Submitted:
30 August 2023
Posted:
31 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
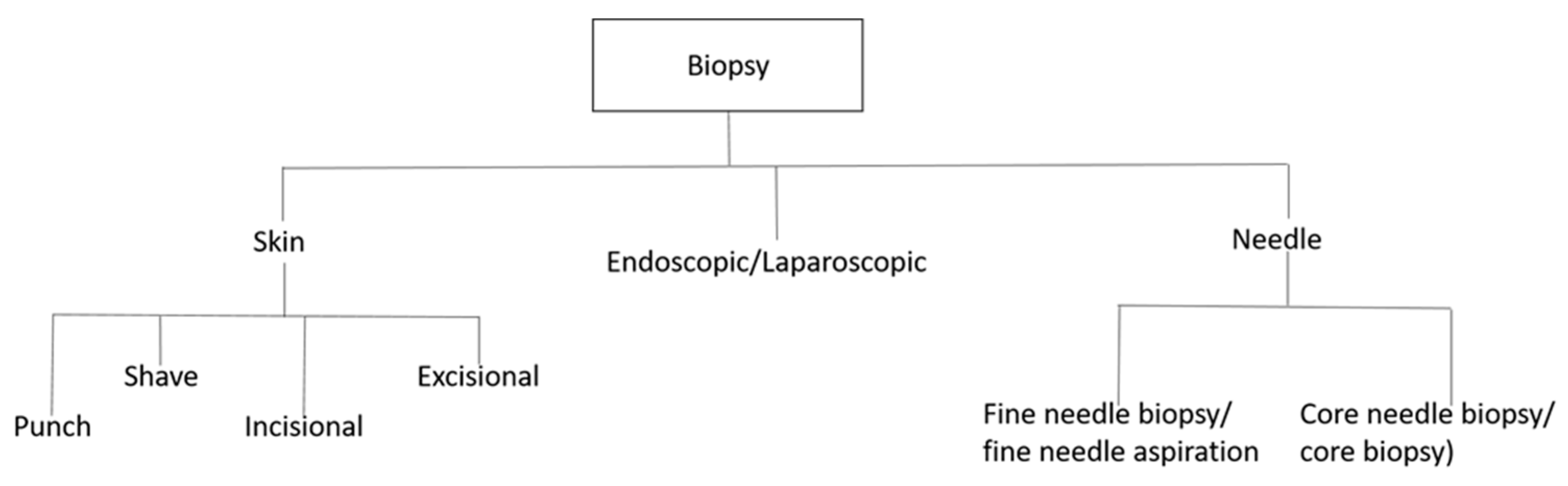
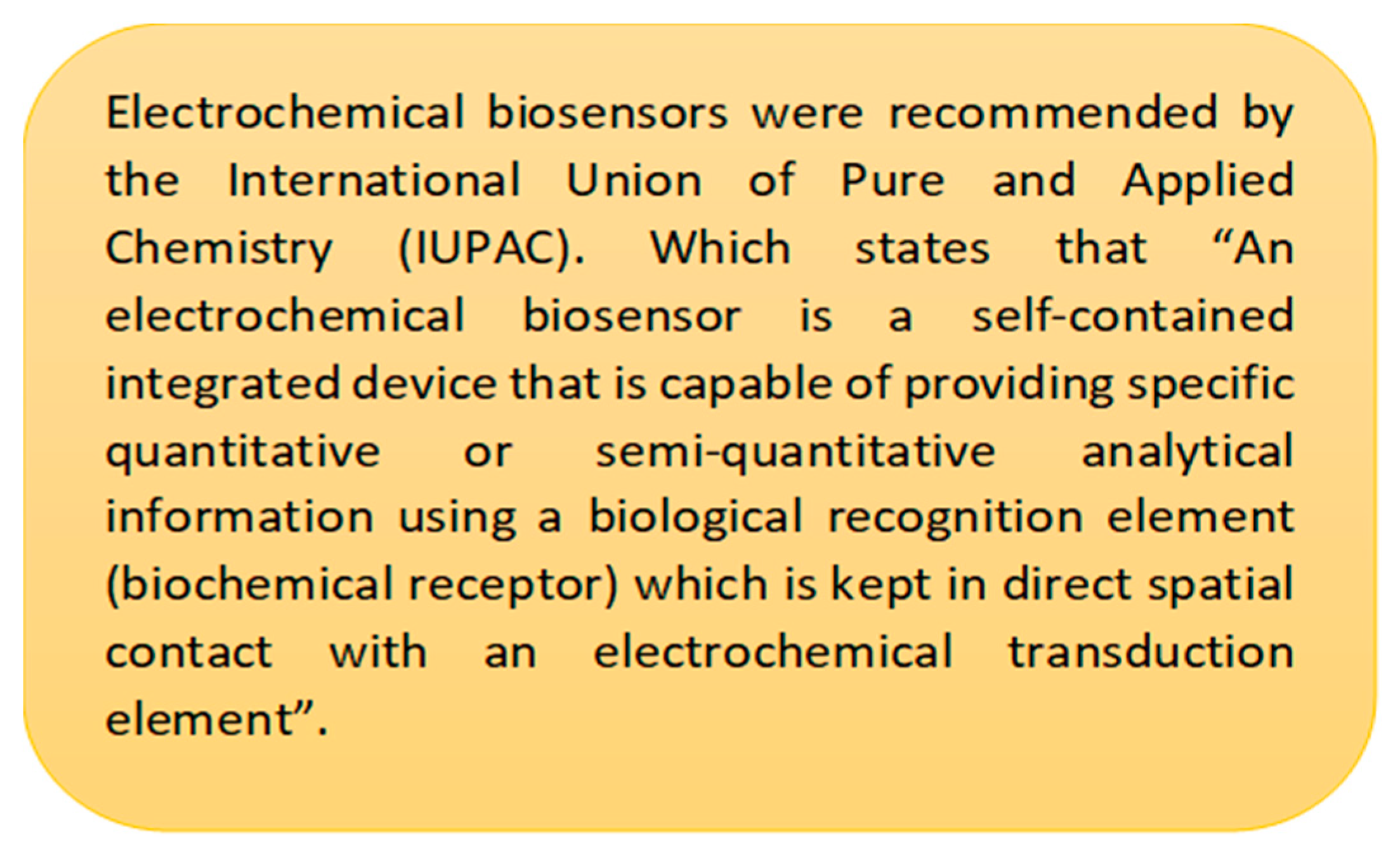

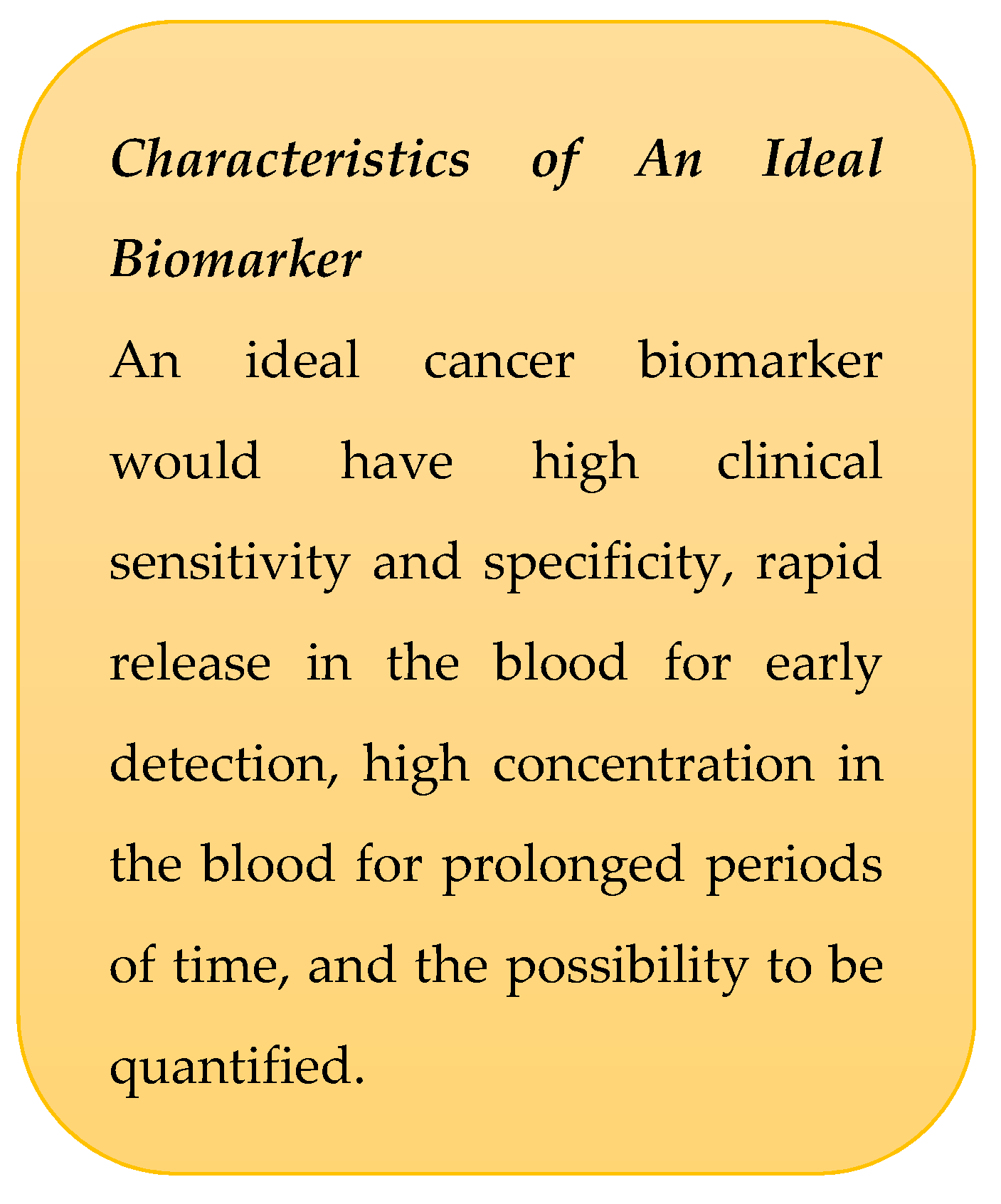
1.1. Essential Biomarkers for Liquid Biopsy Detection and Monitoring
| Biomarker | Biofluid/Sample | Electrochemical method | LOD | Ref. |
|---|---|---|---|---|
| Exosomes | Plasma | Potentiometric | 20pM 106 mL−1 43 particles μL−1 <105 vesicles/ 10 μL |
[20] [21] [22] [23] |
|
Circulating Nucleic acids
|
Human serum Serum |
DPV DPV and EIS |
3.9 x10-22 g/ml 0.45 fM |
[24] [25] [26] [27] |
|
Circulating tumor cells (CTCs) |
Blood Blood Peripheral blood |
Amperometry DPV DPV |
5 cells/ml 27 cells/ml 3 cells/ml |
[28] [29] [30] |
|
Proteins |
Human serum, saliva |
CV and EIS |
3.3 fg m/L |
[31] |
