1. Introduction
The ischemic stroke represents the third cause of overall worldwide morbidity and mortality, more than 80% from patients suffering an episode of cerebral ischemia remaining with a life-altering disability. For these patients, the short therapeutic window available for a complete treatment addressed to cerebral ischemia has led to important researches for a molecule able to limit the destruction of cerebral tissue (1). In recent years, the role of neuro inflammation in the neurologic pathology was studied, with highlight on pro inflammatory cytokines such as IL-1, IL-6, and TNF-alpha. IL-1 is a pro inflammatory cytokine, with importance in local inflammation, systemic inflammation this making it a central factor in immune reaction to infections and other injuries (2,3). The IL-1 family has three main ligands: IL-1 alpha, IL-1 beta, as agonists, and the internal antagonist IL-1Ra. Recent studies demonstrated that IL-1 is quickly expressed in the cerebral tissue during a neuronal injury, associated with multiple inflammatory changes (4,5). On the other hand, other risk factors strongly related with a cerebral ischemic episode such as cerebral and atheromatosis of carotid artery, and post stroke infections are correlated with strong expression of inflammatory markers, namely NLRP3, Il1 beta, TNF alpha and IL6 inflammasome. Considering all this, the inflammation process will be the next therapeutic target for the future (6,7).
2. Materials and methods
2.1. Study Design
The study took place over 12 months. The patient enrolled in the study were patients with acute neurological pathology, ischemic stroke less than 24 hours old, admitted and treated in the Neurology Clinic, Sibiu County Emergency Hospital. At admission, the biological markers were determined, with a reevaluation 7 days after the onset of the stroke. During hospitalisation, we performed ultrasound examination of the carotid arteries, in order to determine the extent of the atheromatosis at this level.
Inclusion Criteria:
- -
Adult patients, admitted in the Neurology Department, Sibiu County Emergency Hospital, with diagnostic suspicion of ischemic stroke
- -
Neurological symptoms and signs strongly suggesting the onset of a ischemic stroke (less than 24 hour)
- -
Cerebral imaging rules out cerebral tumors or hemorrhagic stroke
Exclusion Criteria:
- -
Any medical pathology that can trigger modification of inflammatory markers: infections, autoimmune diseases, neoplasia, hematological disorders (lymphoma, multiple myeloma)
- -
Patients undergoing (in last 30 days) corticosteroid or immunosuppressive therapy
- -
Patients diagnosed over the last 180 days with acute myocardial infarction, myocarditis, or acute ischemic stroke
- -
Patients who suffered brain traumatic injuries documented at the time of admission
-
a.
Collection of biological samples, bio-markers measurement
All the samples were collected in EDTA vacutainers, centrifuged at 1500x for 15 minutes, and frozen at -80°C afterwards. The IL-1 beta biomarker was determined from these samples. The non-standard samples have been excluded.
We used the Carotid Doppler ultrasound as imaging tool, with the staging of carotid atheromatosis as follows:
a. mild carotid atheromatosis – small atheroma plaques in internal and external carotid arteries
b. moderate carotid atheromatosis – carotid stenosis between 50-60%
c. severe carotid atheromatosis – carotid stenosis greater than 70%.
-
b.
Statistical analysis
The database was created using the Microsoft Office Excel 2016 application. The SPSS 25.0 program (SPSS Inc, Chicago, USA) was used for statistical analysis and data description. The normality of the distribution of quantitative data was verified using the Shapiro-Wilk or Kolmogorov-Smirnov tests. The accepted error threshold was α =0.05. To describe normally distributed continuous quantitative data, the arithmetic mean±standard deviation was used, and for those that did not have a Gaussian distribution, the median (quartile 1-quartile 3) was used. Qualitative data were described using frequencies.To compare the means of the quantitative variables of two independent groups, the Student's test (t-test) was used if the variables were normally distributed. The nonparametric Mann–Whitney and Kruskall–Wallis tests were used to compare the means of two independent groups, where the variables had or abnormal distribution. The correlation analysis was done using the Pearson linear correlation coefficient for data with normal distribution, respectively the Spearman correlation coefficient for quantitative data without normal distribution or for ordinal data. Colton's empirical rules were used to interpret the correlation coefficients.
