1. Introduction
In skeletal muscle, a network of signaling pathways regulates protein synthesis and protein degradation, and under certain physiological and pathological conditions this regulation can be disrupted or disturbed leading to skeletal muscle atrophy [
1]. This atrophy results from an imbalance between protein degradation and protein synthesis and can be induced in skeletal muscle under various conditions including physical inactivity, muscle disuse, cancer, and aging [
1,
2]. Several lines of evidence clearly show that muscle wasting, due to reduced muscle mass and strength, is the major contributor to all-cause mortality in chronic diseases such as cancer [
3,
4]. Tumor-induced metabolic dysfunction, together with systemic inflammation [
5], appears to affect protein turnover [
6] and promotes muscle wasting, characterized by a decrease in protein content, fiber diameter, force production, and fatigue resistance, with serious implications for effective response to pharmacological treatment in cancer patients [
7,
8,
9,
10,
11,
12]. There is increasing evidence that loss of muscle mass is an inherent condition in these patients, regardless of the disease stage [
13], and that loss of appendicular lean mass is associated with increased mortality in all cancer sites [
14,
15,
16,
17].
In addition, the ability of skeletal muscle to respond to various stimuli such as exercise, immobilization, and disease, depends on its regenerative capacity, which allows damaged myofibers to be replaced by new ones without altering the original tissue structure [
18]. The ability to repair under stress is achieved by replacing the original tissue with connective tissue (fibrosis), resulting in the loss of the original structure and, therefore its functionality [
19,
20,
21].
Considerable efforts have been made in recent years to understand the mechanisms underlying muscle wasting and fibrosis and to find an effective agent to counteract both. Exercise has been proposed as a possible intervention to mitigate and/or reverse this muscle dysfunction in cancer patients, by downregulating the activity of pro-inflammatory cytokines, and by increasing the phosphorylation of contractile proteins [
22,
23]. However, there are inconsistencies in the studies that have examined the effects of cancer on muscle wasting, and it is not entirely clear which muscles are favored in the process of cancer-induced atrophy. In contrast to inactivity-induced atrophy, which mainly affects slow oxidative muscles, it is generally accepted that glycolytic muscles are more susceptible to cancer-induced muscle wasting [
24,
25], and selective atrophy of type II fibers, which may be accompanied by a fast-to-slow shift, has been reported in mouse models of cancer [
26,
27,
28], although, there are also conflicting results [
29].
The present study used a mouse model of breast cancer to determine the animal’s response to endurance exercise training, using two different phenotypic muscles (soleus and gastrocnemius). The aim is to investigate the role of long-term exercise training on muscle wasting, and collagen disposition, and to assess whether exercise can attenuate this and improve their regenerative capacity.
2. Materials and Methods
2.1. Animal model and experimental design
The animals, 50 female Sprague-Dawley mice (38 days old; 289 ± 17g body weight) from Harlan Interfauna Inc. (Barcelona, Spain), were housed in collective cages (4 animals per cage) and maintained under controlled atmospheric conditions (21-22°C; 60 ± 5 % humidity) with a 12/12-hour light/dark cycle and free access to food (standard laboratory diet 4RF21
® Mucedola, Italy) and water. After one week of acclimatization animals were randomly assigned to one of four experimental groups: sedentary MNU (MNU+SED, n = 15); exercised MNU (MNU+EX, n = 15); sedentary control (CONT+SED, n = 10) and exercised control (CONT+EX, n = 10). Animals in the MNU groups were injected (i.p.) with 1-methyl-1-nitrosureia (ISOPAC
®, Sigma Chemical Co., Madrid, Spain) at a dose of 50 mL/kg, while the other two groups received only the vehicle (saline solution 0,9%). Two days after carcinogen injection animals from the exercised groups started a treadmill running program (TRP) for thirty-five weeks (Treadmill Control LE 8710, Harvard Apparatus, USA). Duration and intensity of running were gradually increased during the first two weeks until 60 m/day, 5 days/week, and 20 m/m of speed were reached (an estimated work rate of 70% of maximal oxygen consumption was reached [
30]). The animal protocol was approved by the Portuguese Ethics Committee for Animal Experimentation (General Directorate for Food and Veterinary) under license number 008961. At the end of the experimental protocol, the animals were euthanized by i.p. injection of ketamine (75mg/Kg, Imalgen
® 1000, Merial SAS, Lyon, France) and xylazine (10 mg/Kg, Rompun
® 2%, Bayer Healthcare S. A., Kiel, Germany). The Gastrocnemius and Soleus muscles were removed, weighed, and prepared for histological analysis. Body weight was determined taking into account baseline and final body weight, and weight gain in the control groups, to characterize overall wasting [
31].
2.2. Histology
Muscles were pinned to their in vivo length in a paraffin plate and fixed in this position with 4% paraformaldehyde (in 0,1M PBS, pH 7,4; with 2,5% w/v Sucrose and 0,1% Glutaraldehyde) for 24 hours. The samples were washed in PBS 0,1M, pH 7,4, dehydrated in graded ethanol solutions, cleared in xylene, and embedded in paraffin. Paraffin blocks were then sectioned at 5 µm using a Leica 2125 rotary microtome (Leica Microsystems Inc.). The paraffin-embedded slides were deparaffinized in xylene and hydrated through a graded series of ethanol.
Light microscope: For light microscopy analysis, the slides of gastrocnemius and soleus muscles from each animal were stained with H&E for quantitative cross-sectional area (CSA) analysis, and with PSR (Picrosirius red) for collagen content (CC) analysis.
