1. Introduction
Chagas disease is a neglected tropical disease and at the same time also a most elusive disease [
1,
2]. Due to the chronic nature of Chagas disease, with an indeterminate phase that is asymptomatic and lasts for decades, the vast majority of the carriers do not know that they are infected. For the same reason, there are no solid data on the prevalence of Chagas disease. The epidemiology of Chagas disease is further complicated by (i) the large zoonotic reservoir of
Trypanosoma cruzi, which infects all kinds of mammals provided they are preyed upon by the triatomine vectors [
3]; (ii) alternative transmission routes, including via the oral mucosa upon consumption of contaminated food [
4], via blood or organ donation [
5], and transplacental to the unborn child [
6]; (iii) the genetic heterogeneity and genomic flexibility of
T. cruzi with its (at least) seven different Discrete Typing Units (DTU) [
7,
8].
The parasites are elusive also in the human body.
Trypanosoma cruzi can infect any type of nucleated cell, and the parasites will replicate intracellularly in the cytosol of the host cell. Infected macrophages distribute the parasites throughout the body. Thus, they can access different tissues and niches to hide in, including the heart and the intestinal tract, the typical sites of chronic pathology [
9,
10]. Trypomastigote
T. cruzi do not proliferate but persist extracellularly in the blood thanks to their elaborate immune evasion strategies [
11]. The intracellular amastigotes, too, can enter a non-replicative state of dormancy [
12,
13]. All this makes Chagas disease difficult to diagnose and even harder to cure, as became apparent in the clinical trials with new antichagasic drug candidates [
14,
15]. In the laboratory, research on
T. cruzi is hampered by the fact that the disease-relevant stages, the amastigotes, are strictly intracellular and require host cells for
in vitro culture. The infectious nature of
T. cruzi renders all experimental investigation resource-intensive in terms of biosafety measures, assay time, and overall cost [
16].
On a positive note, there has been tremendous technological progress in genomics and reverse genetics with
T. cruzi, which has boosted basic research as well as drug discovery. Classical genetic manipulation based on homologous recombination [
17,
18] is being replaced by CRISPR/Cas9-mediated gene editing [
19], which allows for functional genomics in spite of the fact that
T. cruzi lacks the RNA interference machinery [
20]. Genetically engineered reporter strains of
T. cruzi have enabled assay formats that better predict the potential of antichagasic molecules for irreversible and cidal action, both
in vitro and
in vivo [
21,
22]. Here we present a new reference strain,
T. cruzi STIB980, which is useful for all kinds of investigation including genomics, reverse genetics, and drug efficacy testing.
2. Materials and Methods
2.1. Cell cultivation
Trypanosoma cruzi epimastigotes were cultured at 27 °C in LIT medium supplemented with 2 µg/mL hemin and 10% heat-inactivated fetal calf serum (iFCS) [
23]. The cultures were diluted weekly. Metacyclogenesis was stimulated by keeping the epimastigotes for 3 to 4 weeks in the same medium. Mouse embryonic fibroblasts (MEF) were cultured at 37 °C, 5% CO
2 in RPMI medium supplemented with 10% iFCS and >95% humidity. The MEF were subpassaged weekly at a ratio of 1:10 after 5 min treatment with trypsin. Peritoneal mouse macrophages (PMM) were obtained from female CD1 mice. A 2% starch solution in distilled water was injected
i.p., and the macrophages were harvested 24 h later by peritoneal lavage. The cells were washed and resuspended in RPMI medium containing 1× antibiotic cocktail [
24], 10% iFCS, and 15% medium conditioned by LADMAC cells (ATCC® CRL2420™), which secrete colony stimulating factor 1 (CSF-1). The macrophages were kept in this medium at 37 °C for 3 to 4 days and then detached with trypsin treatment and cell scrapers. The isolation of PMM from mice was conducted in accordance with the strict guidelines set out by the Swiss Federal Veterinary Office, under the ethical approval of license number #2374.
