Submitted:
29 August 2023
Posted:
31 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
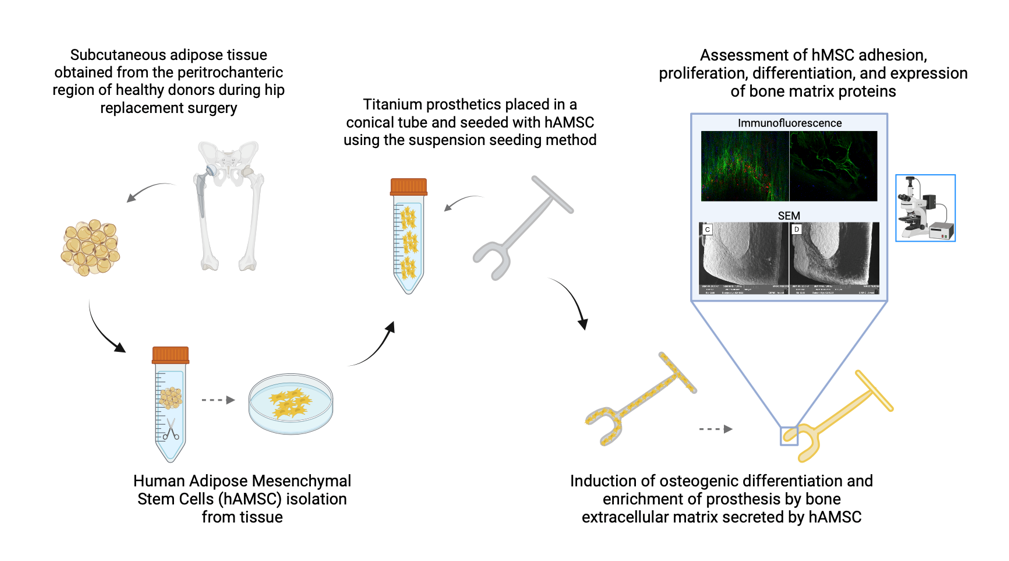
2.1. Production of titanium-biohybrid middle ear prostheses
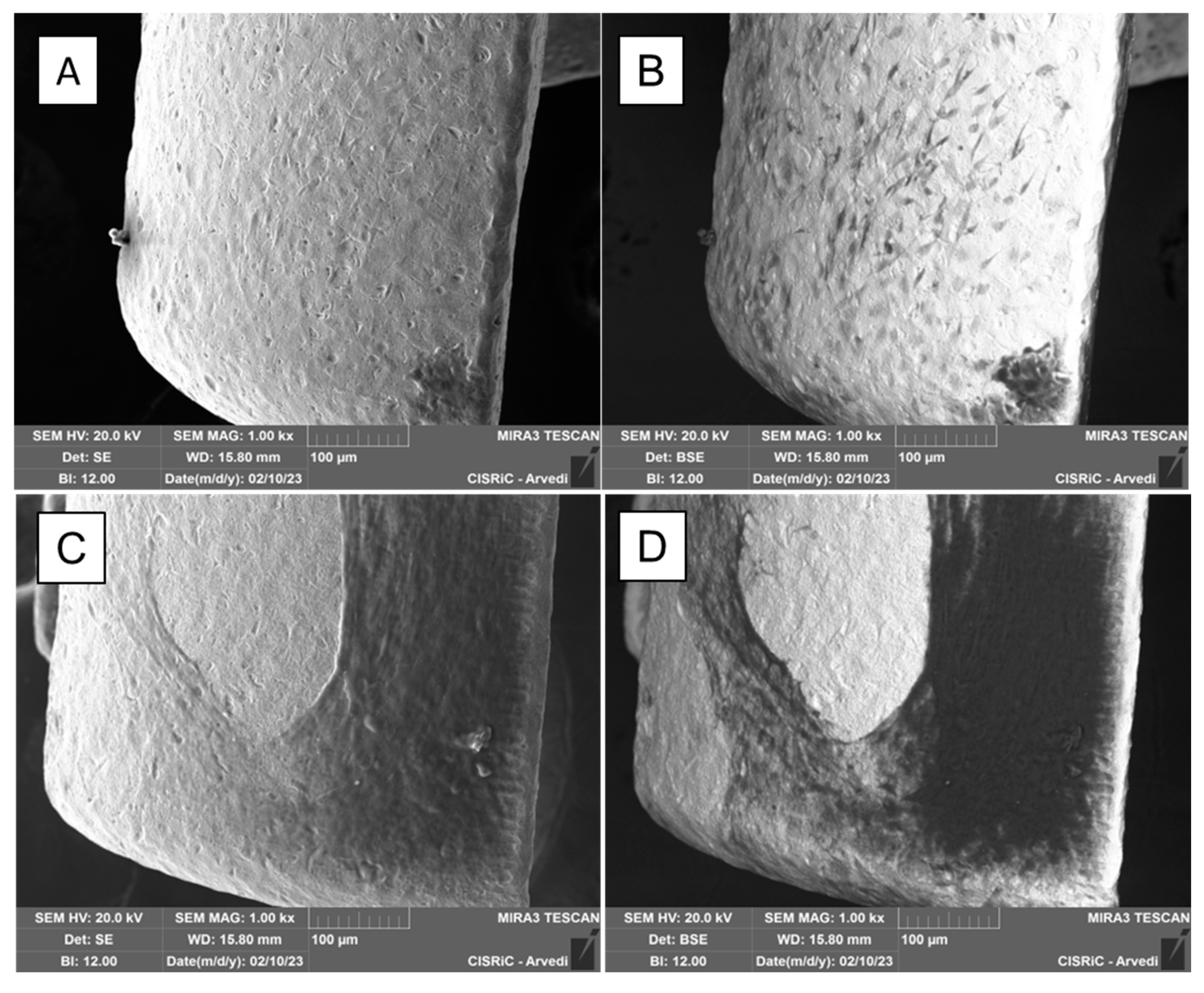
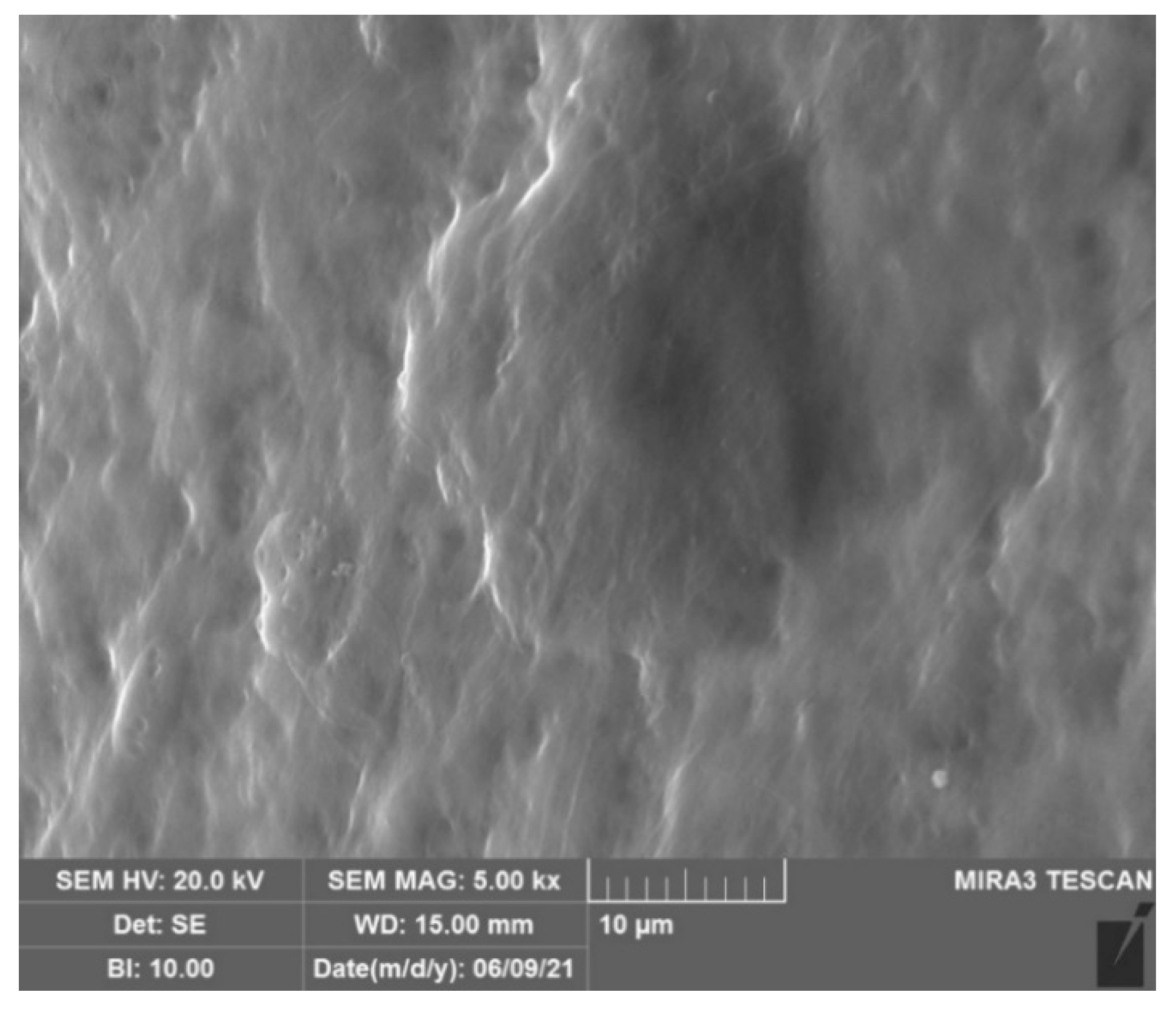
2.2. Titanium-biohybrid middle ear prostheses analyses
2.2.1. Cell proliferation and osteogenic differentiation
2.2.2. Production of extracellular matrix
3. Results
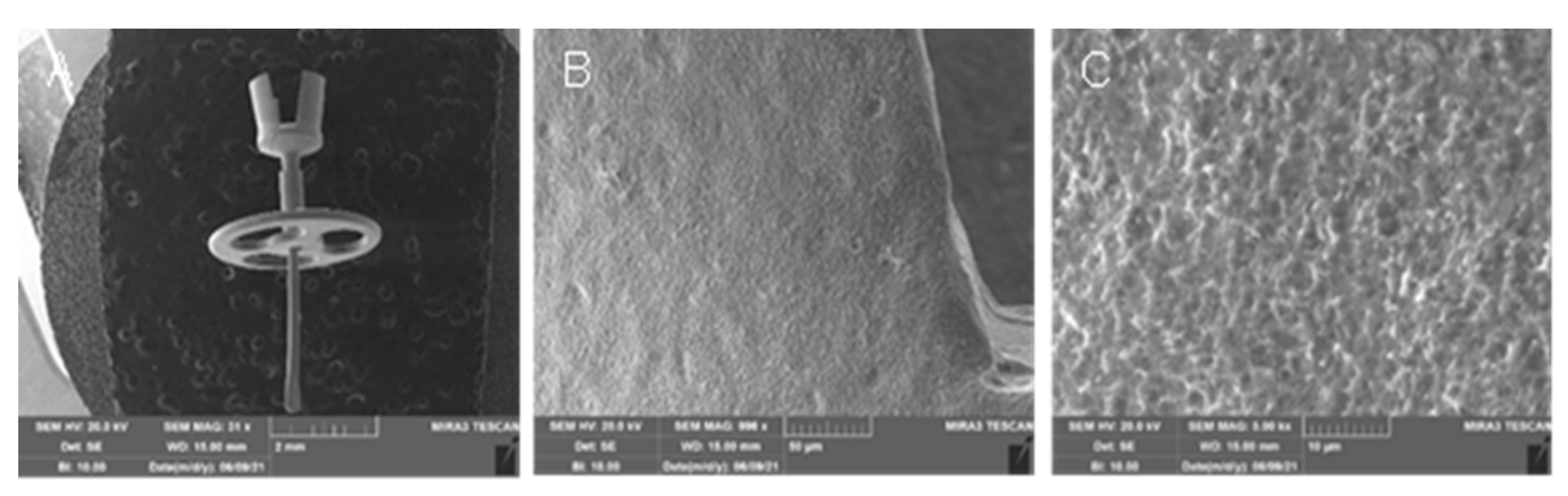
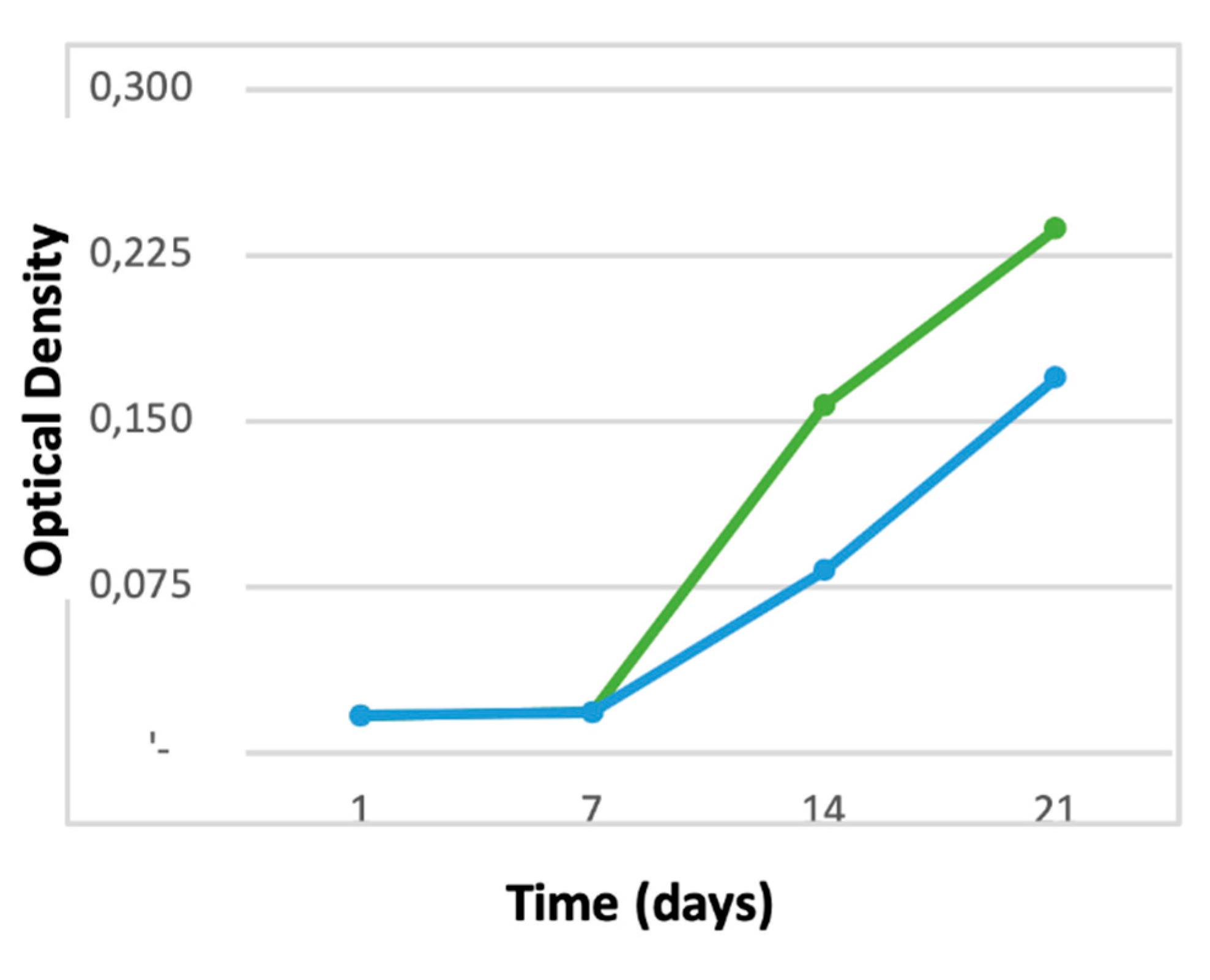
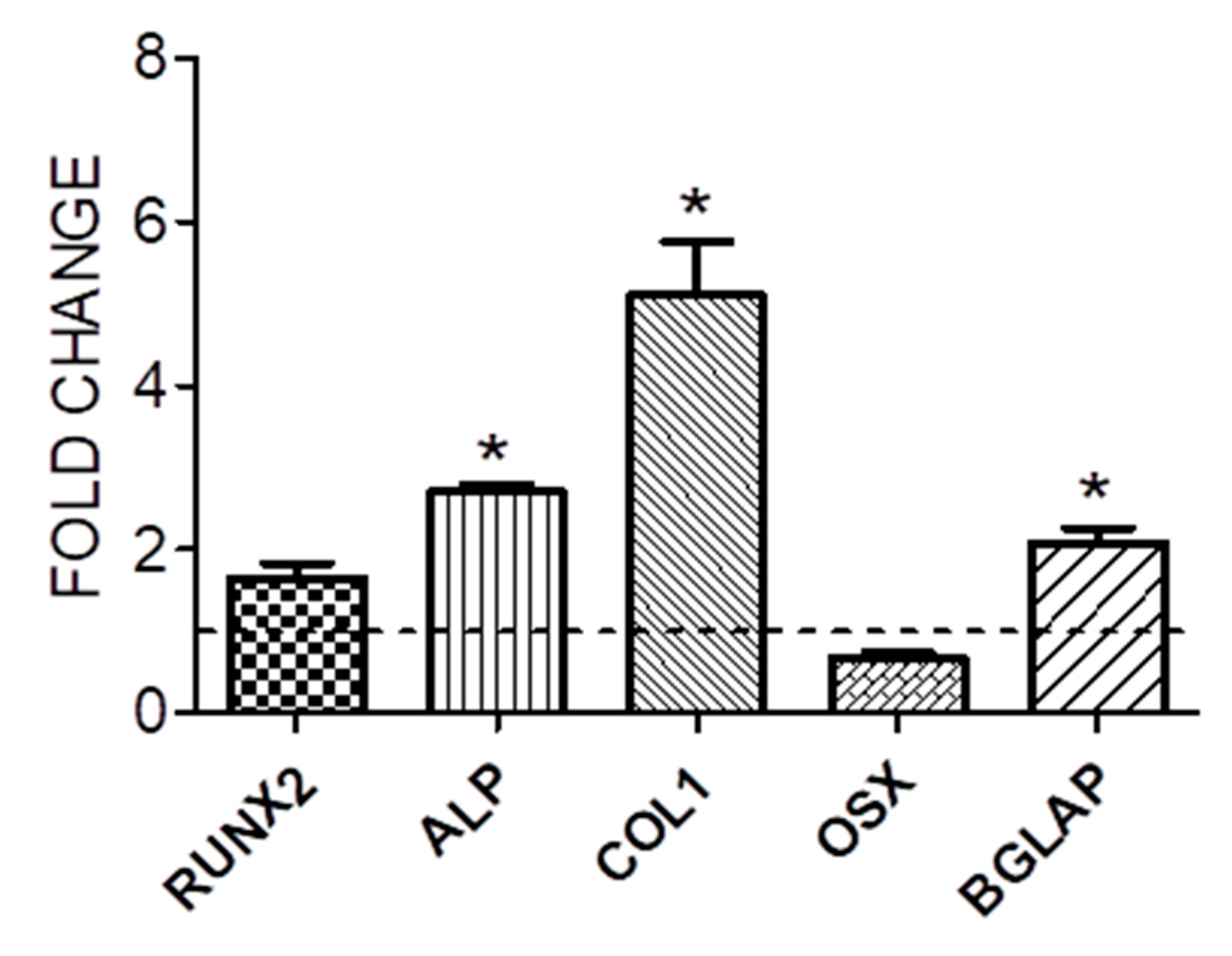
3.1. Cell proliferation and osteogenic differentiation
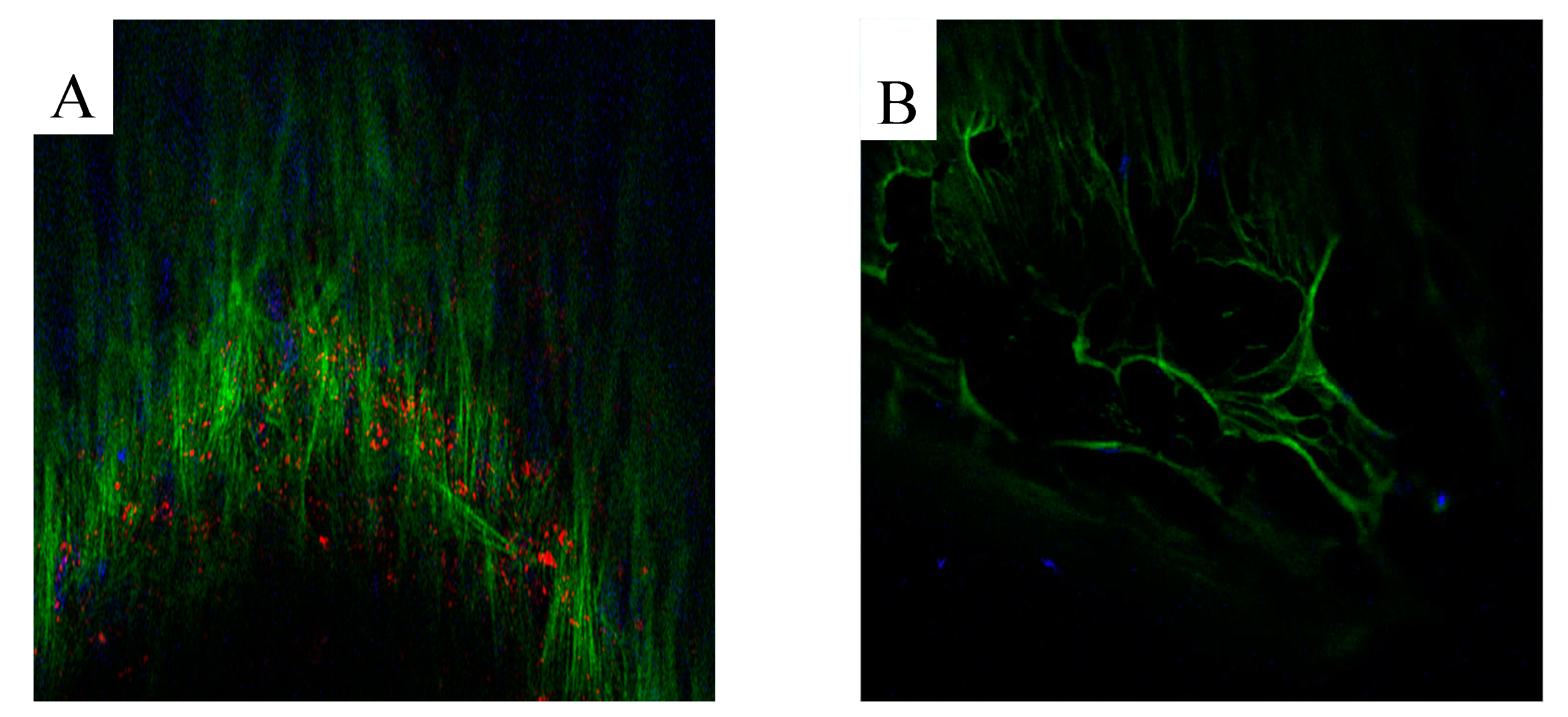
3.2. Production of extracellular matrix
