Submitted:
28 August 2023
Posted:
31 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Thermodynamic Model
2.1. Regular Solution Model (RSM)
2.2. Wilson model
2.3. Nonrandom two-liquid (NRTL) model
2.4. Molecular Interaction Volume Model (MIVM)
3. Pair potential energy polynomials of the binary liquid
4. Result analysis
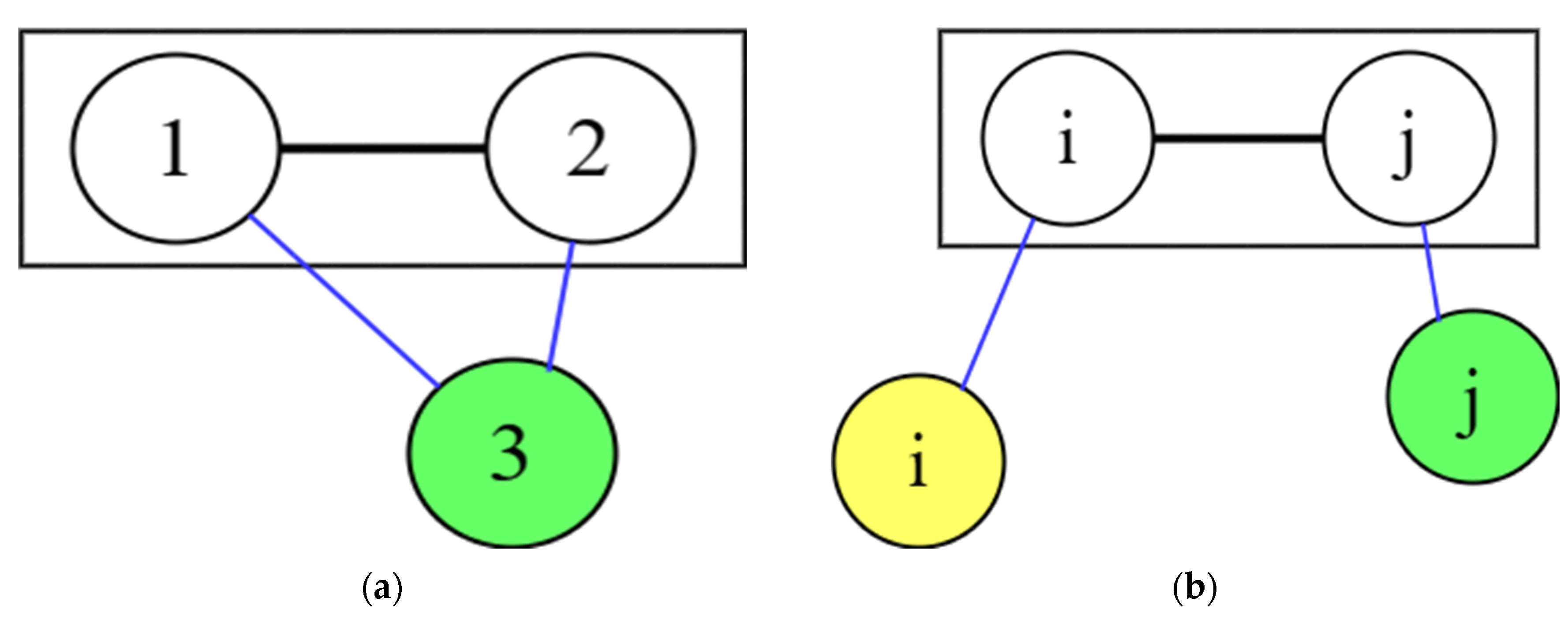
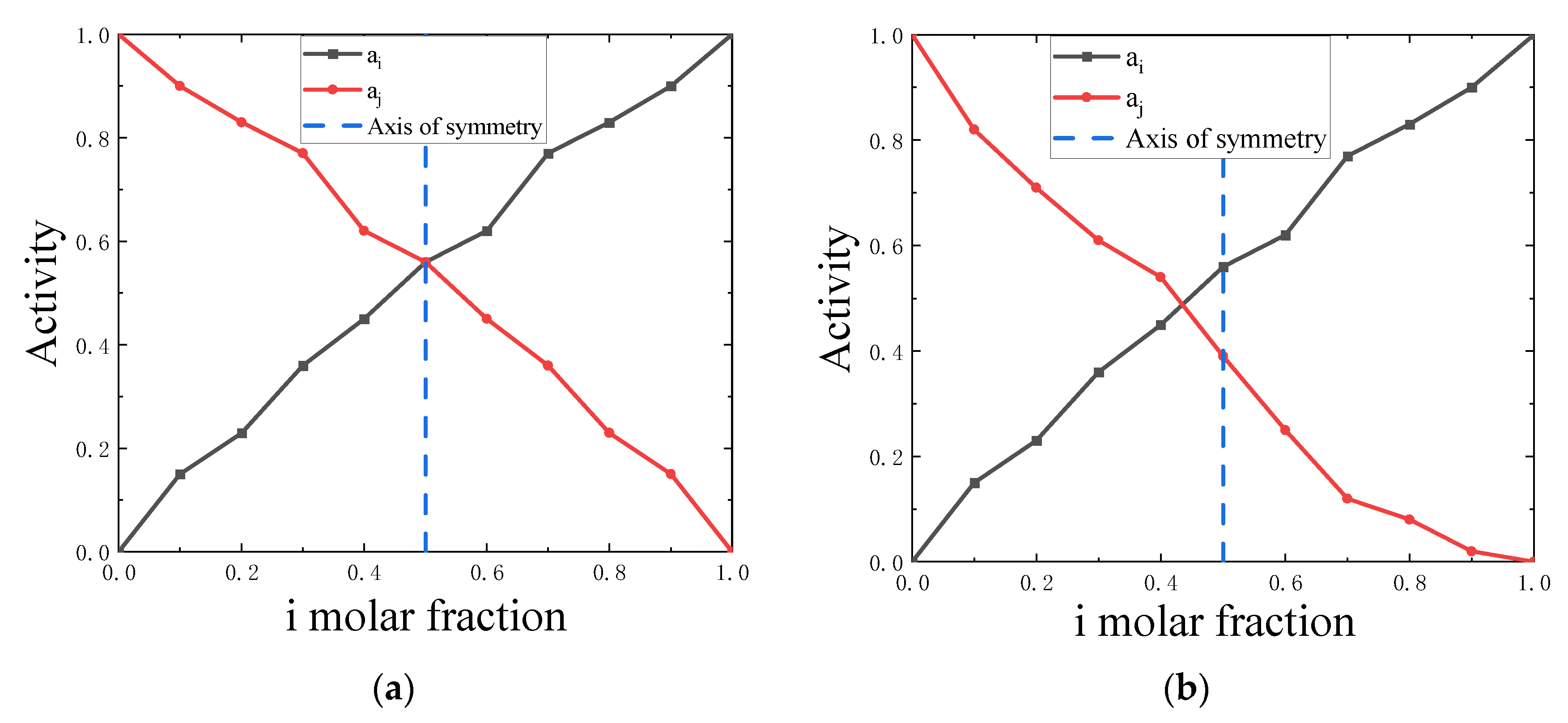
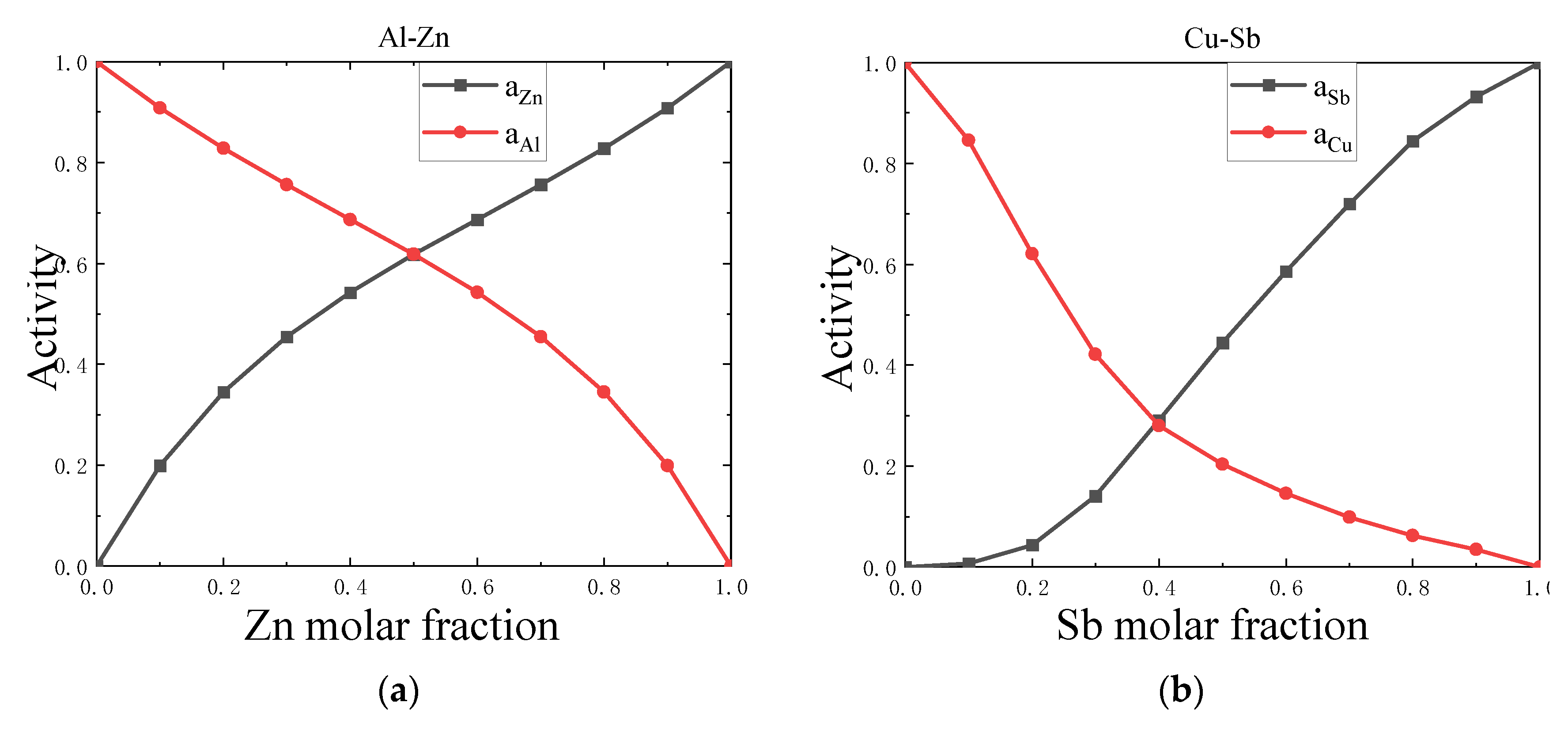
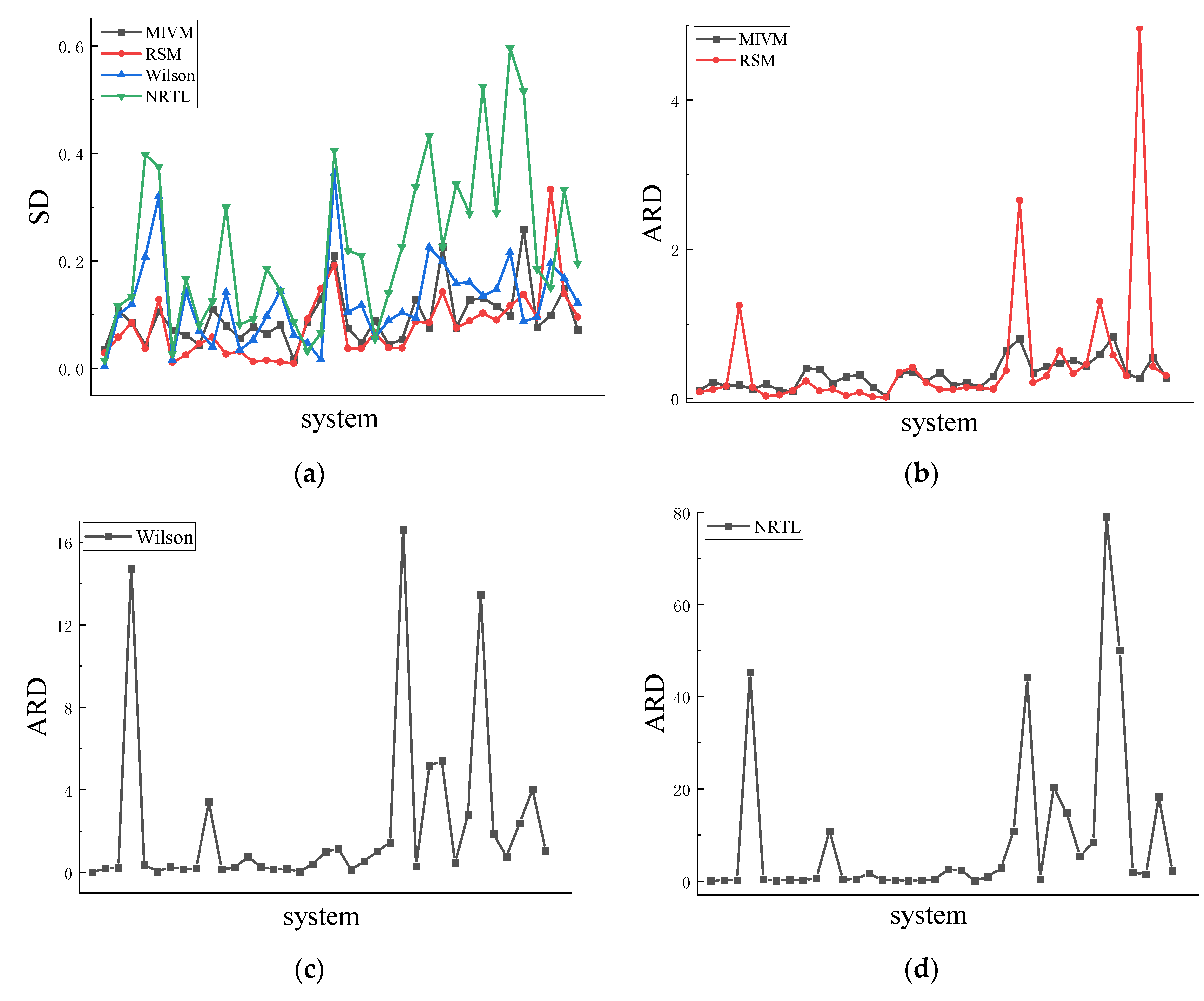
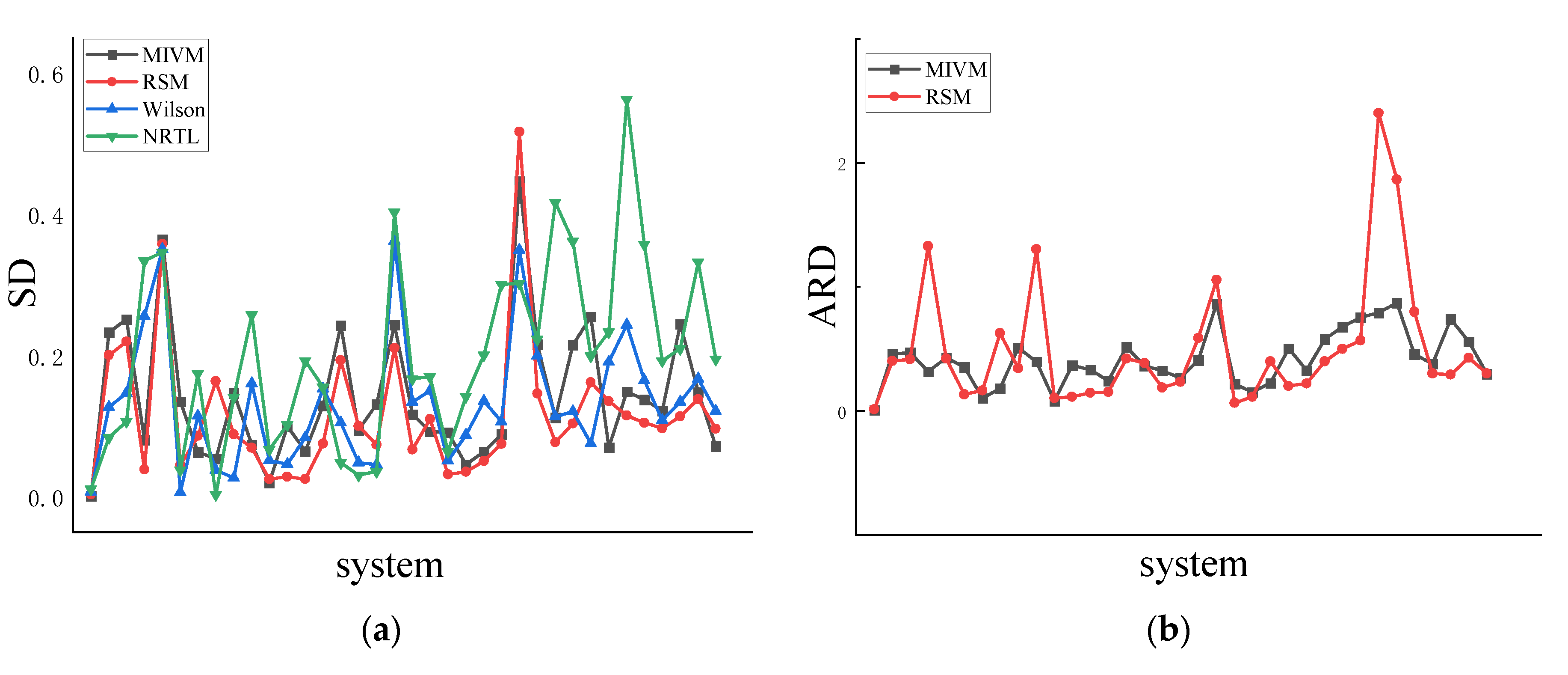
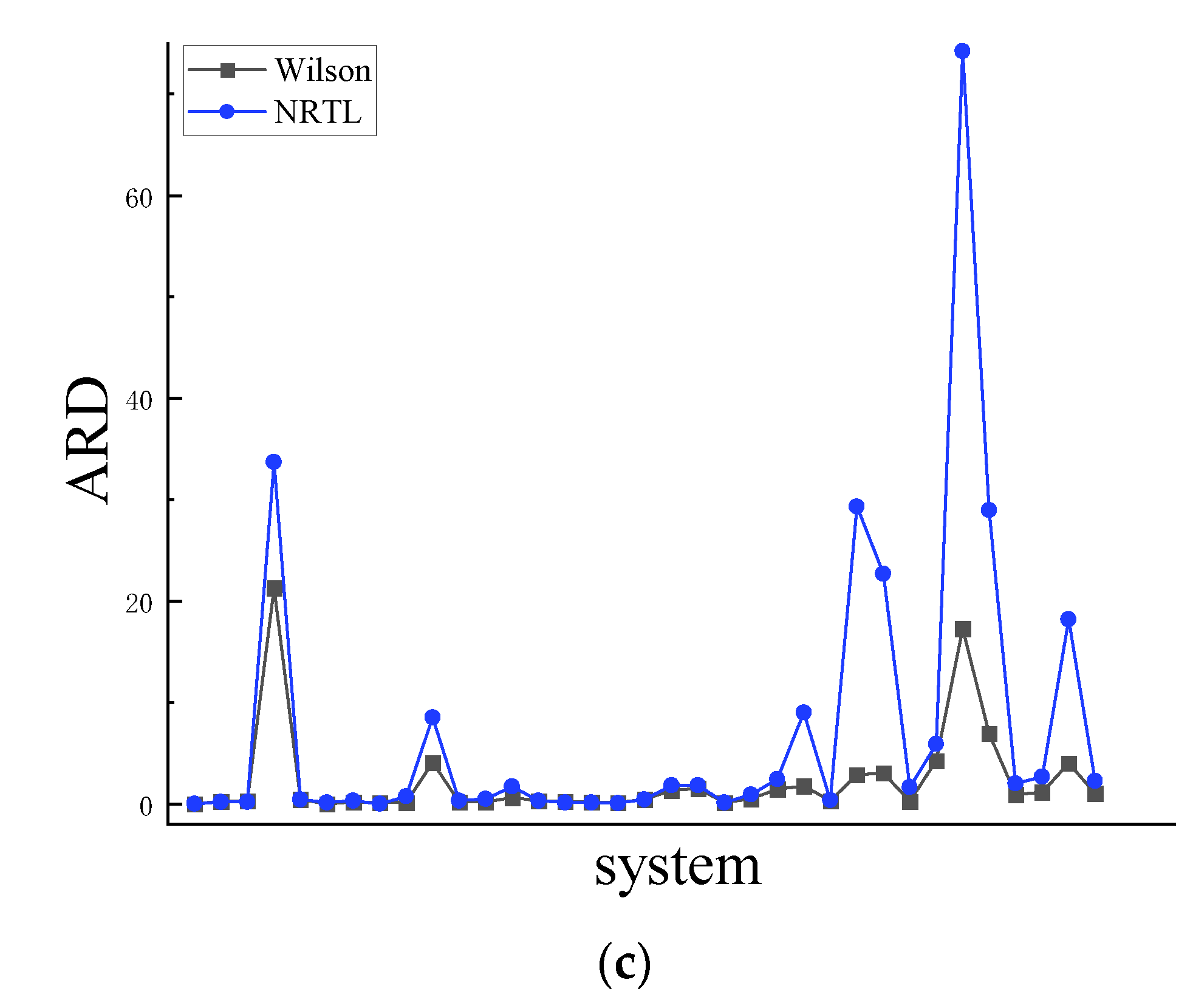
4.1. Symmetry
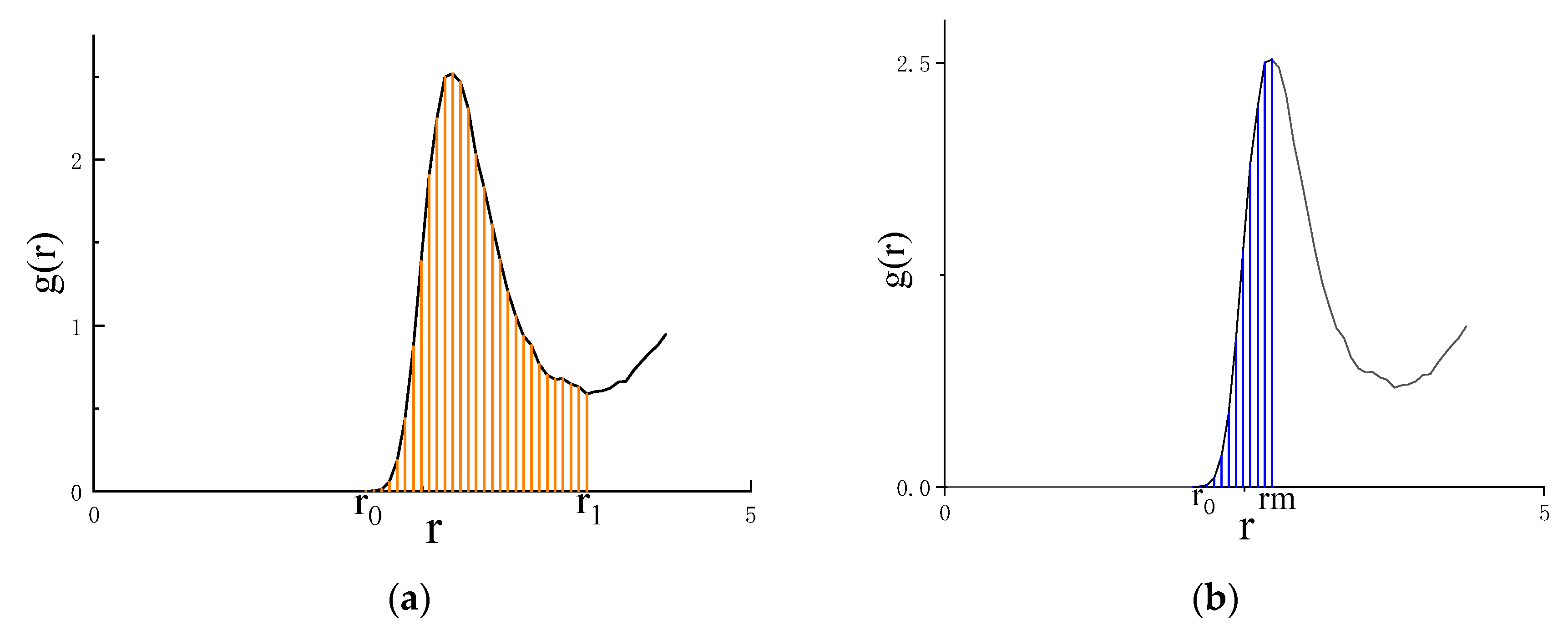
4.2. Asymmetric method for calculating the RDF
4.3. Symmetric method for calculating the RDF
5. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Xie, J.C.; Kar, T.; Xie, R.-H. An Accurate Pair Potential Function for Diatomic Systems. Chemical Physics Letters 2014, 591, 69–77. [Google Scholar] [CrossRef]
- Lennard-Jones, J.E. Cohesion. Proc. Phys. Soc. 1931, 43, 461. [Google Scholar] [CrossRef]
- Morse, P.M. Diatomic Molecules According to the Wave Mechanics. II. Vibrational Levels. Phys. Rev. 1929, 34, 57–64. [Google Scholar] [CrossRef]
- Born, M.; Mayer, J.E. Zur Gittertheorie der Ionenkristalle. Z. Physik 1932, 75, 1–18. [Google Scholar] [CrossRef]
- Ter Horst, M.A.; Schatz, G.C.; Harding, L.B. Potential Energy Surface and Quasiclassical Trajectory Studies of the CN+H 2 Reaction. The Journal of Chemical Physics 1996, 105, 558–571. [Google Scholar] [CrossRef]
- Hirst, D.M. Ab Initio Potential Energy Surfaces for Excited States of the NO2+ Molecular Ion and for the Reaction of N+ with O2. The Journal of Chemical Physics 2001, 115, 9320–9330. [Google Scholar] [CrossRef]
- Grandinetti, F.; Vinciguerra, V. Adducts of NF2+ with Diatomic and Simple Polyatomic Ligands: A Computational Investigation on the Structure, Stability, and Thermochemistry. International Journal of Mass Spectrometry 2002, 216, 285–299. [Google Scholar] [CrossRef]
- Hildebrand, J.H. SOLUBILITY. XII. REGULAR SOLUTIONS 1. J. Am. Chem. Soc. 1929, 51, 66–80. [Google Scholar] [CrossRef]
- Hildebrand, J.H.; Prausnitz, J.M.; Scott, R.L. Regular and Related Solutions : The Solubility of Gases Liquids, and Solids/Joel H. Hildebrand, John M. Prausnitz, Robert L. Scott.
- Wilson, G.M. Vapor-Liquid Equilibrium. XI. A New Expression for the Excess Free Energy of Mixing. J. Am. Chem. Soc. 1964, 86, 127–130. [Google Scholar] [CrossRef]
- Fan, T.X.; Yang, G.J.; Chen, J.Q.; Zhang, D. Model Prediction of Thermodynamics Activity in Multicomponent Liquid Alloy. KEM 2006, 313, 19–24. [Google Scholar] [CrossRef]
- Renon, H.; Prausnitz, J.M. Local Compositions in Thermodynamic Excess Functions for Liquid Mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]
- Tao, D.P. A New Model of Thermodynamics of Liquid Mixtures and Its Application to Liquid Alloys. Thermochimica Acta 2000, 363, 105–113. [Google Scholar] [CrossRef]
- Hill, T.L. Statistical Mechanics : Principles and Selected Applications/Terrell L. Hill. Journal of Chemical Education 1957. [Google Scholar]
- Hu, Y. . Molecular thermodynamics of fluids [M]. Higher Education Press, 1982.
- S.-T. Yeh, Using Trapezoidal Rule for the Area under a Curve Calculation, (n.d.).