2.2. Cloning of T. cruzi
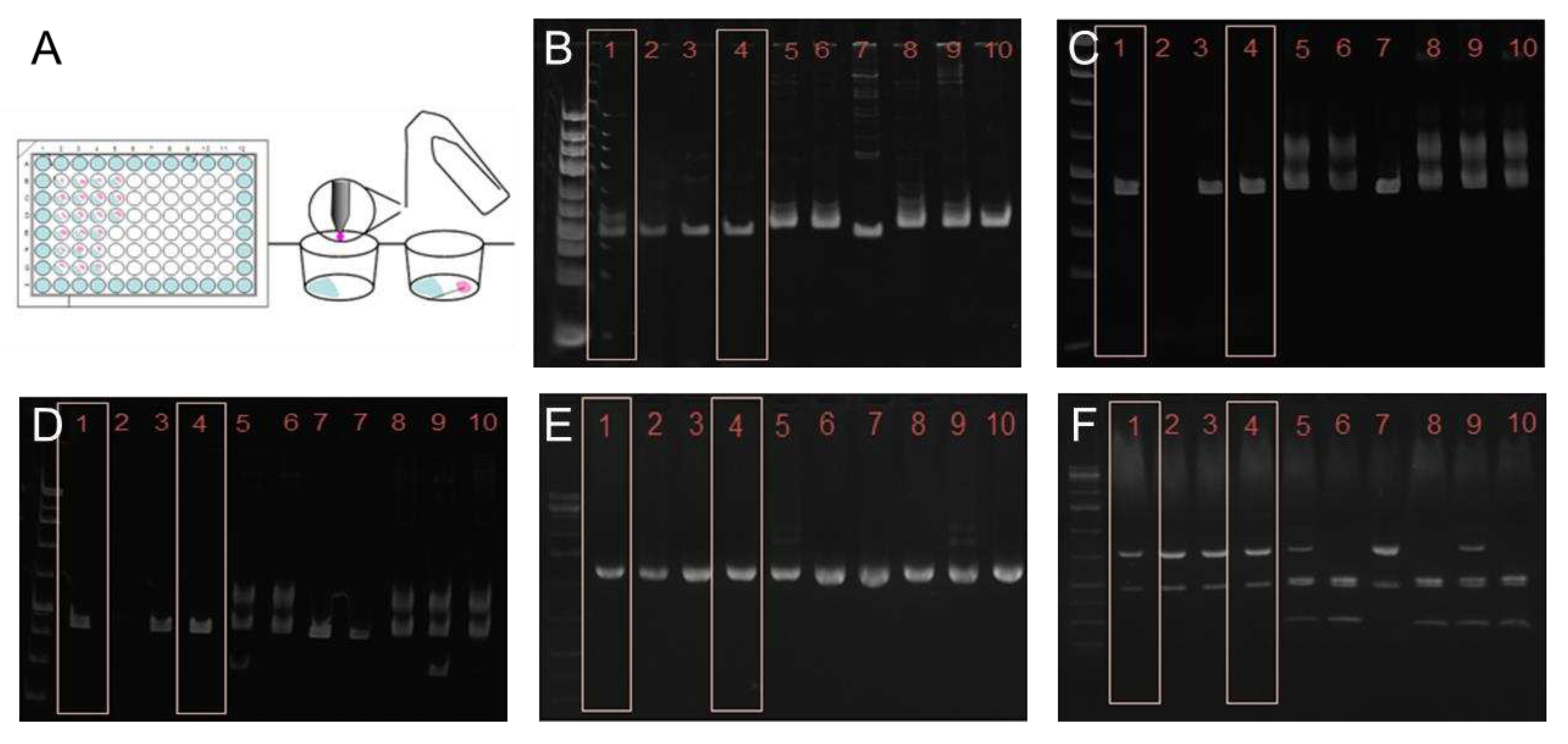
The gilded paper clip method was used for cloning (
Figure 1A). An exponentially growing epimastigote culture was diluted to 5×10
4 cells/mL. The outer wells of a 96-well plate were filled with 100 μL sterile water. 15 μL of conditioned LIT medium supplemented with 10% filtered post-culture medium and 20% iFCS were placed at the edge of the other wells, so that some space of the well remained dry. Using a gold-plated paperclip, a micro-drop of approximately 0.1 μL was transferred from the diluted parasite suspension to the dry space of the well. Two people analysed the droplet under an inverted microscope. Wells that contained only one parasite were supplemented with 35 μL of conditioned LIT. The plates were incubated at 27 °C and assessed regularly for outgrowth of the clones.
2.3. Isolation of genomic DNA
Genomic DNA for genome sequencing was isolated from 108 epimastigotes. The cells were washed and resuspended in 500 µl NTE, and lysed by addition of 25 µL of 10% SDS. The lysate was treated with 50 µl RNase A (10 mg/mL) and 25 µl pronase (20 mg/mL), and incubated over night at 37 °C. The lysate was extracted sequentially with phenol and chloroform:isoamyl alcohol (24:1). The DNA was precipitated by the addition of 1 mL cold absolute ethanol. For Illumina sequencing, the DNA was pelleted by centrifugation; for Oxford Nanopore sequencing, the DNA was collected with a glass hook. The DNA was washed with 70% ethanol, air-dried, and resuspended in 80 µL DNase-free water. For other purposes, genomic DNA was isolated with the QIAGEN DNeasy blood and tissue kit.
2.4. Genome sequencing and assembly
Library preparation and sequencing on the Illumina platform was performed at the Quantitative Genomics Facility Basel (GFB) of the ETH Zürich. Sequencing libraries were prepared using the PCR-free KAPA HyperPrep kit (Illumina). Paired-end sequencing of 125 nucleotides was done on an Illumina HiSeq 2500 sequencer. For Nanopore sequencing, the library was prepared using the Ligation Sequencing kit 108 (SQK-LSK108, Oxford Nanopore Technology) and sequenced using the MinION platform. Basecalling was carried out using Albacore. Quality control for all reads was done with FastQC (version 0.11.3) [
25]. Different assemblers were tested: Velvet [
26] and SOAPdenovo (version 2.04) [
27] for the Illumina reads (with a range of different k-mer sizes from 17 to 73), Canu (version 1.7) [
28] and Flye (release 2.3.3) [
29] for the Nanopore reads. Illumina polishing of the Canu-assembled Nanopore reads was performed using Pilon (version 1.22) [
30]. The Flye assembly was done on the pore-chopped long reads, with an expected genome size of 53 Mb [
29].
2.5. Optimization of electroporation
10
7 epimastigotes from a dense culture were centrifuged and resuspended in 100 µL TbBSF buffer [
31] containing 10 µg of circular (for transient transfection) or linearized (for stable transfection) plasmid DNA. The cells were electroporated with a nucleofector device (Lonza) in a 0.2 mm cuvette (BioRad). After electroporation, the cells were transferred to 10 mL LIT with a fine-tipped Pasteur pipette. The parasites transfected with circular plasmid were incubated for 24 h and then tested for GFP expression with flow cytometry on a FACSCalibur machine (Becton Dickinson and Company, Franklin Lakes, NJ, USA). The parasites transfected with linearized plasmid were incubated for 24 h, diluted 1:10 in medium containing 100 µg/mL G418 (Gibco), and further distributed in a fourfold dilution series in a 48-well plate under antibiotic pressure. Outgrowing epimastigotes were cloned by limiting dilution and assessed for correct integration of the transgene by PCR and Southern blot.