3. Results
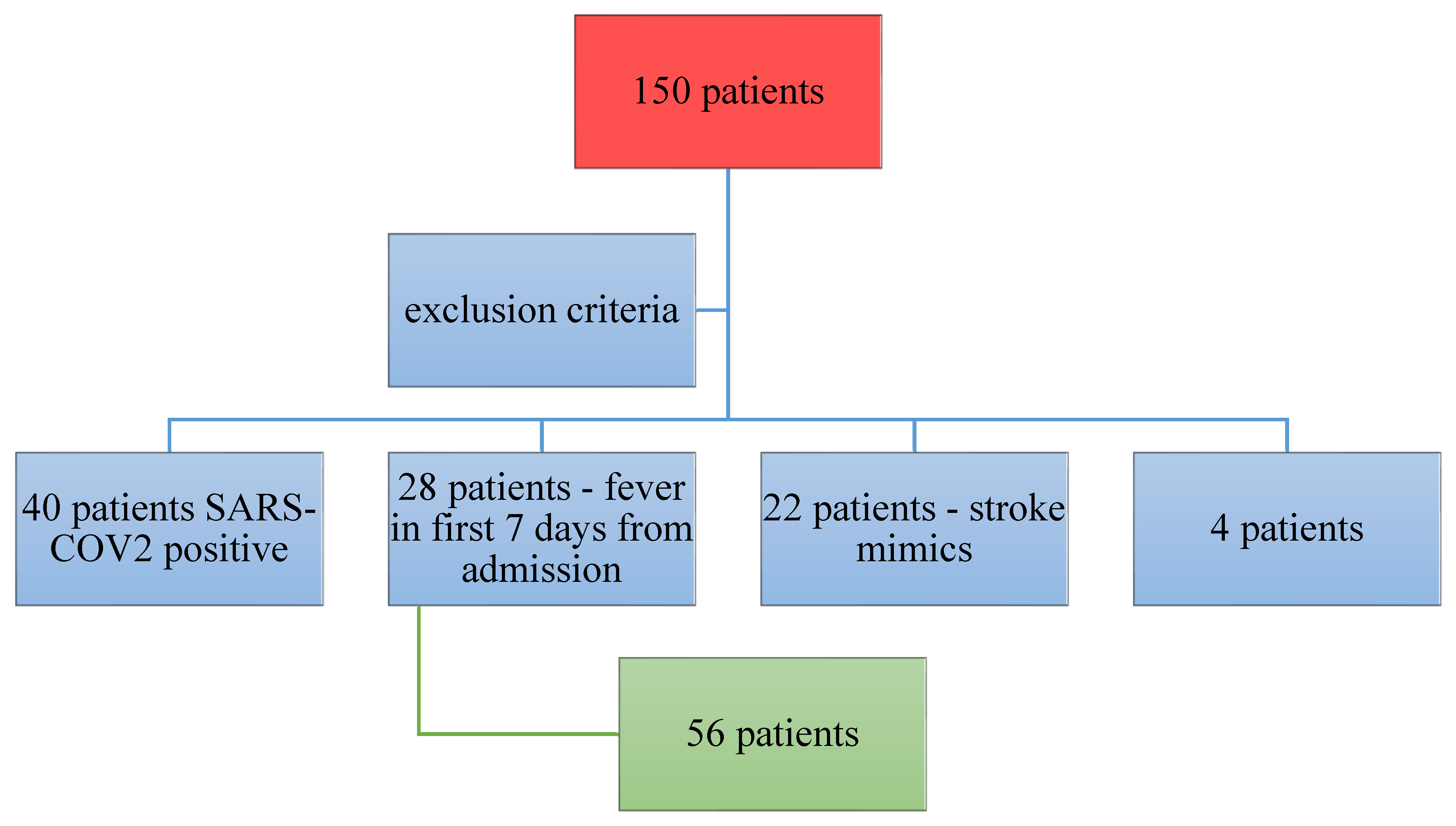
The study was conducted between 1
st January 2021 – 31th December 2021. 150 patients were included in our study. From this initial group, 40 were diagnosed with SARS-COV 2 infection at admission, 28 developed fever in the first 7 days that made the second evaluation of inflammatory markers useless; for 22 patients the diagnosis was a stroke-like syndrome, 4 patients were excluded because of non-standard biological samples, improper harvesting and storage of sample. In all, 56 patients remained enrolled until the end point of the study (
Figure 1).
From the 56 patients that fulfilled the inclusion criteria, 28 were males and 28 females, with a mean age of 74 years. At admission and right before discharge, the NIHSS (National Institute of Health Stroke Scale) was determined by the neurologist. The values at admission were 24 at the highest and 2 at the lowest, while at discharge the values decreased for most of the patients: maximal 20 and minimal 0. IL-1 beta values were determined in the first 24 hours after admission, and in the 7th day of hospitalisation.
The results of the Doppler ultrasound examination were classified in three main categories:
- 0.
Mild atheromatosis
- 1.
Moderate atheromatosis, ICA stenosis under 50%
- 2.
Severe atheromatosis, ICA stenosis greater than 70%
The median age of stroke patients was 74 years years old, ranging from 33 to 91 years. In addition, 80 % suffered an ischemic stroke localized in the anterior vascular territory, the rest of 20 % being localized in the posterior vascular territory. The median Il1beta plasma concentration (quartile 1 – quartile 3) in day 1 was 0,51 (ranging from 0 to 1,56) and in day 7 from the onset of stroke was 0 (ranging from 0 to 1,59). The median NIHSS at admission was 7 – 50% of the patients had NIHSS lower than 7 and 50% of the patients included in the study had NIHSS greater than 7 (ranging from 4 to 11). 25% of the patients included in the study had an NIHSS greater than 11 at admission. The median NIHSS at discharge was 5 (ranging from 2 to 10), meaning that 25% of the patients included in the study were discharged with a NIHS score greater than 10, which means moderate or severe disability.
