1. Introduction
The development of novel nondairy-fermented products utilizing vegetables, fruits, legumes, or cereals has garnered significant attention in recent times [
1]. These products not only exhibit probiotic properties but also serve as abundant sources of phytochemicals and phytonutrients [
2,
3]. Simultaneously, there has been a growing interest in fermented juices made from fruits or vegetables among health-conscious consumers and researchers, driven by the increasing popularity of veganism [
4]. Fermented vegetable juices have emerged as a promising category within the functional foods market, offering not only nutritional benefits but also the potential for innovative flavors [
5]. This trend aligns with the rising consumer demand for healthier and more sustainable food choices, while also addressing the need for convenient and versatile products suited for today's fast-paced lifestyle [
6].
Kale is a vegetable belonging to the genus
Brassica, species
Brassica oleracea, group
acephala, appears frequently in the 'healthiest foods' or 'superfoods' [
7]. This leafy green vegetable is highly regarded for its abundant nutritional value, encompassing flavonoids, antioxidant enzymes, and other trace compounds [
8]. Notably, kale stands out for its rich content of flavonoids, specifically quercetin and kaempferol, which surpass the levels found in other vegetables such as collard greens, mustard greens, sweet potato greens, and green onion [
9]. These components exhibit significant potential, not only for disease prevention but also as therapeutic agents. Previous studies suggested that kale may contribute significantly to the management of chronic conditions such as obesity, cholesterol reduction, and inflammatory bowel disease [
10].
The concept of a starter culture involves the addition of one or more microorganisms to raw materials, facilitating and controlling the fermentation process in production of fermented foods [
11]. Particularly, lactic acid bacteria (LAB) are widely employed as starter culture for vegetable fermentation such as kimchi, sauerkraut, and tempeh [
12]. LAB can enhance the bioavailability and digestibility of nutrients present in the vegetables, making them more easily absorbed by the body, and produce bioactive metabolites that exhibit antioxidant properties [
13,
14]. Moreover, LAB can contribute to sensory properties by breaking down various food components in vegetable into flavor precursors and converting them into aromatic compounds including acetoin and diacetyl [
15].
Inflammation is a crucial process that safeguards the host against external threats, such as bacteria, viruses, and toxins, by eliminating pathogens and promoting tissue recovery [
16]. However, prolonged inflammation can disrupt immune tolerance, cause significant physiological changes in tissues, organs, and normal cells, and increase the risk of various non-communicable diseases [
17]. Kale contains phytochemicals, sulfur-containing indolic glucosinolates, and aliphatic glucosinolates that have demonstrated anti-inflammatory activity [
18]. Phenolic compounds such as kaempferol and quercetin have been shown to possess anti-inflammatory effects by inhibiting the expression of PGE2, COX-2, and mPGES-1, which are upregulated by lipopolysaccharide (LPS)-induced inflammation [
19]. Moreover, probiotics are widely used for the management of chronic diseases due to their ability to modulate the immune system and elicit anti-inflammatory responses [
20,
21]. Probiotic strains have shown anti-inflammatory effects by reducing the expression of pro-inflammatory cytokines, primarily through toll-like receptor (TLR)-mediated mechanisms [
22]. Previous studies have reported that fermentation of kale juice using
L. acidophilus IFO 3025 led to improvements in nutrient content, including calcium, phosphorus, and magnesium [
6]. Fermentation of kale juice by various LAB has also been found to enhance its antioxidant activity and exhibit antibacterial effects, particularly against Gram-negative bacteria, during the fermentation process, thereby enhancing its physiological properties [
23].
This study aimed to develop an enhanced antioxidative and anti-inflammatory product by fermenting kale juice with selected probiotic strains and assessing their health-promoting effects. Specifically, we utilized
Limosilactobacillus reuteri EFEL6901 [
24],
L. fermentum EFEL6800 [
24],
L. fermentum MG7011 [
25], and
Leuconostoc citreum EFEL2061 [
26], which have previously demonstrated probiotic properties with antioxidant and anti-inflammatory activities in relevant research. The fermentation characteristics of these strains were analysed in kale juice and the metabolites and flavonoid contents, such as kaempferol and quercetin, were measured. Moreover, the health functionality of the fermented kale juice was evaluated including its anti-inflammatory and antioxidant activities.
2. Materials and methods
2.1. Bacterial strains and growth conditions
The bacterial strains used in this study are presented in
Table 1. For the assessment of probiotic activity,
Lactiplantibacillus plantarum WCFS1 (WCFS1) and
Lacticaseibacillus rhamnosus GG (LGG) were used as positive controls. All strains were preserved in a deep freezer at -80 ℃ in 50% glycerol (30% v/v). The strains were cultured in MRS broth (BD Difco, Franklin Lakes, NJ, USA) for 24 h under optimal anaerobic conditions. To initiate the juice fermentation process, the cultured strains were harvested through centrifugation (10,000 ×
g for 1 minute), washed twice with sterile 0.85% (w/v) NaCl, and used as inoculums.
2.2. Characteristics of fermented kale juices
Kale (Brassica oleraceae L. var. acephala DC.) was obtained from a local organic market in Cheongju, Korea. The leaves were trimmed to remove damaged portions and washed with tap water. A total of 600 g of kale was then ground using a grinder and mixed with 1800 mL of distilled water. The mixture was pasteurized at 70 ℃ for 30 minutes and inoculated with a 4% starter culture consisting of L. reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Le. citreum EFEL2061 at a final concentration of 106 CFU/mL. The inoculated samples were incubated at 37 ℃ for 72 h. Viable cell counts were determined by serial dilution with 0.85% NaCl and plating on MRS agar medium. The plates were incubated at 37 ℃ for 48 h, and the viable cell counts were expressed as a colony forming unit (CFU)/mL. The pH of the kale juices was measured using a pH meter (Orion Star A211, Thermo Fisher Scientific Inc., MA, USA), and the titratable acidity was determined by titrating with 0.01 N NaOH, expressed as the percentage of lactic acid in the samples. Color measurements of kale juices were performed using a colorimeter (Minolta CR-300, Minolta, Osaka, Japan) with calibration using white and black standard tiles. The samples were placed in transparent cuvettes for color detection, and the chromatic values L*, a*, and b* were recorded. Higher values of L*, a*, and b* indicate higher brightness, redness, and yellowness, respectively. The total color difference (ΔE*) of the samples was calculated using the following formula: . All measurements and analyses were performed in triplicate to ensure accuracy and reproducibility.
2.3. DPPH radical scavenging activity assay
Fermented kale juices were centrifuged at 11,000 ×
g for 10 min at 4 ℃, and the supernatants were collected. The DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activities were measured according to the method of Hur [
27]. A 200 μL volume of 0.2 mM DPPH solution (in methanol) was added to 50 μL of the fermented kale juice sample, followed by incubation in the dark for 30 min. The absorbance was measured at 517 nm. The various concentrations of ascorbic acid (1 mM, 0.5 mM, and 0.25 mM) were used as positive controls. The DPPH radical scavenging activities were calculated according to the following equation: DPPH scavenging activity (%)
where, A sample: absorbance of the sample added group; A control: absorbance of the distilled water added group.
2.4. ABTS radical scavenging activity assay
The ABTS+ scavenging activities of samples were measured by modifying the method of Biglar [
28]. ABTS+ radical cations were generated by reacting 7 mM 2,2'-azobis (2-amidinopropane) dihydrochloride and 2.45 mM potassium persulfate in distilled water at room temperature for 24 h in the dark. Before analysis, the ABTS+ solution was diluted with distilled water to adjust an absorbance of 1.00 ± 0.02 at 734 nm. Consecutively, 10 μL of fermented kale juices and 200 μL of ABTS+ solution was mixed, and the absorbances were measured at 734 nm after reaction for 6 min. The various concentrations of ascorbic acid (1 mM, 0.5 mM, and 0.25 mM) were used as positive controls. The ABTS+ radical scavenging activities were calculated according to the following equation:
ABTS radical scavenging activity (%) where, A sample: absorbance of the sample added group; A control: absorbance of the distilled water added group.
