Submitted:
30 August 2023
Posted:
31 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
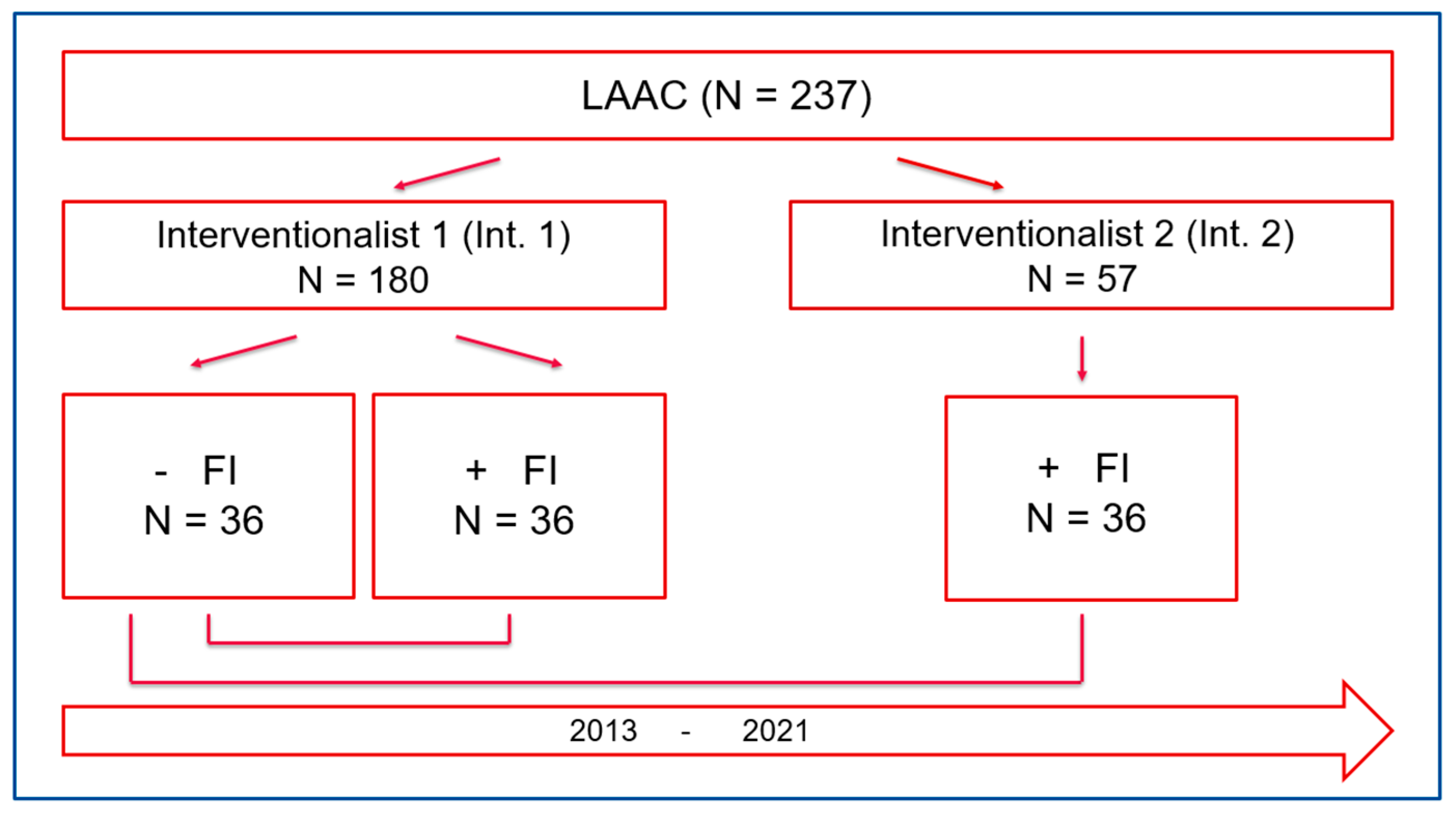
2.1. Study design
2.2. Intervention
