soil lability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of Soil and Fertilizer Phosphorus Use. 2008. [Google Scholar]
- Cordell, D.; Drangert, J.O.; White, S. The Story of Phosphorus: Global Food Security and Food for Thought. Global Environmental Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Smil, V. Phosphorus in the Environment: Natural Flows and Human Interferences. Energy and the Environment 2000, 25, 53–88. [Google Scholar]
- Pavinato, P.S.; Cherubin, M.R.; Soltangheisi, A.; Rocha, G.C.; Chadwick, D.R.; Jones, D.L. Revealing Soil Legacy Phosphorus to Promote Sustainable Agriculture in Brazil. Sci Rep 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Santos, D.R. dos; Gatiboni, L.C.; Kaminski, J. Fatores Que Afetam a Disponibilidade Do Fósforo e o Manejo Da Adubação Fosfatada Em Solos Sob Sistema Plantio Direto. Ciencia Rural 2008, 38, 576–586. [Google Scholar] [CrossRef]
- Doydora, S.; Gatiboni, L.; Grieger, K.; Hesterberg, D.; Jones, J.L.; McLamore, E.S.; Peters, R.; Sozzani, R.; Van den Broeck, L.; Duckworth, O.W. Accessing Legacy Phosphorus in Soils. Soil Syst 2020, 4, 1–22. [Google Scholar] [CrossRef]
- Guedes, R.S.; Melo, L.C.A.; Vergütz, L.; Rodríguez-Vila, A.; Covelo, E.F.; Fernandes, A.R. Adsorption and Desorption Kinetics and Phosphorus Hysteresis in Highly Weathered Soil by Stirred Flow Chamber Experiments. Soil Tillage Res 2016, 162, 46–54. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus Dynamics: From Soil to Plant. Plant Physiol 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Houben, D.; Michel, E.; Nobile, C.; Lambers, H.; Kandeler, E.; Faucon, M.P. Response of Phosphorus Dynamics to Sewage Sludge Application in an Agroecosystem in Northern France. Applied Soil Ecology 2019, 137, 178–186. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Potential Benefits and Risks of Land Application of Sewage Sludge. Waste Management 2008, 28, 347–358. [Google Scholar] [CrossRef]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J.A. Struvite: A Slow-Release Fertiliser for Sustainable Phosphorus Management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef]
- van der Kooij, S.; van Vliet, B.J.M.; Stomph, T.J.; Sutton, N.B.; Anten, N.P.R.; Hoffland, E. Phosphorus Recovered from Human Excreta: A Socio-Ecological-Technical Approach to Phosphorus Recycling. Resour Conserv Recycl 2020, 157, 104744. [Google Scholar] [CrossRef]
- Demirbas, A.; Edris, G.; Alalayah, W.M. Sludge Production from Municipal Wastewater Treatment in Sewage Treatment Plant. Energy Sources, Part A: Recovery, Utilization and Environmental Effects 2017, 39, 999–1006. [Google Scholar] [CrossRef]
- Nascimento, A.L.; de Souza, A.J.; Oliveira, F.C.; Coscione, A.R.; Viana, D.G.; Regitano, J.B. Chemical Attributes of Sewage Sludges: Relationships to Sources and Treatments, and Implications for Sludge Usage in Agriculture. J Clean Prod 2020, 258. [Google Scholar] [CrossRef]
- Sayara, T.; Basheer-Salimia, R.; Hawamde, F.; Sánchez, A. Recycling of Organic Wastes through Composting: Process Performance and Compost Application in Agriculture. Agronomy 2020, 10, 23. [Google Scholar] [CrossRef]
- Kominko, H.; Gorazda, K.; Wzorek, Z. The Possibility of Organo-Mineral Fertilizer Production from Sewage Sludge. Waste Biomass Valorization 2017, 8, 1781–1791. [Google Scholar] [CrossRef]
- Chia, W.Y.; Chew, K.W.; Le, C.F.; Lam, S.S.; Chee, C.S.C.; Ooi, M.S.L.; Show, P.L. Sustainable Utilization of Biowaste Compost for Renewable Energy and Soil Amendments. Environmental Pollution 2020, 267, 115662. [Google Scholar] [CrossRef]
- Weeks, J.J.; Hettiarachchi, G.M. A Review of the Latest in Phosphorus Fertilizer Technology: Possibilities and Pragmatism. J Environ Qual 2019, 48, 1300–1313. [Google Scholar] [CrossRef]
- Adam, C.; Peplinski, B.; Michaelis, M.; Kley, G.; Simon, F.G. Thermochemical Treatment of Sewage Sludge Ashes for Phosphorus Recovery. Waste Management 2009, 29, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Günther, S.; Grunert, M.; Müller, S. Overview of Recent Advances in Phosphorus Recovery for Fertilizer Production. Eng Life Sci 2018, 18, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Vogel, T.; Nelles, M.; Eichler-Löbermann, B. Phosphorus Application with Recycled Products from Municipal Waste Water to Different Crop Species. Ecol Eng 2015, 83, 466–475. [Google Scholar] [CrossRef]
- Hermann, L.; Schaaf, T. Outotec (AshDec®) Process for P Fertilizers from Sludge Ash. In Phosphorus Recovery and Recycling; 2018; pp. 221–233 ISBN 9789811080319.