The extracranial carotid ultrasound was performed to all patients admitted in the study. Moderate to severe carotid stenosis was described in 69,6% of the subjects, 35,7% presented a moderate stenosis (50-60% stenosis) and 33,9% had severe carotid stenosis (>70%).
Table 1.
description of the data included in the study (median – quartile 1 – quartile 3).
Table 1.
description of the data included in the study (median – quartile 1 – quartile 3).
| AGE |
74(70-80) |
| GENRE |
| F |
28(50%) |
| M |
28(50%) |
| NIHSS (ADMISSION) |
7(4-11) |
| NIHSS (DISCHARGE) |
5(2-10) |
| IL 1 BETA (24 hours) |
0,51(0-1,56) |
| IL 1 BETA (DAY 7) |
0(0-1,59) |
| CHOLESTEROL |
196(55,79) |
| CAROTID ULTRASOUND |
|
| 0 |
14(25%) |
| 1 |
20(35,7%) |
| 2 |
19(33,9%) |
As can be noted in
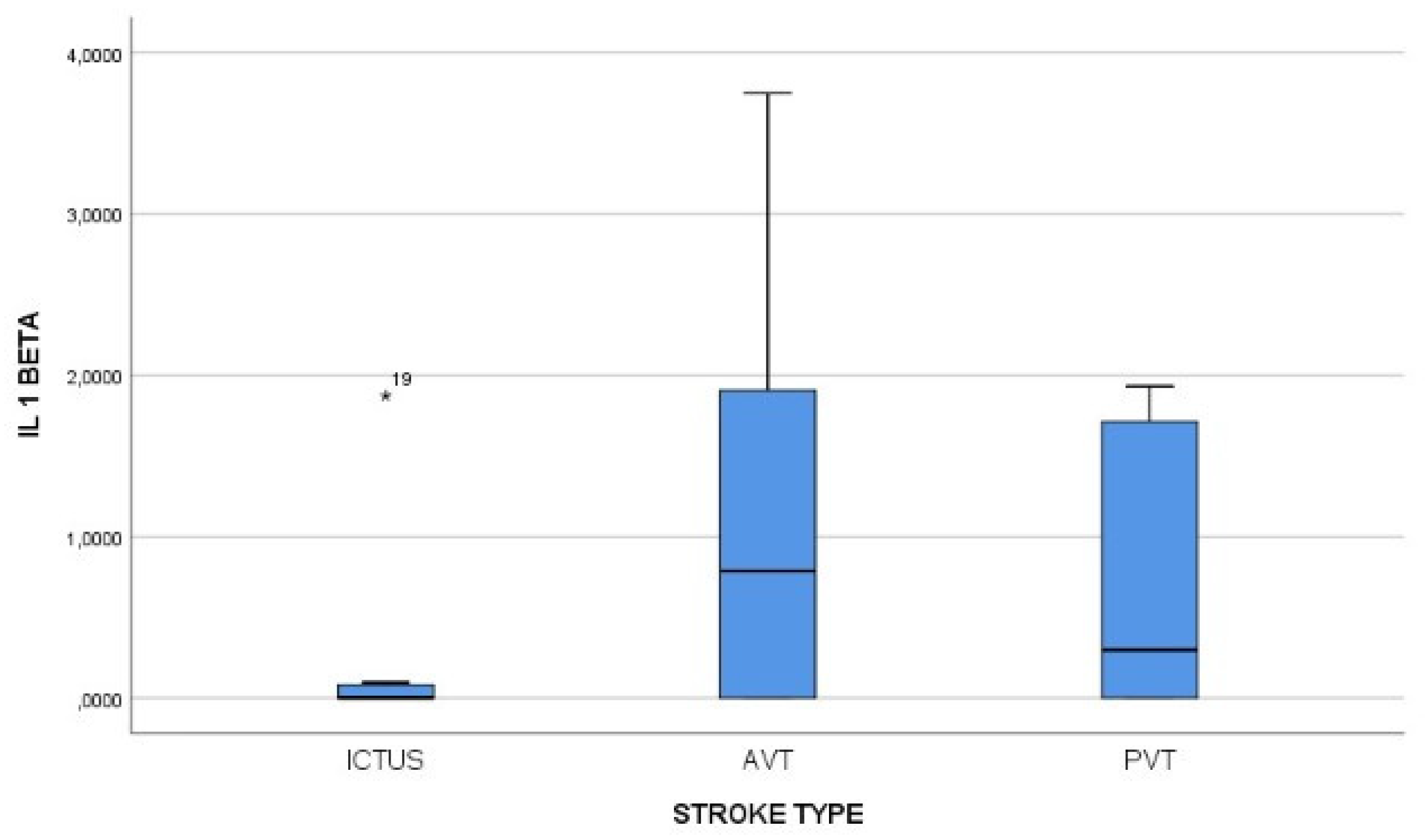
Figure 2, the IL-1 beta value was positively correlated with stroke type, plasma concetration being grater in AVT strokes. Also, Il1beta first measurement concentration was correlated with the NIHSS score at admission, with a Pearson index of 0,424.