2.5. Quercetin and kaempferol analysis
For analysis of quercetin and kaempferol, the lyophilized fermented kale juice sample was extracted with 50% aqueous methanol and 1.5 M HCl (37.5 mL of 66.7% aqueous methanol and 12.5 mL of 6 M HCl) for twice. And then, refluxed at 90 ℃ for 2 h and the extract was filtered through a 0.45 μm PTFE filter for HPLC analysis [
29]. HPLC analysis of the flavonoids was conducted using an Infinity 1260 HPLC (Agilent Technologies, Santa Clara, CA, USA) consisting of autosampler, the Phoroshell C18 column (4.6 × 150 mm, 120 Å, 2.7 µm) (Agilent technologies), and UV detector. The measurement was performed using the method of Rha [
30]. In detail, the flow rate was set at 0.8 mL/min, and the gradient elution was made with mobile phase A (0.1% formic acid in water) and mobile phase B (0.1% formic acid in acetonitrile). The elution gradient was as follows: 0-8% B (0-2 min), 8-12% B (2-3 min), 12-16% B (3-4 min), 16% B (4-12 min), 16-20% B (12-15 min), 20% B (15-18 min), 20-24% B (18-21 min). 24-30% B (21-22 min), 30% B (22-26 min), 30-50% B (26-28 min), 50% B (28-30 min), 50-80% B (30-32 min), 80% B (32-33min), 80-8% B (33-34 min), and 8% B (34-35 min). The UV chromatograms recorded at 365 nm were used for quantification of quercetin (≥95%, Sigma-Aldrich), and kaempferol (≥90%, Sigma-Aldrich), based on external standard calibration curves.
2.61. H-NMR analysis
To analyse the metabolites of fermented kale juices, nuclear magnetic resonance (NMR) spectroscopy analysis was conducted. For the preparation of samples, fermented kale juices were centrifuged (11,000 × g, 10 min), then the supernatants were collected. The pH of supernatants was adjusted to 6.0 ± 0.2 and mixed with 350 μL of 1 mM sodium 2,2-dimethyl-2-silapentane-5-sulfonate (DSS; Sigma-Aldrich) as an internal standard in 10% deuterium oxide (D2O; Sigma-Aldrich). The mixtures (700 μL) were transferred to NMR tubes and subjected to 1H-NMR analysis. The 1H-NMR spectra were recorded on an Avance 500-MHz spectrometer (Bruker BioSpin, Karlsruhe, Germany). The metabolites peaks in the NMR spectra were identified, and its concentrations were calculated using Chenomx NMR suite 8.4 library software (Chenomx Inc, Edmonton, AB, Canada).
2.7. Cytotoxicity of fermented kale juice
The cytotoxicity was evaluated by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide (MTT) assay using RAW 264.7 cell lines. The RAW 264.7 cell lines were obtained from the Korean Cell Line Bank (Seoul, Korea). Briefly, RAW 264.7 cells were seeded at 2.0×105 cells per well with DMEM containing 10% FBS in 96-well plates and cultured at 37 ℃ for 18–20 h. The cells were treated with 6.25-100 μg/mL of fermented kale juice extracted with DMSO. All samples were treated simultaneously with LPS (1 μg/mL)(Sigma-Aldrich). After incubating for 24 h, the medium was removed, and cells were washed twice with PBS. And then, MTT solution (0.25 mg/mL) (HiMedia, Mumbai, India) was added followed by incubation in the dark at 37 ℃ for 1 h. Finally, the formazan crystals were dissolved by adding 150 μL of dimethylsulfoxide, and the absorbance of each well was measured at 570 nm using a microplate spectrophotometer (BioTek, Santa Barbara, CA, USA). The percent cell viability was calculated according to the following equation: Cell viability (%) = () 100.
2.8. Measurement of nitric oxide (NO) production
The effect of kale juices fermented with different starters on the production of NO was determined in LPS-induced RAW 264.7 cell lines using Griess reaction [
31]. RAW 264.7 cells (2×10
5 cells/well) were seeded in 96-well plates and incubated for 18-20 h. RAW 264.7 cells were treated with fermented kale juice at the above-mentioned concentrations followed by stimulation with LPS (1 μg/mL). Methyl arginine was used as the positive control. After 24 h of incubation, 100 μL of culture supernatants were transferred to 96-well plates and mixed with an equal volume of Griess reagent (Sigma-Aldrich) and placed in the dark for 15 min at room temperature. The absorbance of each mixture at 540 nm was measured using a microplate spectrophotometer (BioTek). Nitrite concentration was calculated using dilutions of sodium nitrite as standards, and fresh medium was used as the blank control.
2.9. mRNA expression level of nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and cytokine
The effect of fermented kale juice on the pro-inflammatory transcriptome was assessed in LPS-induced RAW 264.7 cells using an RT-qPCR. RAW 264.7 cells (1×10
6 cells/well) were seeded in 6-well plates and incubated for 24 h. Cells were treated with LPS (1 μg/mL) with kale juice fermented with different strains for 24 h. After incubation, cells were washed twice with sterile PBS, and total RNA was extracted with the with the Trizol RNA isolation reagent (Invitrogen, Carlsbad, USA) according to the manufacturer’s protocol. An adequate amount of RNA was reverse transcribed into cDNA using LeGene Express 1st Strand cDNA Synthesis System Kit (LeGene Biosciences, San Diego, CA, USA). Real-time PCR was conducted using Exicycler 96 Real-Time Quantitative Thermal Block (Exicycler 96; Bioneer, Daejeon, Korea). The mixtures containing synthesized cDNA, 10 pmol of specific primers, and PCR Master Mix containing SYBR Green Mix were amplified as follows: 95 ℃ for 5 min followed by 45 cycles at 95 ℃ for 15 sec, 59 ℃ for 30 sec with a final extension at 59 ℃ for 30 sec. The result was analysed after normalization with GAPDH as a reference gene. Relative expression levels of target genes were calculated with the delta-delta Ct (
ΔΔCt) method. The specific primer sequences used in this study are listed in
Table 2.
2.10. Enzyme and related genes
Genetic analysis was conducted to identify the genes related to utilization of phytochemical by a sequence similarity search using BLASTP. The sequences were obtained from the NCBI database (
https://www.ncbi.nlm.nih.gov/).
2.11. Statistical analysis
Each experiment was conducted in triplicate, and the data were presented as the mean value ± standard deviation (SD). Statistical analysis was performed using IBM SPSS software version 22 (SPSS Inc., USA). Independent t-tests were used to analyse differences between two groups, and one-way analysis of variance (ANOVA) with Tukey’s method was used to analyse differences between multiple groups. Different letters and symbols on the error bars indicate significant differences. Statistical significance was basically set at p < 0.05.