2.3. Real-time echocardiography-fluoroscopy fusion (FI) imaging during LAAC
2.4. Learning curve and procedural parameters
2.5. Statistical analysis
3. Results
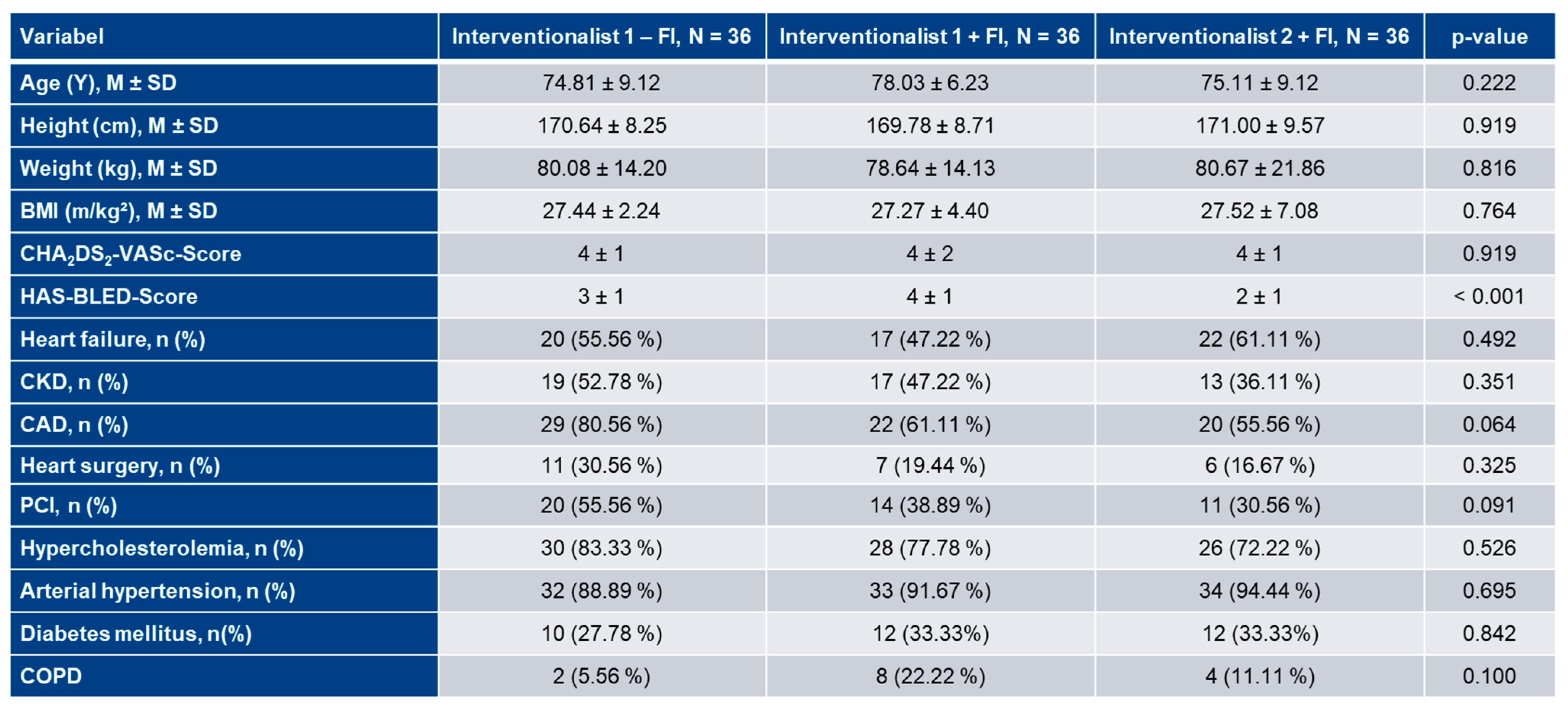