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Ha, J.; Choo, O.-S.; Park, H.; Choung, Y.-H. Which Is Better for Ossiculoplasty following tympanomastoidectomy: Polycel® or Titanium? Ann Otol Rhinol Laryngol 2023, 000348942311599. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, M.; Roosta, S.; Faramarzi, A.; Khera, M. Comparison of partial vs. total ossicular chain reconstruction using titanium prosthesis: a retrospective cohort study. Eur Arch Otorhinolaryngol 2023, 280, 3567–3575. [Google Scholar] [CrossRef] [PubMed]
- Coffey, C.S.; Lee, F.; Lambert, P.R. Titanium versus nontitanium prostheses in ossiculoplasty. Laryngoscope 2008, 118, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Kortebein, S.; Russomando, A.C.; Greda, D.; Cooper, M.; Ledbetter, L.; Kaylie, D. Ossicular chain reconstruction with titanium prostheses: a systematic review and meta-analysis. Otol Neurotol 2023, 44, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Danti, S.; Stefanini, C.; D’Alessandro, D.; Moscato, S.; Pietrabissa, A.; Petrini, M.; Berrettini, S. Novel biological/biohybrid prostheses for the ossicular chain: fabrication feasibility and preliminary functional characterization. Biomed Microdevices 2009, 11, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Danti, S.; D’Alessandro, D.; Pietrabissa, A.; Petrini, M.; Berrettini, S. Development of tissue-engineered substitutes of the ear ossicles: PORP-shaped poly(propylene fumarate)-based scaffolds cultured with human mesenchymal stromal cells. Biomed Mater Res A 2010, 92, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Miceli, E.; Gastaldi, G.; Scaffino, M.F.; Ventura, U.; Fontana, J.M.; Orsenigo, M.N.; Corazza, G.R. Solute