Submitted:
31 August 2023
Posted:
01 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. ihypBase helper plasmid doesn’t increase the transposition efficiencies in Ae. aegypti and D. suzukii embryos
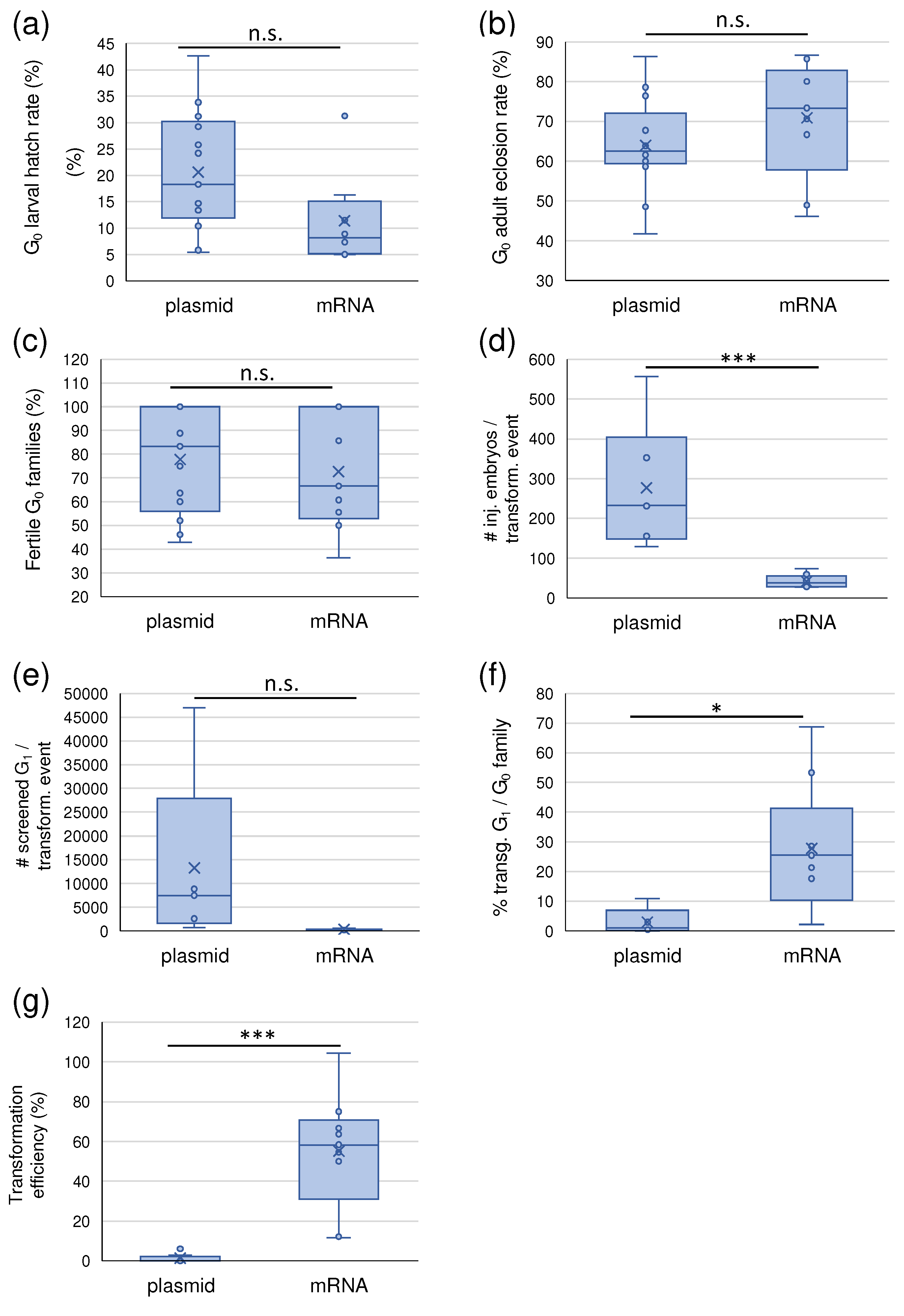
2.2. pBac mRNA boosts the transposition rate in Ae. aegypti
2.3. Preliminary data indicate high transposition rates with pBac mRNA in Ae. albopictus
2.2. Capped mRNA doesn’t improve Cre- or phiC31-RMCE efficiencies in Aedes.
3. Discussion
4. Materials and Methods
4.1. Insect rearing
4.2. In vitro transcription (IVT) of pBac, phiC31, and Cre mRNA for injections
4.2.1. Production of the IVT templates
4.2.2. In vitro transcription reaction and mRNA purification
4.3. Preparation of injection mixes
4.4. Embryonic microinjections of Ae. aegypti
4.5. Embryonic microinjections of Ae. albopictus
4.5.1. Preparation of donor plasmid
4.5.2. Injections for pBac-mediated transformation
4.5.3. Injections for recombinase-mediated cassette exchange
4.6. Embryonic microinjections of D. suzukii
4.7. Copy number variation (CNV) and linkage analysis of transgene integrations by droplet digital PCR
4.8. Analysis of genomic integration sites by inverse PCR
4.9. Transformation efficiency calculation
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bingham, P.M.; Kidwell, M.G.; Rubin, G.M. The molecular basis of P-M hybrid dysgenesis: the role of the P element, a P-strain-specific transposon family. Cell 1982, 29, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.M.; Spradling, A.C. Genetic transformation of Drosophila with transposable element vectors. Science 1982, 218, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.M.; Spradling, A.C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res 1983, 11, 6341–6351. [Google Scholar] [CrossRef] [PubMed]
- O'Brochta, D.A.; Atkinson, P.W. Transformation systems in insects. Methods MolBiol 2004, 260, 227–254. [Google Scholar]
- O'Brochta, D.A.; Sethuraman, N.; Wilson, R.; Hice, R.H.; Pinkerton, A.C.; Levesque, C.S.; et al. Gene vector and transposable element behavior in mosquitoes. J Exp Biol 2003, 206, 3823–3834. [Google Scholar] [CrossRef] [PubMed]
- Handler, A.M. Use of the piggyBac transposon for germ-line transformation of insects. Insect Biochem Mol Biol 2002, 32, 1211–1220. [Google Scholar] [CrossRef]
- Gregory, M.; Alphey, L.; Morrison, N.I.; Shimeld, S.M. Insect transformation with piggyBac: getting the number of injections just right. Insect Mol Biol 2016, 25, 259–271. [Google Scholar] [CrossRef]
- Allen, M.L.; Handler, A.M.; Berkebile, D.R.; Skoda, S.R. piggyBac transformation of the New World screwworm, Cochliomyia hominivorax, produces multiple distinct mutant strains. Medical and Veterinary Entomology 2004, 18, 1–9. [Google Scholar] [CrossRef]
- Handler, A.M.; Harrell, R.A. Germline transformation of Drosophila melanogaster with the piggyBac transposon vector. Insect Molecular Biology 1999, 8, 449–457. [Google Scholar] [CrossRef]
- Handler, A.M.; McCombs, S.D.; Fraser, M.J.; Saul, S.H. The lepidopteran transposon vector, piggyBac, mediates germ-line transformation in the Mediterranean fruit fly. Proceedings of the National Academy of Sciences, USA 1998, 95, 7520–7525. [Google Scholar] [CrossRef]