- Stemann, J.; Adam, C.; Hermann, L. Production of Citrate Soluble Phosphates by Calcination of Secundary Phosphate Sources with a Sodium-Sulfuric Compound 2015, 26.
- Brazil RESOLUÇÃO No 498, DE 19 DE AGOSTO DE 2020; Brazil, 2020; pp. 265–269;
- IFDC Manual for Determining Physical Properties of Fertilizer; International Fertilizer Development Center: Muscle Shoals, 1986; ISBN 2053816600.
- et al. Sistema Brasileiro de Classificação de Solos; 5th ed.; Brasília, 2018; ISBN 978-85-7035-198-2.
- Cantarella, H.; Quaggio, J.A.; Matos Jr., D.; Boaretto, R.M.; Raij, B. van Boletim 100: Recomendações de Adubação e Calagem Para o Estado de São Paulo; Cantarella, H., Quaggio, J.A., Matos Jr., D., Boaretto, R.M., Raij, B. van, Eds.; Campinas, 2022.
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal Chim Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Science Society of America Journal 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Gatiboni, L.C.; Kaminski, J.; Rheinheimer, D. dos S.; Flores, J.P.C. Biodisponibilidade de Formas de Fósforo Acumuladas Em Solo Sob Sistema Plantio Direto. Rev Bras Cienc Solo 2007, 31, 691–699. [Google Scholar] [CrossRef]
- Camargo, M.S. de Solubilidade e Disponibilidade Do Fósforo de Fosfatos Naturais Com Origens Geológicas Diferentes. dissertation, University of São Paulo, 1997.
- Vasconcelos, C.A.; Santos, H.L. Dos; Franca, G.E. De; Pitta, G.V.E.; Bahia Filho, A.F.C. Eficiencia Agronomica de Fosfatos Naturais Para a Cultura Do Sorgo-Granifero. I. Fosforo Total e Soluvel Em Acido Citrico e Granulometria. Rev Bras Cienc Solo 1986, 10, 117–121. [Google Scholar]
- Raniro, H.R.; Soares, T. de M.; Adam, C.; Pavinato, P.S. Waste-Derived Fertilizers Can Increase Phosphorus Uptake by Sugarcane and Availability in a Tropical Soil#. Journal of Plant Nutrition and Soil Science 2022, 1–12. [CrossRef]
- Lemming, C.; Bruun, S.; Jensen, L.S.; Magid, J. Plant Availability of Phosphorus from Dewatered Sewage Sludge, Untreated Incineration Ashes, and Other Products Recovered from a Wastewater Treatment System. Journal of Plant Nutrition and Soil Science 2017, 180, 779–787. [Google Scholar] [CrossRef]
- Nikiema, J.; Cofie, O.; Impraim, R.; Adamtey, N. Processing of Fecal Sludge to Fertilizer Pellets Using a Low-Cost Technology in Ghana. Environment and Pollution 2013, 2. [Google Scholar] [CrossRef]
- Hettiarachchi, L.; Jayathilake, N.; Fernando, S.; Gunawardena, S. Effects of Compost Particle Size, Moisture Content and Binding Agents on Co-Compost Pellet Properties. International Journal of Agricultural and Biological Engineering 2019, 12, 184–191. [Google Scholar] [CrossRef]
- Zafari, A.; Kianmehr, M.H. Factors Affecting Mechanical Properties of Biomass Pellet from Compost. Environmental Technology (United Kingdom) 2014, 35, 478–486. [Google Scholar] [CrossRef]
- Tabil, L.G. Binding and Pelleting Characteristics Os Alfalfa, University of Saskatchewan, 1996.