3.1. Patient characteristics
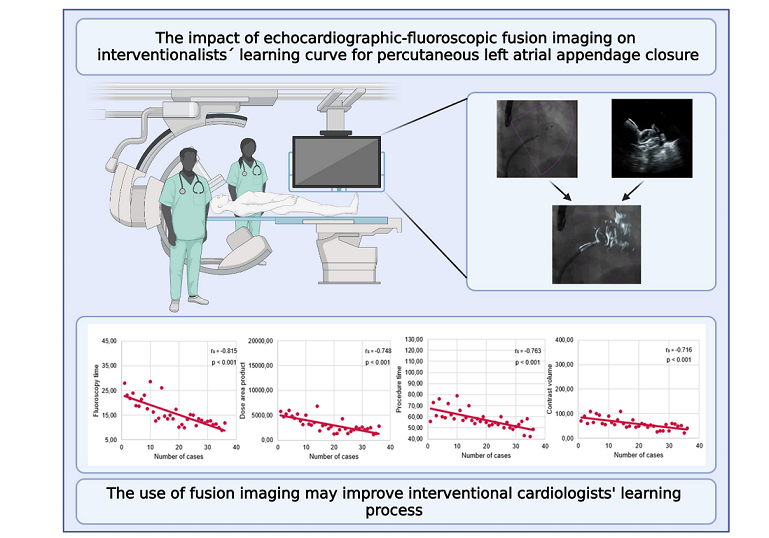
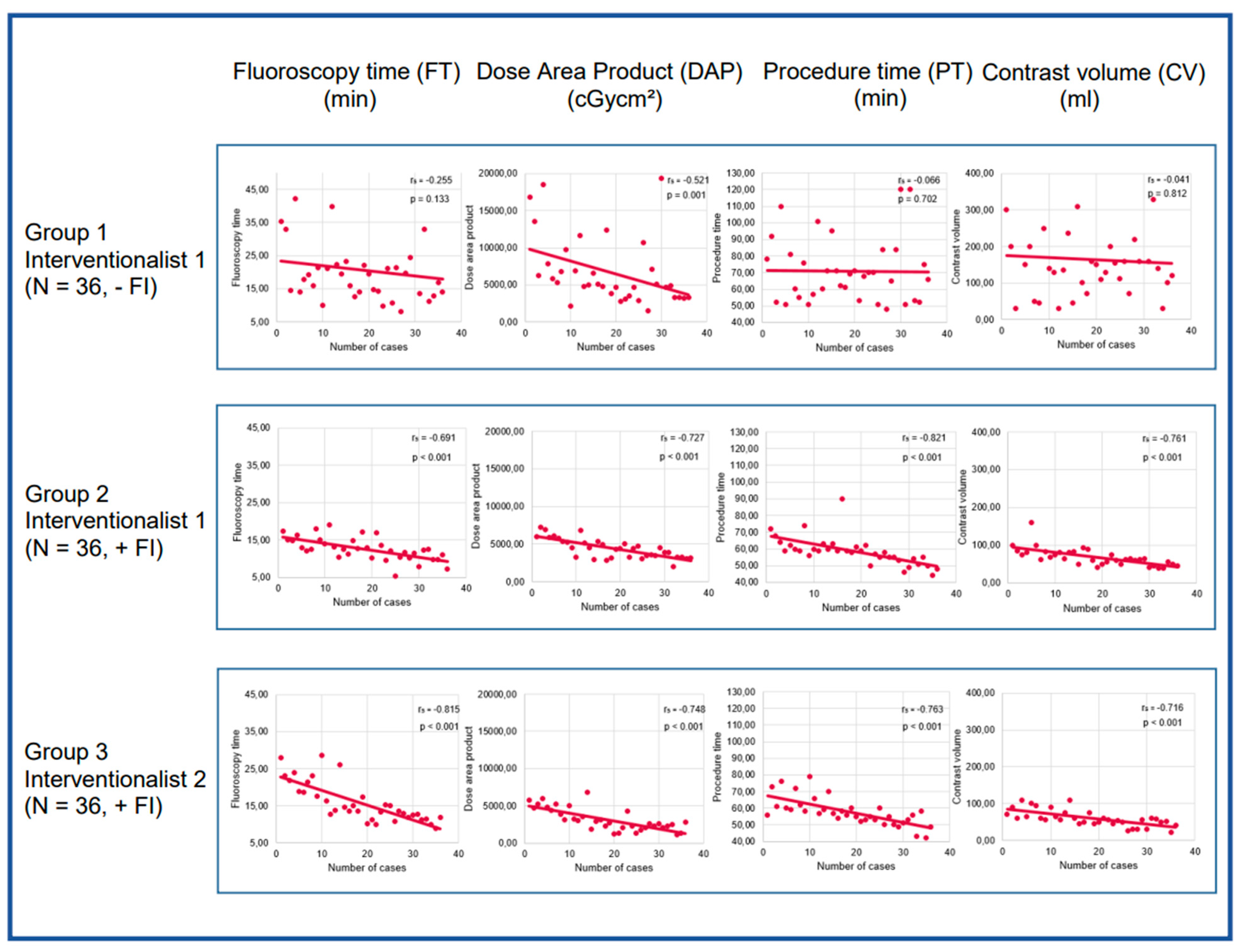
3.2. Learning curve analyses
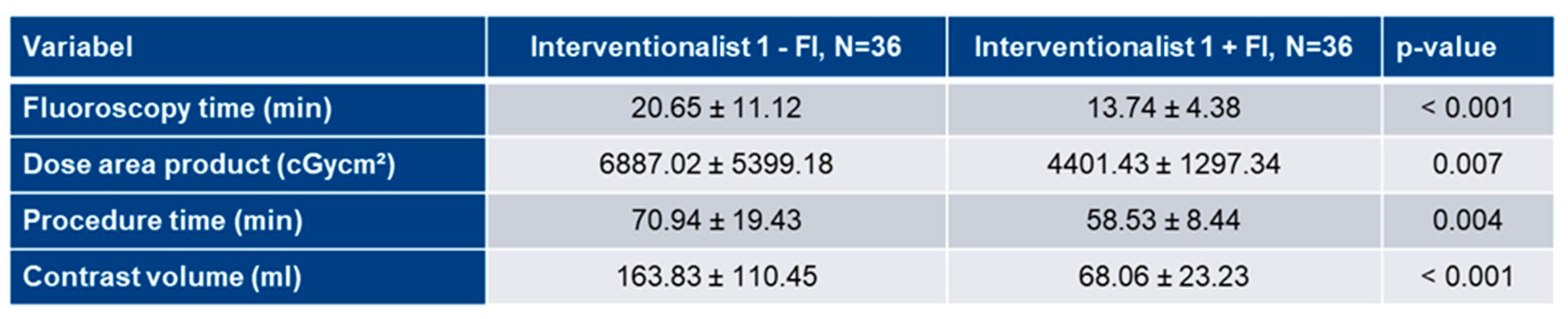