- Schetelig, M.F.; Handler, A.M. Germline transformation of the spotted wing drosophilid, Drosophila suzukii, with a piggyBac transposon vector. Genetica 2013, 141, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Kokoza, V.; Ahmed, A.; Wimmer, E.A.; Raikhel, A.S. Efficient transformation of the yellow fever mosquito Aedes aegypti using the piggyBac transposable element vector pBac[3xP3-EGFPafm]. Insect Biochem Mol Biol 2001, 31, 1137–1143. [Google Scholar] [CrossRef]
- Nolan, T.; Bower, T.M.; Brown, A.E.; Crisanti, A.; Catteruccia, F. piggyBac-mediated germline transformation of the malaria mosquito Anopheles stephensi using the red fluorescent protein dsRED as a selectable marker. Journal of Biological Chemistry 2002, 277, 8759–8762. [Google Scholar] [CrossRef] [PubMed]
- Labbé, G.M.; Nimmo, D.D.; Alphey, L. piggybac- and PhiC31-mediated genetic transformation of the Asian tiger mosquito, Aedes albopictus (Skuse). PLoS neglected tropical diseases 2010, 4, e788. [Google Scholar] [CrossRef] [PubMed]
- Perera, O.P.; Harrell, R.; Handler, A.M. Germ-line transformation of the South American malaria vector, Anopheles albimanus, with a piggyBac/EGFP transposon vector is routine and highly efficient. Insect Molecular Biology 2002, 11, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.G.; Oliveira, S.B.; Rocha, B.C.; Moreira, L.A. Germline transformation of Aedes fluviatilis (Diptera:Culicidae) with the piggyBac transposable element. Mem Inst Oswaldo Cruz 2006, 101, 755–757. [Google Scholar] [CrossRef]
- Grossman, G.L.; Rafferty, C.S.; Clayton, J.R.; Stevens, T.K.; Mukabayire, O.; Benedict, M.Q. Germline transformation of the malaria vector, Anopheles gambiae, with the piggyBac transposable element. Insect Molecular Biology 2001, 10, 597–604. [Google Scholar] [CrossRef]
- O'Brochta D.A.; Alford R.T.; Pilitt K.L.; Aluvihare C.U.; Harrell R.A.; 2nd. piggyBac transposon remobilization and enhancer detection in Anopheles mosquitoes. Proc Natl Acad Sci USA 2011, 108, 16339–16344. [CrossRef]
- O'Brochta D.A.; Pilitt K.L.; Harrell R.A.; 2nd, Aluvihare C.; Alford R.T. Gal4-based enhancer-trapping in the malaria mosquito Anopheles stephensi. G3 (Bethesda) 2012, 2, 1305–1315. [CrossRef]
- Thorpe, H.M.; Smith, M.C. In vitro site-specific integration of bacteriophage DNA catalyzed by a recombinase of the resolvase/invertase family. Proceedings of the National Academy of Sciences, USA 1998, 95, 5505–5510. [Google Scholar] [CrossRef]
- Siegal, M.L.; Hartl, D.L. Application of Cre/loxP in Drosophila. Site-specific recombination and transgene coplacement. Methods in Molecular Biology 2000, 136, 487–495. [Google Scholar]
- Andrews, B.J.; Beatty, L.G.; Sadowski, P.D. Site-specific recombination of the yeast plasmid two-micron circle: intermediates in the binding process. Basic Life Sciences 1986, 40, 407–424. [Google Scholar] [PubMed]
- Horn, C.; Handler, A.M. Site-specific genomic targeting in Drosophila. Proceedings of the National Academy of Sciences, USA 2005, 102, 12483–12488. [Google Scholar] [CrossRef] [PubMed]