2. Miniaturization Strategies for Biosensing
2.1. Micro- and Nanofabrication Methods
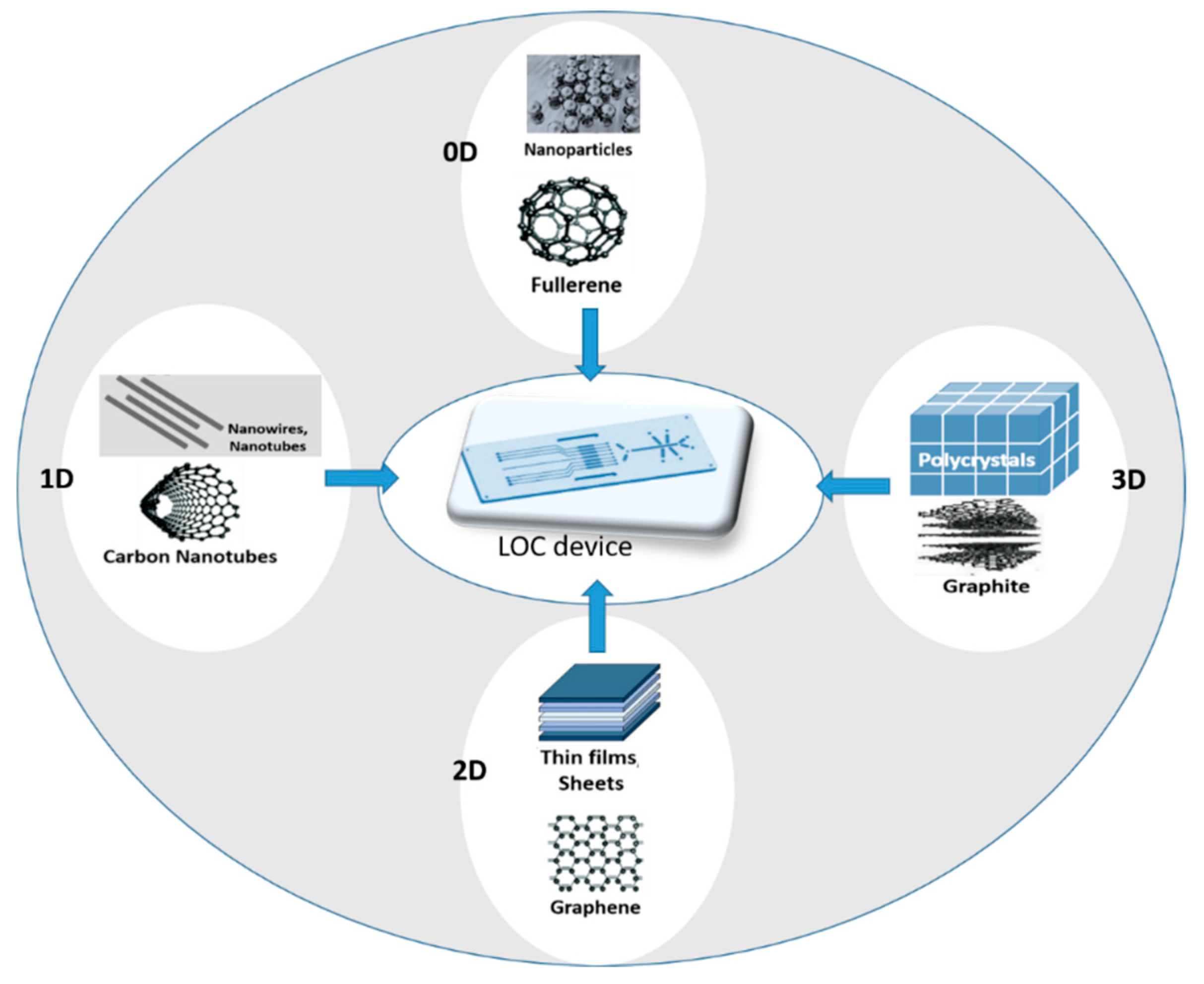
2.2. Nanomaterials in Electrochemical Sensors Integrated in LOC Device: From 2D to 3D Electrodes
3. Electrochemical Biosensors
3.1. Amperometric Method
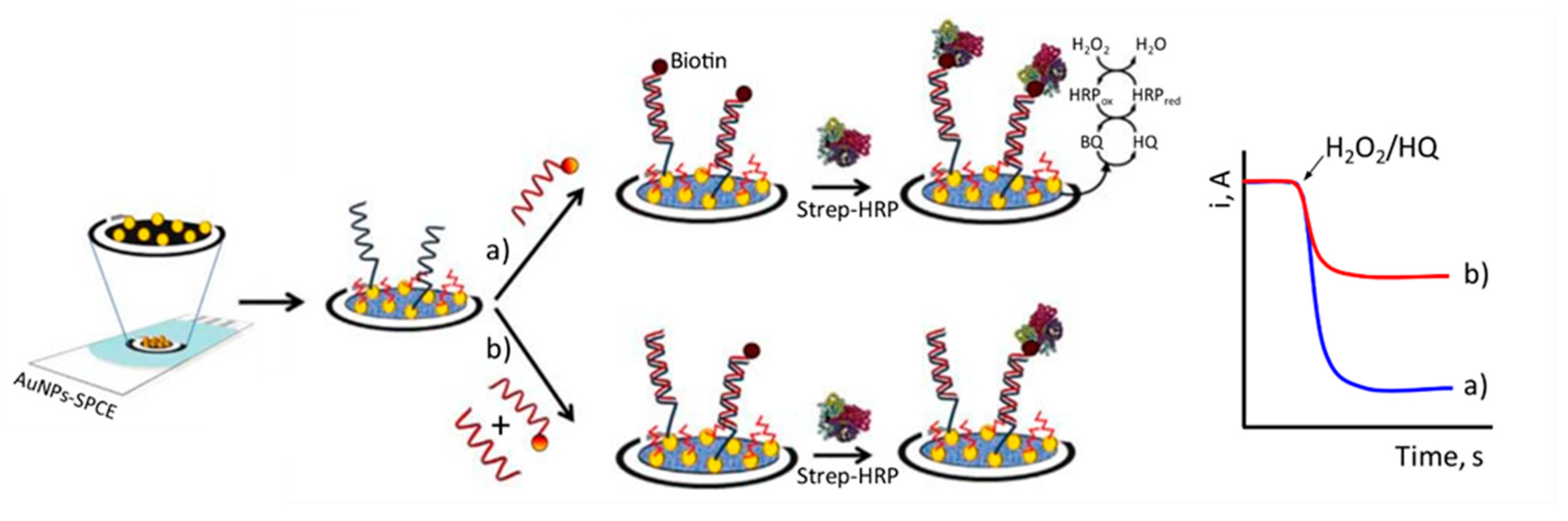
3.2. Potentiometric Method
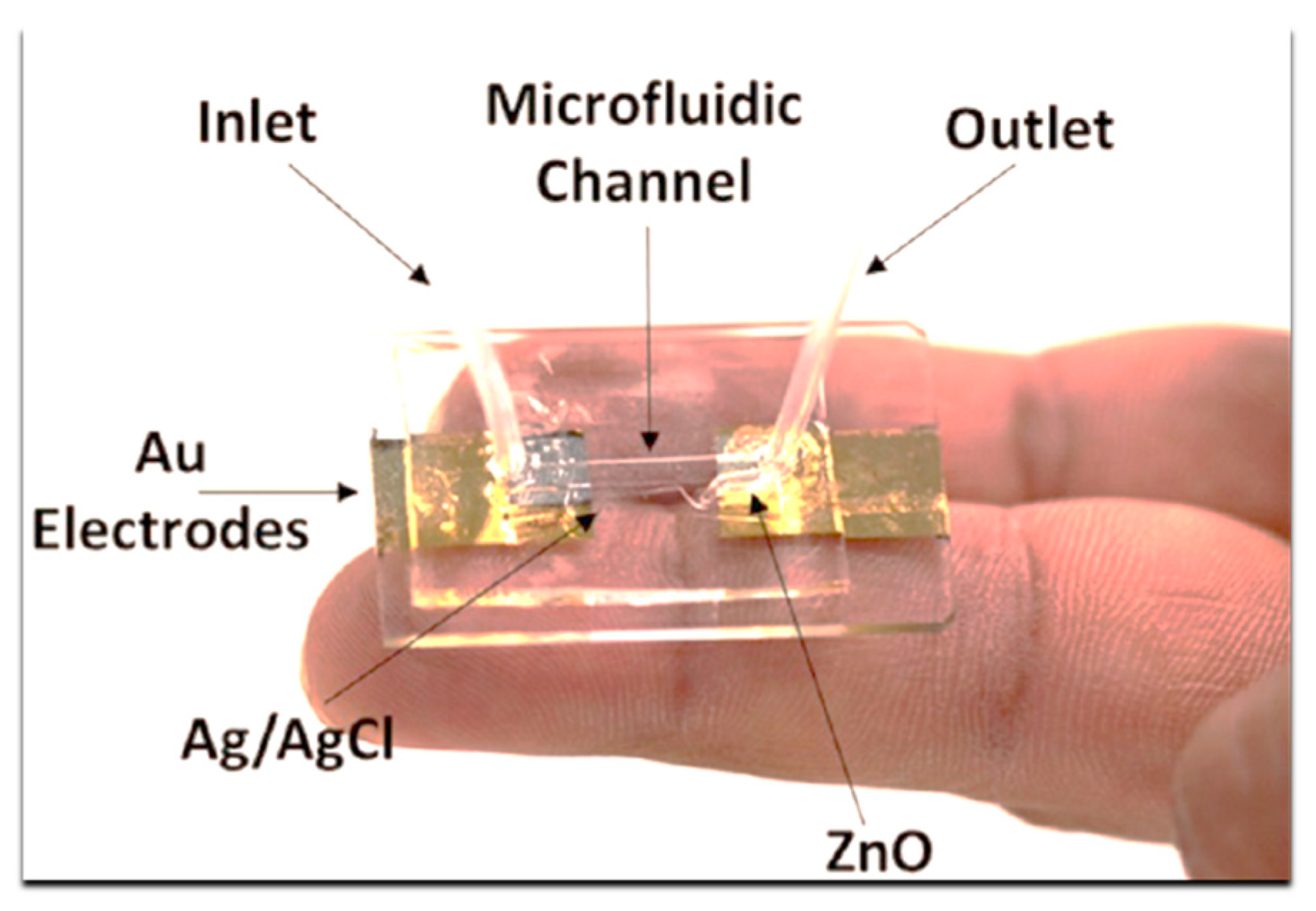
3.3. Impedimetric Method

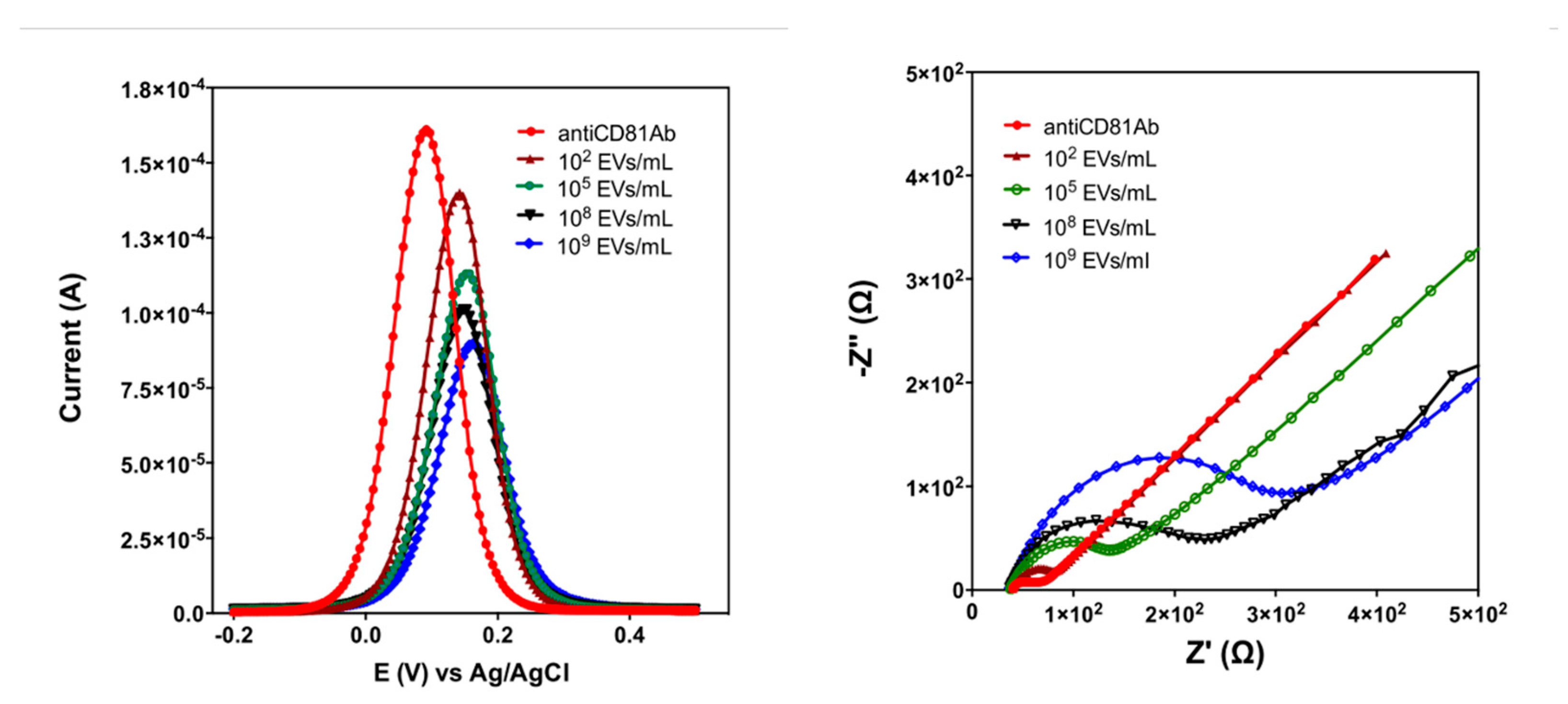
3.4. Conductometric Method
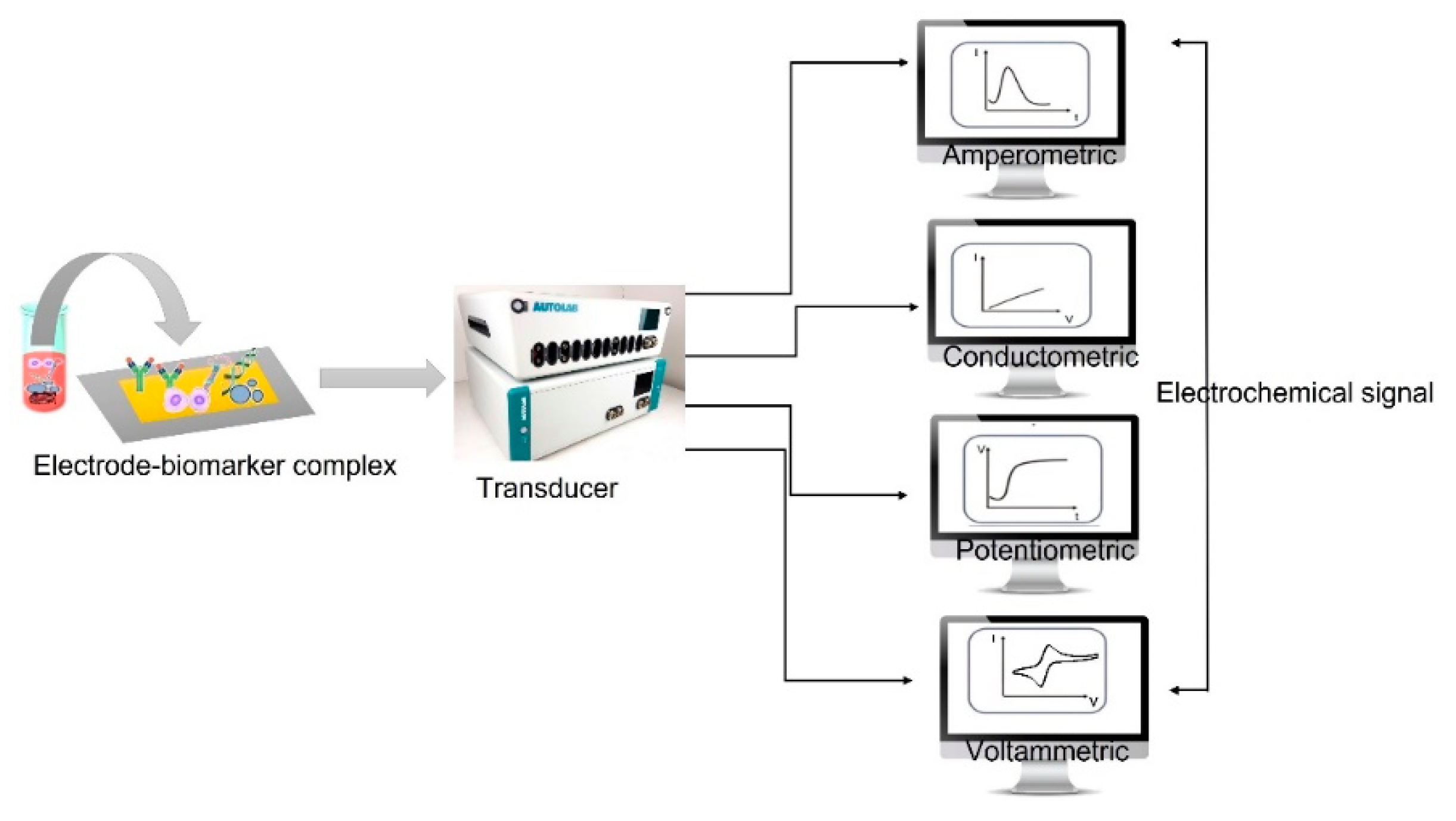
3.5. Voltammetric Method
3.5.1. Cyclic Voltammetry (CV)
3.5.2. Differential Pulse Voltammetry (DPV)
3.5.3. Linear Sweep Voltammetry (LSV)
3.5.4. Square Wave Voltammetry (SWV)
3.5.5. Stripping Voltammetry (SV)
4. Sensors Integration
4.1. Lab-on-Chip Platforms: Wearable and Portable Devices for POC