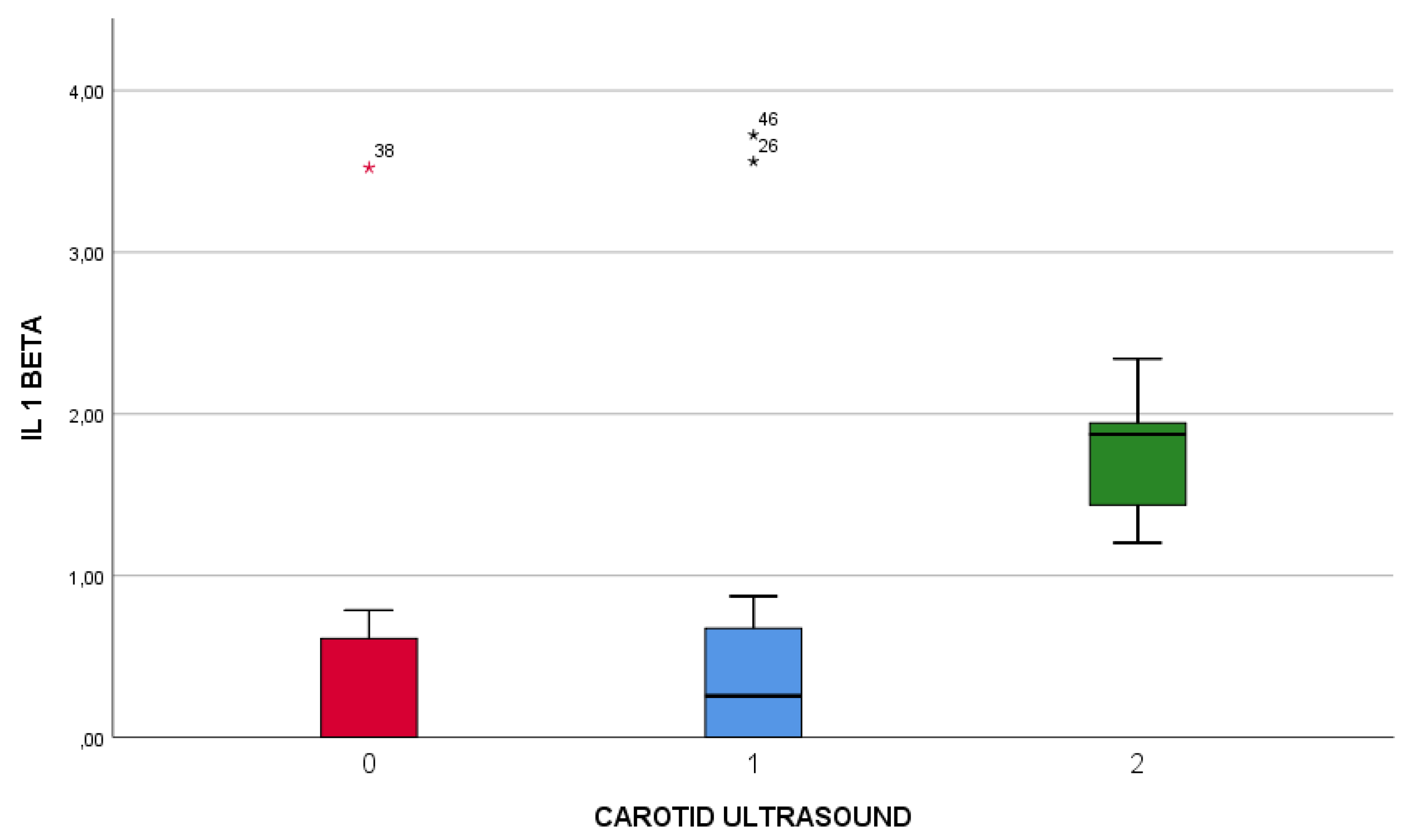
Figure 3 illustrates that, both measurements of IL-1 beta value (day 1, and day 7 from the onset of the symptoms) were positively correlated with the degree of carotid artery atheromatosis, with a Spearman correlation index of 0,529 (first measurement), 0,653 respectively for the second measurement.
Considering the type of the stroke, the patients enrolled in the study were assigned to three groups:
1. Lacunar stroke - patients with cerebral ischemic lesions smaller than 10 mm.
2. Anterior Vascular Territory AVT – patients with cerebral ischemia in anterior vascular territory
3. Posterior Vascular Territory PVT - patients with cerebral ischemia in posterior vascular territory
The initial value of IL-1 beta was bigger than normal in all types of ischemic stroke, with the smallest values in the lacunar stroke group, followed by the group. The AVT type ischemic strokes were correlated with the highest levels of IL-1 beta.
Table 2, shows us how we can evaluate and follow up patients with moderate carotid stenosis. The cut off value of 0,964 in the 2
nd measurement of the Il1beta plasma concentration makes the difference between severe and moderate carotid stenosis in the study group.