- Schetelig, M.F.; Handler, A.M. A Functional Comparison of the 3xP3 Promoter by Recombinase-Mediated Cassette Exchange in Drosophila and a Tephritid Fly, Anastrepha suspensa. G3 (Bethesda) 2013, p. [Google Scholar] [CrossRef]
- Schetelig, M.F.; Scolari, F.; Handler, A.M.; Kittelmann, S.; Gasperi, G.; Wimmer, E.A. Site-specific recombination for the modification of transgenic strains of the Mediterranean fruit fly Ceratitis capitata. Proc Natl Acad Sci U S A 2009, 106, 18171–18176. [Google Scholar] [CrossRef] [PubMed]
- Schetelig, M.F.; Yan, Y.; Zhao, Y.; Handler, A.M. Genomic targeting by recombinase-mediated cassette exchange in the spotted wing drosophila, Drosophila suzukii. Insect Mol Biol 2019, 28, 187–195. [Google Scholar] [CrossRef]
- Haghighat-Khah, R.E.; Scaife, S.; Martins, S.; St John, O.; Matzen, K.J.; Morrison, N.; et al. Site-specific cassette exchange systems in the Aedes aegypti mosquito and the Plutella xylostella moth. PLoS One 2015, 10, e0121097. [Google Scholar] [CrossRef]
- Amenya, D.A.; Bonizzoni, M.; Isaacs, A.T.; Jasinskiene, N.; Chen, H.; Marinotti, O.; et al. Comparative fitness assessment of Anopheles stephensi transgenic lines receptive to site-specific integration. Insect Mol Biol 2010, 19, 263–269. [Google Scholar] [CrossRef]
- Meredith, J.M.; Basu, S.; Nimmo, D.D.; Larget-Thiery, I.; Warr, E.L.; Underhill, A.; et al. Site-specific integration and expression of an anti-malarial gene in transgenic Anopheles gambiae significantly reduces Plasmodium infections. PLoS One 2011, 6, e14587. [Google Scholar] [CrossRef]
- Lobo, N.F.; Hua-Van, A.; Li, X.; Nolen, B.M.; Fraser, M.J., Jr. Germ line transformation of the yellow fever mosquito, Aedes aegypti, mediated by transpositional insertion of a piggyBac vector. Insect Mol Biol 2002, 11, 133–139. [Google Scholar] [CrossRef]
- Adelman, Z.N.; Jasinskiene, N.; Vally, K.J.; Peek, C.; Travanty, E.A.; Olson, K.E.; et al. Formation and loss of large, unstable tandem arrays of the piggyBac transposable element in the yellow fever mosquito, Aedes aegypti. Transgenic Res 2004, 13, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Yusa, K.; Zhou, L.; Li, M.A.; Bradley, A.; Craig, N.L. A hyperactive piggyBac transposase for mammalian applications. Proc Natl Acad Sci USA 2011, 108, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.A.; Smith, R.C.; Li, X.; Craig, N.L.; Atkinson, P.W. IPB7 transposase behavior in Drosophila melanogaster and Aedes aegypti. Insect Biochem Mol Biol 2013, 43, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Eckermann, K.N.; Ahmed, H.M.M.; KaramiNejadRanjbar, M.; Dippel, S.; Ogaugwu, C.E.; Kitzmann, P.; et al. Hyperactive piggyBac transposase improves transformation efficiency in diverse insect species. Insect Biochem Mol Biol 2018, 98, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Häcker, I.; Harrell II, R.A.; Eichner, G.; Pilitt, K.L.; O'Brochta, D.A.; Handler, A.M.; et al. Cre/lox-recombinase-mediated cassette exchange for reversible site-specific genomic targeting of the disease vector, Aedes aegypti. Sci Rep 2017, 7, 43883. [Google Scholar] [CrossRef]