Transporters and Aquaporins Are Impaired in Celiac Disease. Biology of the Cell 2010, 102, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Caliogna, L.; Bina, V.; Botta, L.; Benazzo, F.M.; Medetti, M.; Maestretti, G.; Mosconi, M.; Cofano, F.; Tartara, F.; Gastaldi, G. Osteogenic potential of human adipose derived stem cells (HASCs) seeded on titanium trabecular spinal cages. Sci Rep 2020, 10, 18284. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Das, R.; Patel, A.; Duc Nguyen, T. Physical stimulations for bone and cartilage regeneration. Regen Eng Transl Med 2018, 4, 216–237. [Google Scholar] [CrossRef] [PubMed]
- Canzi, P.; Berrettini, S.; Albera, A.; Barbara, M.; Bruschini, L.; Canale, A.; Carlotto, E.; Covelli, E.; Cuda, D.; Dispenza, F.; et al. Current trends on subtotal petrosectomy with cochlear implantation in recalcitrant chronic middle ear disorders. Acta Otorhinolaryngol Ital 2023, 43, S67–S75. [Google Scholar] [CrossRef] [PubMed]
- Canzi, P.; Avato, I.; Beltrame, M.; Bianchin, G.; Perotti, M.; Tribi, L.; Gioia, B.; Aprile, F.; Malpede, S.; Scribante, A.; et al. Retrosigmoidal placement of an active transcutaneous bone conduction implant: surgical and audiological perspectives in a multicentre study. Acta Otorhinolaryngol Ital 2021, 41, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.S.; Jeong, C.Y.; Shim, M.J.; Kim, W.J.; Yeo, S.W.; Park, S.N. Comparative study of new autologous material, bone-cartilage composite graft, for ossiculoplasty with Polycel ® and Titanium. Clin Otolaryngol 2018, 43, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Van