- Chanda, S.; Ahmed, A.Z.Z.; Bhuiyan, G.M.; Barman, S.K.; Sarker, S. A Test of Distribution Function Method in the Case of Liquid Transition Metals Alloys. Journal of Non-Crystalline Solids 2011, 357, 3774–3780. [Google Scholar] [CrossRef]
- Trybula, M.; Jakse, N.; Gasior, W.; Pasturel, A. Structural and Physicochemical Properties of Liquid Al–Zn Alloys: A Combined Study Based on Molecular Dynamics Simulations and the Quasi-Lattice Theory. The Journal of Chemical Physics 2014, 141, 224504. [Google Scholar] [CrossRef]
- Hongri, C.; Xiufang, B.; Hui, L.; Li, W. Molecular Dynamics Simulation on Structures of Cu-Ni Alloy. cjcp 2002, 15, 288–294. [Google Scholar]
- Belova, I.V.; Ahmed, T.; Sarder, U.; Yi Wang, W.; Kozubski, R.; Liu, Z.-K.; Holland-Moritz, D.; Meyer, A.; Murch, G.E. Computer Simulation of Thermodynamic Factors in Ni-Al and Cu-Ag Liquid Alloys. Computational Materials Science 2019, 166, 124–135. [Google Scholar] [CrossRef]
- Wang, H.; Wei, B. Understanding Atomic-Scale Phase Separation of Liquid Fe-Cu Alloy. Chin. Sci. Bull. 2011, 56, 3416–3419. [Google Scholar] [CrossRef]
- Goto, R.; Shimojo, F.; Munejiri, S.; Hoshino, K. Structural and Electronic Properties of Liquid Ge–Sn Alloys: Ab Initio Molecular-Dynamics Simulations. J. Phys. Soc. Jpn. 2004, 73, 2746–2752. [Google Scholar] [CrossRef]
- Song, B.; Xu, N.; Jiang, W.; Yang, B.; Chen, X.; Xu, B.; Kong, L.; Liu, D.; Dai, Y. Study on Azeotropic Point of Pb–Sb Alloys by Ab-Initio Molecular Dynamic Simulation and Vacuum Distillation. Vacuum 2016, 125, 209–214. [Google Scholar] [CrossRef]
- Qin, J.; Pan, S.; Qi, Y.; Gu, T. The Structure and Thermodynamic Properties of Liquid Al–Si Alloys by Ab Initio Molecular Dynamics Simulation. Journal of Non-Crystalline Solids 2016, 433, 31–37. [Google Scholar] [CrossRef]
- Kbirou, M.; Mazroui, M.; Hasnaoui, A. Atomic Packing and Fractal Behavior of Al-Co Metallic Glasses. Journal of Alloys and Compounds 2018, 735, 464–472. [Google Scholar] [CrossRef]
- Canales, M.; González, D.J.; González, L.E.; Padró, J.A. Static Structure and Dynamics of the Liquid Li-Na and Li-Mg Alloys. Phys. Rev. E 1998, 58, 4747–4757. [Google Scholar] [CrossRef]
- Pu, Z.; Zhang, H.; Li, Y.; Yang, B. Study on Sn-Sb Alloy by Ab-Initio Molecular Dynamic Simulation. IOP Conf. Ser.: Mater. Sci. Eng. 2018, 394, 032098. [Google Scholar] [CrossRef]
- Jakse, N.; Pasturel, A. Local Order and Dynamic Properties of Liquid and Undercooled Cu x Zr 1 − x Alloys by Ab Initio Molecular Dynamics. Phys. Rev. B 2008, 78, 214204. [Google Scholar] [CrossRef]
- Korkmaz, Ş.; Korkmaz, S.D. Structure and Inter-Diffusion Coefficients of Liquid Na x K1−x Alloys. J. Phase Equilib. Diffus. 2010, 31, 15–21. [Google Scholar] [CrossRef]
- Ishii, Y.; Takanaga, T. Atomic and Electronic Structures and Dynamics in Liquid Alloys near Eutectic Point. J. Phys. Soc. Jpn. 2000, 69, 3334–3341. [Google Scholar] [CrossRef]
- Wang, C.C.; Wong, C.H. Short-to-Medium Range Order of Al–Mg Metallic Glasses Studied by Molecular Dynamics Simulations. Journal of Alloys and Compounds 2011, 509, 10222–10229. [Google Scholar] [CrossRef]
- Korkmaz, S.D.; Korkmaz, Ş. A Study for Structure and Inter-Diffusion Coefficient of Liquid K 1− xCs xMetal Alloys. Physics and Chemistry of Liquids 2011, 49, 801–810. [Google Scholar] [CrossRef]
- Costa Cabral, B. First Principles Molecular Dynamics of a Liquid Li–Na Alloy. Journal of Molecular Structure: THEOCHEM 1999, 463, 145–149. [Google Scholar] [CrossRef]
- Bai, Y.W.; Zhao, X.L.; Bian, X.F.; Song, K.K.; Zhao, Y. Structure Evolution of Au50Cu50 Alloy from Melt to the Disordered Solid Solution. MSF 2020, 993, 273–280. [Google Scholar] [CrossRef]
- Shi, L.; Jia, L.; Ning, P.; Sun, X.; Wang, C.; Ma, Y.; Wang, F.; Qu, T.; Li, K. Vacuum Distillation and Ab Initio Molecular Dynamic Simulation of Al–Li Alloys. Vacuum 2023, 210, 111877. [Google Scholar] [CrossRef]
- Yang, S.J.; Hu, L.; Wang, L.; Wei, B. Molecular Dynamics Simulation of Liquid Structure for Undercooled Zr-Nb Alloys Assisted with Electrostatic Levitation Experiments. Chemical Physics Letters 2018, 701, 109–114. [Google Scholar] [CrossRef]
- Özdemir Kart, S.; Tomak, M.; Uludoğan, M.; Çağın, T. Liquid Properties of Pd–Ni Alloys. Journal of Non-Crystalline Solids 2004, 337, 101–108. [Google Scholar] [CrossRef]
- Liu, D.; Qin, J.Y.; Gu, T.K. The Structure of Liquid Mg–Cu Binary Alloys. Journal of Non-Crystalline Solids 2010, 356, 1587–1592. [Google Scholar] [CrossRef]
- Rao, R.V.G.; Venkatesh, R. Investigations of the Dynamic Properties of the Liquid Phase of Glassy CaAl Alloys. phys. stat. sol. (b) 1992, 170, 39–46. [Google Scholar] [CrossRef]
- Mendelev, M.I.; Kramer, M.J.; Hao, S.G.; Ho, K.M.; Wang, C.Z. Development of Interatomic Potentials Appropriate for Simulation of Liquid and Glass Properties of NiZr 2 Alloy. Philosophical Magazine 2012, 92, 4454–4469. [Google Scholar] [CrossRef]
- Roik, O.S.; Yakovenko, O.M.; Kazimirov, V.P.; Sokol’skii, V.E.; Golovataya, N.V.; Kashirina, Ya.O. Structure of Liquid Al Sn Alloys. Journal of Molecular Liquids 2021, 330, 115570. [Google Scholar] [CrossRef]
- Roik, O.S.; Samsonnikov, O.V.; Kazimirov, V.P.; Sokolskii, V.E.; Galushko, S.M. Medium-Range Order in Al-Based Liquid Binary Alloys. Journal of Molecular Liquids 2010, 151, 42–49. [Google Scholar] [CrossRef]
- Chen, F.; Cao, C.; Zhong, Q.; Liu, J.; Yang, L.; Chen, Z. Ab Initio Molecular Dynamics Study on Local Structure and Dynamic Properties of Liquid Ni62Nb38 Alloy. Materials Today Communications 2021, 27, 102207. [Google Scholar] [CrossRef]
- Gruner, S.; Kaban, I.; Kleinhempel, R.; Hoyer, W.; Jóvári, P.; Delaplane, R.G. Short-Range Order and Atomic Clusters in Liquid Cu–Sn Alloys. Journal of Non-Crystalline Solids 2005, 351, 3490–3496. [Google Scholar] [CrossRef]
- Jakse, N.; Nguyen, T.L.T.; Pasturel, A. Local Order and Dynamic Properties of Liquid Au x Si1− x Alloys by Molecular Dynamics Simulations. The Journal of Chemical Physics 2012, 137, 204504. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-Y.; Kim, H.; Hwang, G.S. A Comparative First-Principles Study of the Structure, Energetics, and Properties of Li–M (M = Si, Ge, Sn) Alloys. J. Phys. Chem. C 2011, 115, 20018–20026. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Qin, J.; Dong, B.; Pan, S. The Reassessment of the Structural Transition Regions along the Liquidus of Fe–Si Alloys and a Possible Liquid–Liquid Structural Transition in FeSi2 Alloy. Physics Letters A 2018, 382, 2655–2661. [Google Scholar] [CrossRef]
- Bhuiyan, G.M.; Ali, I.; Rahman, S.M.M. Atomic Transport Properties of AgIn Liquid Binary Alloys. Physica B: Condensed Matter 2003, 334, 147–159. [Google Scholar] [CrossRef]
- Weber, H.; Schumacher, M.; Jóvári, P.; Tsuchiya, Y.; Skrotzki, W.; Mazzarello, R.; Kaban, I. Experimental and Ab Initio Molecular Dynamics Study of the Structure and Physical Properties of Liquid GeTe. Phys. Rev. B 2017, 96, 054204. [Google Scholar] [CrossRef]
- Peng, H.L.; Voigtmann, Th.; Kolland, G.; Kobatake, H.; Brillo, J. Structural and Dynamical Properties of Liquid Al-Au Alloys. Phys. Rev. B 2015, 92, 184201. [Google Scholar] [CrossRef]
- Guo, F.; Tian, Y.; Qin, J.; Xu, R.; Zhang, Y.; Zheng, H.; Lv, T.; Qin, X.; Tian, X.; Sun, Y. Structure of Liquid Cu–Sb Alloys by Ab Initio Molecular Dynamics Simulations, High Temperature X-Ray Diffraction, and Resistivity. J Mater Sci 2013, 48, 4438–4445. [Google Scholar] [CrossRef]
- Dorini, T.T.; Eleno, L.T.F. Liquid Bi–Pb and Bi–Li Alloys: Mining Thermodynamic Properties from Ab-Initio Molecular Dynamics Calculations Using Thermodynamic Models. Calphad 2019, 67, 101687. [Google Scholar] [CrossRef]
- Franke, P.L. , & Neuschütz, D. Binary Systems. “Binary Systems. Part 1-5.