3.3. Learning curve analysis of Interventionalist 1:
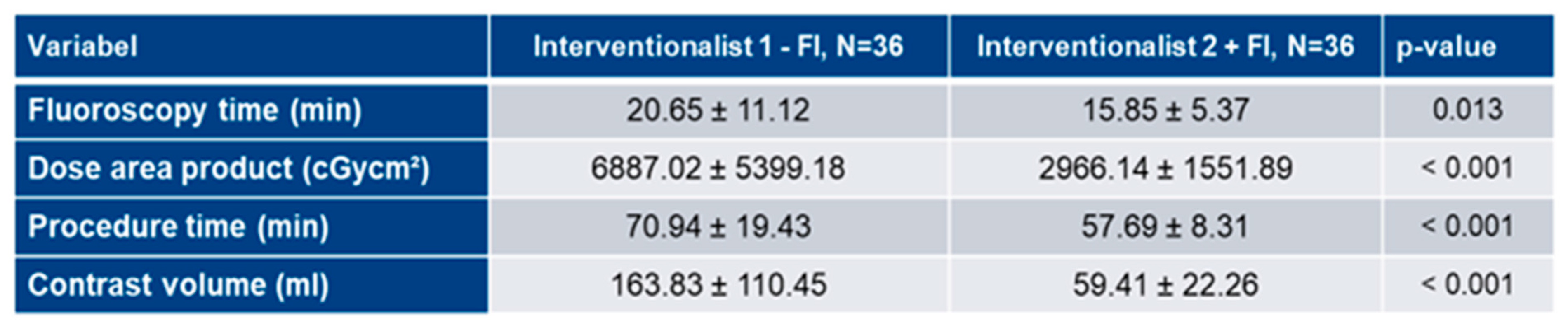
3.4. Learning curve analysis of Interventionalist 2
3.5. Analysis of FI’s impact on learning curves