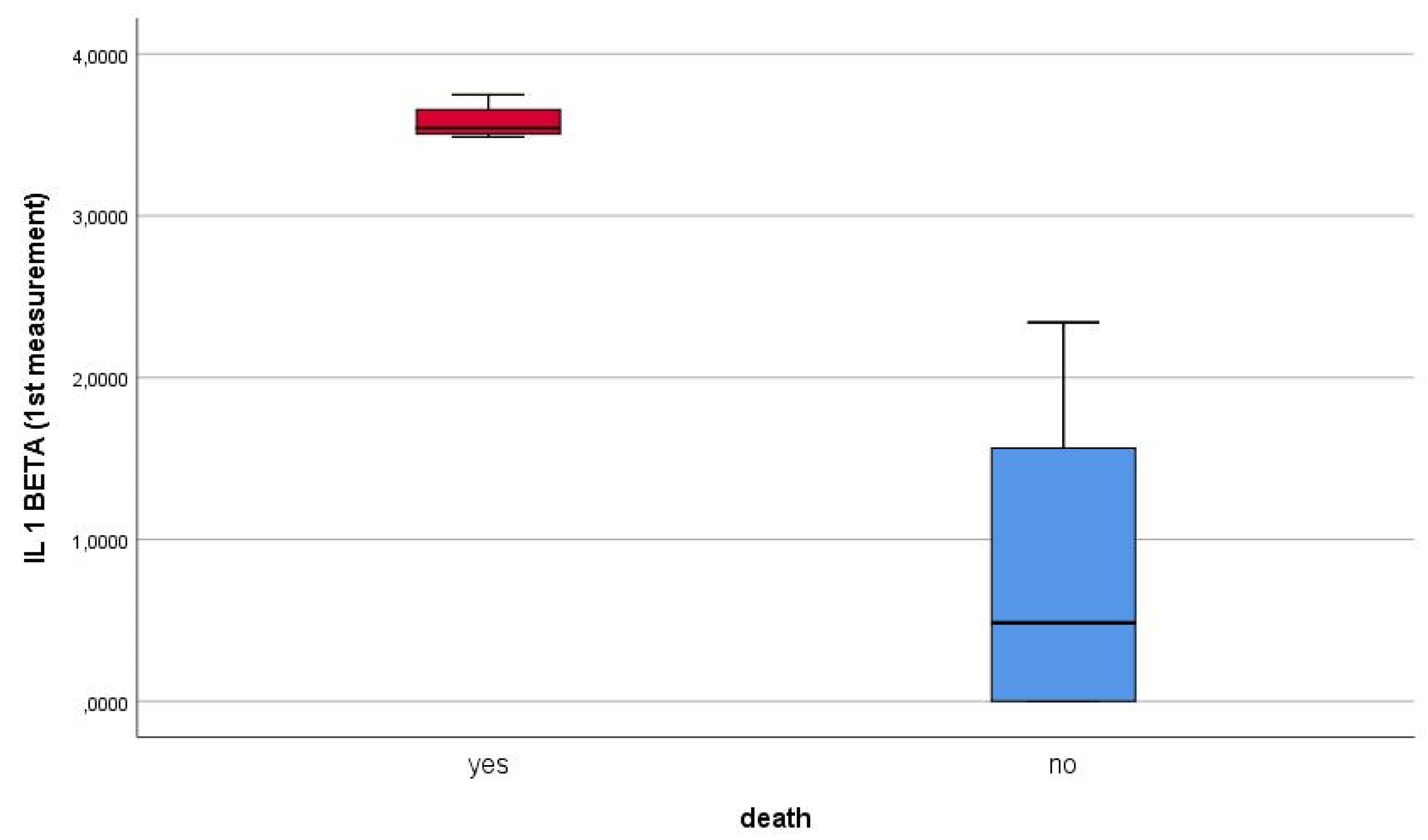
Furthermore, blood levels of Il1beta were positively correlated with patients` mortality (p value < 0.001).
Figure 4 highlights how first measurement of Il1beta was correlated with patients` death. At the same time, the first measurement of IL1beta correlated positively, directly proportionally, with the second measurement of the biomarker.
4. Discussion
IL-1 beta is a pro inflammatory cytokine, with a controversial role in the pathology of ischemic stroke, due to non-consistent results of previous clinical studies (8).
Recent studies have demonstrated that IL-1 beta is firstly stimulating the secretion of pro inflammatory mediators, such as TNF-alpha cytokines and IL-6, but also the secretion of adhesion molecules. In the same time, this cytokine is involved in the astrocytes activation, that leads to the production of survival-promoting factors and according to literature, has a role in the development of risk factors for ischemic stroke, such as atheromatosis (9,10).
Clinical studies designed similar with ours demonstrated that patients with ischemic stroke have elevated levels of IL-1 beta in the first 24 hours since the beginning of the event (same as the results of our study). Boutin et al, Smith et al concluded after stage II, single center randomized studies that the administration of IL-1 alpha in mice led to the extension of cerebral ischemic lesions, and the fact that mice with deficiency of Il-1 beta presented smaller volume cerebral infarction. The IL-1 receptor antagonist, namely IL-1Ra was tested in multiple clinical studies (11-13). The patients who received Anakinra - IL-1 receptors antagonist (100 mg loading dose, followed by 2mg/kgc/hour continuously IV for 72 hours ) have had a reduced inflammatory response, with lower values for WBC count, hsCRP and IL-1, probably through a peripheral immunosuppression mechanism (14). Likewise, the subcutaneous administration of IL-1Ra significantly reduced the inflammation level in patients included in SCIL-STROKE study, designed by Smith et al, without an improvement of clinical status at discharge (quantified with RANKIN scale) (15).