2.6. Generation of transgenic lines
Exponentially growing
T. cruzi epimastigotes were synchronized for 24 h with 20 mM of hydroxyurea (Sigma) [
32]. Following hydroxyurea removal by washing twice with PBS, 10
7 epimastigotes were electroporated with 2.5 μg of pTRIX2 Luc::Neon-HYG plasmid linearized with
AscI and
SacI (New England Biolabs) or pLEW-Cas9 plasmid linearized with
NotI (New England Biolabs). The pTRIX2 Luc::Neon-HYG plasmid had been derived from pTRIX-REh9 [
33]. CRISPR-Cas9 mediated genetic knock-out was performed according to [
34,
35]. The resistance cassette was amplified by PCR from plasmid pPOTcruzi v1 blast-blast mNeonGreen with primers 5’ aacatcaagaagggaccagcccccttctacggtagttaagagctcggacccac (forward) and 5’ acgagtgctggggcgtcggtttccactatcccaatttgagagacctgtgc (reverse). sgRNA were amplified using a gene specific forward primer (5’ gaaattaatacgactcactatagggagtacttctacacagccatgttttagagctagaaatagc and 5’ gaaattaatacgactcactataggcggctgtgccgtcctccagggttttagagctagaaatagc) and the G00 (sgRNA scaffold) reverse primer. 24 h after transfection, the parasites were diluted 1:10 in medium containing 100 μg/mL G418 (Gibco).
2.7. Drug sensitivity assay with epimastigotes
In a 96-well microtiter plate, 100 µL epimastigotes at a starting density of 5×10
6/mL, 10
5/mL, or 2×10
4/mL were incubated with test compound in threefold serial dilution with 11 dilution steps. After 69 h or 165 h of incubation at 27 °C, 10 µL of resazurin (Sigma) solution (12.5 mg in 100 mL water) were added to each well. After another 3 h of incubation, the plates were read with a SpectraMAX GeminiXS fluorescence reader (Molecular Devices, San Jose, CA). 50% inhibitory values (IC50) were determined in R version 3.5.1 (R Core Team 2018) using the “drc” package [
36].
2.8. Flow cytometry
For flow cytometry, 105 epimastigotes were fixed with 10% formalin (Sigma) for 15 min and then analyzed for the green fluorescence levels (FL1) on a BD FACSCalibur (Becton Dickinson and Company, Franklin Lakes, NJ, USA). The threshold for GFP expression was set above the autofluorescence level of 99.6% of the untransfected control cells. The proportion of GFP expressing cells was defined as the proportion of cells exhibiting a higher level of fluorescence than the threshold.
2.9. High-content drug efficacy assay
Assays were performed with two technical and two biological replicates. For the standard assay, 10
4 PMM were seeded into the central wells of a black 96-well plate (Greiner, uClear, black, REF 655090, Lot E1803364) in 100 µl of RPMI medium containing 1% antibiotic mix [
24], 10% iFCS and 15% RPMI containing LADMAC growth factors per well. The border wells were filled with 100 µl of water. After 48 hours, the PMM were infected with 10
4 trypomastigotes from either the wildtype or the transgenic STIB980 line. After 24 h, the remaining trypomastigotes were washed off twice with 200 µl RPMI per well. The infected PMM were kept in 100 µL RPMI containing 1% antibiotic mix and 10% iFCS. Drugs were added in three-fold serial dilution 24 h post-infection. 96 h after addition of drugs, the plates were fixed with 10% formalin for 15 min at room temperature. Subsequently, the plates were stained with 50 µl of 5 µM Draq5 (Merck, Darmstadt, Germany) per well for 30 min at room temperature in the dark. The plates were stored at 4 °C for at least 24 h and then imaged using an ImageXpress Micro XLS microscope (Molecular Devices, San Jose, CA) with a 20× Zeiss objective with the Cy5 filter cube for 300 ms per image on 9 sites per well. Image analysis was performed with the MetaXpress 6 software. Statistical analysis and graphs were done in R version 3.5.1 (R Core Team 2018) using the packages “tidyverse” [
37] and “readxl” [
38].
3. Results and Discussion
3.1. Genotyping and cloning of T. cruzi STIB980
Trypanosoma cruzi STIB980, originally received in 1983 from Prof. Antonio Osuna, University of Granada, is one of the standard strains used for drug efficacy testing at the Parasite Chemotherapy Unit of the Swiss TPH. Amastigote and epimastigote forms are readily cultured as described under Methods. A fresh clone of
T. cruzi STIB980 was made with epimastigotes, employing the gilded paperclip method (
Figure 1A).
This clone of
T. cruzi STIB980 was used for all further analyses. Genotyping based on restriction fragment length polymorphisms of three target loci (the large ribosomal RNA subunit, heat-shock protein 60, and glucose-6-phosphate isomerase) [
39] placed
T. cruzi STIB980 in DTU TcI (
Figure 1B to F). TcI is the DTU that circulates most broadly among humans, and is correlated mostly with cardiomyopathic symptoms [
40,
41]. Therefore, a TcI strain is highly relevant as an assay strain.