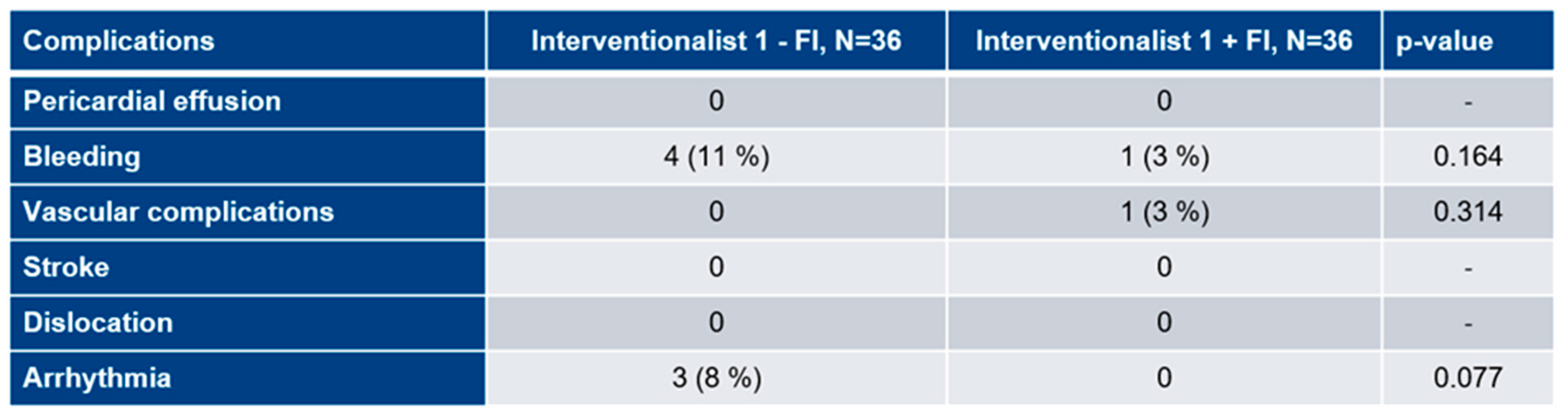
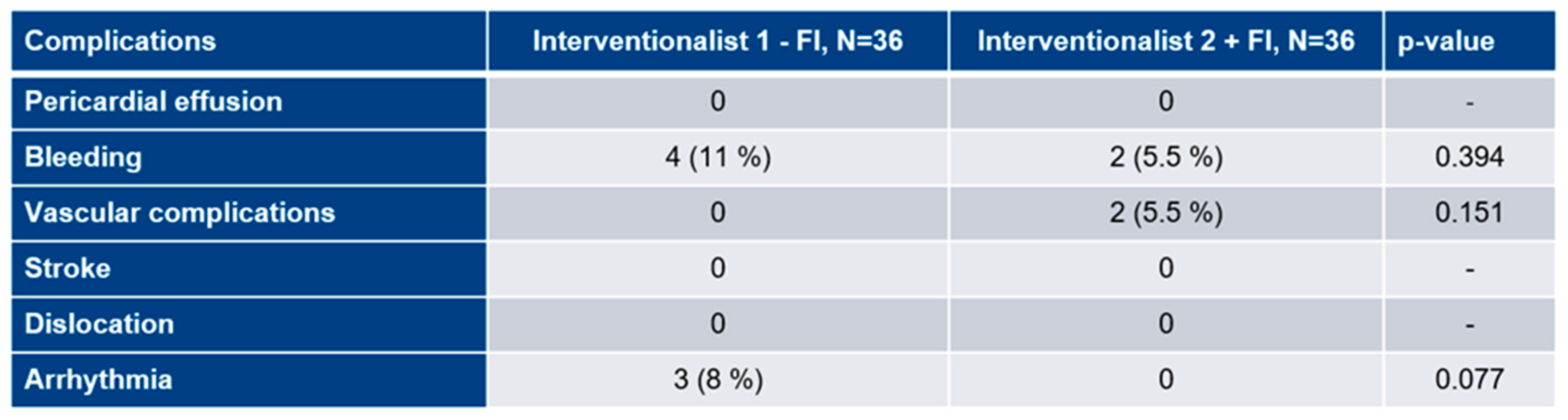
3.6. Procedural complications
4. Discussion
- Left atrial appendage closure learning curve has a flat course without FI.