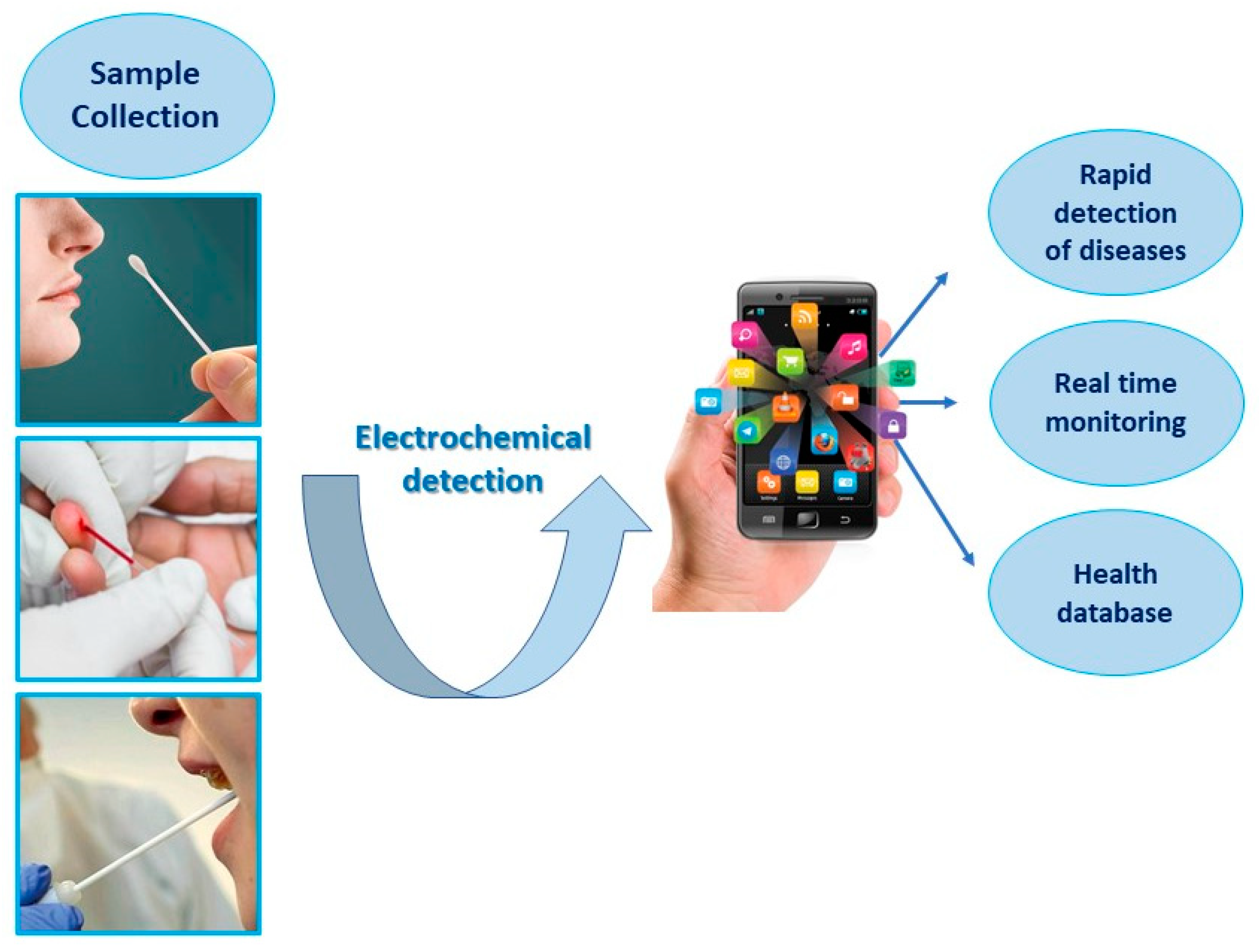
5. Comparison of Liquid Biopsy Electrochemical Methods: Advantages and Limitations
| EC Method | Advantages | Limitations |
|---|---|---|
| Potentiometric |
|
|
| Impedimetric |
|
|
| Conductometric |
|
|
| Cyclic Voltammetry (CV) |
|
|
| Differential PulseVoltammetry (DPV) |
|
|
| Stripping Voltammetry (SV) |
|
|
6. Future Perspectives and Concluding Remarks
Acknowledgements
References
- Ferrara, F.; Zoupanou, S.; Primiceri, E.; Ali, Z.; Chiriacò, M.S. Beyond liquid biopsy: Toward non-invasive assays for distanced cancer diagnostics in pandemics. Biosens. Bioelectron. 2022, 196, 113698–113698. [Google Scholar] [CrossRef] [PubMed]
- Nič M., Jirát J., Košata B., Jenkins A., McNaught A., 1997, editors. Compendium of chemical terminology. Oxford: Blackwell Scientific Publications., 1997.