4. Discussion
Fermented foods are good carriers for delivering probiotics while having health-promoting effects [
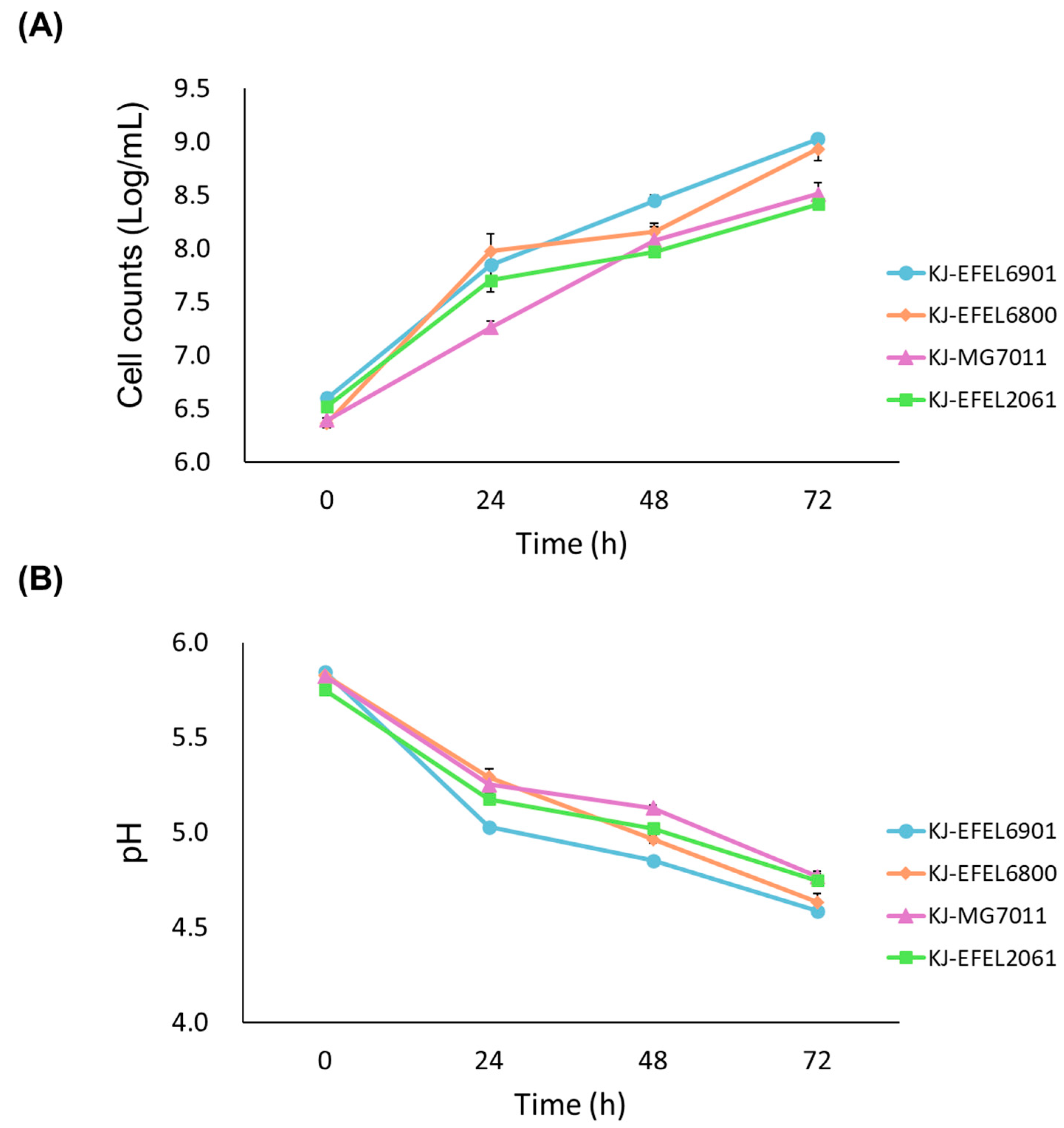
32]. In this study, EFEL6901 and EFEL6800 strains were selected as starters for kale juice fermentation. The two strains exhibited substantial cell growths in kale juice, reaching cell counts of > 9.0 log CFU/mL (a 200-fold increment), pH of 4.5~4.7 after 72 h of fermentation (
Figure 1). Comparing these values with the growth of
Streptococcus thermophilus, a commonly used starter culture for milk yogurt, which reached a cell count of 8.78 log CFU/mL and a pH of 4.3 [
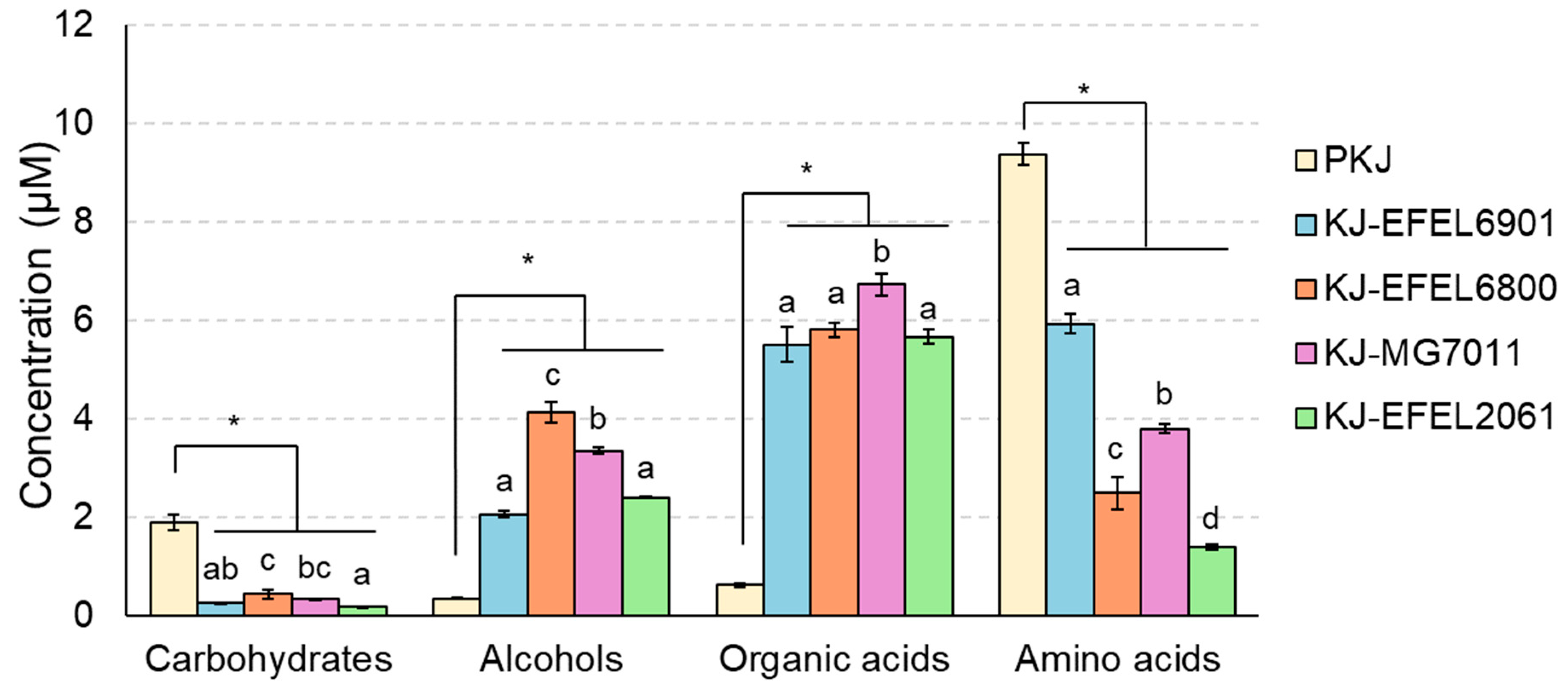
33], the fermentation characteristics of EFEL6901 and EFEL6800 in kale juice were comparable to the commercial product. The suitability of kale juice as a culture medium for selected LAB may be attributed to its rich nutrient composition. The pasteurized kale juice before fermentation contained various carbon sources such as glucose, fructose, mannose, sucrose, acetate, and citrate and different nitrogen sources of 18 amino acids with large amounts of aspartate and glutamate (
Table 3). This result indicates that kale juice can serve not only as a suitable medium for the cell growth of the selected LAB strains but also as an excellent carrier for delivering probiotics.