3.2. Genome sequence of T. cruzi STIB980
Genomic DNA of
T. cruzi STIB980 was sequenced on the Illumina HiSeq 2500 and the Oxford Nanopore MinION platforms. Illumina sequencing was done with a 125-bp paired end protocol and yielded 67,187,531 reads that passed quality control. Assuming a diploid genome size of 53.3 Mb as reported for
T. cruzi Dm28c [
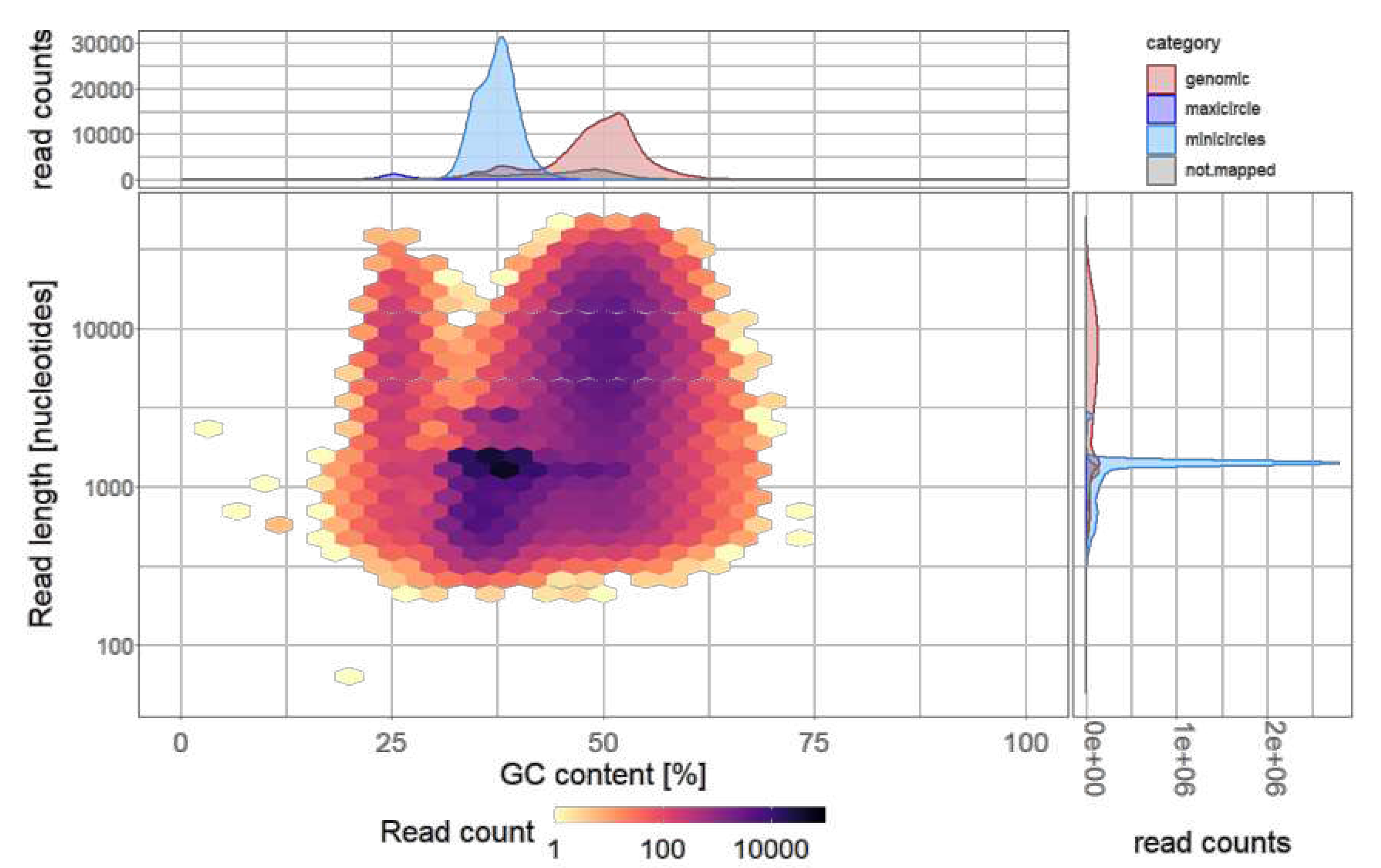
42], this corresponds to a coverage of 158-fold. With Nanopore sequencing we obtained 250,005 reads and a median length of 1.4 kb. The estimated coverage, again assuming a genome size of 53.3 Mb, was 12-fold. The reads were categorized according to their size and GC content (
Figure 2) into nuclear genome, maxicircle (assembled to a single contig), minicircles, and sequences of unknown origin (
Table 1).
The best results for genome assembly (as judged by the mapping rate) was obtained by first assembling the long Nanopore reads (using Canu v1.7 [
28]), followed by fixing errors with the short Illumina reads (using Pilon v1.22 [
30]). This combination of Nanopore and Illumina reads led to drastic improvements compared to the assembly based on Illumina reads alone: the number of contigs was reduced 23-fold, the N50 increased 30-fold, and the numbers of gaps (n=13,000) and undetermined nucleotides (5 Mb) were reduced to zero. The total assembly amounted to 28.2 Mb in 492 contigs; the nuclear genome had a haploid size of 27.9 Mb in 397 contigs (
Table 1).
Gene prediction was performed using GLIMMER [
43] with the standard codon table. The genome of
T. cruzi Dm28c [
42] served as training set. This resulted in 10,043 open-reading frames (ORFs) with a median length of 1077 bp. The amino acid sequences were queried against UniProt KnowledgeBase [
44] using blastp [
45] with an expectancy (E-value) cut-off of 10
-8. This allowed the functional annotation of 3,505 genes.
3.3. Antibiotic sensitivity profile of epimastigote T. cruzi STIB980
In order to determine the best selection markers for use in genetic manipulation, we tested the sensitivity of
T. cruzi STIB980 epimastigotes to commonly used antibiotics: blasticidin, G418, hygromycin, phleomycin, and puromycin. Benznidazole and nifurtimox were included as benchmark drugs, DMSO as the most commonly used solvent of test compounds. Drug sensitivity was tested for 72 h and 168 h of incubation. For the latter, we used two different inocula: a lower starting density (2×10
4 epimastigotes/mL) to assess the inhibition of proliferation, a higher density (10
5 epimastigotes/mL) to measure cidality. However, the obtained IC
50 values were similar across all the tested conditions (
Table 2). The STIB980 epimastigotes had comparably high IC
50 values for G418, which is in agreement with the high concentrations (100 to 500 µg/mL) of G418 that are generally used for epimastigote
T. cruzi [
32] and in stark contrast to the 1 to 5 µg/mL used in the genetic manipulation of procyclic
T. brucei [
31].