- Ragavan, K.; Kumar, S.; Swaraj, S.; Neethirajan, S. Advances in biosensors and optical assays for diagnosis and detection of malaria. Biosens. Bioelectron. 2018, 105, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Sadasivuni, K.K., Ponnamma, D., Kim J., Cabibihan J.J., AlMaadeed M.A., 2017, editors. Biopolymer composites in elec-tronics. Elsevier Inc, 544.
- Kelley, S. O., 2015. Disease Detector. Sci. Am., 313, 48-51.
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Brazaca, L., Ribovski, L., Janegitz, B. & Zucolotto, V., 2017. Medical Biosensors for Point of Care (POC) Applications. Elsevier, 229–254.
- Naresh, V., Lee, N., 2021. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Bio-sensors, Sensors, 21(4), 1109.
- Garzarelli, V., Ferrara, F., Primiceri, E., Chiriacò, M.S., 2022. Biofluids manipulation methods for liquid biopsy in mini-mally-invasive assays. MethodsX., 9, 101759.
- de Andrade, A. C., Resende, R. R., Marques, A. P. A., 2020. Lab-on-chip technologies for point-of-care diagnostics: ad-vances and challenges for clinical applications. Biosensors and Bioelectronics, 1(2), 63-73.
- Primiceri, E., Chiriacò, M.S., Ionescu, R.E., D’Amone, E., Cingolani, R., Rinaldi, R., Maruccio, G., 2009. Development of EIS cell chips and their application for cell analysis. Microelectronic Engineering., 86, 1477–1480.
- Gamal, W., Wu, H., Underwood, I., Jia, J., Smith, S., Bagnaninchi, PO., 2018. Impedance-based cellular assays for regen-erative medicine, Phil. Trans. R. Soc. B., 373, 20170226.
- Evangelos, S., Evangelos, A. , George, K., Emmanouil A.V.K., Angeliki, T., Dimitris, T., 2022. Flow determination via na-noparticle strain sensors for easy Lab on Chip integration, Sensors & Actuators: A. Physical, 344, 113765.
- Lucile, A., Amel, B., Iago, P., Madad, A., Simon, D., Laurent, M., Thanh, D. M., Stéphanie, D., 2022. Modular microfluidic system for on-chip extraction, preconcentration and detection of the cytokine biomarker IL-6 in biofluid, Sci. Rep., 12, 9468.
- Shen, C.; Liu, S.; Li, X.; Yang, M. Electrochemical Detection of Circulating Tumor Cells Based on DNA.
- Generated Electrochemical Current and Rolling Circle Amplification, Anal. Chem., 91, 11614−11619.
- Daulton, E., Wicaksono, A. N., Tielea, A., Kocher, Silvan, H.M., Debernardi, S., Crnogorac-Jurcevic, T., Covington, J.A., 2021. Volatile organic compounds (VOCs) for the non-invasive detection of pancreatic cancer from urine, Talanta, 221, 121604.
- Gilany, K., Minai-Tehrani, A., Savadi-Shiraz, E., Rezadoost, H., Lakpour, N., 2015. Exploring the human seminal plasma proteome: An unexplored gold mine of biomarker for male Infertility and male reproduction disorder (Review), J. Reprod. Infertil., 16 (2).
- Park, J.; Kim, N.-E.; Yoon, H.; Shin, C.M.; Kim, N.; Lee, D.H.; Park, J.Y.; Choi, C.H.; Kim, J.G.; Kim, Y.-K.; et al. Fecal Microbiota and Gut Microbe-Derived Extracellular Vesicles in Colorectal Cancer. Front. Oncol. 2021, 11, 650026. [Google Scholar] [CrossRef]
- Garzarelli, V., Chiriacò, M.S., Cereda, M., Gigli, G., Ferrara, F., 2023. Ultrasensitive qPCR platform for rapid detection of bacterial contamination of raw biological samples at the point of care. PubMed., 9, e16229.
- Goda, T., Masuno, K., Nishida, J., Kosaka, N., Ochiya, T., Matsumoto, A., Miyahara, Y. A., 2012. label-free electrical de-tection of exosomal microRNAs using microelectrode array. Chem. Commun., 48, 11942-11944.
- Bari, S.M.I.; Hossain, F.B.; Nestorova, G.G. Advances in Biosensors Technology for Detection and Characterization of Extracellular Vesicles. Sensors 2021, 21, 7645. [Google Scholar] [CrossRef]
- Zhuang, L.; You, Q.; Su, X.; Chang, Z.; Ge, M.; Mei, Q.; Yang, L.; Dong, W.; Li, L. High-Performance Detection of Exosomes Based on Synergistic Amplification of Amino-Functionalized Fe3O4 Nanoparticles and Two-Dimensional MXene Nanosheets. Sensors 2023, 23, 3508. [Google Scholar] [CrossRef]
- Jeong, S.; Park, J.; Pathania, D.; Castro, C.M.; Weissleder, R.; Lee, H. Integrated Magneto–Electrochemical Sensor for Exosome Analysis. ACS Nano 2016, 10, 1802–1809. [Google Scholar] [CrossRef]
- Ruiyi, L., Ling, L., Hongxia, B., Zaijun, L., 2016. Nitrogen-doped multiple graphene aerogel/gold nanostar as the electro-chemical sensing platform for ultrasensitive detection of circulating free DNA in human serum. Biosens. Bioelectron., 79, 457.
- Wang, K.; Peng, Z.; Lin, X.; Nian, W.; Zheng, X.; Wu, J. Electrochemical Biosensors for Circulating Tumor DNA Detection. Biosensors 2022, 12, 649. [Google Scholar] [CrossRef]
- Shen, C.; Liu, S.; Li, X.; Yang, M. Electrochemical Detection of Circulating Tumor Cells Based on DNA Generated Electrochemical Current and Rolling Circle Amplification. Anal. Chem. 2019, 91, 11614–11619. [Google Scholar] [CrossRef]
- Su, S.; Cao, W.; Liu, W.; Lu, Z.; Zhu, D.; Chao, J.; Weng, L.; Wang, L.; Fan, C.; Wang, L. Dual-mode electrochemical analysis of microRNA-21 using gold nanoparticle-decorated MoS2 nanosheet. Biosens. Bioelectron. 2017, 94, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Gurudatt, N.; Chung, S.; Kim, J.-M.; Kim, M.-H.; Jung, D.-K.; Han, J.-Y.; Shim, Y.-B. Separation detection of different circulating tumor cells in the blood using an electrochemical microfluidic channel modified with a lipid-bonded conducting polymer. Biosens. Bioelectron. 2019, 146, 111746. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Qi, J.; Qian, K.; Oderinde, O.; Liu, Q.; Yao, C.; Song, W.; Wang, Y. Copper (II) oxide nanozyme based electrochemical cytosensor for high sensitive detection of circulating tumor cells in breast cancer. J. Electroanal. Chem. 2018, 812, 1–9. [Google Scholar] [CrossRef]
- Tang, S., Shen, H., Hao, Y., Huang, Z., Tao, Y., Peng, Y., Guo, Y., Xie, G., Feng, W., 2018. Biosens. Bioelectron., 104, 72–78.
- Aydın, M., Aydın, E. B., Sezgintürk, M. K., 2018. A highly selective electrochemical immunosensor based on conductive carbon black and star PGMA polymer composite material for IL-8 biomarker detection in human serum and saliva. Biosens. Bioelectron., 117, 720–728.
- Soleymani, L.; Li, F. Mechanistic Challenges and Advantages of Biosensor Miniaturization into the Nanoscale. ACS Sensors 2017, 2, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Martinsson, H.; Sandstrom, T.; Bleeker, A.; Hintersteiner, J.D. Current status of optical maskless lithography. J. Micro/Nanolithography, MEMS, MOEMS 2005, 4, 011003–011003. [Google Scholar] [CrossRef]
- Chen, C., Liu, Y.J., Jiang, Z.-Y., Shen, C., Zhang, Y., Zhong, F., Chen, L., Zhu, S., Liu, H., 2022. Large-area long-wave in-frared broadband all-dielectric metasurface absorber based on maskless laser direct writing lithography. Optics Express., 30, 13391.
- Heiskanen, S.; Maasilta, I.J. Superconducting tunnel junction fabrication on three-dimensional topography based on direct laser writing. Appl. Phys. Lett. 2020, 117, 232601. [Google Scholar] [CrossRef]
- Ye, J.; Tan, H.; Wu, S.; Ni, K.; Pan, F.; Liu, J.; Tao, Z.; Qu, Y.; Ji, H.; Simon, P.; et al. Direct Laser Writing of Graphene Made from Chemical Vapor Deposition for Flexible, Integratable Micro-Supercapacitors with Ultrahigh Power Output. Adv. Mater. 2018, 30, 1801384. [Google Scholar] [CrossRef]
- Dotan, T.; Berg, Y.; Migliorini, L.; Villa, S.M.; Santaniello, T.; Milani, P.; Shacham-Diamand, Y. Soft and flexible gold microelectrodes by supersonic cluster beam deposition and femtosecond laser processing. Microelectron. Eng. 2021, 237, 111478. [Google Scholar] [CrossRef]
- van der Velden, G.; Fan, D.; Staufer, U. Fabrication of a microfluidic device by using two-photon lithography on a positive photoresist. Micro Nano Eng. 2020, 7, 100054. [Google Scholar] [CrossRef]
- Viehrig, M., Anil, H.Thilsted., Matteucci, M., Wu, K., Catak, D., Schmidt, Michael S., Zór, K., Boisen, A., 2018, Injec-tion-Molded Microfluidic Device for SERS Sensing Using Embedded Au-Capped Polymer Nanocones, ACS Applied Ma-terials & Interfaces.,10 (43), 37417-37425.