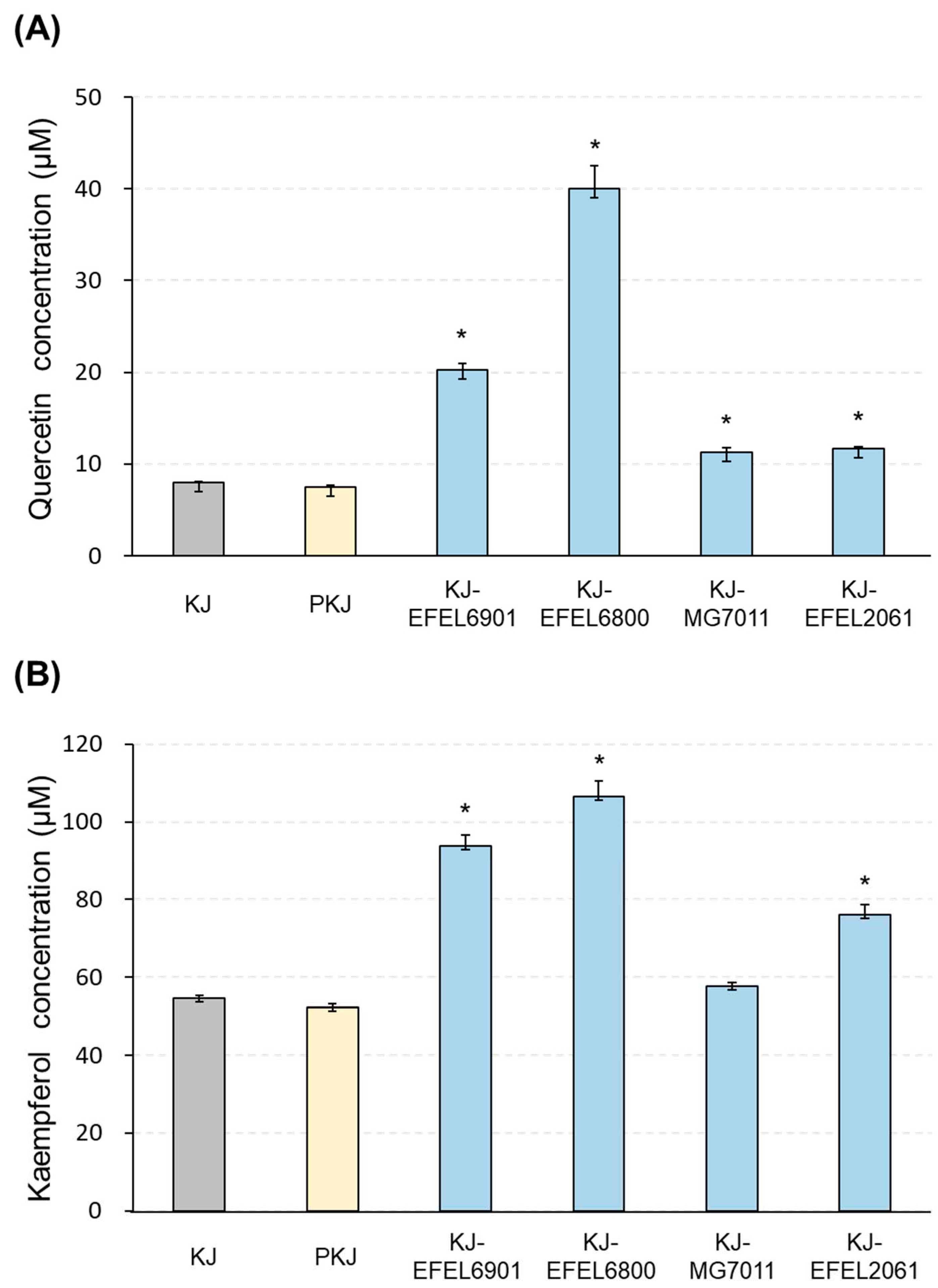
Kale has abundant flavonoid content, specifically quercetin and kaempferol [
29]. In this study, the initial concentrations of quercetin and kaempferol in kale juice were 7.87 μM (45.03 mg/100 g) and 54.46 μM (311.78 mg/100 g), respectively. Those levels are significantly higher than that of collard greens (12.4 and 43.3 mg/100g, respectively), mustard greens (8.8 and 38.3 mg/100g, respectively), sweet potato greens (27.9 and 5.0 mg/100g, respectively), okra (11.1 mg/100g quercetin and non-detectable kaempferol), green onion (non-detectable quercetin and 4.8 mg/100g kaempferol) [
9]. Furthermore, this study shows that, after fermentation of kale juice with KJ-EFEL6901 and KJ-EFEL6800, the quercetin contents were increased three- and five-folds, respectively, and the kaempferol contents were increased about two-folds in both samples (
Figure 2). Consequently, the higher level of flavonol contents in the kale juice was remarkably enhanced to the superior levels by a simple fermentation process using selected LAB.
The phenolic compounds found in plants can be divided into two categories: free phenolic compounds, which are located in the vacuoles of plant cells, and bound phenolic compounds that are covalently linked to structural components of the cell wall [
34]. Fermentation has emerged as a favorable method for obtaining phenolic extracts of high quality and activity from different plant sources, utilizing techniques that are both economically and environmentally friendly [
35]. Changes in phenolic compounds by fermentation process are associated with several enzymatic reactions: hydrolysis of ester bonds which link phenolic compounds to the cell wall matrix with esterase [
36], the oxidative degradation of lignin with laccase or peroxidase [
37], or deglycosylation of flavonoids to produce aglycone with β-glucosidase [
38]. Several enzymes produced by microorganism can hydrolyse the glycosidic bonds in alkyl and aryl-β-D-glucoside, and β-glucosidase is a representative enzyme that produces aglycone [
39]. For example, in soybean fermentation,
L. plantarum WCFS1 could convert soybean isoflavones from glucosides to aglycones by β-glucosidase [
40]. Also,
L. plantarum CECT 748T had the ability to convert arylglucosides into their bioactive aglycone, increasing the antioxidant activity of plant foods during fermentation [
41]. Likewise, the genetic analysis of EFEL6901 and EFEL6800 revealed the presence of multiple enzymes, such as glycoside hydrolase or glucosidase (
Table 5), indicating their potential involvement in the utilization of kale's abundant glycosides. These enzymes likely played a significant role in facilitating the growth of microorganisms by releasing sugars and potentially contributed to the increased levels of quercetin and kaempferol after fermentation. These findings suggest that the efficiency of bio-conversion can be influenced by the strain's adaptability to kale juice and its ability to produce hydrolytic enzymes [
42].
Antioxidants are major contributors to the functionality of the vegetable product [
43]. Overall, antioxidant activity was significantly increased in fermented kale juice, and this result might be associated with the increased flavonol contents after fermentation (
Figure 3). Notably, flavonol aglycones, quercetin and kaempferol, in plants are potent antioxidants that serve to protect the plant from reactive oxygen species (ROS) [
44].
The anti-inflammatory effects of quercetin and kaempferol have been demonstrated through the inhibition of PGE2, COX-2, and mPGES-1 expression, which are upregulated during LPS-induced inflammation [
19]. Additionally, probiotic strains have been shown to reduce the expression of pro-inflammatory cytokines, primarily TLR-mediated mechanisms, thus exerting their anti-inflammatory effects [
22]. In this study, KJ-EFEL6901 and KJ-EFEL6800 resulted in a strong anti-inflammatory effect on LPS-induced RAW 264.7 cells by inhibiting NO production via down-regulating pro-inflammatory cytokines and up-regulating anti-inflammatory cytokine (
Figure 4 and
Figure 5). LPS activation in the mouse macrophage cell line RAW 264.7 induces an inflammatory response, producing inflammatory cytokines such as reactive oxygen species, IL-6, and TNF-α [
45]. IL-6 and IL-1β are pro-inflammatory cytokines, and IL-1β induces several genes involved with inflammation and tissue destruction, such as Th1 cell responses [
46]. And IL-6 acts as a chronic stressor that can accelerate the risk of age-related diseases by promoting the differentiation and inflammation of immune cells such as T and B cells [
47]. Meanwhile, IL-10 plays an anti-inflammatory role in returning the immune system to a dormant state by inhibiting activated macrophage. Notably, the results of this study suggest that the anti-inflammatory effect of LBRE6901-KJ and LBF6800-KJ was improved compared to that of kale juice, possibly due to LAB themselves and/or their metabolites synthesized during fermentation. In our previous studies, strong antioxidative and anti-inflammatory activities of
L. reuteri EFEL6901 and
L. fermentum EFEL6800 have been demonstrated in
in vitro and
in vivo experiments [
24,
25]. Therefore, the superior levels of antioxidant and an-inflammatory activities observed in KJ-EFEL6901 and KJ-EFEL6800 were attributed to the dual sources that are kale, a flavonoid-rich vegetable, and
L. reuteri EFEL6901 and
L. fermentum EFEL6800, probiotic starters.
Figure 1.
Cell growth (A), pH changes (B), titratable acidity (C) of kale juices fermented at 37 ℃. KJ-EFEL6901, KJ-EFEL6800, KJ-MG7011, and KJ-EFEL2061 corresponded to the kale juices fermented with Limosilactobacillus reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Leuconostoc citreum EFEL2061, respectively. Results are expressed as means ± standard deviations (n=3).
Figure 1.
Cell growth (A), pH changes (B), titratable acidity (C) of kale juices fermented at 37 ℃. KJ-EFEL6901, KJ-EFEL6800, KJ-MG7011, and KJ-EFEL2061 corresponded to the kale juices fermented with Limosilactobacillus reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Leuconostoc citreum EFEL2061, respectively. Results are expressed as means ± standard deviations (n=3).