Rompaey, V.; Farr, M.R.B.; Hamans, E.; Mudry, A.; Van De Heyning, P.H. Allograft tympanoplasty: a historical perspective. Otol Neurotol 2013, 34, 180–188. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.; Danti, S.; De Vito, A.; Forli, F.; Bruschini, L.; Berrettini, S. Histologic characterization of human ear ossicles for the development of tissue-engineered replacements. Otol Neurotol 2012, 33, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, M.; Pitel, K.S.; Withers, S.G.; Drissi, H.; Hawse, J.R. TIEG1 enhances Osterix expression and mediates its induction by TGFβ and BMP2 in osteoblasts. Biochem Biophys Res Commun 2016, 470, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Caliogna, L.; Bina, V.; Brancato, A.M.; Gastaldi, G.; Annunziata, S.; Mosconi, M.; Grassi, F.A.; Benazzo, F.; Pasta, G. The role of PEMFs on bone healing: an in vitro study. IJMS 2022, 23, 14298. [Google Scholar] [CrossRef] [PubMed]
- Canzi, P.; Avato, I.; Marconi, S.; Del Maestro, M.; Lucifero, A.G.; Magnetto, M.; Carlotto, E.; Auricchio, F.; Luzzi, S.; Benazzo, M. A 3D printed custom-made mask model for frameless neuronavigation during retrosigmoid craniotomy. A preclinical cadaveric feasibility study. Ann Ital Chir 2020, 91, 526–533. [Google Scholar] [PubMed]
- Canzi, P.; Magnetto, M.; Marconi, S.; Morbini, P.; Mauramati, S.; Aprile, F.; Avato, I.; Auricchio, F.; Benazzo, M. New frontiers and emerging applications of 3D printing in ENT surgery: a systematic review of the literature. Acta Otorhinolaryngol Ital 2018, 38, 286–303. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