- Perrone, E.; Cesaria, M.; Zizzari, A.; Bianco, M.; Ferrara, F.; Raia, L.; Guarino, V.; Cuscunà, M.; Mazzeo, M.; Gigli, G.; et al. Potential of CO2-laser processing of quartz for fast prototyping of microfluidic reactors and templates for 3D cell assembly over large scale. Mater. Today Bio 2021, 12, 100163. [Google Scholar] [CrossRef]
- Guascito, M.; Filippo, E.; Malitesta, C.; Manno, D.; Serra, A.; Turco, A. A new amperometric nanostructured sensor for the analytical determination of hydrogen peroxide. Biosens. Bioelectron. 2008, 24, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, G.E., Corvaglia, S., Pompa, P.P., Mattei, G., 2021. An innovative and simple all electrochemical approach to functionalize electrodes with a carbon nanotubes/polypyrrole molecularly imprinted nanocomposite and its application for sulfamethoxazole analysis. Journal of Colloid and Interface Science., 599, 676–685.
- Guascito, M.; Chirizzi, D.; Malitesta, C.; Mazzotta, E.; M. Siciliano, M.V.; Siciliano, T.; Tepore, A.; Turco, A. Low-potential sensitive H2O2 detection based on composite micro tubular Te adsorbed on platinum electrode. Biosens. Bioelectron. 2011, 26, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Paul, A., Chiriacò, M.S., Primiceri, E., Srivastava, D., Maruccio, G., 2019. Picomolar detection of retinol binding protein 4 for early management of type II diabetes. Biosensors and Bioelectronics., 128, 122–128.
- Timilsina, S.S. , Durr, N., Yafia, M., Sallum, H.M., Jolly, P., Ingber, D.E., 2021. Ultrarapid Method for Coating Electro-chemical Sensors with Antifouling Conductive Nanomaterials Enables Highly Sensitive Multiplexed Detection in Whole Blood. Advanced Healthcare Materials., 11, 2102244.
- Li, X.; Zhan, C.; Huang, Q.; He, M.; Yang, C.; Yang, C.; Huang, X.; Chen, M.; Xie, X.; Chen, H.-J. Smart Diaper Based on Integrated Multiplex Carbon Nanotube-Coated Electrode Array Sensors for In Situ Urine Monitoring. ACS Appl. Nano Mater. 2022, 5, 4767–4778. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Kung, C.-T.; Chen, R.-F.; Tsai, M.-H.; Chao, H.-R.; Wang, Y.-N.; Fu, L.-M. Recent advances in microfluidic paper-based assay devices for diagnosis of human diseases using saliva, tears and sweat samples. Sensors Actuators B: Chem. 2021, 342, 130078. [Google Scholar] [CrossRef]
- Dong, T.; Pires, N.M.M.; Yang, Z.; Jiang, Z. Advances in Electrochemical Biosensors Based on Nanomaterials for Protein Biomarker Detection in Saliva. Adv. Sci. 2022, 10, 2205429. [Google Scholar] [CrossRef]
- De Benedetto, G.E. , Corvaglia, S., Pompa, P.P., Mattei, G., 2021b. An innovative and simple all electrochemical approach to functionalize electrodes with a carbon nanotubes/polypyrrole molecularly imprinted nanocomposite and its application for sulfamethoxazole analysis. Journal of Colloid and Interface Science 599, 676–685.
- Zhang, Q.; Xu, J.-J.; Liu, Y.; Chen, H.-Y. In-situ synthesis of poly(dimethylsiloxane)–gold nanoparticles composite films and its application in microfluidic systems. Lab a Chip 2007, 8, 352–357. [Google Scholar] [CrossRef]
- Anshori, I.; Harimurti, S.; Rizalputri, L.N.; Hartono, M.S.; Althof, R.R.; Handayani, M.; Mengko, T.L.E.R.; Yuliarto, B. Modified screen-printed electrode using graphene ink for electrochemical sensor application. J. Physics: Conf. Ser. 2021, 1912, 012022. [Google Scholar] [CrossRef]
- Pachauri, N. , Lakshmi, G.B.V.S., Sri, S., Gupta, P.K., Solanki, P.R., 2020. Silver molybdate nanoparticles based im-munosensor for the non-invasive detection of Interleukin-8 biomarker. Materials Science and Engineering., C 113, 110911.
- Ko, E.; Tran, V.-K.; Geng, Y.; Chung, W.S.; Park, C.H.; Kim, M.K.; Jin, G.H.; Seong, G.H. Continuous electrochemical detection of hydrogen peroxide by Au-Ag bimetallic nanoparticles in microfluidic devices. J. Electroanal. Chem. 2017, 792, 72–78. [Google Scholar] [CrossRef]
- Xuecui, M. , Jiao, Y., Xinge, Y., Zhengchun, P., Guanghui, Z., Yingchun, L., 2023, Wearable molecularly imprinted elec-trochemical sensor with integrated nanofiber-based microfluidic chip for in situ monitoring of cortisol in sweat, Sensors and Actuators B: Chemical., 381, 133451.
- Zheng, F. , Pu, Z., He, E., Huang, J., Yu, B., Li, D., Li, Z., 2018. From functional structure to packaging: full-printing fab-rication of a microfluidic chip. Lab on a Chip., 18, 1859–1866.
- Cinti, S.; Arduini, F.; Moscone, D.; Palleschi, G.; Gonzalez-Macia, L.; Killard, A.J. Cholesterol biosensor based on inkjet-printed Prussian blue nanoparticle-modified screen-printed electrodes. Sensors Actuators B: Chem. 2015, 221, 187–190. [Google Scholar] [CrossRef]
- Lee, C.-W.; Chang, H.-Y.; Wu, J.-K.; Tseng, F.-G. Ultra-sensitive electrochemical detection of bacteremia enabled by redox-active gold nanoparticles (raGNPs) in a nano-sieving microfluidic system (NS-MFS). Biosens. Bioelectron. 2019, 133, 215–222. [Google Scholar] [CrossRef]
- Beck, F. , Horn, C., Baeumner, A.J., 2021. Dry-reagent microfluidic biosensor for simple detection of NT-proBNP via Ag nanoparticles. Analytica Chimica Acta., 1191, 339375.
- Gu, S.; Lu, Y.; Ding, Y.; Li, L.; Song, H.; Wang, J.; Wu, Q. A droplet-based microfluidic electrochemical sensor using platinum-black microelectrode and its application in high sensitive glucose sensing. Biosens. Bioelectron. 2014, 55, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xianyu, Y.; Jiang, X. Point-of-care biochemical assays using gold nanoparticle-implemented microfluidics. Chem. Soc. Rev. 2014, 43, 6239–6253. [Google Scholar] [CrossRef] [PubMed]
- Aravamudhan, S.; Kumar, A.; Mohapatra, S.; Bhansali, S. Sensitive estimation of total cholesterol in blood using Au nanowires based micro-fluidic platform. Biosens. Bioelectron. 2007, 22, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, X.; He, Z.; Hou, L.; Ge, C.; Wang, L.; Li, S.; Xu, Y. Detection of prostate specific antigen in whole blood by microfluidic chip integrated with dielectrophoretic separation and electrochemical sensing. Biosens. Bioelectron. 2022, 204, 114057. [Google Scholar] [CrossRef] [PubMed]
- Vural, T.; Yaman, Y.T.; Ozturk, S.; Abaci, S.; Denkbas, E.B. Electrochemical immunoassay for detection of prostate specific antigen based on peptide nanotube-gold nanoparticle-polyaniline immobilized pencil graphite electrode. J. Colloid Interface Sci. 2018, 510, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhatia, V. Magnetic nanoparticles in microfluidics-based diagnostics: an appraisal. Nanomedicine 2021, 16, 1329–1342. [Google Scholar] [CrossRef]
- Kurbanoglu, S. , Mayorga-Martinez, C.C., Medina-Sánchez, M., Rivas, L., Ozkan, S.A., Merkoçi, A., 2015. Antithyroid drug detection using an enzyme cascade blocking in a nanoparticle-based lab-on-a-chip system. Biosensors and Bioelec-tronics., 67, 670–676.
- Liu, Z.; Jin, M.; Cao, J.; Niu, R.; Li, P.; Zhou, G.; Yu, Y.; Berg, A.v.D.; Shui, L. Electrochemical sensor integrated microfluidic device for sensitive and simultaneous quantification of dopamine and 5-hydroxytryptamine. Sensors Actuators B: Chem. 2018, 273, 873–883. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J.-H.; Strickler, J.R.; Chang, W.-J.; Gunasekaran, S. Nickel nanoparticle–chitosan-reduced graphene oxide-modified screen-printed electrodes for enzyme-free glucose sensing in portable microfluidic devices. Biosens. Bioelectron. 2013, 47, 530–538. [Google Scholar] [CrossRef]
- Cincotto, F.H.; Fava, E.L.; Moraes, F.C.; Fatibello-Filho, O.; Faria, R.C. A new disposable microfluidic electrochemical paper-based device for the simultaneous determination of clinical biomarkers. Talanta 2019, 195, 62–68. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Tang, W.; Wang, C.; Han, Y.; Qiang, L.; Gao, J.; Liu, H.; Han, L. Ultrasensitive, high-throughput and multiple cancer biomarkers simultaneous detection in serum based on graphene oxide quantum dots integrated microfluidic biosensing platform. Anal. Chim. Acta 2021, 1178, 338791. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J.-H.; Strickler, J.R.; Chang, W.-J.; Gunasekaran, S. Nickel nanoparticle–chitosan-reduced graphene oxide-modified screen-printed electrodes for enzyme-free glucose sensing in portable microfluidic devices. Biosens. Bioelectron. 2013, 47, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Wisitsoraat, A.; Sritongkham, P.; Karuwan, C.; Phokharatkul, D.; Maturos, T.; Tuantranont, A. Fast cholesterol detection using flow injection microfluidic device with functionalized carbon nanotubes based electrochemical sensor. Biosens. Bioelectron. 2010, 26, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Materón, E.M.; Lima, R.S.; Joshi, N.; Shimizu, F.M.; Oliveira, O.N. Graphene-Containing Microfluidic and Chip-Based Sensor Devices for Biomolecules. , in: Elsevier EBooks., 2019; pp. 321–336. [CrossRef]
- Narang, J.; Malhotra, N.; Singhal, C.; Mathur, A.; Chakraborty, D.; Anil, A.; Ingle, A.; Pundir, C.S. Point of care with micro fluidic paper based device integrated with nano zeolite–graphene oxide nanoflakes for electrochemical sensing of ketamine. Biosens. Bioelectron. 2017, 88, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Dolati, A. , AbdelFatah, T., Sanati, A., Jalali, M., Flynn, S.J., Mahshid, S., Mahshid, S., 2020. A Nanostructured Gold/Graphene Microfluidic Device for Direct and Plasmonic-Assisted Impedimetric Detection of Bacteria. ACS Applied Materials & Interfaces., 12, 23298–23310.