Figure 2.
Quantification of quercetin (A) and kaempferol (B) in kale juices fermented at 37 ℃. KJ-EFEL6901, KJ-EFEL6800, KJ-MG7011, and KJ-EFEL2061 corresponded to the kale juices fermented with Limosilactobacillus reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Leuconostoc citreum EFEL2061, respectively. Quercetin and kaempferol contents were measured by HPLC analysis. Results are expressed as means ± SD (n = 3). PKJ, pasteurized kale juice before fermentation. The statistical analysis was performed using the independent T-test compared to PKJ (*p<0.001).
Figure 2.
Quantification of quercetin (A) and kaempferol (B) in kale juices fermented at 37 ℃. KJ-EFEL6901, KJ-EFEL6800, KJ-MG7011, and KJ-EFEL2061 corresponded to the kale juices fermented with Limosilactobacillus reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Leuconostoc citreum EFEL2061, respectively. Quercetin and kaempferol contents were measured by HPLC analysis. Results are expressed as means ± SD (n = 3). PKJ, pasteurized kale juice before fermentation. The statistical analysis was performed using the independent T-test compared to PKJ (*p<0.001).
Figure 3.
Changes in metabolite concentrations of kale juices fermented at 37 ℃. Metabolites were analyzed by 1H-NMR. PKJ, pasteurized kale juice before fermentation. KJ-EFEL6901, KJ-EFEL6800, KJ-MG7011, and KJ-EFEL2061 corresponded to the kale juices fermented with Limosilactobacillus reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Leuconostoc citreum EFEL2061, respectively. Results are expressed as means ± standard deviations (n=3). The statistical analysis was performed using the independent T-test compared to PKJ (*p<0.05). Different letters indicate a significant difference in the same group at p<0.05 according to Tukey’s multiple range test.
Figure 3.
Changes in metabolite concentrations of kale juices fermented at 37 ℃. Metabolites were analyzed by 1H-NMR. PKJ, pasteurized kale juice before fermentation. KJ-EFEL6901, KJ-EFEL6800, KJ-MG7011, and KJ-EFEL2061 corresponded to the kale juices fermented with Limosilactobacillus reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Leuconostoc citreum EFEL2061, respectively. Results are expressed as means ± standard deviations (n=3). The statistical analysis was performed using the independent T-test compared to PKJ (*p<0.05). Different letters indicate a significant difference in the same group at p<0.05 according to Tukey’s multiple range test.
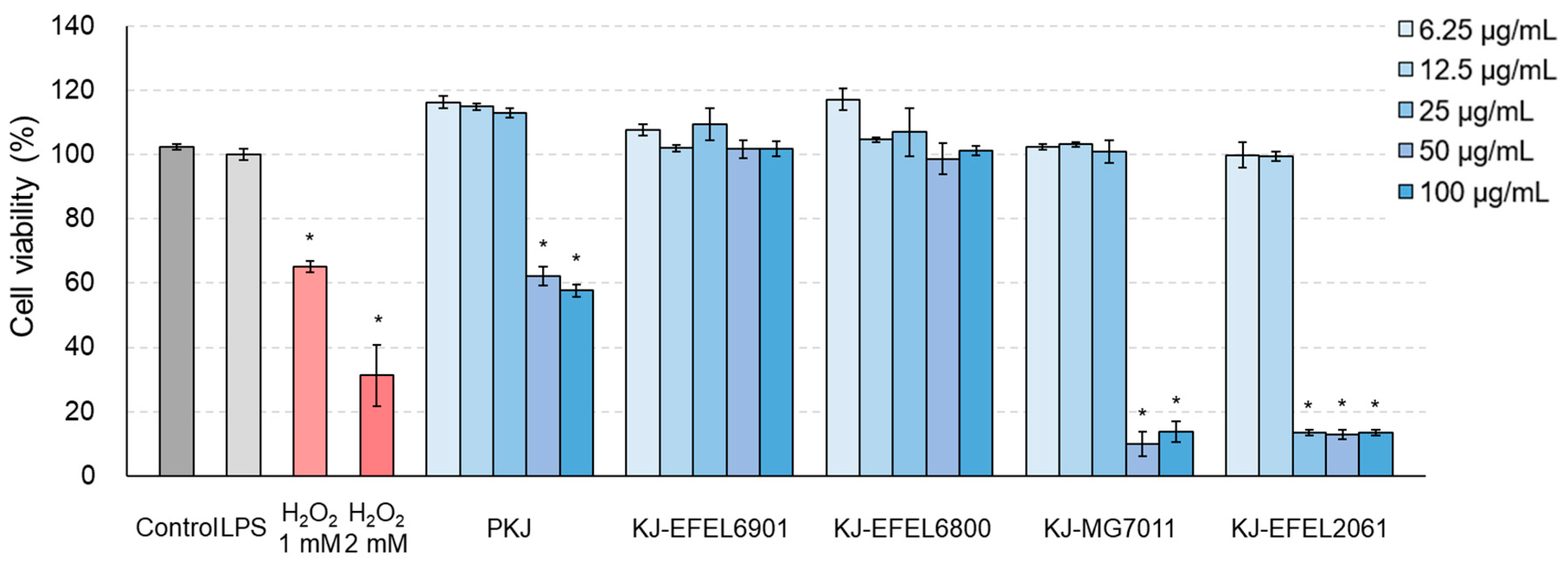
Figure 4.
Effects of fermented kale juice on cell viability of RAW 264.7 cells. RAW 264.7 cells (2×105 cells/well) were treated with 6.25, 12.5, 25, 50, and 100 μg/mL of fermented kale juice powder with 1 μg/mL LPS for 24 h, and cell viability was determined by the MTT assay. Dimethylsulfoxide was used as a vehicle. H2O2 was used as a negative control. LPS, lipopolysaccharide; PKJ, pasteurized kale juice before fermentation. KJ-EFEL6901, KJ-EFEL6800, KJ-MG7011, and KJ-EFEL2061 corresponded to the kale juices fermented at 37 ℃ for 72 h with Limosilactobacillus reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Leuconostoc citreum EFEL2061, respectively. Data are the mean ± SD (n = 3). The statistical analysis was performed using the independent T-test compared to LPS (*p<0.05).
Figure 4.
Effects of fermented kale juice on cell viability of RAW 264.7 cells. RAW 264.7 cells (2×105 cells/well) were treated with 6.25, 12.5, 25, 50, and 100 μg/mL of fermented kale juice powder with 1 μg/mL LPS for 24 h, and cell viability was determined by the MTT assay. Dimethylsulfoxide was used as a vehicle. H2O2 was used as a negative control. LPS, lipopolysaccharide; PKJ, pasteurized kale juice before fermentation. KJ-EFEL6901, KJ-EFEL6800, KJ-MG7011, and KJ-EFEL2061 corresponded to the kale juices fermented at 37 ℃ for 72 h with Limosilactobacillus reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Leuconostoc citreum EFEL2061, respectively. Data are the mean ± SD (n = 3). The statistical analysis was performed using the independent T-test compared to LPS (*p<0.05).