2.3. Tissue analysis
Slides from one section of each muscle stained with PSR were scanned using a Virtual Slide System VS110 (Olympus, Japan). The digital slides were analyzed to quantify collagen deposition using Image Pro (Media Cybernetics, version 6.0). For CSA quantification, images were captured with a coupled digital camera (Axio Imager A1, Carl Zeiss; Germany) and analyzed using NIH ImageJ software (Image Processing and Analysis in Java). An average of 1468 ± 189 fibers from each muscle and each group were analyzed for CSA quantification, all digitized sections were used for CC.
To analyze the frequency of the fiber-size distribution, the fibers were divided into different quartiles and categorized according to their size. In the gastrocnemius muscle, the fibers measuring less than 792 μm2 and 977 μm2, for CONT+SED and CONT+EX respectively, were categorized as small (quartile 1 – Q1), and those measuring more than 1616 μm2/1964 μm2, for CONT+SED and CONT+EX respectively, were categorized as large (quartile 3 - Q3). The same procedure was used for the soleus muscle (CONT+SED - Q1 < 950 μm2; Q3 > 1589 μm2 and CONT+EX – Q1 < 1030 μm2; Q3 > 1751).
2.4. Statistical analysis
Graph Pad Prism software (version 10.0) was used for all analyses. Kruskall-Wallis one-way analyses of variance, followed by Dunn’s multiple comparisons test were used to test differences between groups in the absence of normality (muscle CSA and CC, and fiber diameter). Results are presented as mean ± standard deviation (SD), for weight, and as median and interquartile range (IQR) for CSA and CC. Differences were considered significant at p < 0.05.
3. Results
3.1. Morphometric analysis of gastrocnemius and soleus muscles
3.1.1. Cross-sectional area
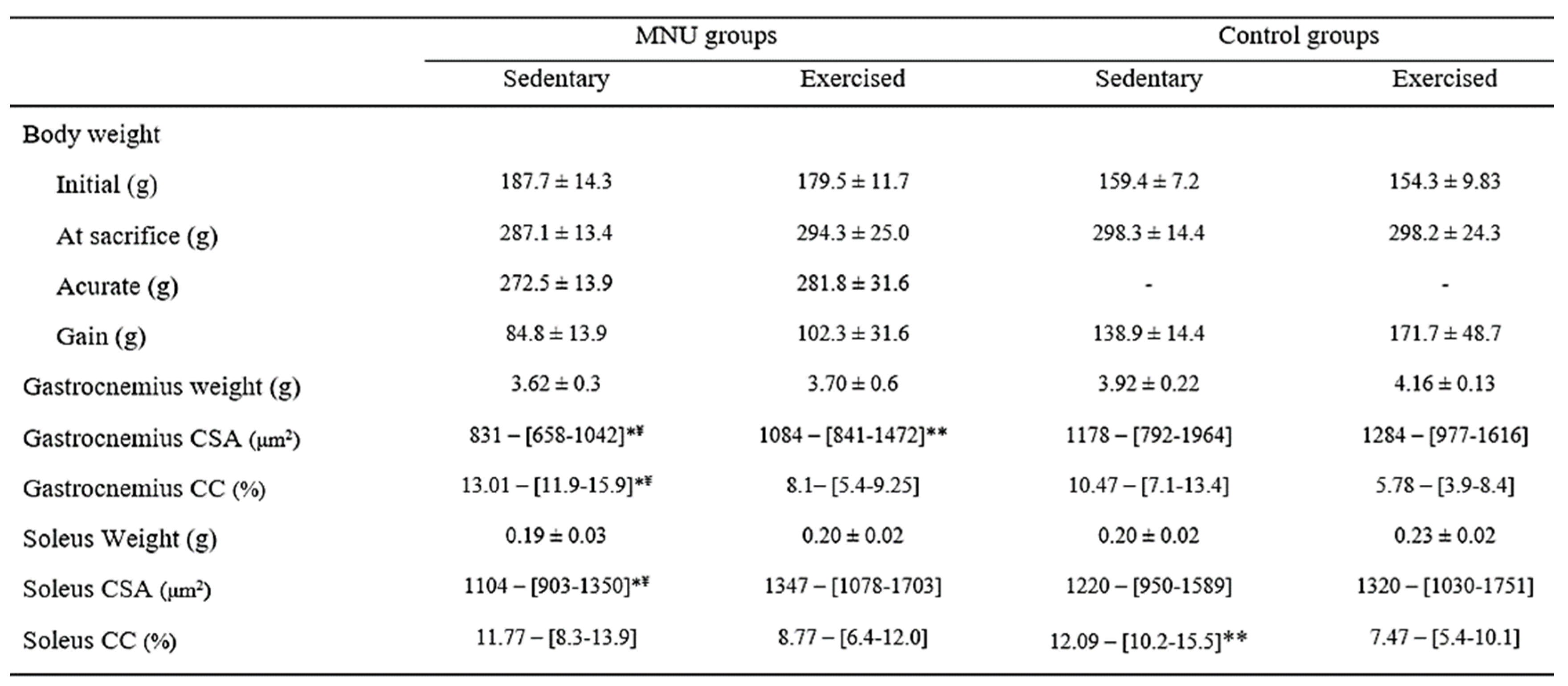
Although not significant, catabolic effects were observed in the gastrocnemius muscle weight of MNU animals, along with a smaller decrease in soleus muscle (
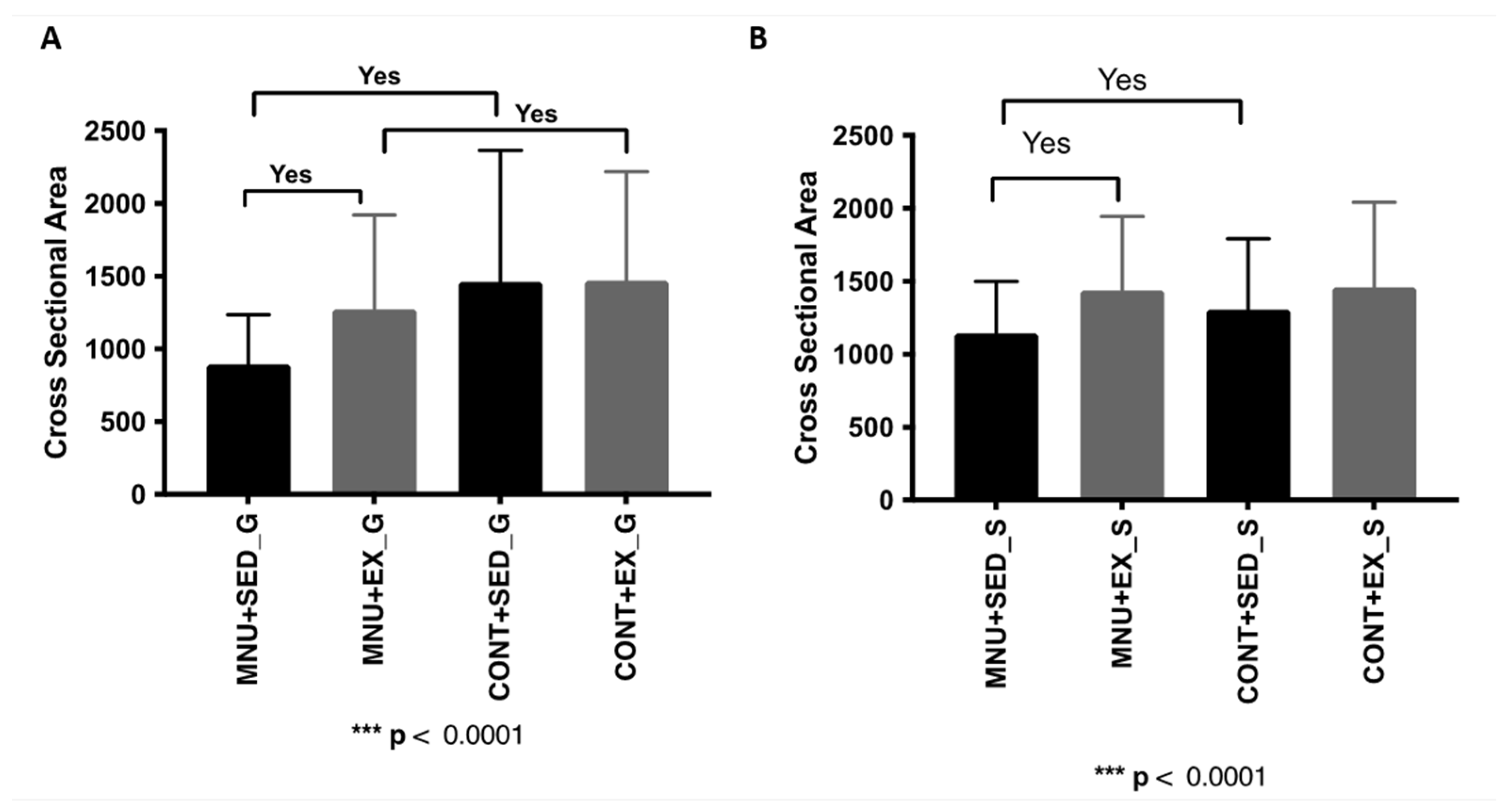
Table 1). In addition, a significant reduction in gastrocnemius cross-sectional area was observed (
Figure 1A) in MNU-treated animals (p < 0.0001), which was greater in sedentary animals (39% vs. 13%), and was prevented in 30% by exercise training. The fiber cross-sectional area of the soleus muscle (
Table 1) also shows a significant reduction but only in MNU sedentary animals (12% Vs. 1%), probably related to physical inactivity rather than cancer status (
Figure 1B).
3.1.2. Fiber-size distribution
To analyze the frequency of fiber-size distribution, the fibers were divided into different quartiles and categorized by measures.