Besides the sensitivity of the untransfected trypanosomes, other factors will determine the optimal concentration of antibiotics to select positive transfectants. The expression level of the resistance gene will be affected by its copy number (especially in episomal transfections) and by the strength of the promoter, by the RNA polymerase (RNAPolII usually resulting in a lower level of transcription than RNAPolI), and – in case of the ribosomal locus – by the exact site of integration [
46]. Overall, we recommend blasticidin or puromycin to select for
T. cruzi STIB980 transfectants, rather than G418, hygromycin, or phleomycin.
3.4. Optimal transfection protocol for T. cruzi STIB980
The Lonza nucleofector 2b is a widely used electroporation device for genetic transfection. It provides excellent results also with trypanosomes but is a black box, as the provider does not disclose the characteristics of the electric discharge nor the composition of the buffers. Tests on nucleofector programs had already been published for
T. brucei [
31] and
T. cruzi [
47]. We investigated which program is best suited for
T. cruzi STIB980. Epimastigotes were transfected with a circular pTcRG plasmid, kindly provided by Santuza Teixeira (Federal University of Minas Gerais, Brazil), that contained the green fluorescent protein (GFP) gene plus the 3’ UTR of the GAPDH gene, which confers constitutive expression. 4×10
7 epimastigotes in the exponential growth phase were transfected with 10 µg plasmid DNA using nine different nucleofector programs. Immediately after transfection, we counted the surviving parasites. Then, we incubated them for 24 h in 10 mL LIT medium at 27 °C. Finally, the proportion of GFP expressing parasites was quantified by flow cytometry [
31]. The transfection efficiency was calculated as the product of cell survival and GFP positivity (
Table 3). The programs U-033, X-001, and Z-001 had the best overall efficiencies. The lower survival rates with Z-001 and U-033 were compensated by higher fractions of GFP expression. Program X-001 was recommended for the transfection of
Leishmania mexicana promastigotes [
48]. For subsequent transfections, we have used the Nucleofector programs U-033 or X-014 [
47].
3.5. Transgenic T. cruzi STIB980 lines for drug testing and reverse genetics
The levels of cytosolic GFP obtained after stable transfection of pTcRG were too low for high-content fluorescence microscopy. Most of the parasite signal was below three times the background level (i.e. the autofluorescence of untransfected epimastigotes). For better use of
T. cruzi STIB980 in drug efficacy testing and molecular genetics, we generated stable transgenic lines expressing a
LucNeon reporter gene,
Cas9 nuclease gene, or both [
34].
LucNeon is a chimeric gene encoding a fusion protein of mNeonGreen, suitable for fluorescence-based
in vitro imaging, plus a red-shifted luciferase that is suitable for bioluminescence-based
in vivo imaging [
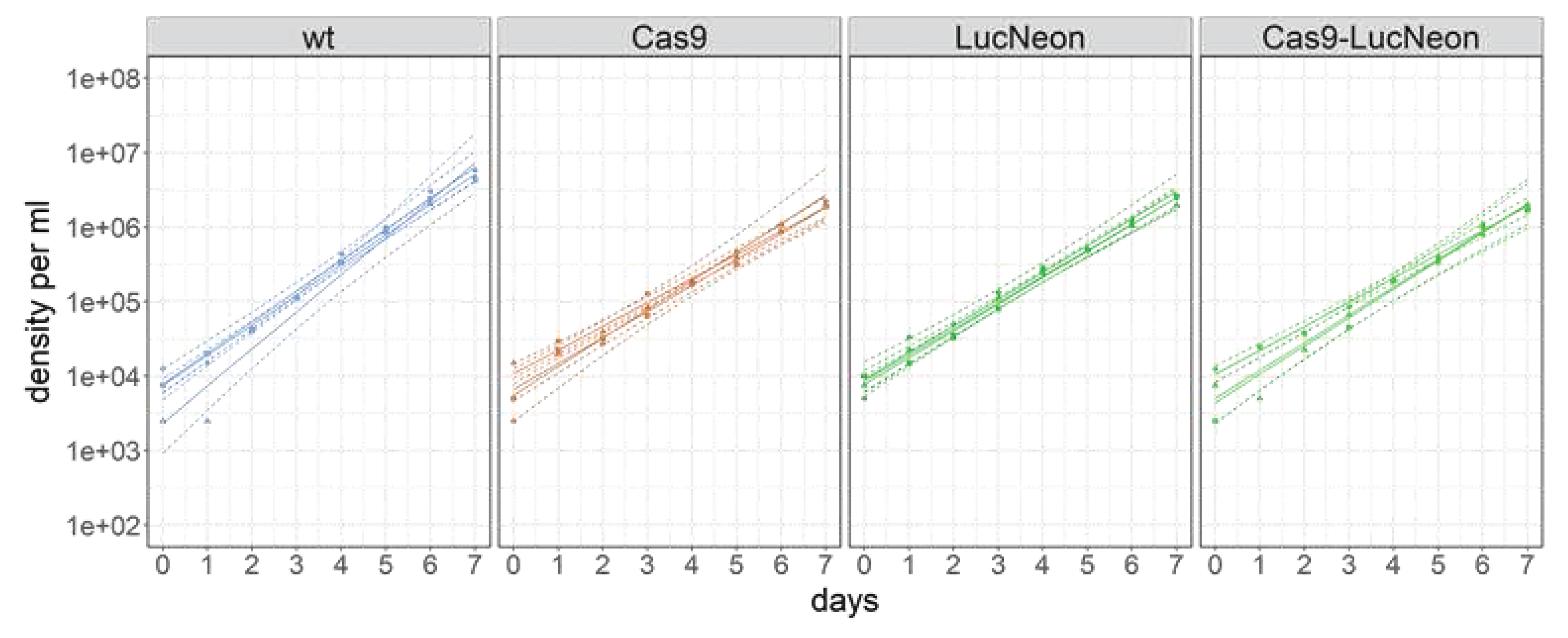
34]. The plasmids had been kindly provided by John Kelly (LSHTM). Epimastigotes were transfected as described in the Methods section. The three resulting transgenic lines all had similar growth rates with population doubling times around 20 h, slightly higher than the 17 h of the parental
T. cruzi STIB980 (
Figure 3).
The sensitivity profiles to reference drugs (benznidazole and nifurtimox) and drug candidates (posaconazole, fexinidazole, and the oxaborole DNDi-6148) of parental
T. cruzi STIB980 and STIB980-LucNeon were determined by high content imaging of intracellular amastigotes in expanded mouse peritoneal macrophages. The IC
50 values were calculated by two different methods, either based on the number of infected host cells or the total number of intracellular amastigotes (
Table 4).