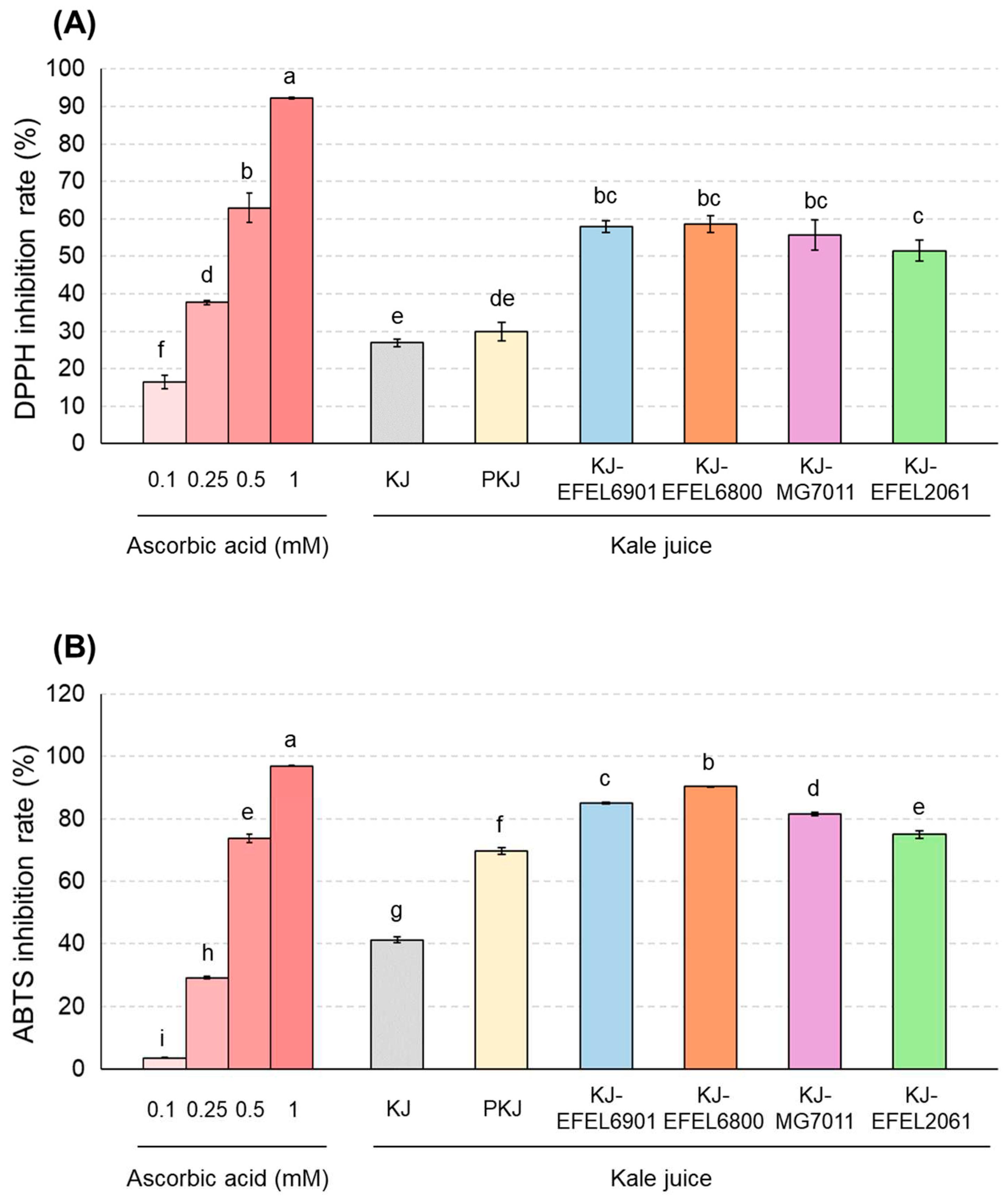
Figure 5.
DPPH free radical scavenging activity (A) and ABTS+ free radical scavenging activity (B) of fermented kale juices. KJ, kale juice; PKJ, pasteurized kale juice before fermentation. KJ-EFEL6901, KJ-EFEL6800, KJ-MG7011, and KJ-EFEL2061 corresponded to the kale juices fermented at 37 ℃ for 72 h with Limosilactobacillus reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Leuconostoc citreum EFEL2061, respectively. Different letters indicate a significant difference at p<0.05 according to Tukey’s multiple range test.
Figure 5.
DPPH free radical scavenging activity (A) and ABTS+ free radical scavenging activity (B) of fermented kale juices. KJ, kale juice; PKJ, pasteurized kale juice before fermentation. KJ-EFEL6901, KJ-EFEL6800, KJ-MG7011, and KJ-EFEL2061 corresponded to the kale juices fermented at 37 ℃ for 72 h with Limosilactobacillus reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Leuconostoc citreum EFEL2061, respectively. Different letters indicate a significant difference at p<0.05 according to Tukey’s multiple range test.
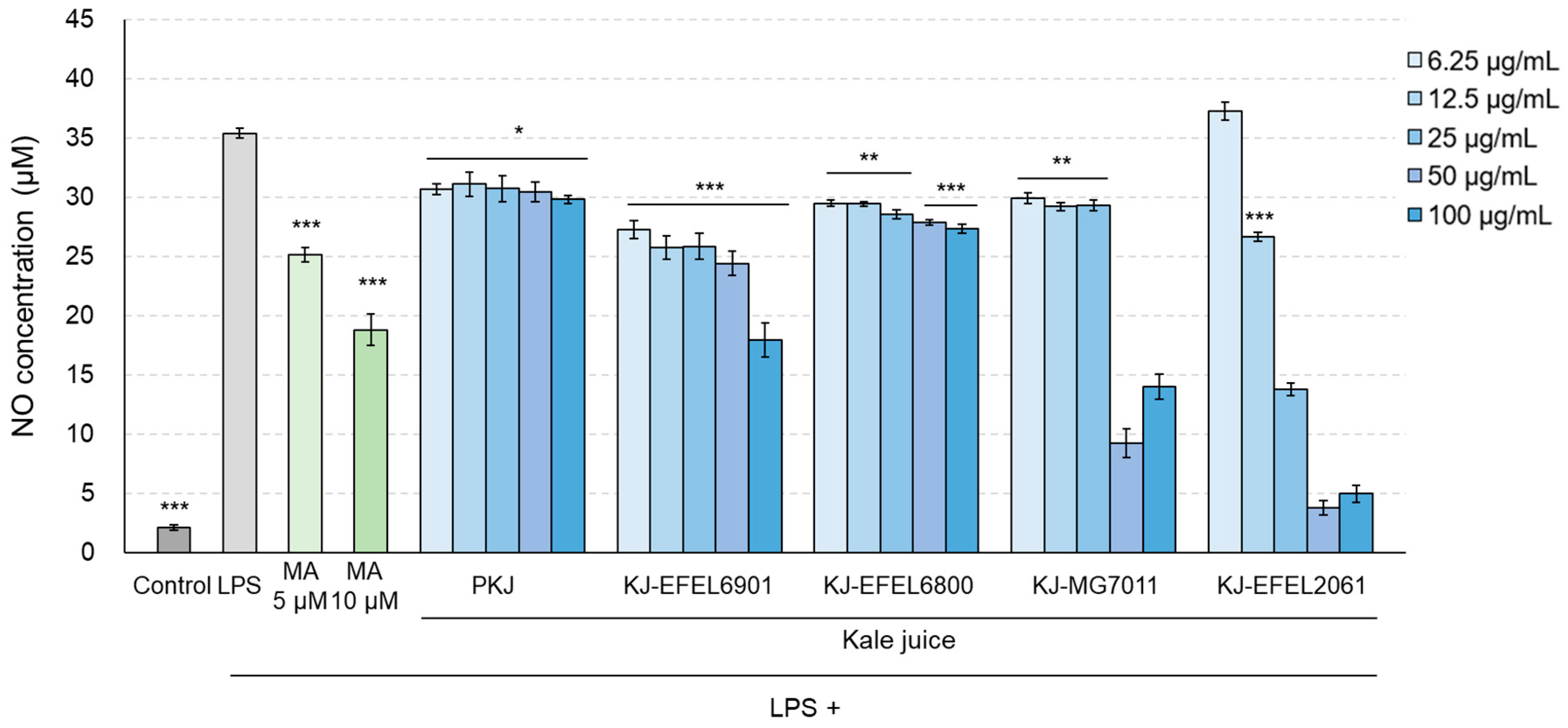
Figure 6.
Effects of fermented kale juice on the production of NO in LPS-induced RAW 264.7 cells. KJ-EFEL6901, KJ-EFEL6800, KJ-MG7011, and KJ-EFEL2061 corresponded to the kale juices fermented at 37 ℃ for 72 h with Limosilactobacillus reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Leuconostoc citreum EFEL2061, respectively. RAW 264.7 cells were incubated with 6.25, 12.5, 25, 50, and 100 μg/mL of fermented kale juice powder with 1 μg/mL LPS for 24 h. Dimethylsulfoxide was used as a vehicle. NO was measured according to the Griess reaction. LPS, lipopolysaccharide; MA, methyl arginine (positive control); PKJ, pasteurized kale juice before fermentation. Data are the mean ± SD (n = 3). The statistical analysis was performed using the independent T-test compared to LPS (* p < 0.05, ** p < 0.01, *** p < 0.001).