| System | Co-Ni [17] | Al-Zn [18] | Cu-Ni [19] | Al-Ni [20] | Cu-Fe [21] | Ge-Sn [22] | Ag-Cu [20] | Pb-Sb [23] | Al-Si [24] | Al-Co [25] |
| System | Li-Mg [26] | Sb-Sn [27] | Cu-Zr [28] | K-Na [29] | Pb-Sn [30] | Al-Mg [31] | Cs-K [32] | Li-Na [33] | Au-Cu [34] | Al-Li [35] |
| System | Nb-Zr [36] | Ni-Pd [37] | Cu-Mg [38] | Al-Ca [39] | Ni-Zr [40] | Al-Sn [41] | Al-Cu [42] | Nb-Ni [43] | Cu-Sn [44] | Au-Si [45] |
| System | Li-Sn [46] | Fe-Si [47] | Ag-In [48] | Ge-Te [49] | Al-Au [50] | Cu-Sb [51] |
| System | Co-Ni | Al-Zn | Cu-Ni | Al-Ni | Cu-Fe | Ge-Sn | Ag-Cu | Pb-Sb | Al-Si | Al-Co |
| 0 | 0 | 0.0028 | 0.0034 | 0.0048 | 0.007 | 0.0088 | 0.0096 | 0.0102 | 0.0114 | |
| System | Li-Mg | Sb-Sn | Cu-Zr | K-Na | Pb-Sn | Al-Mg | Cs-K | Li-Na | Au-Cu | Al-Li |
| 0.0114 | 0.0115 | 0.0119 | 0.0136 | 0.0158 | 0.0177 | 0.0228 | 0.0336 | 0.0364 | 0.0452 | |
| System | Nb-Zr | Ni-Pd | Cu-Mg | Al-Ca | Ni-Zr | Al-Sn | Al-Cu | Nb-Ni | Cu-Sn | Au-Si |
| 0.0473 | 0.0494 | 0.0597 | 0.0724 | 0.0807 | 0.0823 | 0.1116 | 0.1334 | 0.1342 | 0.139 | |
| System | Li-Sn | Fe-Si | Ag-In | Ge-Te | Al-Au | Cu-Sb | ||||
| 0.14 | 0.1454 | 0.1483 | 0.1548 | 0.160 | 0.208 |
| System | MIVM | RSM | Wilson | NRTL | |||
|---|---|---|---|---|---|---|---|
| Co-Ni | 0.964 | 0.977 | 0.343 | 0.964 | 0.977 | -0.036 | -0.023 |
| Al-Zn | 1.246 | 0.743 | 0.423 | 1.166 | 0.795 | 0.220 | -0.297 |
| Cu-Ni | 1.086 | 0.863 | 0.374 | 0.999 | 0.938 | 0.082 | -0.147 |
| Al-Ni | 1.493 | 1.649 | -5.203 | 0.991 | 2.485 | 0.401 | 0.500 |
| Cu-Fe | 0.943 | 0.805 | 1.512 | 0.943 | 0.805 | -0.059 | -0.217 |
| Ge-Sn | 0.740 | 1.273 | 0.266 | 1.214 | 0.776 | -0.302 | 0.241 |
| Ag-Cu | 0.675 | 1.194 | 1.225 | 0.471 | 1.709 | -0.394 | 0.177 |
| Pb-Sb | 1.558 | 0.517 | 1.046 | 1.457 | 0.553 | 0.443 | -0.660 |
| Al-Si | 1.405 | 1.077 | -1.856 | 1.226 | 1.235 | 0.340 | 0.074 |
| Al-Co | 1.112 | 1.884 | -4.213 | 0.802 | 2.612 | 0.106 | 0.633 |
| Li-Mg | 1.357 | 0.914 | -1.095 | 1.469 | 0.844 | 0.305 | -0.090 |
| Sb-Sn | 0.769 | 1.545 | -0.847 | 0.735 | 1.618 | -0.262 | 0.435 |
| Cu-Zr | 0.908 | 1.669 | -2.277 | 1.782 | 0.851 | -0.096 | 0.512 |
| K-Na | 0.700 | 1.176 | 1.018 | 0.366 | 2.252 | -0.357 | 0.162 |
| Pb-Sn | 1.044 | 0.912 | 0.267 | 0.932 | 1.021 | 0.043 | -0.092 |
| Al-Mg | 0.820 | 1.123 | 0.459 | 1.162 | 0.793 | -0.198 | 0.116 |
| Cs-K | 1.204 | 0.635 | 1.310 | 0.801 | 0.955 | 0.185 | -0.454 |
| Li-Na | 0.764 | 0.999 | 1.347 | 1.416 | 0.539 | -0.270 | -0.001 |
| Au-Cu | 1.163 | 1.444 | -2.877 | 0.812 | 2.068 | 0.151 | 0.368 |
| Al-Li | 1.136 | 1.379 | -2.356 | 1.485 | 1.055 | 0.128 | 0.321 |
| Nb-Zr | 0.814 | 1.255 | -0.110 | 1.048 | 0.975 | -0.206 | 0.227 |
| Ni-Pd | 1.168 | 1.087 | -1.307 | 1.590 | 0.798 | 0.153 | 0.080 |
| Cu-Mg | 1.312 | 1.258 | -2.781 | 2.575 | 0.641 | 0.272 | 0.230 |
| Al-Ca | 2.202 | 1.259 | -5.761 | 5.826 | 0.476 | 0.789 | 0.230 |
| Ni-Zr | 1.522 | 1.730 | -5.373 | 3.247 | 0.811 | 0.420 | 0.548 |
| Al-Sn | 0.624 | 1.440 | 0.598 | 1.024 | 0.877 | -0.471 | 0.365 |
| Al-Cu | 1.568 | 1.530 | -5.209 | 2.192 | 1.152 | 0.476 | 0.450 |
| Nb-Ni | 1.772 | 1.071 | -3.558 | 1.070 | 1.774 | 0.572 | 0.069 |
| Cu-Sn | 1.987 | 0.849 | -2.904 | 0.874 | 1.932 | 0.687 | -0.163 |
| Au-Si | 1.224 | 1.684 | -3.129 | 1.034 | 1.993 | 0.202 | 0.521 |
| Li-Sn | 3.396 | 2.186 | -10.225 | 4.263 | 1.742 | 1.223 | 0.782 |
| Fe-Si | 5.678 | 1.776 | -9.822 | 4.695 | 2.148 | 1.737 | 0.574 |
| Ag-In | 1.595 | 0.925 | -2.227 | 2.476 | 0.596 | 0.467 | -0.078 |
| Ge-Te | 0.409 | 1.891 | 1.285 | 0.912 | 0.848 | -0.894 | 0.637 |
| Al-Au | 1.680 | 1.660 | -5.740 | 2.287 | 1.219 | 0.519 | 0.507 |
| Cu-Sb | 1.804 | 0.881 | -2.315 | 0.757 | 2.098 | 0.590 | -0.127 |
| System | MIVM | RSM | Wilson | NRTL | ||||
|---|---|---|---|---|---|---|---|---|
| SD | ARD/% | SD | ARD/% | SD | ARD/% | SD | ARD/% | |
| Co-Ni | 0.036 | 10.8 | 0.029 | 8.8 | 0.003 | 0.8 | 0.015 | 4.4 |