- Zhang, S.; Zahed, A.; Sharifuzzaman; Yoon, S. ; Hui, X.; Barman, S.C.; Sharma, S.; Yoon, H.S.; Park, C.; Park, J.Y. A wearable battery-free wireless and skin-interfaced microfluidics integrated electrochemical sensing patch for on-site biomarkers monitoring in human perspiration. Biosens. Bioelectron. 2021, 175, 112844. [Google Scholar] [CrossRef]
- Ciftci, S.; Cánovas, R.; Neumann, F.; Paulraj, T.; Nilsson, M.; Crespo, G.A.; Madaboosi, N. The sweet detection of rolling circle amplification: Glucose-based electrochemical genosensor for the detection of viral nucleic acid. Biosens. Bioelectron. 2020, 151, 112002. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Xie, H.; Zhao, L.; Zheng, L.; Ye, H. Applications of Catalytic Hairpin Assembly Reaction in Biosensing. Small 2019, 15, e1902989. [Google Scholar] [CrossRef]
- He, R. , Niu, Y., Li, Z., Li, A., Yang, H.-Y., Xu, F., Li, F., 2020. A Hydrogel Microneedle Patch for Point-of-Care Testing Based on Skin Interstitial Fluid. Advanced Healthcare Materials., 9, 1901201.
- Lin, Y. , Bariya, M., Nyein, H.Y.Y., Kivimäki, L., Uusitalo, S., Jansson, E., Ji, W., Yuan, Z., Happonen, T., Liedert, C., Hil-tunen, J., Fan, Z., Javey, A., 2019. Porous Enzymatic Membrane for Nanotextured Glucose Sweat Sensors with High Sta-bility toward Reliable Noninvasive Health Monitoring. Advanced Functional Materials., 29, 1902521.
- Zhong, R.; Tang, Q.; Wang, S.; Zhang, H.; Zhang, F.; Xiao, M.; Man, T.; Qu, X.; Li, L.; Zhang, W.; et al. Self-Assembly of Enzyme-Like Nanofibrous G-Molecular Hydrogel for Printed Flexible Electrochemical Sensors. Adv. Mater. 2018, 30, e1706887. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, Y.; Pan, L.; Yu, G. Multifunctional Nanostructured Conductive Polymer Gels: Synthesis, Properties, and Applications. Accounts Chem. Res. 2017, 50, 1734–1743. [Google Scholar] [CrossRef]
- Li, L. , Wang, Y., Pan, L., Shi, Ye, Cheng, W., Shi, Yi, Yu, G., 2015b. A Nanostructured Conductive Hydrogels-Based Bio-sensor Platform for Human Metabolite Detection. Nano Letters., 15, 1146–1151.
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, e1806133. [Google Scholar] [CrossRef]
- Xu, J.; Xu, K.; Han, Y.; Wang, D.; Li, X.; Hu, T.; Yi, H.; Ni, Z. A 3D porous graphene aerogel@GOx based microfluidic biosensor for electrochemical glucose detection. Anal. 2020, 145, 5141–5147. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, S.; Zhang, K.; Zhang, L.; Liu, C.; Shi, H.; Wang, C.; Wang, N.; Zhu, A. A facile integrated microfluidic chip based on Chitosan-Gold Nanoparticles-Anchored Three-Dimensional graphene fiber film for monitoring prostate specific antigen. Microchem. J. 2023, 184, 108171. [Google Scholar] [CrossRef]
- Chand, R.; Neethirajan, S. Microfluidic platform integrated with graphene-gold nano-composite aptasensor for one-step detection of norovirus. Biosens. Bioelectron. 2017, 98, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y. , Shi, S., Ma, J., Guo, Y., 2021b. Smartphone-based electrochemical system with multi-walled carbon nano-tubes/thionine/gold nanoparticles modified screen-printed immunosensor for cancer antigen 125 detection. Microchemical Journal., 174, 107044.
- Di Giulio, T.; Barca, A.; Verri, T.; De Gennaro, M.; Giancane, G.; Mazzotta, E.; Malitesta, C. Molecular imprinting based on metal-ion mediated recognition: Electrosynthesis of artificial receptors for the selective detection of peptides. Sensors Actuators B: Chem. 2023, 383, 133589. [Google Scholar] [CrossRef]
- Mazouz, Z.; Rahali, S.; Fourati, N.; Zerrouki, C.; Aloui, N.; Seydou, M.; Yaakoubi, N.; Chehimi, M.M.; Othmane, A.; Kalfat, R. Highly Selective Polypyrrole MIP-Based Gravimetric and Electrochemical Sensors for Picomolar Detection of Glyphosate. Sensors 2017, 17, 2586. [Google Scholar] [CrossRef] [PubMed]
- Yarman, A.; Kurbanoglu, S.; Jetzschmann, K.J.; Ozkan, S.A.; Wollenberger, U.; Scheller, F.W. Electrochemical MIP-Sensors for Drugs. Curr. Med. Chem. 2018, 25, 4007–4019. [Google Scholar] [CrossRef] [PubMed]
- Mazouz, Z.; Mokni, M.; Fourati, N.; Zerrouki, C.; Barbault, F.; Seydou, M.; Kalfat, R.; Yaakoubi, N.; Omezzine, A.; Bouslema, A.; et al. Computational approach and electrochemical measurements for protein detection with MIP-based sensor. Biosens. Bioelectron. 2020, 151, 111978. [Google Scholar] [CrossRef]
- Liustrovaite, V.; Pogorielov, M.; Boguzaite, R.; Ratautaite, V.; Ramanaviciene, A.; Pilvenyte, G.; Holubnycha, V.; Korniienko, V.; Diedkova, K.; Viter, R.; et al. Towards Electrochemical Sensor Based on Molecularly Imprinted Polypyrrole for the Detection of Bacteria—Listeria monocytogenes. Polymers 2023, 15, 1597. [Google Scholar] [CrossRef]
- Wang, L.; Pagett, M.; Zhang, W. Molecularly imprinted polymer (MIP) based electrochemical sensors and their recent advances in health applications. Sensors Actuators Rep. 2023, 5. [Google Scholar] [CrossRef]
- Li, M.-X.; Wang, X.-H.; Zhang, L.-M.; Wei, X.-P. A high sensitive epitope imprinted electrochemical sensor for bovine serum albumin based on enzyme amplifying. Anal. Biochem. 2017, 530, 68–74. [Google Scholar] [CrossRef]
- Majd, S.M.; Mirzapour, F.; Shamsipur, M.; Manouchehri, I.; Babaee, E.; Pashabadi, A.; Moradian, R. Design of a novel aptamer/molecularly imprinted polymer hybrid modified Ag–Au@Insulin nanoclusters/Au-gate-based MoS2 nanosheet field-effect transistor for attomolar detection of BRCA1 gene. Talanta 2023, 257, 124394. [Google Scholar] [CrossRef]
- Choi, D.Y.; Yang, J.C.; Hong, S.W.; Park, J. Molecularly imprinted polymer-based electrochemical impedimetric sensors on screen-printed carbon electrodes for the detection of trace cytokine IL-1β. Biosens. Bioelectron. 2022, 204, 114073. [Google Scholar] [CrossRef] [PubMed]
- Ratautaite, V. , Boguzaite, R., Brazys, E., Plausinaitis, D., Ramanavicius, S., Samukaite-Bubniene, U., Bechelany, M., Ra-manavicius, A., 2023. Evaluation of the interaction between SARS-CoV-2 spike glycoproteins and the molecularly im-printed polypyrrole. Talanta., 253, 123981.
- Choi, D.Y.; Yang, J.C.; Hong, S.W.; Park, J. Molecularly imprinted polymer-based electrochemical impedimetric sensors on screen-printed carbon electrodes for the detection of trace cytokine IL-1β. Biosens. Bioelectron. 2022, 204, 114073. [Google Scholar] [CrossRef] [PubMed]
- Shumyantseva, V.V. , Bulko, T.V., Sigolaeva, L.V., Kuzikov, A.V., Pogodin, P.V., Archakov, A.I., 2018. Molecular im-printing coupled with electrochemical analysis for plasma samples classification in acute myocardial infarction diagnostic. Biosensors and Bioelectronics., 99, 216–222.
- Hussein, H. , Kandeil, A., Gomaa, M.R., Nashar, R.M.E., El-Sherbiny, I.M., Hassan, R.Y.A., 2021. SARS-CoV-2- Impedi-metric Biosensor: Virus-Imprinted Chips for Early and Rapid Diagnosis. ACS Sensors., 6, 4098–4107.
- Liu, W.; Ma, Y.; Sun, G.; Wang, S.; Deng, J.; Wei, H. Molecularly imprinted polymers on graphene oxide surface for EIS sensing of testosterone. Biosens. Bioelectron. 2017, 92, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Motia, S.; Bouchikhi, B.; El Bari, N. An electrochemical molecularly imprinted sensor based on chitosan capped with gold nanoparticles and its application for highly sensitive butylated hydroxyanisole analysis in foodstuff products. Talanta 2020, 223, 121689. [Google Scholar] [CrossRef]
- Shoaie, N.; Daneshpour, M.; Azimzadeh, M.; Mahshid, S.; Khoshfetrat, S.M.; Jahanpeyma, F.; Gholaminejad, A.; Omidfar, K.; Foruzandeh, M. Electrochemical sensors and biosensors based on the use of polyaniline and its nanocomposites: a review on recent advances. Microchim. Acta 2019, 186, 465. [Google Scholar] [CrossRef]
- Shoaie, N.; Forouzandeh, M.; Omidfar, K. Voltammetric determination of the Escherichia coli DNA using a screen-printed carbon electrode modified with polyaniline and gold nanoparticles. Microchim. Acta 2018, 185, 217. [Google Scholar] [CrossRef]
- Eggins, B. R. , 2002. Chemical Sensors and Biosensors; John Wiles & Sons: New York.
- Zhang, X.; Li, F.; Wei, Q.; Du, B.; Wu, D.; Li, H. Ultrasensitive nonenzymatic immunosensor based on bimetallic gold–silver nanoclusters synthesized by simple mortar grinding route. Sensors Actuators B: Chem. 2014, 194, 64–70. [Google Scholar] [CrossRef]
- Zouari, M. , Campuzano, S., Pingarrón, J.M., Raouafi, N., 2017. Competitive RNA-RNA hybridization-based integrated nanostructured disposable electrode for highly sensitive determination of miRNAs in cancer cells. Biosens. Bioelectron., 91, 40–45.