The controversies concerning IL-1 beta gained momentum when clinical studies denied the role of this particular cytokine in the pathogenesis of ischemic stroke. In the same time, at least two studies reported IL-1 beta levels within the base levels at 12, 24, and 72 hours after the onset of ischemic stroke (16,17). During the years, reports were published suggesting that the genetic polymorphism of IL-1Ra is a risk factor for the ischemic stroke, and also that the inhibition of IL-1 beta can prevent or delay the onset of cerebral ischemia. Chiba et al tried to inhibit IL-1 beta by giving the mice a polyclonal antibody targeted against IL-1 beta (600 micrograms/day) (18). Results were not as expected, the use of antibody did slightly delayed, but without signification the onset of ischemic stroke in lab mice (18,19).
The discrepancies concerning the role of IL1 beta in the onset of ischemic stroke arise from the design of the studies, because the pre-clinical studies showed good results, since the clinical studies demonstrated unfavorable, conflicting results. The differences are the result of the fact that the risk factors (smoking, alcohol consumption, stress, age) cannot be reproduced in the laboratory, where the ischemic stroke on animals is induced by simple mechanical occlusion of the artery, with subsequent thrombus inflammation, and micro thrombosis (with Treg cells activation). Other factors are the heterogeneity of ischemic strokes on human subjects, and the fact that in animal experimental models the complete arterial occlusion is followed by reperfusion, which is not the case in human subjects, excepting the cases of patients with thrombectomy (20,21).
We have to underline the contribution of chronic inflammation, especially by IL1 beta to the pathologies considered as risk factors for ischemic stroke, such as elevated blood pressure, obesity, infections and atheromatosis.
The atheromatosis is recognised as a chronic inflammatory condition, defined by the formation of plaque in the arterial intima, which restricts the blood flow and in the same time promotes the onset of ruptures and erosions at this level, favoring the thrombotic occlusions (7, 22). IL1 beta induces an inflammatory reaction in the endothelial cells, resulting in the increased secretion of adhesion factors and chemokines. In the same time, this pro inflammatory cytokine helps the accumulation of pro inflammatory cells in the affected blood vessel, promoting their invasion in the intima, hence the start of atheroma plaque formation (23).
One of the results of our study, namely the correlation between the levels of IL1beta with the severity of carotid atheromatosis concurs with the literature data. In another similar study Galea et al compared the IL1 beta levels with the coronarian atheromatosis in healthy patients and patients with cardiovascular pathology, concluding that there is a correlation with the severity of atheromatosis (24,25). In the same time, IL1 beta levels are correlated with the production of more inflammatory mediators, such as the cyclo oxygenase 2 ( COX 2), leading to the formation of prostaglandins.
The synthesis of IL6 and matrix metalloproteinases (MMPs) are also mediated by IL1 beta, leading to an elevation in the levels of acute phase reactants (C reactive protein, fibrinogen) with a role in the development of atheromatosis (26). The metalloproteinases (MMP1, MMP8, MMP13) are collagenases, deeply related with the rupture of the fibrous cap of the atheroma plaque by their ability of breaking the collagen fibers. It is very important to underline the fact that IL1 beta plays a big role in the growth of the already settled atheroma plaque (27,28). In about 80% of our patients, the plaques from the severely occluded arteries were old, fibrous, with a hyperechogenic appearance.
The pro atherogenic feature of IL1 beta has been demonstrated in many animal model studies. The chronic administration of IL1 beta in pigs, in a study by Shimokawa et al, resulted in elevated arterial intima media thickness IMT and in the same time the inhibition of IL1 beta by administration of IL1Ra limited the development of atheromatosis (29). Similarly, other studies, such as the one of Chamberlain et al, demonstrated that the IL1Ra deficiency can led to neo intima formation after the endothelial lesions. The neo intima formation was reduced by the administration of IL1Ra or in the case if a IL1 beta deficiency, without a causality between this and IL1 alpha levels (30).
Elhage et al and Devlin et al targeted the role of IL1Ra, showing that its deficiency is leading to trans mural arterial inflammation, underlining by this the contribution of IL1 in the initial stages of atheromatosis development (31,32).
The deficiency of IL1 beta decreased the spontaneous development of atherosclerotic lesions in mice, while the transplantation of bone marrow with lower levels of IL1 beta , IL1 alpha resulted in a lower degree of diet induced atheromatosis (33,34).
Recent studies concluded that monoclonal antibodies targeting IL1 beta are capable of reducing the diet induced atheromatosis in Apoe+ mice, hence the need for bigger cohort studies on human subjects in order to establish the molecule best suited (monoclonal antibodies, anti-inflammatory agents) to stop the development of atheromatous lesions. We are reviewing some of these studies (Cantos, Colcort, Cirt, Lodoco, Convince) in the
Table 3 (35,37).