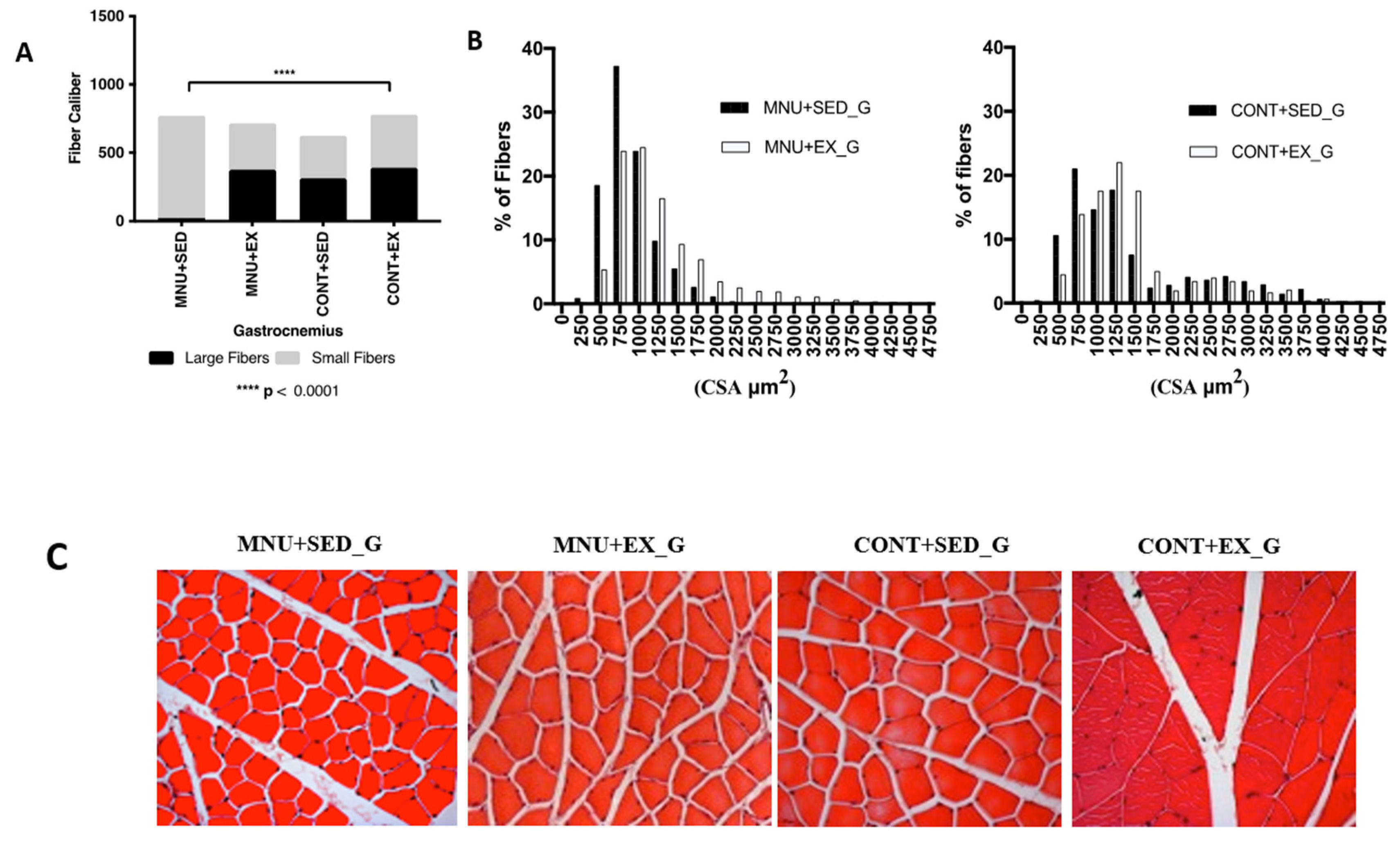
Gastrocnemius: The frequency distribution of the gastrocnemius fibers (
Figure 2A) showed, that the tumor animals suffered atrophy mainly in the large fibers. The mean fiber diameter shows a significant (p < 0.0001) decrease in the MNU-treated animals when compared with their control counterparts (2164 ± 244.8 μm2 vs. 2846 ± 577.5 μm2, and 2311 ± 634.5 vs. 2547 ± 677.6 μm2 for sedentary and exercising animals respectively). In addition, sedentary animals with tumors had 96% fewer large fibers (1% vs. 25%), than controls. Fibers sized ≥ 1750 μm2 were almost absent and completely disappeared around 2000 μm2 (
Figure 2B).
The effect of exercise training was observed in the higher expression of large fibers in MNU-exercised animals (
Figure 2C), with 16% less than in CONT+EX (21% vs. 25%), but with 95% more than in the MNU+SED ones (21% vs. 1%).
There appears to be a shift towards smaller fibers seems to occur in the gastrocnemius muscle of the MNU animals, which was partially reduced by exercise. The MNU+SED animals have more than 44% smaller fibers than the controls (45% vs. 25%), while the MNU+EX animals show a difference of 11%. We believe that this may explain the differences observed in the gastrocnemius cross-sectional area of the MNU+SED animals.