- Francisco, G.O. , German, E.G., Chiara B., Ines, C.G., Carmen, G.N., Richard, F.D., María P.M.V., Maria, J.S., Germ, A. M., Jose, E.H., Martin, A. F.B., 2022. Microfluidic amperometric immunosensor based on porous nanomaterial towards claudin7 determination for colorectal cancer diagnosis, Talanta., 251, 123766.
- Bard, A. J. , Faulkner, L. R., 2008. Electrochemical Methods: Fundamentals and Applications, 2nd ed. Wiley, New York.
- Grieshaber, D. , MacKenzie, R., Vörös, J., Reimhult, E., 2008. Electrochemical Biosensors - Sensor Principles and Architec-tures. Sensors., 8, 1400-1458.
- Jia, Y.; Qin, M.; Zhang, H.; Niu, W.; Li, X.; Wang, L.; Li, X.; Bai, Y.; Cao, Y.; Feng, X. Label-free biosensor: A novel phage-modified Light Addressable Potentiometric Sensor system for cancer cell monitoring. Biosens. Bioelectron. 2007, 22, 3261–3266. [Google Scholar] [CrossRef]
- Wang, Y. , Zhang, Z., Jain, V., Yi, J., Mueller, S., Sokolov, J., Liu, Z., Levon, K., Rigas, B., Rafailovich, M.H., 2010. Poten-tiometric sensors based on surface molecular imprinting: Detection of cancer biomarkers and viruses. Sensors Actuators, B Chem., 146, 381.
- Mathur, A.; Blais, S.; Goparaju, C.M.V.; Neubert, T.; Pass, H.; Levon, K. Development of a Biosensor for Detection of Pleural Mesothelioma Cancer Biomarker Using Surface Imprinting. PLOS ONE 2013, 8, e57681. [Google Scholar] [CrossRef]
- Goda, T. , Masuno, K., Nishida, J., Kosaka, N., Ochiya, T., Matsumoto, A., Miyahara, Y., 2012. A label-free electrical de-tection of exosomal microRNAs using microelectrode array. Chem. Commun., 48, 11942.
- Shaibani, P.M.; Etayash, H.; Naicker, S.; Kaur, K.; Thundat, T. Metabolic Study of Cancer Cells Using a pH Sensitive Hydrogel Nanofiber Light Addressable Potentiometric Sensor. ACS Sensors 2017, 2, 151–156. [Google Scholar] [CrossRef]
- Gu, Y.; Ju, C.; Li, Y.; Shang, Z.; Wu, Y.; Jia, Y.; Niu, Y. Detection of circulating tumor cells in prostate cancer based on carboxylated graphene oxide modified light addressable potentiometric sensor. Biosens. Bioelectron. 2015, 66, 24–31. [Google Scholar] [CrossRef]
- Mani, G.K. , Morohoshi, M., Yasoda, Y., Yokoyama, S., Kimura, H., Tsuchiya, K., 2017. ZnO-Based Microfluidic pH Sensor: A Versatile Approach for Quick Recognition of Circulating Tumor Cells in Blood. ACS Applied Mater, Interfaces, 9, 5193–5203.
- Elshafey, R. , Tavares, A.C., Siaj, M., Zourob, M., 2013. Electrochemical impedance immunosensor based on gold nano-particles–protein G for the detection of cancer marker epidermal growth factor receptor in human plasma and brain tissue, Biosens. Bioelectron., 50, 143149.
- Han, L.; Liu, P.; Petrenko, V.A.; Liu, A. A Label-Free Electrochemical Impedance Cytosensor Based on Specific Peptide-Fused Phage Selected from Landscape Phage Library. Sci. Rep. 2016, 6, 22199. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zuo, P.; Ye, B.-C. Label-free electrochemical impedance spectroscopy biosensor for direct detection of cancer cells based on the interaction between carbohydrate and lectin. Biosens. Bioelectron. 2012, 43, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Azzouzi, S.; Mak, W.C.; Kor, K.; Turner, A.P.; Ben Ali, M.; Beni, V. An integrated dual functional recognition/amplification bio-label for the one-step impedimetric detection of Micro-RNA-21. Biosens. Bioelectron. 2017, 92, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kilic, T. , De Sousa Valinhas, A. T., Wall, I., Renaud, P., Carrara, S., 2018. Label-free detection of hypoxiainduced extra-cellular vesicle secretion from MCF-7 cells, Sci. Rep., 9, 27203.
- Ronkainen, N.J. , Halsall, H. B., Heineman, W. R., 2010. Electrochemical biosensors, Chem. Soc. Rev., 39, 1747-1763.
- Labib, M.; Hedström, M.; Amin, M.; Mattiasson, B. A capacitive immunosensor for detection of cholera toxin. Anal. Chim. Acta 2009, 634, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, J.; Zhang, L.; Wang, S.; Yao, C.; Zhang, Z. Conductometric immunoassay of alpha-fetoprotein in sera of liver cancer patients using bienzyme-functionalized nanometer-sized silica beads. Anal. 2018, 144, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.K.; Sharma, A.L.; Bhardwaj, N.; Kukkar, M.; Gill, A.A.; Kim, K.-H.; Deep, A. TCNQ-doped Cu-metal organic framework as a novel conductometric immunosensing platform for the quantification of prostate cancer antigen. Sensors Actuators B: Chem. 2017, 240, 10–17. [Google Scholar] [CrossRef]
- Lin, Y.H. , Lin, W.S., Wong, J.C., Hsu, W.C., Peng, Y.S., Chen, C.L., 2017. Bottom-up assembly of silicon nanowire con-ductometric sensors for the detection of apolipoprotein A1, a biomarker for bladder cancer, Microchim. Acta, 184, 2419.
- Pourali, A.; Rashidi, M.R.; Barar, J.; Pavon-Djavid, G.; Omidi, Y. Voltammetric biosensors for analytical detection of cardiac troponin biomarkers in acute myocardial infarction. TrAC Trends Anal. Chem. 2020, 134, 116123. [Google Scholar] [CrossRef]
- Malhotra, B.D. , 2017., Biosensors: Fundamentals Applications, Smithers Rapra.
- Kumar, S. , Kumar, S., Tiwari, S., Srivastava, M., Srivastava, B.K., Yadav, et al., 2015. Biofunctionalized nanostructured zirconia for biomedical application: a smart approach for oral cancer detection, Adv. Sci. 2 (8), 1500048.
- Wang, H. , Zhang, Y., Yu, H., Wu, D., Ma, H., Li, H. et al., 2013. Label-free electrochemical immunosensor for pros-tate-specific antigen based on silver hybridized mesoporous silica nanoparticles, Anal. Biochem., 434 (1), 123127.
- Kumar, S.; Lei, Y.; Alshareef, N.H.; Quevedo-Lopez, M.; Salama, K.N. Biofunctionalized two-dimensional Ti3C2 MXenes for ultrasensitive detection of cancer biomarker. Biosens. Bioelectron. 2018, 121, 243–249. [Google Scholar] [CrossRef]
- Taleat, Z. , Cristea, C., Marrazza, G., Mazloum-Ardakani, M., Sa˘ndulescu, R., 2014. Electrochemical immunoassay based on aptamer–protein interaction and functionalized polymer for cancer biomarker detection, J. Electroanalytical Chem., 717, 119124.
- Feng, X.; Gan, N.; Zhang, H.; Yan, Q.; Li, T.; Cao, Y.; Hu, F.; Yu, H.; Jiang, Q. A novel strategy for multiplexed immunoassay of tumor markers based on electrochemiluminescence coupled with cyclic voltammetry using graphene-polymer nanotags. Electrochimica Acta 2015, 170, 292–299. [Google Scholar] [CrossRef]
- Lin, C.-W.; Wei, K.-C.; Liao, S.-S.; Huang, C.-Y.; Sun, C.-L.; Wu, P.-J.; Lu, Y.-J.; Yang, H.-W.; Ma, C.-C.M. A reusable magnetic graphene oxide-modified biosensor for vascular endothelial growth factor detection in cancer diagnosis. Biosens. Bioelectron. 2015, 67, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Khoshraj, J.M.; Majidi, M.R.; Baradaran, B.; de la Guardia, M. Evaluation of Flavonoid Derivative and Doxorubicin Effects in Lung Cancer Cells (A549) Using Differential Pulse Voltammetry Method. Adv. Pharm. Bull. 2018, 8, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; He, M.-Q.; Zhai, F.-H.; He, R.-H.; Yu, Y.-L. A novel electrochemical biosensor based on polyadenine modified aptamer for label-free and ultrasensitive detection of human breast cancer cells. Talanta 2017, 166, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.P.G.; Silva, M.S.V.; Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C. Molecularly imprinted electrochemical sensor for the point-of-care detection of a breast cancer biomarker (CA 15-3), Sens. Actuators B: Chem., 256, 905912.
- Fakunle, E.S.; Fritsch, I. Low-temperature co-fired ceramic microchannels with individually addressable screen-printed gold electrodes on four walls for self-contained electrochemical immunoassays. Anal. Bioanal. Chem. 2010, 398, 2605–2615. [Google Scholar] [CrossRef]
- Zani, A.; Laschi, S.; Mascini, M.; Marrazza, G. A New Electrochemical Multiplexed Assay for PSA Cancer Marker Detection. Electroanalysis 2010, 23, 91–99. [Google Scholar] [CrossRef]
- Erdem, A.; Congur, G. Label-free voltammetric detection of MicroRNAs at multi-channel screen printed array of electrodes comparison to graphite sensors. Talanta 2014, 118, 7–13. [Google Scholar] [CrossRef]
- Ahn, J.M.; Cho, J.Y. Current Serum Lung Cancer Biomarkers. J. Mol. Biomarkers Diagn. 2013, s4. [Google Scholar] [CrossRef]
- Alatas, F. , Alatas O¨., Metintas, M., Colak, O., Harmanci, E., Demir, S., 2001. Diagnostic value of CEA, CA 15-3, CA 19-9, CYFRA 21-1, NSE and TSA assay in pleural effusions, Lung Cancer, 31 (1), 916.