Figure 6.
Effects of fermented kale juice on the production of NO in LPS-induced RAW 264.7 cells. KJ-EFEL6901, KJ-EFEL6800, KJ-MG7011, and KJ-EFEL2061 corresponded to the kale juices fermented at 37 ℃ for 72 h with Limosilactobacillus reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Leuconostoc citreum EFEL2061, respectively. RAW 264.7 cells were incubated with 6.25, 12.5, 25, 50, and 100 μg/mL of fermented kale juice powder with 1 μg/mL LPS for 24 h. Dimethylsulfoxide was used as a vehicle. NO was measured according to the Griess reaction. LPS, lipopolysaccharide; MA, methyl arginine (positive control); PKJ, pasteurized kale juice before fermentation. Data are the mean ± SD (n = 3). The statistical analysis was performed using the independent T-test compared to LPS (* p < 0.05, ** p < 0.01, *** p < 0.001).
Figure 7.
Effects of fermented kale juice on mRNA expression of iNOS (A), COX-2 (B), IL-6 (C), IL-1ß (D), and IL-10 (E) in LPS-induced RAW 264.7 cells. RAW 264.7 cells were incubated with 6.25 and 12.5 μg/mL of fermented kale juice powder with 1 μg/mL LPS for 24 h. Dimethylsulfoxide was used as a vehicle. The mRNA expression levels of iNOS and COX-2 were determined by real-time PCR. LPS, lipopolysaccharide; PKJ, pasteurized kale juice before fermentation. KJ-EFEL6901, KJ-EFEL6800, KJ-MG7011, and KJ-EFEL2061 corresponded to the kale juices fermented at 37 ℃ for 72 h with Limosilactobacillus reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Leuconostoc citreum EFEL2061, respectively. Data are the mean ± SD (n = 3). The statistical analysis was performed using the independent T-test compared to 6 μg/mL of PKJ (#p<0.05, ##p<0.01) or 12.5 μg/mL of PKJ (* p < 0.05, ** p < 0.01). Different letters indicate a significant difference at p<0.05 according to Tukey’s multiple range test.
Figure 7.
Effects of fermented kale juice on mRNA expression of iNOS (A), COX-2 (B), IL-6 (C), IL-1ß (D), and IL-10 (E) in LPS-induced RAW 264.7 cells. RAW 264.7 cells were incubated with 6.25 and 12.5 μg/mL of fermented kale juice powder with 1 μg/mL LPS for 24 h. Dimethylsulfoxide was used as a vehicle. The mRNA expression levels of iNOS and COX-2 were determined by real-time PCR. LPS, lipopolysaccharide; PKJ, pasteurized kale juice before fermentation. KJ-EFEL6901, KJ-EFEL6800, KJ-MG7011, and KJ-EFEL2061 corresponded to the kale juices fermented at 37 ℃ for 72 h with Limosilactobacillus reuteri EFEL6901, L. fermentum EFEL6800, L. fermentum MG7011, and Leuconostoc citreum EFEL2061, respectively. Data are the mean ± SD (n = 3). The statistical analysis was performed using the independent T-test compared to 6 μg/mL of PKJ (#p<0.05, ##p<0.01) or 12.5 μg/mL of PKJ (* p < 0.05, ** p < 0.01). Different letters indicate a significant difference at p<0.05 according to Tukey’s multiple range test.
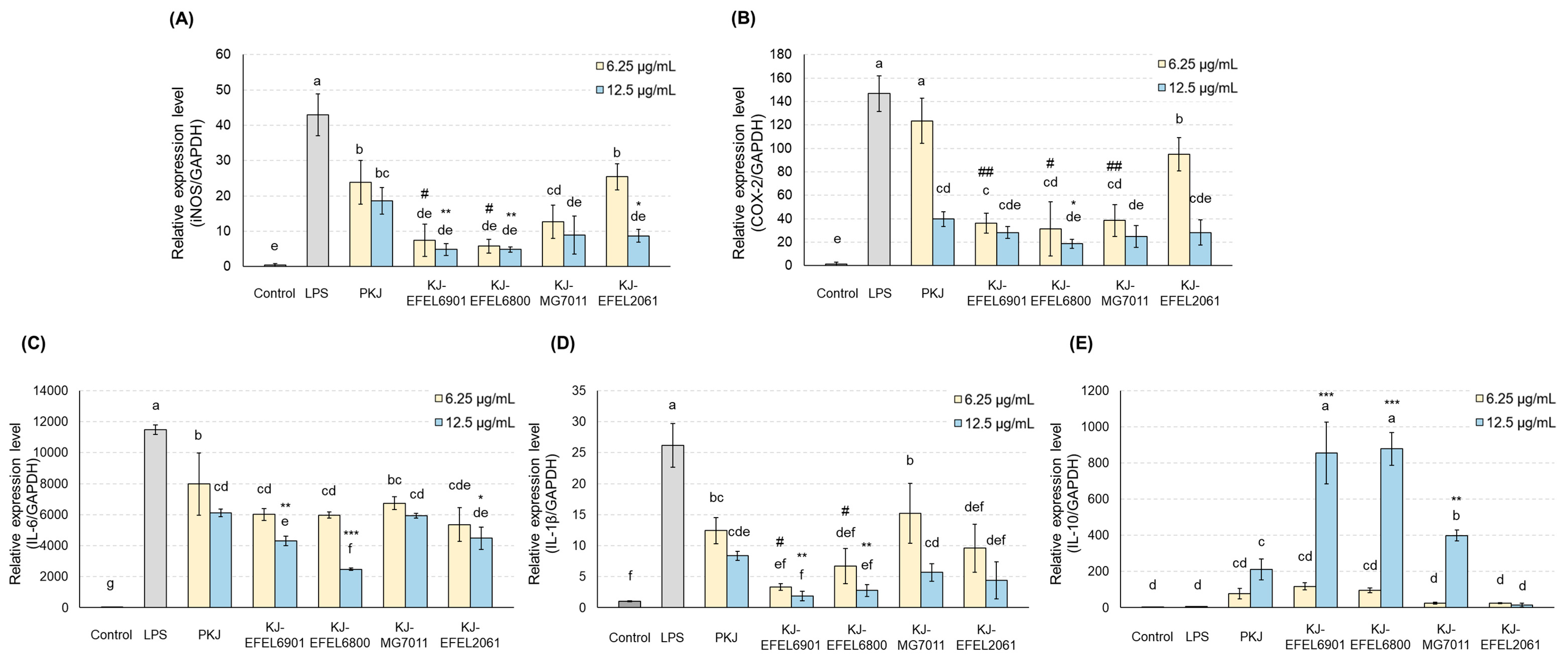
Table 1.
List of strains used in this study.
Table 1.
List of strains used in this study.
| Species |
Collection |
Abbreviation |
Culture condition |
|
Limosilactobacillus reuteri EFEL6901 |
KACC 81105BP |
EFEL6901 |
37 ℃, MRS |
|
L. fermentum EFEL6800 |
KACC 81106BP |
EFEL6800 |
37 ℃, MRS |
|
L. fermentum MG7011 |
KACC 81147BP |
MG7011 |
37 ℃, MRS |
|
Leuconostoc citreum EFEL2061 |
KACC 92070P |
EFEL2601 |
37 ℃, MRS |
|
Lactiplantibacillus plantarum WCFS1 |
ATCC BAA-793 |
WCFS1 |
37 ℃, MRS |
|
Lacticaseibacillus rhamnosus GG |
KCTC 5033 |
LGG |
37 ℃, MRS |
Table 2.
Primer sequences for mouse-specific PCR.
Table 2.
Primer sequences for mouse-specific PCR.
| Gene |
Forward primer (5’-3’) |
Reverse primer (5’-3’) |
| GAPDH |
TTGTCTCCTGCGACTTCAACA |
GCTGTAGCCGTATTCATTGTCATA |
| iNOS |
ACCATGGAGCATCCCAAGTA |
CCATGTACCAACCATTGAAGG |
| COX-2 |
AGCATTCATTCCTCTACATAAGC |
GTAACAACACTCACATATTCATACAT |
| TNF-α |
ATGATCCGCGACGTGGAA |
ACCGCCTGGAGTTCTGGA |
| IL-1β |
GTTGACGGACCCCAAAAGAT |
CACACACCAGCAGGTTATCA |
| IL-10 |
GGACAACATACTGCTAACCGACTC |
AAAATCACTCTTCACCTGCTCCAC |
Table 3.