- Kaliyan, N.; Morey, R.V. Factors Affecting Strength and Durability of Densified Biomass Products. Biomass Bioenergy 2009, 33, 337–359. [Google Scholar] [CrossRef]
- Gilvari, H.; de Jong, W.; Schott, D.L. Quality Parameters Relevant for Densification of Bio-Materials: Measuring Methods and Affecting Factors - A Review. Biomass Bioenergy 2019, 120, 117–134. [Google Scholar] [CrossRef]
- Jiang, L.; Liang, J.; Yuan, X.; Li, H.; Li, C.; Xiao, Z.; Huang, H.; Wang, H.; Zeng, G. Co-Pelletization of Sewage Sludge and Biomass: The Density and Hardness of Pellet. Bioresour Technol 2014, 166, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Racz, G.J.; Soper, R.J. Reaction Products of Orthophosphates in Soils Containing Varying Amounts of Calcium and Magnesium. Can J Soil Sci 1967, 47. [Google Scholar] [CrossRef]
- Lombi, E.; McLaughlin, M.J.; Johnston, C.; Armstrong, R.D.; Holloway, R.E. Mobility and Lability of Phosphorus from Granular and Fluid Monoammonium Phosphate Differs in a Calcareous Soil. Soil Science Society of America Journal 2004, 68, 682–689. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, Z.S. Carbon and Nitrogen Mineralization of Sewage Sludge Compost in Soils with a Different Initial PH. Soil Sci Plant Nutr 2009, 55, 715–724. [Google Scholar] [CrossRef]
- Jouraiphy, A.; Amir, S.; El Gharous, M.; Revel, J.C.; Hafidi, M. Chemical and Spectroscopic Analysis of Organic Matter Transformation during Composting of Sewage Sludge and Green Plant Waste. Int Biodeterior Biodegradation 2005, 56, 101–108. [Google Scholar] [CrossRef]
- Moretti, S.M.L.; Bertoncini, E.I.; Abreu-Junior, C.H. Composting Sewage Sludge with Green Waste from Tree Pruning. Sci Agric 2015, 72, 432–439. [Google Scholar] [CrossRef]
- Raniro, H.R.; Bettoni Teles, A.P.; Adam, C.; Pavinato, P.S. Phosphorus Solubility and Dynamics in a Tropical Soil under Sources Derived from Wastewater and Sewage Sludge. J Environ Manage 2022, 302, 113984. [Google Scholar] [CrossRef]
- Brännvall, E.; Wolters, M.; Sjöblom, R.; Kumpiene, J. Elements Availability in Soil Fertilized with Pelletized Fly Ash and Biosolids. J Environ Manage 2015, 159, 27–36. [Google Scholar] [CrossRef]
- Weihrauch, C.; Opp, C. Ecologically Relevant Phosphorus Pools in Soils and Their Dynamics: The Story so Far. Geoderma 2018, 325, 183–194. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; McBeath, T.M.; Smernik, R.; Stacey, S.P.; Ajiboye, B.; Guppy, C. The Chemical Nature of P Accumulation in Agricultural Soils-Implications for Fertiliser Management and Design: An Australian Perspective. Plant Soil 2011, 349, 69–87. [Google Scholar] [CrossRef]
- Antille, D.L.; Sakrabani, R.; Godwin, R.J. Phosphorus Release Characteristics from Biosolids-Derived Organomineral Fertilizers. Commun Soil Sci Plant Anal 2014, 45, 2565–2576. [Google Scholar] [CrossRef]
- Gatiboni, L.C. Disponibilidade de Formas de Fósforo Do Solo Às Plantas, Universidade Federal de Santa Maria, 2003.