- FI may improve the left atrial appendage closure learning curve.
- Even highly experienced interventionalists may benefit from FI guidance in their early phase of left atrial appendage closure training.
5. Limitations
6. Summary
Authors Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CAD | Coronary Artery Disease |
| CKD | Chronic Kidney Disease |
| COPD | Chronic Obstructive Pulmonary Disease |
| CV | Contrast Volume |
| DAP | Dose Area Product |
| EAPCI | European Association of Percutaneous Cardiovascular Interventions |
| FI | Fusion Imaging |
| FT | Fluoroscopy Time |
| IC | Interventional Cardiologist |
| IQR | Interquartile Range |
| LA | Left Atrium |
| LAA | Left Atrial Appendage |
| LAAC | Left Atrial Appendage Closure |
| MACE | Major Adverse Cardiac Events |
| NOAC | Novel oral anticoagulant |
| PT | Procedure Time |
| RAO | Right Anterior Oblique |
| SD | Standard Deviation |
| TEE | Transoesophageal Echocardiography |
| TSP | Transseptal Puncture |
| VKA | Vitamin K Antagonist |
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Doshi, S.K.; Kar, S.; Gibson, D.N.; Price, M.J.; Huber, K.; Horton, R.P.; Buchbinder, M.; Neuzil, P.; Gordon, N.T.; et al. 5-Year Outcomes After Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J Am Coll Cardiol 2017, 70, 2964–2975. [Google Scholar] [CrossRef] [PubMed]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. 4-Year Outcomes After Left Atrial Appendage Closure Versus Nonwarfarin Oral Anticoagulation for Atrial Fibrillation. J Am Coll Cardiol 2022, 79, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, N.C.; Beigel, R.; Swaans, M.J.; Ho, S.Y.; Siegel, R.J. Percutaneous interventions for left atrial appendage exclusion: options, assessment, and imaging using 2D and 3D echocardiography. JACC Cardiovasc Imaging 2015, 8, 472–488. [Google Scholar] [CrossRef] [PubMed]
- Biaggi, P.; Sager, D.F.; Kulling, J.; Kuest, S.; Wyss, C.; Hurlimann, D.; Reho, I.; Buhler, I.; Noll, G.; Huber, M.; et al. Potential Value of Fusion Imaging and Automated Three-Dimensional Heart Segmentation During Transcatheter Aortic Valve Replacement. J Am Soc Echocardiogr 2020, 33, 516–517.e511. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Korsholm, K.; Rodés-Cabau, J.; Saw, J.; Berti, S.; Alkhouli, M.A. Left atrial appendage occlusion. EuroIntervention 2023, 18, e1038–e1065. [Google Scholar] [CrossRef]
- Qamar, S.R.; Jalal, S.; Nicolaou, S.; Tsang, M.; Gilhofer, T.; Saw, J. Comparison of cardiac computed tomography angiography and transoesophageal echocardiography for device surveillance after left atrial appendage closure. EuroIntervention 2019, 15, 663–670. [Google Scholar] [CrossRef]
- Freixa, X.; Aminian, A.; Tzikas, A.; Saw, J.; Nielsen-Kudsk, J.E.; Ghanem, A.; Schmidt, B.; Hildick-Smith, D. Left atrial appendage occlusion with the Amplatzer Amulet: update on device sizing. J Interv Card Electrophysiol 2020, 59, 71–78. [Google Scholar] [CrossRef]
- Simsek, B.; Kostantinis, S.; Karacsonyi, J.; Hakeem, A.; Prasad, A.; Prasad, A.; Bortnick, A.E.; Elbarouni, B.; Jneid, H.; Abbott, J.D.; et al. Educational Experience of Interventional Cardiology Fellows in the United States and Canada. JACC Cardiovasc Interv 2023, 16, 247–257. [Google Scholar] [CrossRef]