Colormetric properties of fermented kale juice.
Table 3.
Colormetric properties of fermented kale juice.
| Sample |
L* |
a* |
b* |
ΔE* |
| Kale juice |
29.95±0.02 |
-6.56±0.02 |
10.91±0.02 |
- |
| PKJ |
34.61±0.00 a
|
-6.80±0.01 e
|
15.14±0.03a
|
6.30±0.04 |
| KJ-EFEL6901 |
34.02±0.01b
|
-0.01±0.01a
|
13.73±0.02a
|
6.80±0.02a
|
| KJ-EFEL6800 |
34.59±0.03a
|
-1.36±0.01d
|
15.25±0.09a
|
5.67±0.04b
|
| KJ-MG7011 |
34.61±0.04a
|
-0.76±0.04b
|
15.70±1.44a
|
6.49±0.47a
|
| KJ-EFEL2061 |
34.64±0.02a
|
-0.85±0.01c
|
14.65±0.01a
|
6.07±0.04ab
|
Table 4.
Metabolite concentrations in fermented kale juice analyzed by 1H-NMR.
Table 4.
Metabolite concentrations in fermented kale juice analyzed by 1H-NMR.
| Group |
Metabolites |
PKJ |
KJ-EFEL6901 |
KJ-EFEL6800 |
KJ-MG7011 |
KJ-EFEL2061 |
| Carbohydrates |
Fructose |
0.65±0.12 |
0.08±0.01c* |
0.20±0.03a* |
0.14±0.00b* |
0.04±0.00d* |
| Glucose |
0.90±0.04 |
0.06±0.01* |
0.06±0.01* |
0.05±0.00* |
0.04±0.00* |
| Mannose |
0.16±0.05 |
0.09±0.01* |
0.13±0.04 |
0.09±0.00* |
0.07±0.00* |
| Sucrose |
0.14±0.01 |
0.02±0.00* |
0.05±0.04* |
0.05±0.00* |
0.02±0.00* |
| Alcohols |
Mannitol |
0.10±0.01 |
1.23±0.03a* |
1.14±0.05ab* |
1.10±0.02b* |
0.98±0.02c* |
| Ethanol |
0.24±0.01 |
1.74±0.06c* |
2.98±0.24a* |
2.25±0.05b* |
1.42±0.01c* |
| Organic acids |
Acetate |
0.19±0.01 |
3.24±0.33* |
3.54±0.11b* |
2.63±0.13b* |
1.76±0.25b* |
| Succinate |
0.09±0.01 |
0.85±0.04b* |
0.30±0.01c* |
1.30±0.17a* |
1.13±0.03 a* |
| Lactate |
0.09±0.01 |
1.69±0.21a* |
1.86±0.03a* |
0.97±0.01b* |
0.49±0.02c* |
| Propionate |
0.03±0.01 |
0.54±0.00bc* |
0.71±0.06b* |
0.63±0.04bc* |
0.84±0.03a* |
| Pyruvate |
0.07±0.01 |
0.08±0.04 |
0.07±0.01 |
0.06±0.01* |
0.04±0.00* |
| Butyrate |
0.04±0.00 |
0.30±0.01b* |
0.18±0.01b* |
0.23±0.40b* |
0.19±0.01a* |
| Citrate |
0.12±0.02 |
0.02±0.01ab* |
0.04±0.01a* |
0.02±0.00ab* |
0.01±0.00b* |
| Amino acids |
Aspartate |
1.40±0.04 |
1.08±0.08a* |
0.04±0.01b* |
0.03±0.01b* |
0.06±0.01b* |
| Glutamate |
1.13±0.11 |
1.35±0.07a* |
0.33±0.02b* |
0.27±0.01b* |
0.06±0.00c* |
| Cysteine |
0.06±0.01 |
0.17±0.01a* |
0.12±0.03b* |
0.12±0.00b* |
0.07±0.00bc
|
| Glycine |
0.08±0.03 |
0.13±0.01ab* |
0.16±0.02a* |
0.18±0.02a* |
0.06±0.00b
|
| Histidine |
0.01±0.00 |
0.01±0.01 |
0.01±0.00 |
0.00±0.00 |
0.01±0.00 |
| Alanine |
0.91±0.03 |
0.58±0.01a* |
0.62±0.13a* |
0.48±0.03a* |
0.09±0.01b* |
| Serine |
1.18±0.21 |
0.09±0.01b* |
0.12±0.01a* |
0.12±0.01ab* |
0.05±0.01c* |
| Threonine |
0.30±0.03 |
0.02±0.00b* |
0.08±0.04b* |
0.18±0.03a* |
0.03±0.00b* |
| Arginine |
0.78±0.04 |
0.05±0.01ab* |
0.03±0.01bc* |
0.02±0.00c* |
0.07±0.00a* |
| Proline |
0.44±0.15 |
0.37±0.10 |
0.26±0.03 |
0.30±0.04 |
0.17±0.00 |
| Tyrosine |
0.19±0.01 |
0.15±0.00b* |
0.03±0.00d* |
0.19±0.01a* |
0.06±0.00c* |
| Valine |
0.53±0.21 |
0.47±0.08a* |
0.07±0.01b* |
0.40±0.01a* |
0.20±0.01ab* |
| Methionine |
0.08±0.01 |
0.06±0.01 |
0.02±0.01a* |
0.01±0.00a* |
0.02±0.00a* |
| Isoleucine |
0.43±0.05 |
0.29±0.02a* |
0.05±0.04b* |
0.11±0.01b* |
0.05±0.01b* |
| Leucine |
0.48±0.02 |
0.31±0.07* |
0.14±0.00* |
0.16±0.00* |
0.10±0.01* |
| Phenylalanine |
0.56±0.05 |
0.16±0.01b* |
0.05±0.03c* |
0.38±0.01a* |
0.10±0.01bc* |
| Tryptophan |
0.23±0.02 |
0.21±0.01a* |
0.07±0.00c* |
0.14±0.01b* |
0.07±0.00c* |
| Lysine |
0.63±0.03 |
0.43±0.05b* |
0.30±0.02c* |
0.70±0.01a
|
0.09±0.00d* |
Table 5.
Genetic analysis results of EFEL6901 and EFEL6800 revealing the presence of glycoside hydrolase or glucosidase enzymes.
Table 5.
Genetic analysis results of EFEL6901 and EFEL6800 revealing the presence of glycoside hydrolase or glucosidase enzymes.
| Related enzyme |
EFEL6901 |
EFEL6800 |
| Gene annotation |
Protein ID |
Gene annotation |
Protein ID |
| Glycoside hydrolase |
Glycoside hydrolase family 13 protein |
WP_003668994.1 |
Glycoside hydrolase family 13 protein |
WP_023466491.1 |
| Glycoside hydrolase family 65 protein |
WP_003669571.1 |
Glycoside hydrolase family 65 protein |
WP_023465690.1 |
| Glycoside hydrolase family 70 protein |
WP_229392148.1 |
|
|
| Glycoside hydrolase family 73 protein |
WP_003674860.1WP_003669289.1 |
Glycoside hydrolase family 73 protein |
WP_023466547.1WP_003681382.1WP_004562972.1 |
| Glycoside hydrolase family 2 TIM barrel-domain containing protein |
WP_003667314.1 |
Glycoside hydrolase family 2 TIM barrel-domain containing protein |
WP_282347715.1WP_282348494.1 |
| |
|
Glycoside hydrolase family 3 N-terminal domain-containing protein |
WP_075667436.1WP_240824081.1 |
| |
|
Family 78 glycoside hydrolase catalytic domain |
WP_282348481.1 |
| Glucosidase |
Alpha-glucosidase |
WP_003669620.1 |
Alpha-glucosidase |
WP_023466314.1 |
| Glycosyl hydrolase 53 family protein |
WP_003668597.1 |
|
|