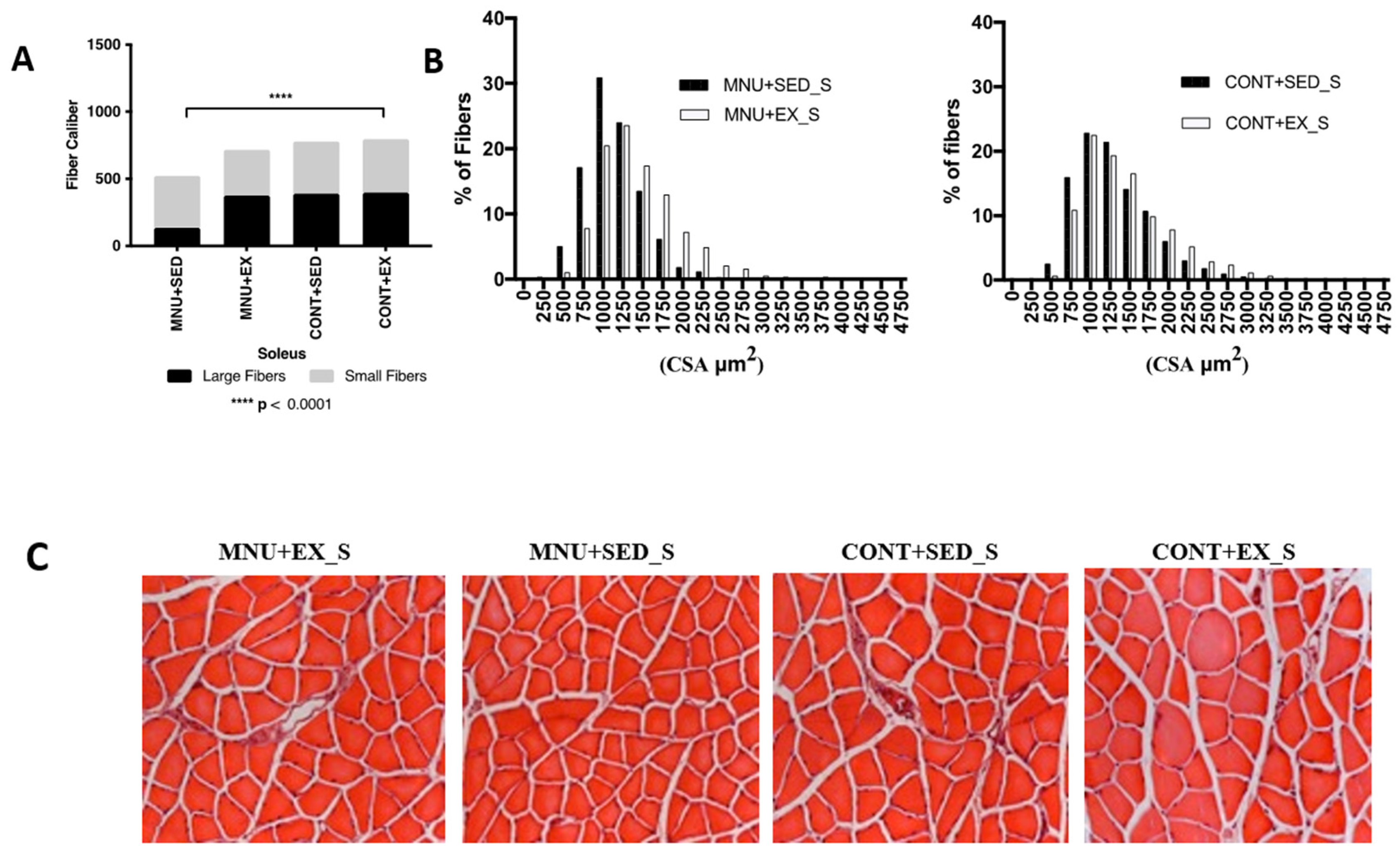
Soleus: The frequency of fiber-size distributions in the slow soleus (
Figure 1B) also shows a significant (p < 0.0001) atrophy of large fibers in MNU+SED animals, although less pronounced (42%) than in the gastrocnemius.
A lower expression of large fibers was observed in MNU sedentary animals (
Figure 3A) when compared to control (11% vs. 25%), and a small difference (8%), probably induced by exercise, was observed between active animals (23% vs. 25%).
A shift towards smaller fibers also seems to occur in sedentary animals with tumors, although much less pronounced than in the gastrocnemius (
Figure 3B), with 19% more expression of small fibers in MNU+SED animals compared to CONT+SED (31% vs. 25%) (
Figure 3C).
The opposite results were observed between trained animals, where CONT+EX animals had 20% more small fibers than the MNU+EX ones (25% vs. 20%), which can be another argument in favor of inactivity-induced atrophy of the slow soleus.
3.1.3. Muscle collagen content
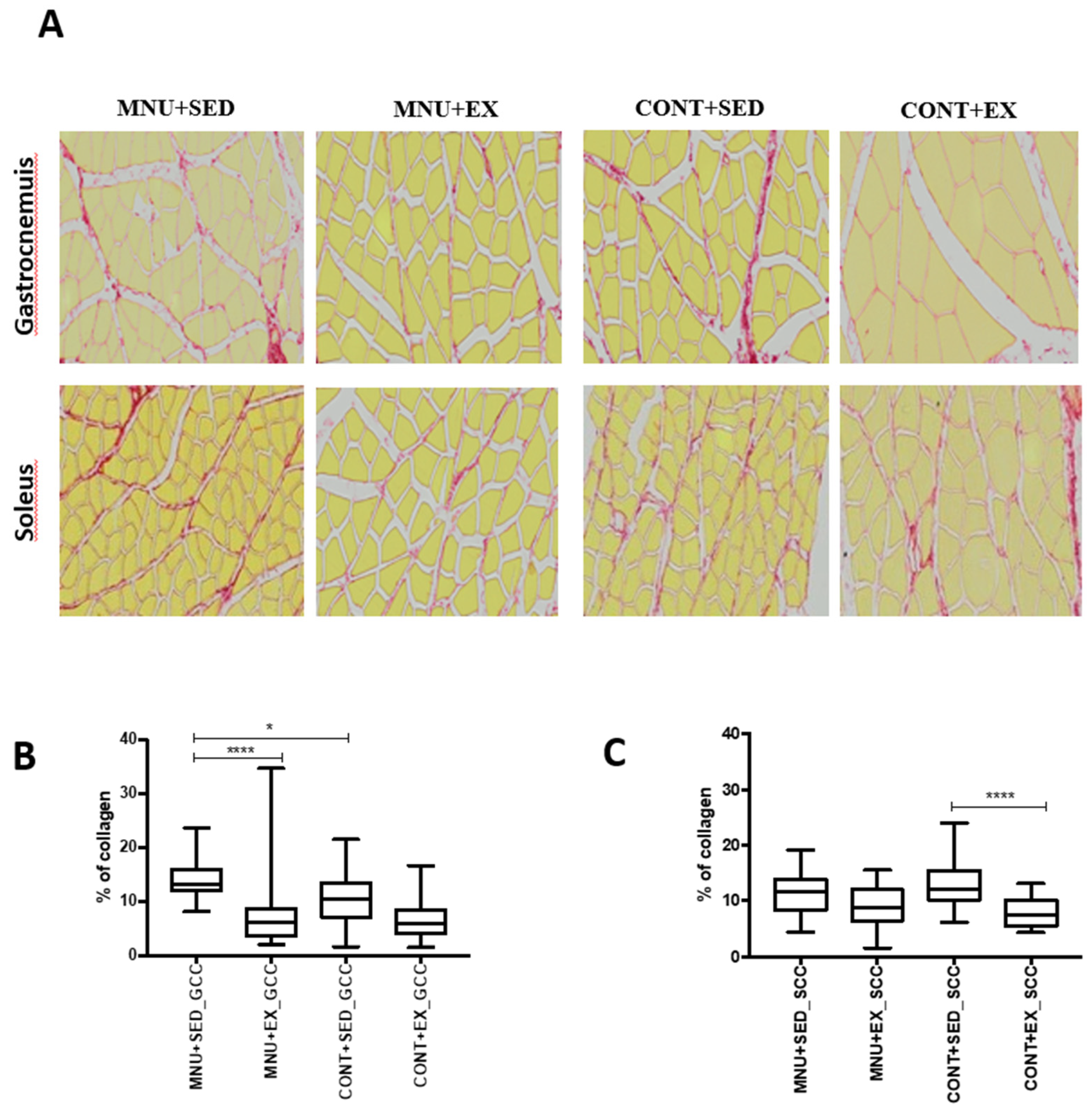
To verify the effect of exercise training on the regeneration versus fibrosis process, an analysis of collagen content was performed. The replacement of functional tissue with dense connective tissue occurred with a higher expression in the gastrocnemius than in the soleus, and exercise training seems to increase their regenerative capacity. (
Figure 4A).
The results showed a higher aggressiveness of tumorigenesis in the interstitial fibrosis of the gastrocnemius than in the soleus, expressed by a significant (p = 0.0006) increase in collagen content (45%) in MNU + SED animals (
Figure 4B), when compared to trained animals (26% vs. 14%) and to controls (26%). No significant differences (p = 0.0980) were observed in the soleus muscle collagen deposition of the MNU groups (
Figure 4C).
4. Discussion.
These results show that even without significant differences in body weight (
Table 1), the response to the cancer stimulus has significantly affected the capacity and function of the muscles analyzed. The present results also show that although there was no significant evidence of muscle weight, there was evidence of muscle wasting in MNU animals, which could be prevented by exercise training. Considering that the interest in muscle function in oncology is mainly related to the study of cachexia (a multifactorial syndrome that can account for a large number of deaths in the cancer population), associated with cancer and that a loss of more than 5-10% of body weight is generally considered to be the starting point for a cachectic state, these results confirm that the physiological changes may be present before this cut-off point is reached [
32]. Furthermore, it appears that the use of body weight as a measure to determine cachexia is arbitrary and that the diagnostic criteria should go beyond weight loss to include direct measures of body composition, particularly by monitoring muscle loss [
16,
33].