| Al-Zn | 0.108 | 22 | 0.058 | 12.1 | 0.100 | 20.5 | 0.116 | 23.7 |
| Cu-Ni | 0.085 | 16.5 | 0.085 | 16.5 | 0.120 | 23.1 | 0.134 | 25.7 |
| Al-Ni | 0.044 | 18.3 | 0.037 | 125 | 0.208 | 1472 | 0.398 | 4520 |
| Cu-Fe | 0.106 | 12.5 | 0.128 | 15.1 | 0.321 | 37.5 | 0.375 | 44 |
| Ge-Sn | 0.071 | 19.4 | 0.011 | 3.1 | 0.016 | 4.1 | 0.027 | 7.5 |
| Ag-Cu | 0.062 | 10.8 | 0.025 | 4.6 | 0.143 | 26.1 | 0.167 | 30.5 |
| Pb-Sb | 0.044 | 9.9 | 0.047 | 10.5 | 0.070 | 15.9 | 0.080 | 18.1 |
| Al-Si | 0.110 | 40.5 | 0.058 | 23.2 | 0.041 | 18.5 | 0.125 | 63.3 |
| Al-Co | 0.080 | 39.2 | 0.027 | 10.5 | 0.142 | 341 | 0.300 | 1085 |
| Li-Mg | 0.056 | 20.7 | 0.032 | 12.3 | 0.035 | 14.2 | 0.082 | 34.9 |
| Sb-Sn | 0.078 | 29.3 | 0.012 | 3.6 | 0.054 | 24.5 | 0.093 | 44.2 |
| Cu-Zr | 0.064 | 31.6 | 0.015 | 8.4 | 0.098 | 74.4 | 0.185 | 162 |
| K-Na | 0.081 | 15.3 | 0.012 | 2 | 0.144 | 27.8 | 0.145 | 28 |
| Pb-Sn | 0.016 | 3.2 | 0.009 | 1.7 | 0.063 | 14.3 | 0.087 | 19.6 |
| Al-Mg | 0.087 | 32.9 | 0.092 | 34.9 | 0.048 | 17 | 0.033 | 11.2 |
| Cs-K | 0.129 | 36 | 0.148 | 41.7 | 0.016 | 3.3 | 0.066 | 16.9 |
| Li-Na | 0.210 | 22.9 | 0.192 | 21.3 | 0.364 | 40.5 | 0.405 | 45.2 |
| Au-Cu | 0.076 | 34.5 | 0.038 | 12.1 | 0.105 | 101 | 0.219 | 255 |
| Al-Li | 0.048 | 17 | 0.037 | 12.2 | 0.118 | 115 | 0.209 | 236 |
| Nb-Zr | 0.089 | 21.1 | 0.066 | 15.1 | 0.058 | 13 | 0.056 | 12.4 |
| Ni-Pd | 0.044 | 15 | 0.039 | 14.1 | 0.090 | 53.7 | 0.140 | 91 |
| Cu-Mg | 0.054 | 30.2 | 0.038 | 12.4 | 0.104 | 103 | 0.226 | 286 |
| Al-Ca | 0.129 | 64.3 | 0.087 | 37.4 | 0.094 | 144 | 0.338 | 1081 |
| Ni-Zr | 0.076 | 80.2 | 0.085 | 266 | 0.225 | 1661 | 0.433 | 4415 |
| Al-Sn | 0.226 | 34.4 | 0.142 | 21.3 | 0.199 | 30.4 | 0.226 | 34.5 |
| Al-Cu | 0.076 | 43 | 0.075 | 30.1 | 0.158 | 516 | 0.343 | 2031 |
| Nb-Ni | 0.128 | 47.2 | 0.089 | 64 | 0.161 | 540 | 0.288 | 1478 |
| Cu-Sn | 0.131 | 51.2 | 0.103 | 33.3 | 0.135 | 48 | 0.524 | 544 |
| Au-Si | 0.116 | 44 | 0.090 | 45.6 | 0.147 | 279 | 0.290 | 847 |
| Li-Sn | 0.098 | 59.1 | 0.117 | 130 | 0.216 | 1347 | 0.596 | 7906 |
| Fe-Si | 0.259 | 82.9 | 0.137 | 58.3 | 0.088 | 186 | 0.516 | 5003 |
| Ag-In | 0.077 | 32.8 | 0.095 | 30.3 | 0.095 | 76 | 0.185 | 191 |
| Ge-Te | 0.099 | 27.3 | 0.333 | 496 | 0.195 | 239 | 0.150 | 148 |
| Al-Au | 0.149 | 55.9 | 0.139 | 42.7 | 0.168 | 405 | 0.333 | 1822 |
| Cu-Sb | 0.072 | 28 | 0.096 | 30.4 | 0.122 | 104 | 0.196 | 229 |
| Ave | 0.095 | 32.2 | 0.078 | 47.4 | 0.124 | 226 | 0.225 | 911 |
| System | MIVM | RSM | Wilson | NRTL | |||
|---|---|---|---|---|---|---|---|
| Co-Ni | 1.089 | 0.900 | 0.118 | 1.089 | 0.900 | 0.085 | -0.106 |
| Al-Zn | 1.317 | 0.938 | -1.162 | 1.232 | 1.003 | 0.275 | -0.064 |
| Cu-Ni | 1.391 | 0.877 | -1.138 | 1.280 | 0.953 | 0.330 | -0.132 |
| Al-Ni | 1.155 | 2.099 | -5.114 | 0.766 | 3.163 | 0.144 | 0.742 |
| Cu-Fe | 0.890 | 1.145 | -0.103 | 0.890 | 1.145 | -0.117 | 0.135 |
| Ge-Sn | 0.562 | 1.573 | 0.545 | 0.923 | 0.958 | -0.576 | 0.453 |
| Ag-Cu | 1.309 | 0.582 | 1.538 | 0.914 | 0.833 | 0.269 | -0.542 |
| Pb-Sb | 1.231 | 0.810 | 0.017 | 1.151 | 0.866 | 0.207 | -0.211 |
| Al-Si | 1.534 | 1.140 | -2.499 | 1.338 | 1.307 | 0.428 | 0.131 |
| Al-Co | 0.830 | 1.774 | -2.218 | 0.551 | 2.673 | -0.186 | 0.573 |
| Li-Mg | 1.087 | 0.988 | -0.363 | 1.177 | 0.912 | 0.083 | -0.012 |
| Sb-Sn | 0.825 | 1.576 | -1.288 | 0.788 | 1.650 | -0.192 | 0.455 |
| Cu-Zr | 1.206 | 1.206 | 1.206 | 1.206 | 1.206 | 1.206 | 1.206 |
| K-Na | 0.562 | 1.364 | 1.390 | 0.293 | 2.613 | -0.577 | 0.311 |
| Pb-Sn | 1.601 | 0.830 | -1.549 | 1.430 | 0.929 | 0.471 | -0.186 |
| Al-Mg | 0.808 | 1.126 | 0.528 | 1.144 | 0.796 | -0.213 | 0.119 |
| Cs-K | 1.642 | 0.660 | -0.391 | 1.092 | 0.992 | 0.496 | -0.416 |
| Li-Na | 0.728 | 1.069 | 1.245 | 1.350 | 0.577 | -0.317 | 0.067 |
| Au-Cu | 2.423 | 0.515 | -1.229 | 1.692 | 0.737 | 0.885 | -0.664 |