- Joshi, A.; Wragg, A. Simulator Training in Interventional Cardiology. Interv Cardiol 2016, 11, 70–73. [Google Scholar] [CrossRef]
- Ledwoch, J.; Krollmann, C.; Staubach, S.; Hug, M.; Strohm, H.; Mudra, H. Learning Curve Assessment for Percutaneous Left Atrial Appendage Closure With the WATCHMAN Occluder. J Interv Cardiol 2016, 29, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.G.; Simard, T.; Killu, A.; Harris, A.A.; Hohmann, S.F.; Holmes, D.R.; Alkhouli, M. Learning Curve and Outcomes of Left Atrial Appendage Closure. JACC Cardiovasc Interv 2021, 14, 2750–2752. [Google Scholar] [CrossRef] [PubMed]
- Berti, S.; Pastormerlo, L.E.; Korsholm, K.; Saw, J.; Alkhouli, M.; Costa, M.P.; Odenstedt, J.; Packer, E.J.; Tondo, C.; Santoro, G.; et al. Intracardiac echocardiography for guidance of transcatheter left atrial appendage occlusion: An expert consensus document. Catheter Cardiovasc Interv 2021, 98, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Jungen, C.; Zeus, T.; Balzer, J.; Eickholt, C.; Petersen, M.; Kehmeier, E.; Veulemans, V.; Kelm, M.; Willems, S.; Meyer, C. Left Atrial Appendage Closure Guided by Integrated Echocardiography and Fluoroscopy Imaging Reduces Radiation Exposure. PLoS One 2015, 10, e0140386. [Google Scholar] [CrossRef]
- Balzer, J.; Zeus, T.; Hellhammer, K.; Veulemans, V.; Eschenhagen, S.; Kehmeier, E.; Meyer, C.; Rassaf, T.; Kelm, M. Initial clinical experience using the EchoNavigator(®)-system during structural heart disease interventions. World J Cardiol 2015, 7, 562–570. [Google Scholar] [CrossRef]
- Zorinas, A.; Zakarkaitė, D.; Janušauskas, V.; Austys, D.; Puodžiukaitė, L.; Zuozienė, G.; Samalavičius, R.S.; Jovaišienė, I.; Davidavičius, G.; Ručinskas, K.; et al. Technical Recommendations for Real-Time Echocardiography and Fluoroscopy Imaging Fusion in Catheter-Based Mitral Valve Paravalvular Leak and Other Procedures. J Clin Med 2022, 11. [Google Scholar] [CrossRef]
- Afzal, S.; Piayda, K.; Hellhammer, K.; Veulemans, V.; Wolff, G.; Heidari, H.; Stuwe, D.; Kanschik, D.; Polzin, A.; Kelm, M.; et al. Real-time echocardiography-fluoroscopy fusion imaging for left atrial appendage closure: prime time for fusion imaging? Acta Cardiol 2021, 76, 1004–1012. [Google Scholar] [CrossRef]
- Van Belle, E.; Teles, R.C.; Pyxaras, S.A.; Kalpak, O.; Johnson, T.W.; Barbash, I.M.; De Luca, G.; Kostov, J.; Parma, R.; Vincent, F.; et al. EAPCI Core Curriculum for Percutaneous Cardiovascular Interventions (2020): Committee for Education and Training European Association of Percutaneous Cardiovascular Interventions (EAPCI). A branch of the European Society of Cardiology. EuroIntervention 2021, 17, 23–31. [Google Scholar] [CrossRef]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion - an update. EuroIntervention 2020, 15, 1133–1180. [Google Scholar] [CrossRef]
- Jone, P.N.; Haak, A.; Petri, N.; Ross, M.; Morgan, G.; Wiktor, D.M.; Gill, E.; Quaife, R.A.; Messenger, J.C.; Salcedo, E.E.; et al. Echocardiography-Fluoroscopy Fusion Imaging for Guidance of Congenital and Structural Heart Disease Interventions. JACC Cardiovasc Imaging 2019, 12, 1279–1282. [Google Scholar] [CrossRef]
- Afzal, S.; Piayda, K.; Hellhammer, K.; Veulemans, V.; Wolff, G.; Heidari, H.; Stüwe, D.; Kanschik, D.; Polzin, A.; Kelm, M.; et al. Real-time echocardiography-fluoroscopy fusion imaging for left atrial appendage closure: prime time for fusion imaging? Acta Cardiol 2021, 76, 1004–1012. [Google Scholar] [CrossRef]