- Wu, J.; Zhang, Z.; Fu, Z.; Ju, H. A disposable two-throughput electrochemical immunosensor chip for simultaneous multianalyte determination of tumor markers. Biosens. Bioelectron. 2007, 23, 114–120. [Google Scholar] [CrossRef]
- Wu, D.; Guo, A.; Guo, Z.; Xie, L.; Wei, Q.; Du, B. Simultaneous electrochemical detection of cervical cancer markers using reduced graphene oxide-tetraethylene pentamine as electrode materials and distinguishable redox probes as labels. Biosens. Bioelectron. 2014, 54, 634–639. [Google Scholar] [CrossRef]
- Xu, T.; Jia, X.; Chen, X.; Ma, Z. Simultaneous electrochemical detection of multiple tumor markers using metal ions tagged immunocolloidal gold. Biosens. Bioelectron. 2014, 56, 174–179. [Google Scholar] [CrossRef]
- Zhou, Y.-G.; Kermansha, L.; Zhang, L.; Mohamadi, R.M. Miniaturized Electrochemical Sensors to Facilitate Liquid Biopsy for Detection of Circulating Tumor Markers, in: M. Tokeshi (Ed.), Applications of Microfluidic Systems in Biology and Medicine, Springer Singapore, Singapore, 7198. [CrossRef]
- Moscovici, M.; Bhimji, A.; Kelley, S.O. Rapid and specific electrochemical detection of prostate cancer cells using an aperture sensor array. Lab a Chip 2013, 13, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hui, A.; Pampalakis, G.; Soleymani, L.; Liu, F.-F.; Sargent, E.H.; Kelley, S.O. Direct, Electronic MicroRNA Detection for the Rapid Determination of Differential Expression Profiles. Angew. Chem. Int. Ed. 2009, 48, 8461–8464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. , Shan, X., Fu, Y., Liu, P., Li, X., Liu, B. et al., 2017. Electrochemical determination of the anticancer drug cape-citabine based on a graphene-gold nanocomposite-modified glassy carbon electrode, Int. J. Electrochem. Sci., 12, 1077310782.
- Venu, M.; Venkateswarlu, S.; Reddy, Y.V.M.; Reddy, A.S.; Gupta, V.K.; Yoon, M.; Madhavi, G. Highly Sensitive Electrochemical Sensor for Anticancer Drug by a Zirconia Nanoparticle-Decorated Reduced Graphene Oxide Nanocomposite. ACS Omega 2018, 3, 14597–14605. [Google Scholar] [CrossRef]
- Yan, M.; Zang, D.; Ge, S.; Ge, L.; Yu, J. A disposable electrochemical immunosensor based on carbon screen-printed electrodes for the detection of prostate specific antigen. Biosens. Bioelectron. 2012, 38, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-T.; Huang, H.-J. Femtomolar immunoassay based on coupling gold nanoparticle enlargement with square wave stripping voltammetry. Anal. Chim. Acta 2005, 538, 159–164. [Google Scholar] [CrossRef]
- Liu, X.; Duckworth, P.A.; Wong, D.K. Square wave voltammetry versus electrochemical impedance spectroscopy as a rapid detection technique at electrochemical immunosensors. Biosens. Bioelectron. 2010, 25, 1467–1473. [Google Scholar] [CrossRef]
- Anderson, J.L.; Coury, L.A.; Leddy, J. Dynamic Electrochemistry: Methodology and Application. Anal. Chem. 1998, 70, 519–590. [Google Scholar] [CrossRef]
- Akcakoca, I.; Ghorbanpoor, H.; Blair, E.; Ozturk, Y.; Dizaji, A.N.; Kocagoz, T.; Avci, H.; Corrigan, D.; Guzel, F.D. An electrochemical biosensor with integrated microheater to improve the sensitivity of electrochemical nucleic acid biosensors. J. Micromechanics Microengineering 2022, 32, 045008. [Google Scholar] [CrossRef]
- Kasturi, S.; Torati, S.R.; Eom, Y.; Kim, C. Microvalve-controlled miniaturized electrochemical lab-on-a-chip based biosensor for the detection of β-amyloid biomarker. J. Ind. Eng. Chem. 2021, 97, 349–355. [Google Scholar] [CrossRef]
- Liu, E.; Cai, Z.; Ye, Y.; Zhou, M.; Liao, H.; Yi, Y. An Overview of Flexible Sensors: Development, Application, and Challenges. Sensors 2023, 23, 817. [Google Scholar] [CrossRef]
- Chen, S.; Qi, J.; Fan, S.; Qiao, Z.; Yeo, J.C.; Lim, C.T. Flexible Wearable Sensors for Cardiovascular Health Monitoring. Adv. Heal. Mater. 2021, 10, 2100116. [Google Scholar] [CrossRef] [PubMed]
- Vaghasiya, J.V.; Mayorga-Martinez, C.C.; Pumera, M. Wearable sensors for telehealth based on emerging materials and nanoarchitectonics. npj Flex. Electron. 2023, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R. , Lin, M., Yin, L., De La Paz, E., Pei, K., Sonsa-Ard, T., De Loyola E Silva, A.N., Khorshed, A.A., Zhang, F., Tostado, N., Xu, S., Wang, J., 2021. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nature Biomedical Engineering., 5, 737–748.
- Subramaniam, S. , 2021b. The smartphone biosensors for point-of-care detection of human infectious diseases: Overview and perspectives—A systematic review. Current Opinion in Electrochemistry., 32, 100925.
- Rauf, S. , Lahcen, A.A., Aljedaibi, A., Beduk, T., De Oliveira Filho, J.P., Salama, K.N., 2021b. Gold nanostructured la-ser-scribed graphene: A new electrochemical biosensing platform for potential point-of-care testing of disease biomarkers. Biosensors and Bioelectronics., 180, 113116.
- Chugh, B. , Thakur, S., Singh, A.K., Joany, R.M., Rajendran, S., Nguyen, T.V., 2022b. Electrochemical sensors for agricul-tural application, in: Elsevier EBooks., pp. 147–164.
- Liu, S.; Shen, Z.; Deng, L.; Liu, G. Smartphone assisted portable biochip for non-invasive simultaneous monitoring of glucose and insulin towards precise diagnosis of prediabetes/diabetes. Biosens. Bioelectron. 2022, 209, 114251. [Google Scholar] [CrossRef] [PubMed]
- Umapathi, R.; Ghoreishian, S.M.; Sonwal, S.; Rani, G.M.; Huh, Y.S. Portable electrochemical sensing methodologies for on-site detection of pesticide residues in fruits and vegetables. Co-ord. Chem. Rev. 2021, 453, 214305. [Google Scholar] [CrossRef]
- Low, S.S. , Pan, Y., Ji, D., Li, Y., Lu, Y., He, Y., Chen, Q., Liu, Q., 2020b. Smartphone-based portable electrochemical bio-sensing system for detection of circulating microRNA-21 in saliva as a proof-of-concept. Sensors and Actuators B-chemical., 308, 127718.
- Fan, Y. , Shi, S., Ma, J., Guo, Y., 2021d. Smartphone-based electrochemical system with multi-walled carbon nano-tubes/thionine/gold nanoparticles modified screen-printed immunosensor for cancer antigen 125 detection. Microchemical Journal., 174, 107044.
- Talukder, N.; Furniturewalla, A.; Le, T.; Chan, M.; Hirday, S.; Cao, X.; Xie, P.; Lin, Z.; Gholizadeh, A.; Orbine, S.; et al. A portable battery powered microfluidic impedance cytometer with smartphone readout: towards personal health monitoring. Biomed. Microdevices 2017, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rosati, G. , Urban, M., Zhao, L., Yang, Q., De Carvalho Castro E Silva, C., Bonaldo, S., Parolo, C., Nguyen, E.P., Ortega, G., Fornasiero, P., Paccagnella, A., Merkoçi, A., 2022b. A plug, print & play inkjet printing and impedance-based biosensing technology operating through a smartphone for clinical diagnostics. Biosensors and Bioelectronics., 196, 113737.
- Tang, X.; Li, Y.; Ma, J.; Wang, X.; Zhao, W.; Hossain, A.; Yang, Y. Adenovirus-mediated specific tumor tagging facilitates CAR-T therapy against antigen-mismatched solid tumors. Cancer Lett. 2020, 487, 1–9. [Google Scholar] [CrossRef]
- Kumar, A.; Deep, G. Exosomes in hypoxia-induced remodeling of the tumor microenvironment. Cancer Lett. 2020, 488, 1–8. [Google Scholar] [CrossRef]
- Gai, P.-P.; Ji, Y.-S.; Wang, W.-J.; Song, R.-B.; Zhu, C.; Chen, Y.; Zhang, J.-R.; Zhu, J.-J. Ultrasensitive self-powered cytosensor. Nano Energy 2016, 19, 541–549. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Shao, B.; Liu, X.; Hu, Z.; Wang, C.; Li, H.; Zhu, L.; Li, P.; Yang, Y. HER2 status of CTCs by peptide-functionalized nanoparticles as the diagnostic biomarker of breast cancer and predicting the efficacy of anti-HER2 treatment. Front. Bioeng. Biotechnol. 2022, 10, 1015295. [Google Scholar] [CrossRef]
- Zoupanou, S. , Volpe, A., Primiceri, E., Gaudiuso, C., Ancona, A., Ferrara, F., Chiriacò, M.S., 2021. SMILE Platform: An Innovative Microfluidic Approach for On-Chip Sample Manipulation and Analysis in Oral Cancer Diagnosis. Micromachines., 12, 885.
- Tran, H.-V.; Ngo, N.M.; Medhi, R.; Srinoi, P.; Liu, T.; Rittikulsittichai, S.; Lee, T.R. Multifunctional Iron Oxide Magnetic Nanoparticles for Biomedical Applications: A Review. Materials 2022, 15, 503. [Google Scholar] [CrossRef]
- Khoshfetrat, S.M.; Mehrgardi, M.A. Amplified detection of leukemia cancer cells using an aptamer-conjugated gold-coated magnetic nanoparticles on a nitrogen-doped graphene modified electrode. Bioelectrochemistry 2017, 114, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Salahandish, R.; Ghaffarinejad, A.; Naghib, S.M.; Majidzadeh-A, K.; Zargartalebi, H.; Sanati-Nezhad, A. Nano-biosensor for highly sensitive detection of HER2 positive breast cancer. Biosens. Bioelectron. 2018, 117, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhong, L.; Xiong, L.; Liu, C.; Yu, L.; Chu, X.; Luo, X.; Zhao, M.; Liu, B. A novel sandwich-like cytosensor based on aptamers-modified magnetic beads and carbon dots/cobalt oxyhydroxide nanosheets for circulating tumor cells detection. Sensors Actuators B: Chem. 2021, 331, 129399. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, J.; Lai, Y.; Wu, B.; Sun, L.; Weng, J. Ultrasensitive label-free detection of circulating tumor cells using conductivity matching of two-dimensional semiconductor with cancer cell. Biosens. Bioelectron. 2019, 142, 111520. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. , Zhao, X., Liu, F.-F., Younis, M., Xia, X.-H., Wang, C., 2019. Direct Plasmon-Enhanced Electrochemistry for En-abling Ultrasensitive and Label-Free Detection of Circulating Tumor Cells in Blood. Analytical Chemistry., 91, 4413–4420.
- Cai, J.; Shen, H.; Wang, Y.; Peng, Y.; Tang, S.; Zhu, Y.; Liu, Q.; Li, B.; Xie, G.; Feng, W. A dual recognition strategy for accurate detection of CTCs based on novel branched PtAuRh trimetallic nanospheres. Biosens. Bioelectron. 2020, 176, 112893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, H.; Yang, M.; Liao, L. Electrochemical assay for detection of circulating tumor cells based on LiFePO4 as electrochemical probe. Mater. Lett. 2020, 276, 128219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