The first method resulted in slightly higher IC
50 values, which was to be expected as the total number of parasites is reduced more readily than the host cells cured of the infection. Overall, the drug sensitivities of the parental STIB980 and transgenic derivative were very similar using either method (
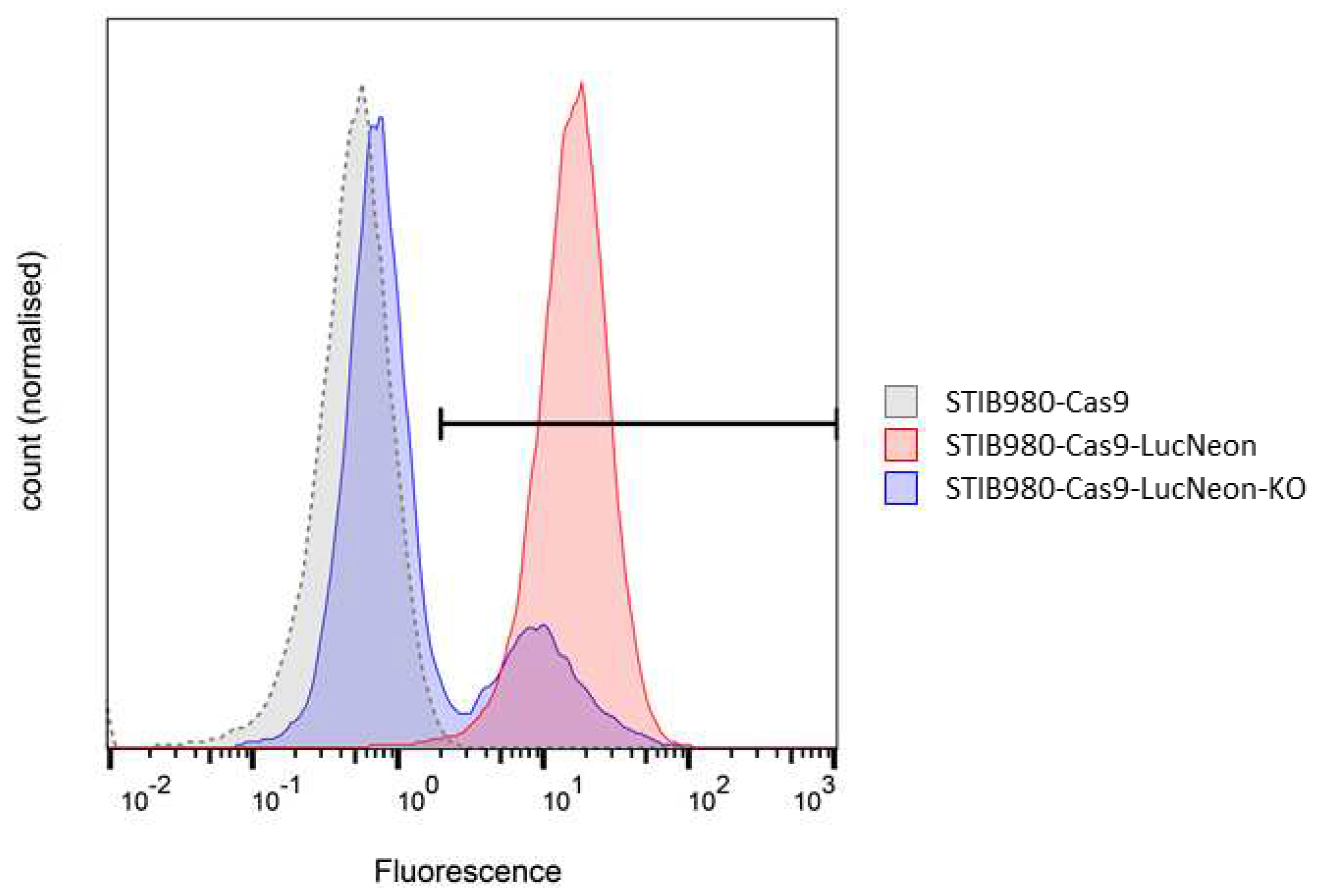
Table 4). The function of the Cas9 nuclease was validated by CRISPR/Cas9-mediated deletion of the fluorescence reporter using specific guide RNAs for the
LucNeon gene (
Figure 4).
4. Conclusions
Trypanosoma cruzi STIB980 is a useful new assay strain in the toolbox of antichagasic drug discovery. It is a DTU TcI strain that is readily cultured in vitro and amenable to genetic manipulation. We provide optimized electroporation conditions and the antibiotic sensitivity profile of epimastigotes to facilitate genetic transfection. The genome sequence of T. cruzi STIB980 was assembled by combining short reads generated by Illumina sequencing and long reads generated by Oxford Nanopore sequencing, demonstrating the power of combining both technologies, in particular for a genome with a high degree of repetitive regions like that of T. cruzi. We further provide T. cruzi STIB980 derivatives that express reporter genes (eGFP, LucNeon) for imaging in vitro and in vivo. The reporter genes are stable in the absence of selective pressure in epimastigotes but much less so in amastigotes, underlining the importance of frequently resorting to a new stabilate, e.g. when running drug testing campaigns against intracellular amastigotes. To facilitate CRISPR/Cas9-mediated gene editing, we have also constructed a line of T. cruzi STIB980-LucNeon with a stably integrated Cas9 gene, and validated that line by knocking-out the LucNeon gene as a proof-of-principle. Thus T. cruzi STIB980 can serve not only as a reference strain for drug efficacy testing but also as a tool for molecular genetics.
Author Contributions
Conceptualization, A.F., S.B., M.K., R.S.S. and P.M.; methodology, A.F., M.K. and R.S.S.; formal analysis, A.F., S.B. and R.S.S.; investigation, A.F., S.B. and R.S.S.; data curation, A.F. and R.S.S.; writing—original draft preparation, A.F. and P.M.; writing—review and editing, A.F., S.B., M.K., R.S.S. and P.M.; visualization, A.F., S.B. and R.S.S.; supervision, A.F., M.K. and P.M.; funding acquisition, A.F. and P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swiss National Science Foundation (grants No. CRSII5_183536 and IZRJZ3_164172), the Nikolaus und Bertha Burckhardt-Bürgin-Stiftung, and the Freiwillige Akademische Gesellschaft.