The animals’ response to tumor burden was different between the muscles studied, showing greater aggressiveness in the gastrocnemius, consistent with the results of previous studies showing that muscle atrophy in a cancerous state appears to be a phenotype-dependent phenomenon. [
24,
25,
34,
35].
Cross-sectional area analysis revealed significantly increased atrophy in both muscles in MNU sedentary animals compared to all other groups, and collagen accumulation was significantly higher in the gastrocnemius but not in the soleus. Sedentary animals with tumors had a total fiber CSA that was 30% lower in the gastrocnemius and 10% lower in the soleus than in the respective controls. In the gastrocnemius of control sedentary animals, atrophy is concentrated in smaller fibers, suggesting that disuse atrophy is greater in this fiber size. In contrast, in cancer-sedentary animals, atrophy is Given that the mechanisms regulating exercise training adaptations may be fiber-type-dependent [
36], it is possible to speculate that the fact that the gastrocnemius is predominantly composed of fast-type fibers (15), and although we have no data allowing us to see if there was a shift in the fiber profile, that considerable atrophy in fast-type fibers of gastrocnemius would be found accompanied by a fast-to-slow shift since similar changes are seen in muscle biopsies of human cancer patients and mouse models of cancer cachexia [
25,
27,
37,
38].
Regarding the soleus, the lack of differences observed between the exercised animals (MNU+EX and CONT+EX) in the frequency of large fibers, and the lowest expression in the small fibers of the same animals, could indicate that the higher differences found in the large fibers of the sedentary animals may not reveal a cancer-induced muscle wasting but a disuse-induced muscle wasting. Furthermore, the marked interstitial fibrosis found in the soleus between control groups, and the lack of differences between the MNU animals seem to confirm this. Indeed, the replacement of muscle fiber by collagenous tissue could be promoted by inactivity resulting in the inefficiency of the regenerative capacity of skeletal muscle [
18,
19]. In addition, the overexpression of collagen tissue found in the extracellular matrix of the gastrocnemius muscle could be related to the reduced expression of dystrophin [
39,
40], which has been reported to be a type II fiber-specific condition in tumor-bearing animals [
26].
Furthermore, satellite cells, which are known to play an important role in muscle regeneration, are altered in their proliferation and differentiation activity during cancer status [
41]. As one of the most efficient stimuli to induce satellite cell activation is exercise training, this may be another reason to explain the reduction of fibrosis in exercised animals with tumor [
42]. These contrasting data on fibrosis content between the analyzed muscles is probably a further reinforcement in favor of a selective atrophic state of type II fibers in response to tumor burden.