- Tzikas, A.; Holmes, D.R., Jr.; Gafoor, S.; Ruiz, C.E.; Blomstrom-Lundqvist, C.; Diener, H.C.; Cappato, R.; Kar, S.; Lee, R.J.; Byrne, R.A.; et al. Percutaneous left atrial appendage occlusion: the Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies. Europace 2017, 19, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Rodeghiero, F.; Tosetto, A.; Abshire, T.; Arnold, D.M.; Coller, B.; James, P.; Neunert, C.; Lillicrap, D. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost 2010, 8, 2063–2065. [Google Scholar] [CrossRef] [PubMed]
- Aeckersberg, G.; Gkremoutis, A.; Schmitz-Rixen, T.; Kaiser, E. The relevance of low-fidelity virtual reality simulators compared with other learning methods in basic endovascular skills training. J Vasc Surg 2019, 69, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Bhatt, D.L.; Kleiman, N.S. A 24-Month Interventional Cardiology Fellowship: Learning Motor Skills Through Blocked Repetition. JACC Cardiovasc Interv 2017, 10, 210–211. [Google Scholar] [CrossRef]
- Kleiman, N.S.; Welt, F.G.P.; Truesdell, A.G.; Sherwood, M.; Kadavath, S.; Shah, P.B.; Klein, L.W.; Hogan, S.; Kavinsky, C.; Rab, T. Should Interventional Cardiologists Super-Subspecialize?: Moving From Patient Selection to Operator Selection. JACC Cardiovasc Interv 2021, 14, 97–100. [Google Scholar] [CrossRef]
- Salemi, A.; Sedrakyan, A.; Mao, J.; Elmously, A.; Wijeysundera, H.; Tam, D.Y.; Di Franco, A.; Redwood, S.; Girardi, L.N.; Fremes, S.E.; et al. Individual Operator Experience and Outcomes in Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2019, 12, 90–97. [Google Scholar] [CrossRef]
- Chhatriwalla, A.K.; Vemulapalli, S.; Szerlip, M.; Kodali, S.; Hahn, R.T.; Saxon, J.T.; Mack, M.J.; Ailawadi, G.; Rymer, J.; Manandhar, P.; et al. Operator Experience and Outcomes of Transcatheter Mitral Valve Repair in the United States. J Am Coll Cardiol 2019, 74, 2955–2965. [Google Scholar] [CrossRef]
- Bertsche, D.; Pfisterer, M.; Dahme, T.; Schneider, L.M.; Metze, P.; Vernikouskaya, I.; Rasche, V. MRI-based training model for left atrial appendage closure. Int J Comput Assist Radiol Surg 2023. [Google Scholar] [CrossRef]
- Sawant, A.C.; Seibolt, L.; Sridhara, S.; Rodriguez, J.; Distler, E.; Murarka, S.; Lazkani, M.; Kumar, A.; Kanwar, N.; Prakash, M.P.H.; et al. Operator Experience and Outcomes after Transcatheter Left Atrial Appendage Occlusion with the Watchman Device. Cardiovasc Revasc Med 2020, 21, 467–472. [Google Scholar] [CrossRef]
- Wang, D.D.; Eng, M.; Kupsky, D.; Myers, E.; Forbes, M.; Rahman, M.; Zaidan, M.; Parikh, S.; Wyman, J.; Pantelic, M.; et al. Application of 3-Dimensional Computed Tomographic Image Guidance to WATCHMAN Implantation and Impact on Early Operator Learning Curve: Single-Center Experience. JACC Cardiovasc Interv 2016, 9, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Veulemans, V.; Balzer, J.; Rassaf, T.; Hellhammer, K.; Polzin, A.; Kelm, M.; Zeus, T. Safety and efficacy of transseptal puncture guided by real-time fusion of echocardiography and fluoroscopy. Neth Heart J 2017, 25, 131–136. [Google Scholar] [CrossRef] [PubMed]
 |
 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