5. Conclusion
All these clinical studies have had promising results in stopping the evolution of cerebral ischemic lesions and atheromatosis, but the incidence of lethal infection was higher in the group treated with monoclonal antibodies or immunosuppressant medication. For this reason, this research should be continued and extended towards other molecules targetting the pro inflammatory cytokines, such as IL1 beta.
Author contributions
Conceptualization: G.V. and R.M. Methodology: G.V. Formal Analysis: M.V. Investigation: G.C. Data Curation: R.M., C.RF. Writing – Original Draft Preparation: GV.; Writing – Review & Editing: C.RF., R.M.; Visualization: G.V, R.M., C.RF., A.P.; Supervision: R.M. Article`s translation in English: AP.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Emergency Clinical County Hospital of Sibiu (protocol code XXX and date of approval).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgements
Not applicable
Conflict of interests
Nothing to declare
References
- Jayaraj, R. L., Azimullah, S., Beiram, R., Jalal, F. Y., & Rosenberg, G. A. (2019). Neuroinflammation: friend and foe for ischemic stroke. Journal of neuroinflammation, 16(1), 1-24. [CrossRef]
- Kim, J. Y., Kawabori, M., & Yenari, M. A. (2014). Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Current medicinal chemistry, 21(18), 2076-2097. [CrossRef]
- Denes, A., Pinteaux, E., Rothwell, N. J., & Allan, S. M. (2011). Interleukin-1 and stroke: biomarker, harbinger of damage, and therapeutic target. Cerebrovascular Diseases, 32(6), 517-527. [CrossRef]
- Banwell, V., Sena, E. S., & Macleod, M. R. (2009). Systematic review and stratified meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke. Journal of Stroke and Cerebrovascular Diseases, 18(4), 269-276. [CrossRef]
- Pinteaux, E., Trotter, P., & Simi, A. (2009). Cell-specific and concentration-dependent actions of interleukin-1 in acute brain inflammation. Cytokine, 45(1), 1-7. [CrossRef]
- Libby, P. (2021). Inflammation in atherosclerosis—no longer a theory. Clinical chemistry, 67(1), 131-142. [CrossRef]
- Nguyen, M. T., Fernando, S., Schwarz, N., Tan, J. T., Bursill, C. A., & Psaltis, P. J. (2019). Inflammation as a therapeutic target in atherosclerosis. Journal of clinical medicine, 8(8), 1109. [CrossRef]
- Mendiola, A. S., & Cardona, A. E. (2018). The IL-1β phenomena in neuroinflammatory diseases. Journal of Neural Transmission, 125, 781-795. [CrossRef]
- Simats, A., & Liesz, A. (2022). Systemic inflammation after stroke: implications for post-stroke comorbidities. EMBO Molecular Medicine, 14(9), e16269. [CrossRef]
- Krishnan, S., O'Boyle, C., Smith, C. J., Hulme, S., Allan, S. M., Grainger, J. R., & Lawrence, C. B. (2021). A hyperacute immune map of ischaemic stroke patients reveals alterations to circulating innate and adaptive cells. Clinical & Experimental Immunology, 203(3), 458-471. [CrossRef]
- Jurcau, A., & Simion, A. (2021). Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: from pathophysiology to therapeutic strategies. International journal of molecular sciences, 23(1), 14. [CrossRef]
- Boutin, H., LeFeuvre, R. A., Horai, R., Asano, M., Iwakura, Y., & Rothwell, N. J. (2001). Role of IL-1α and IL-1β in ischemic brain damage. Journal of Neuroscience, 21(15), 5528-5534. [CrossRef]
- Smith, C. J., Emsley, H. C., Udeh, C. T., Vail, A., Hoadley, M. E., Rothwell, N. J., ... & Hopkins, S. J. (2012). Interleukin-1 receptor antagonist reverses stroke-associated peripheral immune suppression. Cytokine, 58(3), 384-389. [CrossRef]
- Pawluk, H., Woźniak, A., Grześk, G., Kołodziejska, R., Kozakiewicz, M., Kopkowska, E., ... & Kozera, G. (2020). The role of selected pro-inflammatory cytokines in pathogenesis of ischemic stroke. Clinical interventions in aging, 469-484. [CrossRef]
- Smith, C.J.; Hulme, S.; Vail, A.; Heal, C.; Parry-Jones, A.R.; Scarth, S.; Hopkins, K.; Hoadley, M.; Allan, S.M.; Rothwell, N.J.; et al.SCIL-STROKE (Subcutaneous interleukin-1 receptor antagonist in ischemic stroke): A randomized controlled phase 2 trial. Stroke 2018, 49, 1210–1216. [CrossRef]
- Emsley, H. C., Smith, C. J., Gavin, C. M., Georgiou, R. F., Vail, A., Barberan, E. M., ... & Hopkins, S. J. (2007). Clinical outcome following acute ischaemic stroke relates to both activation and autoregulatory inhibition of cytokine production. BMC neurology, 7(1), 1-12. [CrossRef]