- Rheinheimer, D.S.; Anghinoni, I.; Kaminski, J. Depleção Do Fósforo Inorgânico de Diferentes Frações Provocada Pela Extração Sucessiva Com Resina Em Diferentes Solos e Manejos. Rev Bras Cienc Solo 2000, 24, 345–354. [Google Scholar] [CrossRef]
- Nanzer, S.; Oberson, A.; Huthwelker, T.; Eggenberger, U.; Frossard, E. The Molecular Environment of Phosphorus in Sewage Sludge Ash: Implications for Bioavailability. J Environ Qual 2014, 43, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L. Organic Acids in the Rhizosphere – a Critical Review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Almeida, D.S.; Delai, L.B.; Sawaya, A.C.H.F.; Rosolem, C.A. Exudation of Organic Acid Anions by Tropical Grasses in Response to Low Phosphorus Availability. Sci Rep 2020, 10, 1–8. [Google Scholar] [CrossRef]
- O’Connor, G.A.; Sarkar, D.; Brinton, S.R.; Elliott, H.A.; Martin, F.G. Phytoavailability of Biosolids Phosphorus. J Chem Inf Model 2013, 53, 1689–1699. [Google Scholar] [CrossRef]
- Baptistella, J.L.C.; Llerena, J.P.P.; Domingues-Júnior, A.P.; Fernie, A.R.; Favarin, J.L.; Mazzafera, P. Differential Responses of Three Urochloa Species to Low Phosphorus Availability. Annals of Applied Biology 2021, 179, 216–230. [Google Scholar] [CrossRef]
- Brazil Instrução Normativa No 61, de 8 de Julho de 2020; Brazil, 2020; p. 33;
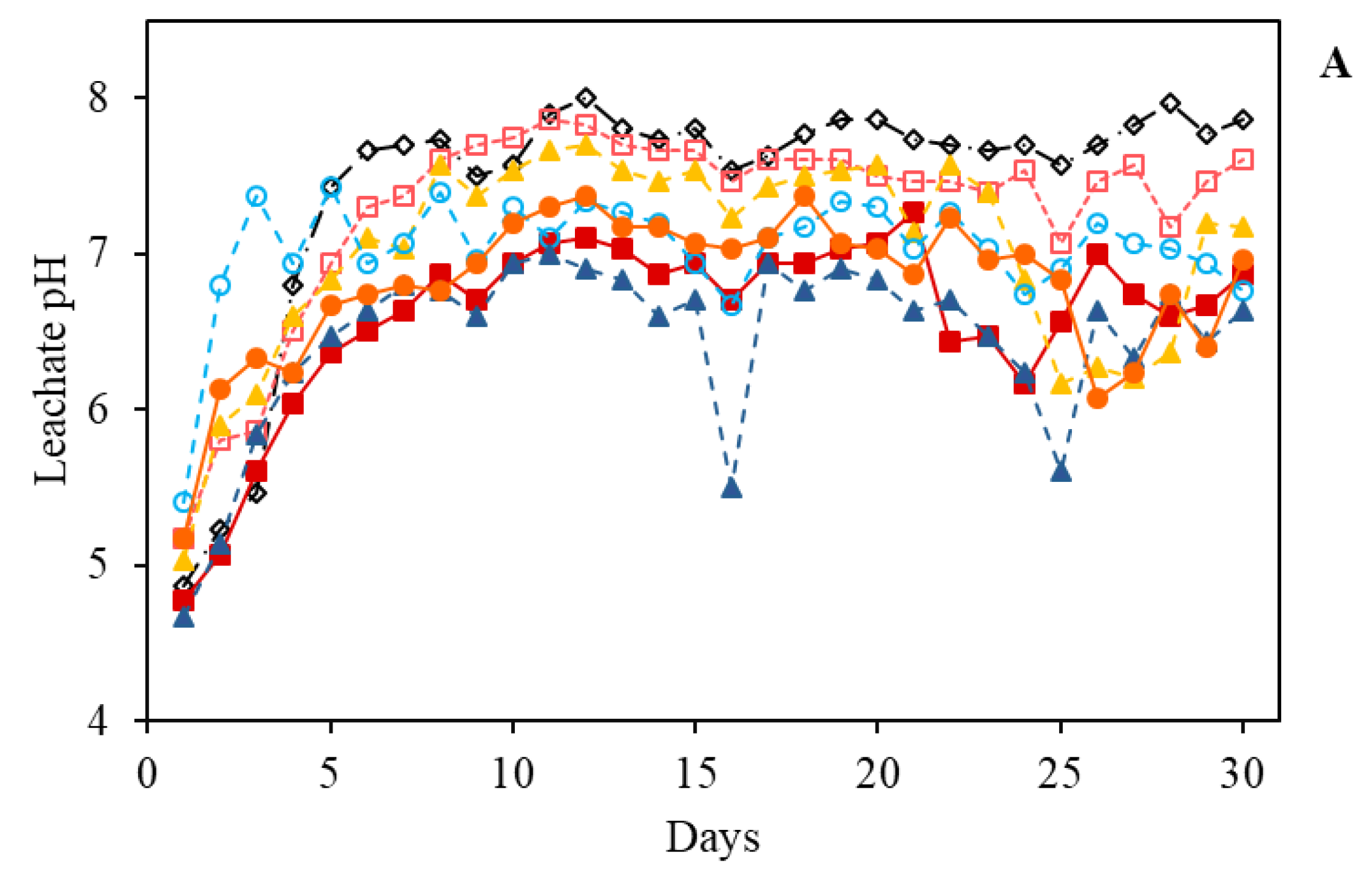
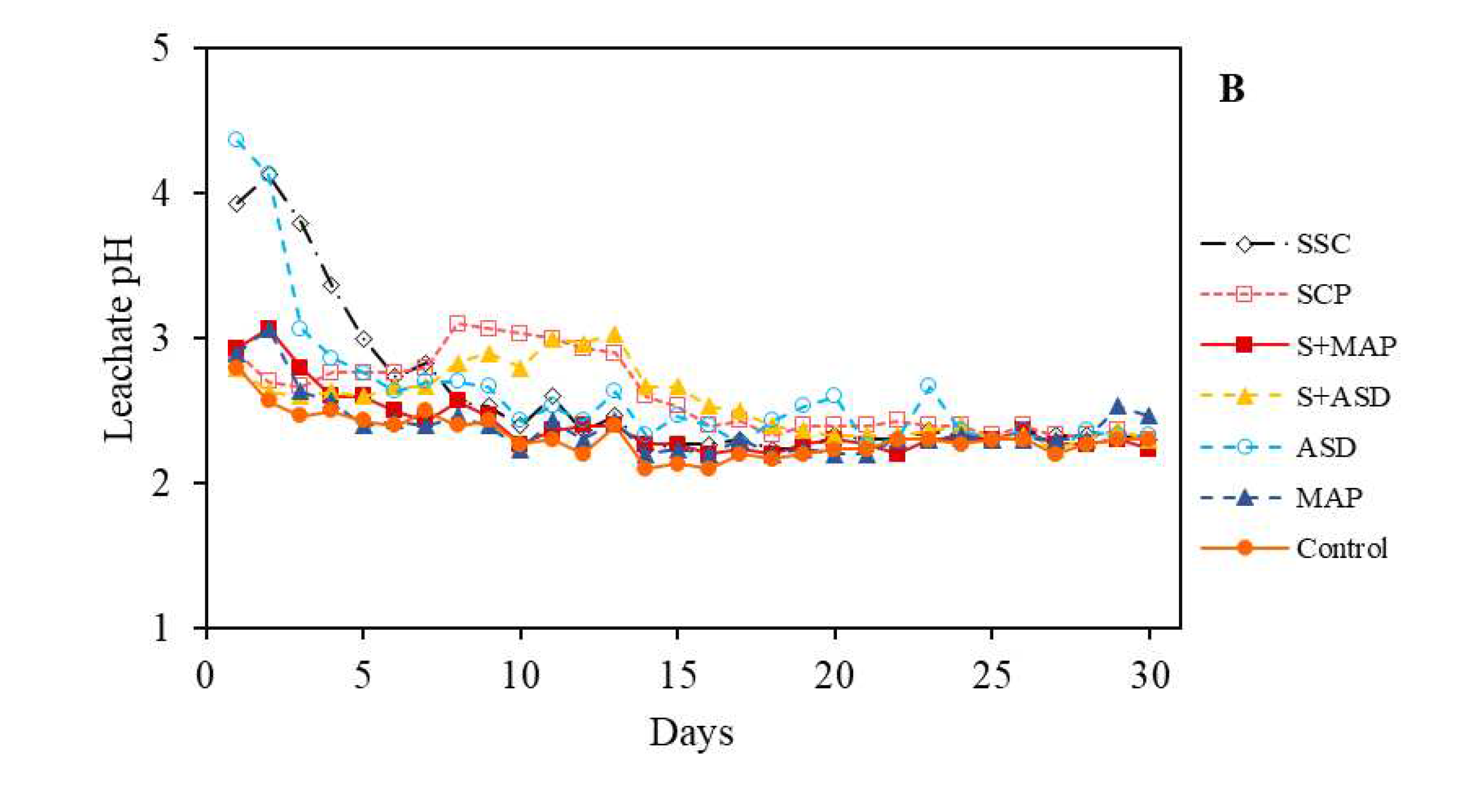
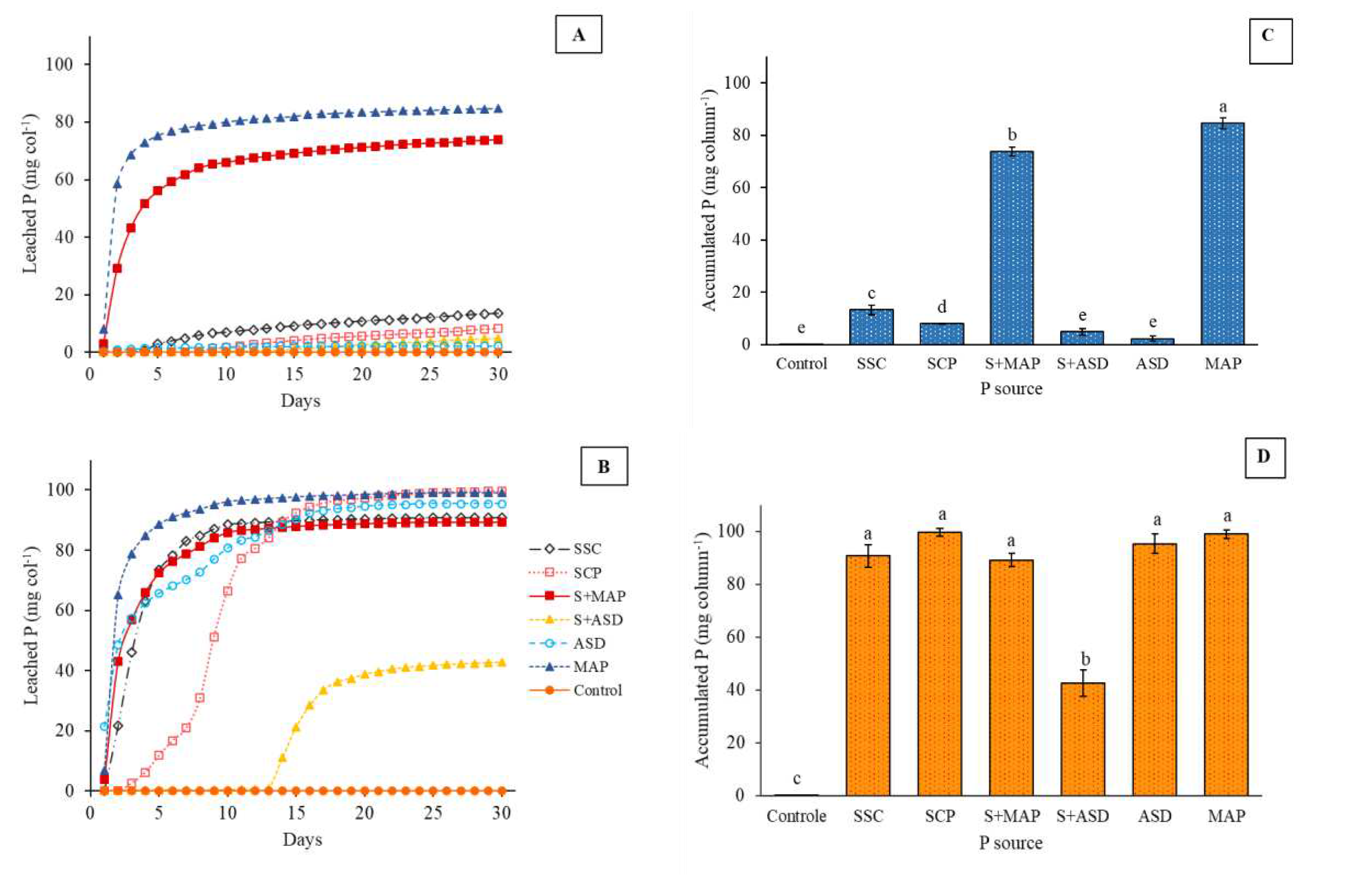
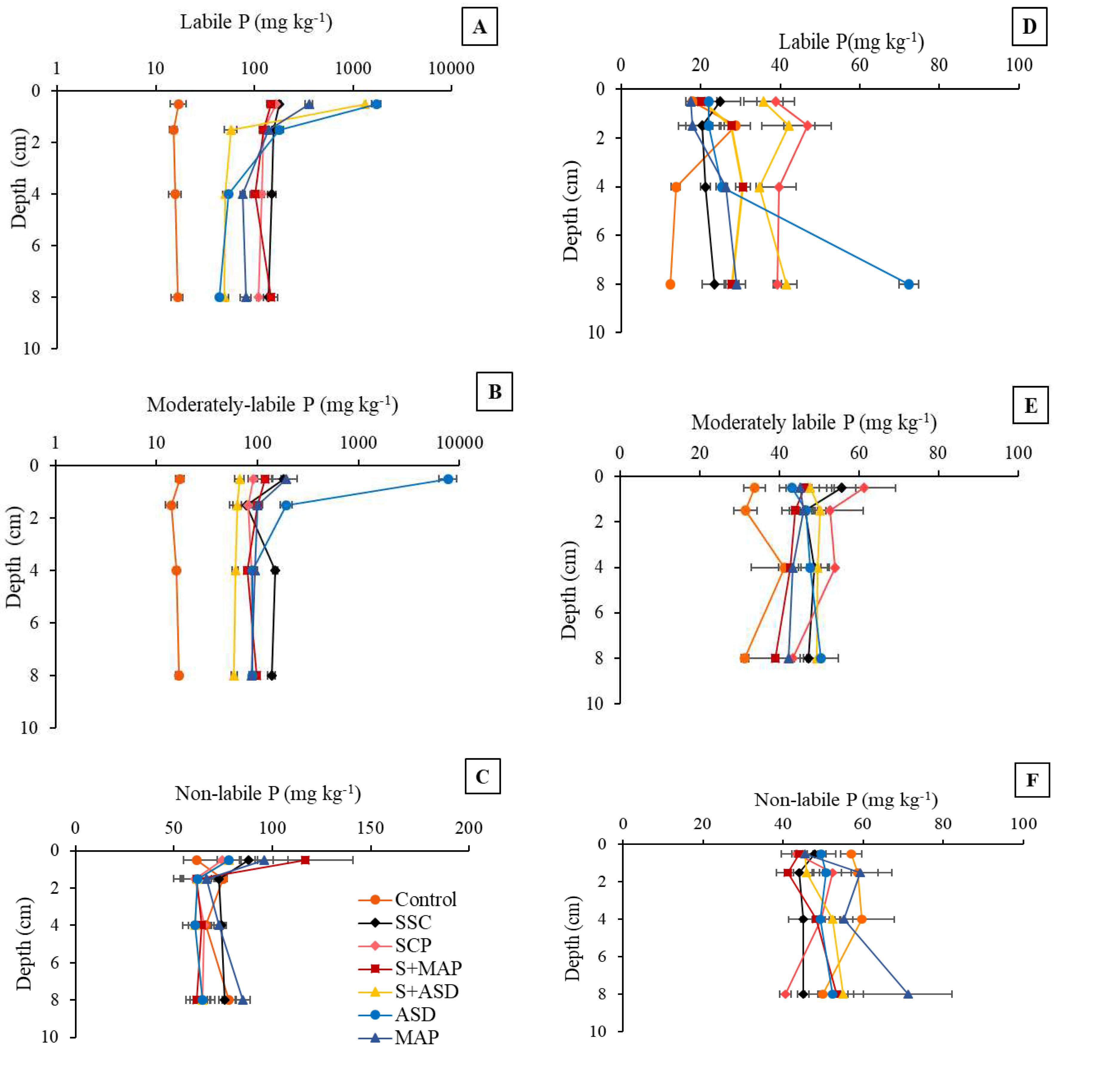