Data Availability Statement
All sequencing reads were deposited at the National Center for Biotechnology Information as BioProject PRJNA1009388. The assay strain T. cruzi STIB980 and its transgenic derivatives described herein are available from the authors.
Acknowledgments
We wish to thank Antonio Osuna for the kind provision of the original T. cruzi strain in 1984; Richard Neher and Nicholas Noll for all their help with Nanopore sequencing; Thomas Otto for advice on genome assembly; Santuza Teixeira for the plasmid pTcRG; John Kelly and Mark Taylor for the plasmid pTRIX2-RE9h; Michael Lewis for DNA of T. cruzi reference strains and recommendations concerning genotyping; and Monica Cal, Christina Kunz and Romina Rocchetti for expert technical assistance. Illumina sequencing was carried out on the University of Basel/ETHZ Genomics Platform, genome assembly was performed on sciCORE, the scientific computing cluster of the University of Basel.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Perez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef] [PubMed]
- De Fuentes-Vicente, J.A.; Santos-Hernandez, N.G.; Ruiz-Castillejos, C.; Espinoza-Medinilla, E.E.; Flores-Villegas, A.L.; de Alba-Alvarado, M.; Cabrera-Bravo, M.; Moreno-Rodriguez, A.; Vidal-Lopez, D.G. What Do You Need to Know before Studying Chagas Disease? A Beginner’s Guide. Trop. Med. Infect. Dis. 2023, 8. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.M.; Xavier, S.; Roque, A.L.R. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasit Vectors 2018, 11, 502. [Google Scholar] [CrossRef]
- Velasquez-Ortiz, N.; Ramirez, J.D. Understanding the oral transmission of Trypanosoma cruzi as a veterinary and medical foodborne zoonosis. Res Vet Sci 2020, 132, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Angheben, A. , Boix, L., Buonfrate, D., Gobbi, F., Bisoffi, Z., Pupella, S., Gandini, G., Aprili, G. Chagas disease and transfusion medicine: a perspective from non-endemic countries. Blood Transfus 2015, 13, 540–550. [Google Scholar] [PubMed]
- Edwards, M.S.; Montgomery, S.P. Congenital Chagas disease: progress toward implementation of pregnancy-based screening. Curr Opin Infect Dis 2021, 34, 538–545. [Google Scholar] [CrossRef]
- Velásquez-Ortiz, N.; Herrera, G.; Hernández, C.; Muñoz, M.; Ramírez, J.D. Discrete typing units of Trypanosoma cruzi: Geographical and biological distribution in the Americas. Sci Data 2022, 9, 360. [Google Scholar] [CrossRef]
- Wang, W.; Peng, D.; Baptista, R.P.; Li, Y.; Kissinger, J.C.; Tarleton, R.L. Strain-specific genome evolution in Trypanosoma cruzi, the agent of Chagas disease. PLoS Pathog 2021, 17, e1009254. [Google Scholar] [CrossRef]
- Matsuda, N.M.; Miller, S.M.; Evora, P.R. The chronic gastrointestinal manifestations of Chagas disease. Clinics 2009, 64, 1219–1224. [Google Scholar] [CrossRef]
- Lewis, M.D.; Francisco, A.F.; Taylor, M.C.; Kelly, J.M. A new experimental model for assessing drug efficacy against Trypanosoma cruzi infection based on highly sensitive in vivo imaging. J Biomol Screen 2015, 20, 36–43. [Google Scholar] [CrossRef]
- Ramírez-Toloza, G.; Ferreira, A. Trypanosoma cruzi Evades the Complement System as an Efficient Strategy to Survive in the Mammalian Host: The Specific Roles of Host/Parasite Molecules and Trypanosoma cruzi Calreticulin. Front Microbiol 2017, 8, 1667. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Valdez, F.J.; Padilla, A.; Wang, W.; Orr, D.; Tarleton, R.L. Spontaneous dormancy protects Trypanosoma cruzi during extended drug exposure. Elife 2018, 7. [Google Scholar] [CrossRef]
- Ward, A.I.; Olmo, F.; Atherton, R.L.; Taylor, M.C.; Kelly, J.M. Trypanosoma cruzi amastigotes that persist in the colon during chronic stage murine infections have a reduced replication rate. Open Biol 2020, 10, 200261. [Google Scholar] [CrossRef]
- Molina, I.; Gomez i Prat, J.; Salvador, F.; Trevino, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; Valerio, L.; et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med 2014, 370, 1899–1908. [Google Scholar] [CrossRef]
- Morillo, C.A.; Waskin, H.; Sosa-Estani, S.; Del Carmen Bangher, M.; Cuneo, C.; Milesi, R.; Mallagray, M.; Apt, W.; Beloscar, J.; Gascon, J.; et al. Benznidazole and Posaconazole in Eliminating Parasites in Asymptomatic T. Cruzi Carriers: The STOP-CHAGAS Trial. J Am Coll Cardiol 2017, 69, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Beilstein, S.; El Phil, R.; Sahraoui, S.S.; Scapozza, L.; Kaiser, M.; Maser, P. Laboratory Selection of Trypanosomatid Pathogens for Drug Resistance. Pharmaceuticals (Basel) 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.C.; Huang, H.; Kelly, J.M. Genetic techniques in Trypanosoma cruzi. Adv Parasitol 2011, 75, 231–250. [Google Scholar]
- Docampo, R. Molecular parasitology in the 21st century. Essays Biochem 2011, 51, 1–13. [Google Scholar] [CrossRef]
- Lander, N.; Chiurillo, M.A. State-of-the-art CRISPR/Cas9 Technology for Genome Editing in Trypanosomatids. J Eukaryot Microbiol 2019. [Google Scholar] [CrossRef]
- Kolev, N.G.; Tschudi, C.; Ullu, E. RNA interference in protozoan parasites: achievements and challenges. Eukaryot Cell 2011, 10, 1156–1163. [Google Scholar] [CrossRef]
- Fesser, A.F.; Braissant, O.; Olmo, F.; Kelly, J.M.; Maser, P.; Kaiser, M. Non-invasive monitoring of drug action: A new live in vitro assay design for Chagas’ disease drug discovery. PLoS Negl Trop Dis 2020, 14, e0008487. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.C.; Francisco, A.F.; Jayawardhana, S.; Mann, G.S.; Ward, A.I.; Olmo, F.; Lewis, M.D.; Kelly, J.M. Exploiting Genetically Modified Dual-Reporter Strains to Monitor Experimental Trypanosoma cruzi Infections and Host-Parasite Interactions. Methods Mol Biol 2019, 1955, 147–163. [Google Scholar] [PubMed]
- Fernandes, J.F.; Castellani, O. Growth Characteristics and Chemical Composition of Trypanosoma cruzi. Experimental Parasitology 1966, 18, 195–202. [Google Scholar] [CrossRef]
- Mäser, P.; Grether-Bühler, Y.; Kaminsky, R.; Brun, R. An anti-contamination cocktail for the in vitro isolation and cultivation of parasitic protozoa. Parasitol Res 2002, 88, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Babraham Bioinformatics. 2022. FastQC A Quality Control tool for High Throughput Sequence Data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/. Accessed 12.05.2022.