- Stone, M. J., Hayward, J. A., Huang, C., E. Huma, Z., & Sanchez, J. (2017). Mechanisms of regulation of the chemokine-receptor network. International journal of molecular sciences, 18(2), 342. [CrossRef]
- Chiba, T., Itoh, T., Tabuchi, M., Nakazawa, T., & Satou, T. (2012). Interleukin-1β accelerates the onset of stroke in stroke-prone spontaneously hypertensive rats. Mediators of Inflammation, 2012. [CrossRef]
- Faura J, Bustamante A, Mir_o-Mur F, Montaner J (2021) Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. J Neuroinflammation 18: 1 – 14. [CrossRef]
- Krishnan S, O’Boyle C, Smith CJ, Hulme S, Allan SM, Grainger JR, Lawrence CB (2021) A hyperacute immune map of ischaemic stroke patients reveals alterations to circulating innate and adaptive cells. Clin Exp Immunol 203 458 – 471. [CrossRef]
- Planas AM, Gomez-Choco M, Urra X, Gorina R, Caballero M, Chamorro A (2012) Brain-derived antigens in lymphoid tissue of patients with acute stroke. J Immunol 188: 2156 – 2163. [CrossRef]
- Mai W and Liao Y (2020) Targeting IL-1b in the Treatment of Atherosclerosis. Front. Immunol. 11:589654. [CrossRef]
- Gomez D, Baylis RA, Durgin BG, Newman A, Alencar GF, Mahan S, et al. Interleukin-1beta has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat Med (2018) 24:1418–29. [CrossRef]
- Galea, J., Armstrong, J., Gadsdon, P., Holden, H., Francis, S. E., & Holt, C. M. (1996). Interleukin-1β in coronary arteries of patients with ischemic heart disease. Arteriosclerosis, thrombosis, and vascular biology, 16(8), 1000-1006. [CrossRef]
- Grebe, A., Hoss, F., & Latz, E. (2018). NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circulation research, 122(12), 1722-1740. [CrossRef]
- Roth S, Cao J, Singh V, Tiedt S, Hundeshagen G, Li T, Boehme JD, Chauhan D, Zhu J, Ricci A et al (2021a) Post-injury immunosuppression and secondary infections are caused by an AIM2 inflammasome-driven signaling cascade. Immunity 54: 648 – 659. [CrossRef]
- Georgakis MK, Bernhagen J, Heitman LH, Weber C, Dichgans M (2022) Targeting the CCL2–CCR2 axis for atheroprotection. Eur Heart J 43: 1799 – 1808. [CrossRef]
- Jiang C, Kong W, Wang Y, Ziai W, Yang Q, Zuo F, Li F, Wang Y, Xu H, Li Q et al (2017) Changes in the cellular immune system and circulating inflammatory markers of stroke patients. Oncotarget 8: 3553 – 3567. [CrossRef]
- Shimokawa, H., Ito, A., Fukumoto, Y., Kadokami, T., Nakaike, R., Sakata, M., ... & Takeshita, A. (1996). Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. The Journal of clinical investigation, 97(3), 769-776. [CrossRef]
- Chamberlain, J., Evans, D., King, A., Dewberry, R., Dower, S., Crossman, D., & Francis, S. (2006). Interleukin-1β and signaling of interleukin-1 in vascular wall and circulating cells modulates the extent of neointima formation in mice. The American journal of pathology, 168(4), 1396-1403. [CrossRef]
- Elhage, R., Maret, A., Pieraggi, M. T., Thiers, J. C., Arnal, J. F., & Bayard, F. (1998). Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E–deficient mice. Circulation, 97(3), 242-244. [CrossRef]
- Devlin, C. M., Kuriakose, G., Hirsch, E., & Tabas, I. (2002). Genetic alterations of IL-1 receptor antagonist in mice affect plasma cholesterol level and foam cell lesion size. Proceedings of the National Academy of Sciences, 99(9), 6280-6285. [CrossRef]
- Kirii, H., Niwa, T., Yamada, Y., Wada, H., Saito, K., Iwakura, Y., ... & Seishima, M. (2003). Lack of interleukin-1β decreases the severity of atherosclerosis in ApoE-deficient mice. Arteriosclerosis, thrombosis, and vascular biology, 23(4), 656-660. [CrossRef]
- Duewell, P., Kono, H., Rayner, K. J., Sirois, C. M., Vladimer, G., Bauernfeind, F. G., ... & Latz, E. (2010). NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature, 464(7293), 1357-1361. [CrossRef]
- Ridker, P. M. (2013). Closing the loop on inflammation and atherothrombosis: why perform the CIRT and CANTOS trials?. Transactions of the American Clinical and Climatological Association, 124, 174.
- Masson W, Lobo M, Molinero G, Masson G, Lavalle-Cobo A (2020) Role of colchicine in stroke prevention: an updated meta-analysis. J Stroke Cerebrovasc Dis 29: 104756. [CrossRef]
- Kelly P, Weimar C, Lemmens R, Murphy S, Purroy F, Arsovska A, Bornstein NM, Czlonkowska A, Fischer U, Fonseca AC et al (2021) Colchicine for prevention of vascular inflammation in Non-CardioEmbolic stroke (CONVINCE) – study protocol for a randomised controlled trial. Eur Stroke J 6: 222 – 228. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).