| pH | O.M. | P | S | K | Ca | Mg | Al | H+Al | SB | CEC | V | m |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaCl2 | g dm-3 | mg dm-3 | ------------------------- mmolc dm-3 ------------------------- | ----- % ----- | ||||||||
| 4.6 | 14.0 | <6 | 9 | 1 | 4 | 3 | 2 | 22 | 8 | 30 | 27 | 20 |
| Sand | Silt | Clay | ||||||||||
| ---------- % ---------- | ||||||||||||
| 74 | 9 | 17 | ||||||||||
| Fertilizer | N | K2O | Ca | Mg | Na | P2O5 Total |
P2O5 H2O |
P2O5 HCi |
P2O5 NaC |
|---|---|---|---|---|---|---|---|---|---|
| ---------------------------------------------- % --------------- -------------------------- | |||||||||
| SSC | 3.73 | 1.10 | 3.08 | 0.63 | 0.09 | 2.46 | 0.4 | 2.20 | 2.30 |
| SCP | 3.73 | 1.10 | 3.08 | 0.63 | 0.09 | 2.46 | 0.4 | 2.20 | 2.30 |
| S+MAP | 5.91 | 0.77 | 2.16 | 0.44 | 0.06 | 17.34 | 13.48 | - | 17.21 |
| S+ASD | 2.61 | 0.64 | 4.36 | 0.76 | 3.34 | 6.75 | 0.49 | 6.22 | - |
| ASD | - | 0.33 | 9.50 | 1.50 | 11.00 | 16.60 | 0.70 | 15.60 | - |
| MAP | 11.00 | - | - | - | 52.00 | 44.00 | - | 52.00 | |
| Fertilizer | Diameter (mm) |
Length (mm) |
Humidity (%) |
Density (g cm-3) |
Resistance (kg) |
|---|---|---|---|---|---|
| SSC | - | - | 6.40 | 0.44 | - |
| SCP | 3.78±0.14 | 15.50±2.02 | 5.19 | 0.61 | 10.24±2.12 |
| S+MAP | 4.16±0.23 | 16.63±2.22 | 3.98 | 0.67 | 8.08±1.38 |
| S+ASD | 3.9±0.12 | 19.12±2.30 | 4.09 | 0.71 | 6.00±1.96 |
| ASD | - | - | 0.00 | 1.17 | - |
| MAP | 3.80±0.48 | - | 1.87 | 1.13 | 6.90±1.19 |
| Treatment | Leached P | Labile P | Moderately labile P | Non-labile P | Recovered P |
|---|---|---|---|---|---|