- Zerbino, D.R.; Birney, E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 2008, 18, 821–829. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, G.; Tang, J.; Luo, R.; Patterson, J.; Liu, S.; Huang, W.; He, G.; Gu, S.; Li, S.; et al. SOAPdenovo-Trans: de novo transcriptome assembly with short RNA-Seq reads. Bioinformatics 2014, 30, 1660–1666. [Google Scholar] [CrossRef]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res 2017, 27, 722–736. [Google Scholar] [CrossRef]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol 2019, 37, 540–546. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; Earl, A.M. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 2014, 9, e112963. [Google Scholar] [CrossRef]
- Burkard, G.; Fragoso, C.M.; Roditi, I. Highly efficient stable transformation of bloodstream forms of Trypanosoma brucei. Mol Biochem Parasitol 2007, 153, 220–223. [Google Scholar] [CrossRef]
- Olmo, F.; Costa, F.C.; Mann, G.S.; Taylor, M.C.; Kelly, J.M. Optimising genetic transformation of Trypanosoma cruzi using hydroxyurea-induced cell-cycle synchronisation. Molecular and Biochemical Parasitology 2018, 226, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.D.; Fortes Francisco, A.; Taylor, M.C.; Burrell-Saward, H.; McLatchie, A.P.; Miles, M.A.; Kelly, J.M. Bioluminescence imaging of chronic Trypanosoma cruzi infections reveals tissue-specific parasite dynamics and heart disease in the absence of locally persistent infection. Cell Microbiol 2014, 16, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.C.; Francisco, A.F.; Jayawardhana, S.; Calderano, S.G.; Lewis, M.D.; Olmo, F.; Beneke, T.; Gluenz, E.; Sunter, J.; Dean, S.; Kelly, J.M.; Taylor, M.C. Expanding the toolbox for Trypanosoma cruzi: A parasite line incorporating a bioluminescence-fluorescence dual reporter and streamlined CRISPR/Cas9 functionality for rapid in vivo localisation and phenotyping. PLoS Negl Trop Dis 2018, 12, e0006388. [Google Scholar] [CrossRef] [PubMed]
- Beneke, T.; Madden, R.; Makin, L.; Valli, J.; Sunter, J.; Gluenz, E. A CRISPR Cas9 high-throughput genome editing toolkit for kinetoplastids. R Soc Open Sci 2017, 4, 170095. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS One 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Wickham, H. 2017. tidyverse: Easily Install and Load the ‘Tidyverse’. https://CRAN.R-project.org/package=tidyverse.
- Wickham, H., & Bryan, J. 2018. readxl: Read Excel Files. https://CRAN.R-project.org/package=readxl.
- Messenger, L.A.; Miles, M.A. Evidence and importance of genetic exchange among field populations of Trypanosoma cruzi. Acta Trop 2015, 151, 150–155. [Google Scholar] [CrossRef]
- Zingales, B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Tropica 2018, 184, 38–52. [Google Scholar] [CrossRef]
- Izeta-Alberdi, A.; Ibarra-Cerdena, C.N.; Moo-Llanes, D.A.; Ramsey, J.M. Geographical, landscape and host associations of Trypanosoma cruzi DTUs and lineages. Parasit Vectors 2016, 9, 631. [Google Scholar] [CrossRef]
- Berná, L.; Rodriguez, M.; Chiribao, M.L.; Parodi-Talice, A.; Pita, S.; Rijo, G.; Alvarez-Valin, F.; Robello, C. Expanding an expanded genome: long-read sequencing of Trypanosoma cruzi. Microb Genom 2018, 4. [Google Scholar] [CrossRef]
- Salzberg, S.L.; Delcher, A.L.; Kasif, S.; White, O. Microbial gene identification using interpolated Markov models. Nucleic Acids Res 1998, 26, 544–548. [Google Scholar] [CrossRef]
- Consortium, U. UniProt: the Universal Protein Knowledgebase in 2023. Nucleic Acids Res 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J Mol Biol 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Alsford, S.; Kawahara, T.; Glover, L.; Horn, D. Tagging a T. brucei RRNA locus improves stable transfection efficiency and circumvents inducible expression position effects. Mol Biochem Parasitol 2005, 144, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Lugo, L.; Díaz-Olmos, Y.; Sáenz-García, J.; Probst, C.M.; DaRocha, W.D. Effective gene delivery to Trypanosoma cruzi epimastigotes through nucleofection. Parasitol Int 2017, 66, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Beneke, T.; Gluenz, E. LeishGEdit: A Method for Rapid Gene Knockout and Tagging Using CRISPR-Cas9. Methods Mol Biol 2019, 1971, 189–210. [Google Scholar] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).