The different degrees of aggressiveness between cancer and disuse stimuli, and the different susceptibility of fiber type, could also be related to differences in reactive oxygen species. Oxidative fibers are more resistant to cancer-induced atrophy than glycolytic fibers, probably because this type of stimulus induces less oxidative stress in the former [
43]. Interestingly, this resistance to oxidative stress appears to be greater in cancer-induced atrophic fibers than in disuse fibers, suggesting that the activation of different protein degradation/synthesis pathways may depend on the type of stimulus [
8,
35].
Our results demonstrated that despite the absence of cachectic evidence, skeletal muscle wasting can occur in cancer and that exercise training can prevent it, which is consistent with the results of several studies suggesting early intervention in the treatment of muscle wasting with a combination of resistance and endurance training [
44,
45] to improve fatigability, reduce inflammation and increase muscle protein synthesis [
46].
5. Conclusions
Skeletal muscle is the most abundant tissue in healthy individuals and its importance extends beyond voluntary movement, and any weakness in skeletal muscle results in some degree of instability and inefficiency. Among other things, disease is one of the factors that can contribute to the impairment of skeletal muscle function, which can adversely impact the Quality of life. Given their important role in health and quality of life, it is crucial to understand the conditions under which muscles respond positively to the atrophic stimulus. Our results show endurance training is an important intervention to reverse cancer-related muscle wasting. These results show that tumor appears to induce greater muscle wasting than disuse, and that endurance exercise appears to reverse it, as active animals with tumor. In addition, it is possible to confirm the different degrees of aggressiveness induced by this cancer status in these fast and slow muscles, and that the larger fibers are the chosen target. The ability to regenerate muscle tissue was also improved in trained animals, showing that exercise is a powerful tool for muscle regeneration and growth.
In Summary, these data show that 35 weeks of endurance training can improve gastrocnemius cancer-induced muscle wasting by modulating a shift in fiber size and reducing fibrosis.
Future studies should focus on understanding whether different types of training, namely resistance training, targeting a specific type of muscle and a specific type of fibers, can prevent the wasting of large fibers in glycolytic muscles.
Author Contributions
Conceptualization, A.C.C.F. and J.A.D.; methodology, A.C.C.F. and J.A.D; software, R.F.; validation, A.C.C.F. and J.A.D and P.O.; formal analysis, L.L.; investigation, A.C.C.F. and J.A.D; resources; A.P..; data curation, L.L.; writing—original draft preparation, A.C.C.F. and J.A.D.; writing—review and editing, A.C.C.F. and J.A.D; visualization, A.C.C.F..; supervision, J.A.D.
Funding
This research received no external funding.
Institutional Review Board Statement
Approved by Portuguese Ethics Committee for Animal Experimentation (General Directorate for Food and Veterinary) under license number 008961.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012, 16, 153–66. [CrossRef]
- Nishimura Y, Musa I, Holm L, Lai YC. Recent advances in measuring and understanding the regulation of exercise-mediated protein degradation in skeletal muscle. Am. J. Physiol., Cell Physiol. 2021, 321, C276–c87. [CrossRef]
- Cheng AJ, Hawke TJ. Wasting away: AJP-Cell Physiology initiates thematic reviews on skeletal muscle wasting. Am. J. Physiol., Cell Physiol. 2021, 321, C38–c9. [CrossRef]
- Mader T, Chaillou T, Alves ES, Jude B, Cheng AJ, Kenne E, et al. Exercise reduces intramuscular stress and counteracts muscle weakness in mice with breast cancer. J. Cachexia Sarcopenia Muscle. 2022, 13, 1151–63. [CrossRef]
- Skipworth RJ, Stewart GD, Ross JA, Guttridge DC, Fearon KC. The molecular mechanisms of skeletal muscle wasting: implications for therapy. Surg-J R Coll Surg E. 2006, 4, 273–83. [CrossRef]
- Fearon KC, Voss AC, Hustead DS, Cancer Cachexia Study G. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am. J. Clin. Nutr. 2006, 83, 1345–50. [CrossRef] [PubMed]
- Argiles JM, Lopez-Soriano FJ, Busquets S. Mechanisms to explain wasting of muscle and fat in cancer cachexia. Curr. Opin. Support. Palliat. Care. 2007, 1, 293–8. [CrossRef] [PubMed]
- Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. DMM Dis. Models Mech. 2013, 6, 25–39. [CrossRef]
- Cao Z, Scott AM, Hoogenraad NJ, Osellame LD. Mediators and clinical treatment for cancer cachexia: a systematic review. JCSM Rapid Communications 2021, 4, 166–86. [CrossRef]
- Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am. J. Physiol., Cell Physiol. 2004, 287, C834–43. [CrossRef]
- Tisdale, MJ. Mechanisms of cancer cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, MJ. Are tumoral factors responsible for host tissue wasting in cancer cachexia? Future Oncol. 2010, 6, 503–13. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010, 39, 412–23. [CrossRef]
- Christensen JF, Jones LW, Andersen JL, Daugaard G, Rorth M, Hojman P. Muscle dysfunction in cancer patients. Ann Oncol. 2014, 25, 947–58. [CrossRef]
- Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010, 29, 154–9. [CrossRef]
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011, 12, 489–95. [CrossRef]
- Villasenor A, Ballard-Barbash R, Baumgartner K, Baumgartner R, Bernstein L, McTiernan A, Neuhouser ML. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012, 6, 398–406. [CrossRef]
- Teixeira E, Duarte JA. Skeletal Muscle Loading Changes its Regenerative Capacity. Sports Med. 2016, 46, 783–92. [CrossRef]
- Moyer AL, Wagner KR. Regeneration versus fibrosis in skeletal muscle. Curr Opin Rheumatol. 2011, 23, 568–73. [CrossRef]
- Sciorati C, Clementi E, Manfredi AA, Rovere-Querini P. Fat deposition and accumulation in the damaged and inflamed skeletal muscle: cellular and molecular players. Cell Mol Life Sci. 2015, 72, 2135–56. [CrossRef]
- Michele, DE. Mechanisms of skeletal muscle repair and regeneration in health and disease. FEBS J. 2022, 289, 6460–2. [Google Scholar] [CrossRef] [PubMed]
- Al-Majid S, Waters H. The biological mechanisms of cancer-related skeletal muscle wasting: the role of progressive resistance exercise. Biol. Res. Nurs. 2008, 10, 7–20. [CrossRef] [PubMed]
- Argiles JM, Busquets S, Lopez-Soriano FJ, Costelli P, Penna F. Are there any benefits of exercise training in cancer cachexia? J Cachexia Sarcopenia Muscle. 2012, 3, 73–6. [CrossRef]
- Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J. Clin. Investig. 2004, 114, 370–8. [CrossRef]
- Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–9. [CrossRef]
- Acharyya S, Butchbach ME, Sahenk Z, Wang H, Saji M, Carathers M, et al. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer cell. 2005, 8, 421–32. [CrossRef] [PubMed]
- Baltgalvis KA, Berger FG, Pena MM, Davis JM, White JP, Carson JA. Muscle wasting and interleukin-6-induced atrogin-I expression in the cachectic Apc ( Min/+ ) mouse. Pflugers Archiv. Eur. J. Appl. Physiol. 2009, 457, 989–1001. [CrossRef]
- Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, et al. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. PNAS. 2006, 103, 16260–5. [CrossRef]
- Diffee GM, Kalfas K, Al-Majid S, McCarthy DO. Altered expression of skeletal muscle myosin isoforms in cancer cachexia. Am. J. Physiol., Cell Physiol. 2002, 283, C1376–82. [CrossRef]
- Lawler JM, Powers SK, Hammeren J, Martin AD. Oxygen cost of treadmill running in 24-month-old Fischer-344 rats. Med. Sci. Sports Exerc. 1993, 25, 1259–64. [CrossRef]
- Guarnier FA, Cecchini AL, Suzukawa AA, Maragno AL, Simao AN, Gomes MD, Cecchini R. Time course of skeletal muscle loss and oxidative stress in rats with Walker 256 solid tumor. Muscle & nerve. 2010, 42, 950–8. [CrossRef]
- Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: mechanisms and clinical implications. Gastroenterol Res Pract. 2011, 601434. [CrossRef]
- Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer-associated weight loss. Am. J. Clin. Oncol 2015, 33, 90–9. [CrossRef] [PubMed]
- Ferreira R, Vitorino R, Neuparth MJ, Appell HJ, Duarte JA, Amado F. Proteolysis activation and proteome alterations in murine skeletal muscle submitted to 1 week of hindlimb suspension. Eur. J. Appl. Physiol. 2009, 107, 553–63. [CrossRef] [PubMed]
- Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. The FEBS J. 2013, 280, 4294–314. [CrossRef]
- Deshmukh AS, Steenberg DE, Hostrup M, Birk JB, Larsen JK, Santos A, et al. Deep muscle-proteomic analysis of freeze-dried human muscle biopsies reveals fiber type-specific adaptations to exercise training. Nat Commun. 2021, 12, 304. [CrossRef]
- Baldwin KM, Haddad F, Pandorf CE, Roy RR, Edgerton VR. Alterations in muscle mass and contractile phenotype in response to unloading models: role of transcriptional/pretranslational mechanisms. Front. Physiol. 2013, 4, 284. [CrossRef]
- Talmadge, RJ. Myosin heavy chain isoform expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle & nerve. 2000, 23, 661–79. [Google Scholar] [CrossRef]
- Klingler W, Jurkat-Rott K, Lehmann-Horn F, Schleip R. The role of fibrosis in Duchenne muscular dystrophy. Acta Myol. 2012, 31, 184–95. [PubMed]
- Acharyya S, Guttridge DC. Cancer cachexia signaling pathways continue to emerge yet much still points to the proteasome. Clin Cancer Res. 2007, 13, 1356–61. [CrossRef]
- Henrot P, Blervaque L, Dupin I, Zysman M, Esteves P, Gouzi F, et al. Cellular interplay in skeletal muscle regeneration and wasting: insights from animal models. J Cachexia Sarcopenia Muscle. 2023, 14, 745–57. [CrossRef] [PubMed]
- Abreu P, Kowaltowski AJ. Satellite cell self-renewal in endurance exercise is mediated by inhibition of mitochondrial oxygen consumption. J Cachexia Sarcopenia Muscle. 2020, 11, 1661–76. [CrossRef] [PubMed]
- Yu Z, Li P, Zhang M, Hannink M, Stamler JS, Yan Z. Fiber type-specific nitric oxide protects oxidative myofibers against cachectic stimuli. PloS one. 2008, 3, e2086. [CrossRef] [PubMed]
- Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013, 10, 90–9. [CrossRef] [PubMed]
- Fearon, KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur. J. Cancer. 2008, 44, 1124–32. [Google Scholar] [CrossRef]
- Al-Majid S, McCarthy DO. Cancer-induced fatigue and skeletal muscle wasting: the role of exercise. Biol. Res. Nurs. 2001, 2, 186–97. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).