| ---------------------------------- (%) -------------------------------- | |||||
| Leaching with deionized water | |||||
| Control | 1.9 | 12.3 | 31.3 | 54.5 | 100.0 |
| SSC | 42.7 | 23.8 | 21.5 | 12.0 | 29.0 |
| SCP | 36.9 | 27.6 | 20.5 | 15.1 | 20.2 |
| S+MAP | 84.1 | 7.1 | 5.1 | 3.7 | 82.3 |
| S+ASD | 24.5 | 43.3 | 15.4 | 16.3 | 18.5 |
| ASD | 3.5 | 19.0 | 69.4 | 8.0 | 60.3 |
| MAP | 85.2 | 5.7 | 5.1 | 4.0 | 93.2 |
| Leaching with deionized citric acid | |||||
| Control | 0.2 | 14.4 | 33.4 | 52.0 | 100.0 |
| SSC | 94.0 | 1.2 | 2.5 | 2.3 | 91.7 |
| SCP | 94.0 | 1.8 | 2.2 | 2.0 | 100.7 |
| S+MAP | 91.9 | 3.6 | 2.1 | 2.5 | 90.2 |
| S+ASD | 85.9 | 3.9 | 5.0 | 5.3 | 47.3 |
| ASD | 92.8 | 2.2 | 2.4 | 2.6 | 97.7 |
| MAP | 93.9 | 1.2 | 2.0 | 2.9 | 100.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