| Al-Li | 0.813 | 1.374 | -0.580 | 1.062 | 1.051 | -0.208 | 0.318 |
| Nb-Zr | 0.671 | 1.414 | 0.279 | 0.864 | 1.098 | -0.399 | 0.346 |
| Ni-Pd | 1.174 | 1.105 | 11.250 | -1.445 | 1.599 | 0.811 | 0.159 |
| Cu-Mg | 0.882 | 1.538 | -1.689 | 1.731 | 0.783 | -0.126 | 0.430 |
| Al-Ca | 2.423 | 0.515 | -1.229 | 1.692 | 0.737 | 0.885 | -0.664 |
| Ni-Zr | 0.607 | 1.260 | 1.484 | 1.296 | 0.591 | -0.499 | 0.231 |
| Al-Sn | 0.649 | 1.393 | 0.566 | 1.065 | 0.849 | -0.433 | 0.332 |
| Al-Cu | 1.963 | 1.534 | -6.647 | 1.472 | 2.180 | 0.714 | 0.453 |
| Nb-Ni | 3.602 | 1.064 | -7.456 | 2.174 | 1.763 | 1.281 | 0.062 |
| Cu-Sn | 4.758 | 0.617 | -5.972 | 2.091 | 1.403 | 1.560 | -0.484 |
| Au-Si | 1.438 | 0.875 | -0.992 | 1.214 | 1.036 | 0.363 | -0.134 |
| Li-Sn | 2.077 | 2.672 | -8.741 | 2.608 | 2.129 | 0.731 | 0.983 |
| Fe-Si | 1.534 | 1.843 | -4.414 | 1.268 | 2.228 | 0.428 | 0.611 |
| Ag-In | 1.066 | 1.467 | -2.560 | 1.654 | 0.946 | 0.064 | 0.384 |
| Ge-Te | 0.636 | 3.025 | -3.272 | 1.419 | 1.356 | -0.452 | 1.107 |
| Al-Au | 1.903 | 0.844 | -2.370 | 0.799 | 2.011 | 0.643 | -0.169 |
| Cu-Sb | 1.743 | 1.017 | -3.207 | 1.743 | 1.017 | 0.556 | 0.017 |
| System | MIVM | RSM | Wilson | NRTL | ||||
|---|---|---|---|---|---|---|---|---|
| SD | ARD/% | SD | ARD/% | SD | ARD/% | SD | ARD/% | |
| Co-Ni | 0.002 | 0.4 | 0.004 | 1.1 | 0.007 | 2.1 | 0.011 | 3.3 |
| Al-Zn | 0.233 | 45.8 | 0.201 | 40 | 0.128 | 26 | 0.085 | 17.5 |
| Cu-Ni | 0.252 | 46.9 | 0.220 | 41.3 | 0.147 | 28.3 | 0.107 | 20.8 |
| Al-Ni | 0.081 | 31.7 | 0.039 | 133 | 0.257 | 2130 | 0.335 | 3370 |
| Cu-Fe | 0.365 | 42.8 | 0.359 | 42 | 0.351 | 41.2 | 0.347 | 40.7 |
| Ge-Sn | 0.135 | 35.4 | 0.045 | 13.2 | 0.007 | 1.6 | 0.039 | 10.8 |
| Ag-Cu | 0.063 | 10.2 | 0.087 | 16.3 | 0.115 | 21.2 | 0.175 | 31.8 |
| Pb-Sb | 0.054 | 17.9 | 0.164 | 62.6 | 0.038 | 13.3 | 0.004 | 0.9 |
| Al-Si | 0.147 | 50.8 | 0.089 | 34.2 | 0.027 | 12.1 | 0.142 | 72.6 |
| Al-Co | 0.074 | 39.6 | 0.070 | 130 | 0.161 | 411 | 0.258 | 852 |
| Li-Mg | 0.020 | 7.9 | 0.026 | 10.2 | 0.052 | 21.7 | 0.067 | 28.5 |
| Sb-Sn | 0.100 | 36.5 | 0.029 | 11.3 | 0.046 | 20.7 | 0.103 | 49.6 |
| Cu-Zr | 0.065 | 33 | 0.026 | 14.4 | 0.084 | 62.1 | 0.193 | 171 |
| K-Na | 0.130 | 24.3 | 0.076 | 15.2 | 0.154 | 29.6 | 0.156 | 29.9 |
| Pb-Sn | 0.244 | 51.4 | 0.194 | 42 | 0.105 | 23.7 | 0.049 | 11 |
| Al-Mg | 0.095 | 36.2 | 0.101 | 38.4 | 0.049 | 17.5 | 0.031 | 10.7 |
| Cs-K | 0.132 | 32.3 | 0.074 | 18.8 | 0.046 | 11.5 | 0.036 | 8.9 |
| Li-Na | 0.244 | 26.7 | 0.211 | 23.4 | 0.363 | 40.4 | 0.404 | 45.1 |
| Au-Cu | 0.117 | 40.7 | 0.067 | 58.8 | 0.135 | 137 | 0.168 | 181 |
| Al-Li | 0.093 | 86.2 | 0.110 | 106 | 0.151 | 156 | 0.171 | 182 |
| Nb-Zr | 0.092 | 21.6 | 0.032 | 6.3 | 0.052 | 11.5 | 0.065 | 14.7 |
| Ni-Pd | 0.046 | 15.2 | 0.036 | 11.3 | 0.088 | 52.4 | 0.143 | 93.2 |
| Cu-Mg | 0.065 | 22.3 | 0.051 | 40 | 0.136 | 144 | 0.202 | 245 |
| Al-Ca | 0.089 | 50.3 | 0.075 | 20 | 0.107 | 175 | 0.301 | 901 |
| Ni-Zr | 0.447 | 4520 | 0.518 | 5760 | 0.350 | 3200 | 0.303 | 2560 |
| Al-Sn | 0.216 | 32.8 | 0.147 | 21.9 | 0.200 | 30.6 | 0.224 | 34.3 |
| Al-Cu | 0.113 | 57.6 | 0.078 | 39.9 | 0.114 | 289 | 0.417 | 2930 |
| Nb-Ni | 0.216 | 67.7 | 0.104 | 49.9 | 0.121 | 302 | 0.363 | 2270 |
| Cu-Sn | 0.255 | 75.4 | 0.163 | 56.7 | 0.076 | 28 | 0.200 | 167 |
| Au-Si | 0.070 | 79 | 0.136 | 240 | 0.192 | 425 | 0.234 | 591 |
| Li-Sn | 0.150 | 86.9 | 0.116 | 187 | 0.244 | 1730 | 0.564 | 7420 |
| Fe-Si | 0.138 | 45.8 | 0.105 | 79.9 | 0.165 | 693 | 0.358 | 2900 |
| Ag-In | 0.122 | 37.9 | 0.097 | 30.1 | 0.109 | 90.8 | 0.193 | 204 |
| Ge-Te | 0.245 | 73.9 | 0.114 | 29.1 | 0.135 | 118 | 0.211 | 268 |
| Al-Au | 0.149 | 55.9 | 0.139 | 42.7 | 0.168 | 405 | 0.333 | 1820 |
| Cu-Sb | 0.072 | 29.9 | 0.097 | 30 | 0.122 | 105 | 0.196 | 228 |
| Ave | 0.143 | 165.9 | 0.117 | 208.3 | 0.133 | 305.6 | 0.200 | 771.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