- Schwirz, J.; Yan, Y.; Franta, Z.; Schetelig, M.F. Bicistronic expression and differential localization of proteins in insect cells and Drosophila suzukii using picornaviral 2A peptides. Insect Biochem Mol Biol 2020, 119, 103324. [Google Scholar] [CrossRef]
- Schetelig, M.F.; Schwirz, J.; Yan, Y. A transgenic female killing system for the genetic control of Drosophila suzukii. Sci Rep 2021, 11, 12938. [Google Scholar] [CrossRef]
- Yan, Y.; Schwirz, J.; Schetelig, M.F. Characterization of the Drosophila suzukii beta2-tubulin gene and the utilization of its promoter to monitor sex separation and insemination. Gene 2021, 771, 145366. [Google Scholar] [CrossRef]
- Yan, Y.; Kobayashi, Y.; Huang, C.; Liu, B.; Qian, W.; Wan, F.; et al. Highly Efficient Temperature Inducible CRISPR-Cas9 Gene Targeting in Drosophila suzukii. Int J Mol Sci 2021, 22, p. [Google Scholar] [CrossRef]
- Jasinskiene, N.; Coates, C.J.; Ashikyan, A.; James, A.A. High efficiency, site-specific excision of a marker gene by the phage P1 cre-loxP system in the yellow fever mosquito, Aedes aegypti. Nucleic Acids Res 2003, 31, e147. [Google Scholar] [CrossRef]
- Ahmed, H.M.M.; Heese, F.; Wimmer, E.A. Improvement on the genetic engineering of an invasive agricultural pest insect, the cherry vinegar fly, Drosophila suzukii. BMC Genet 2020, 21, 139. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, E.A. Insect transgenesis by site-specific recombination. Nature Methods 2005, 2, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.R.; Mitchell, C.J. Genetic selection of a flavivirus-refractory strain of the yellow fever mosquito Aedes aegypti. Am J Trop Med Hyg 1991, 45, 399–407. [Google Scholar] [CrossRef]
- Batz, Z.A.; Clemento, A.J.; Fritzenwanker, J.; Ring, T.J.; Garza, J.C.; Armbruster, P.A. Rapid adaptive evolution of the diapause program during range expansion of an invasive mosquito. Evolution 2020, 74, 1451–1465. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, P.; Hutchinson, R.A.; Linvell, T. Equivalent inbreeding depression under laboratory and field conditions in a tree-hole-breeding mosquito. Proc Biol Sci 2000, 267, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
| Exp. no | helper template | [helper/ donor] (ng/µl) | donor construct | donor plasmid (insert) size (bp) | no. injected embryos | larval hatch rate (%) | adult eclosion rate (%) | total no. G0 families | % fertile G0 families | no. transg. G0 families | total no. G1 screened | no. transg. events | transf. eff. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | pBac | 300/150 | AH452 * | (5267) 8649 | 623 | 29.21 | 78.57 | 8 | 100.00 | 4 | n.d. | 4 | 2.80 |
| 2 | pBac | 200/500 | V3 | (3545) 6911 | 234 | 31.20 | 86.30 | 25 | 52.00 | 1 | 8830 | 1 | 1.25 |
| 3 | pBac | 400/600 | V285 | (5180) 8682 | 705 | 25.82 | 64.29 | 18 | 88.89 | 2 | 14966 | 2 | 1.71 |
| 4 | pBac | 300/500 | V286 | (5600) 9102 | 680 | 13.38 | 41.76 | 15 | 60.00 | 0 | 8772 | 0 | 0.00 |
| 5 | pBac | 160/185 | V258 | (6190) 9690 | 922 | 10.41 | 37.50 | 18 | 83.33 | 0 | 6631 | 0 | 0.00 |
| 6 | pBac | 400/600; 228/342 | V258 | (6190) 9690 | 599 | 24.21 | 34.48 | 11 | 100.00 | 0 | 18721 | 0 | 0.00 |
| 7 | pBac | 300/150 | V257 | (7088) 10587 | 1113 | 42.59 | 76.37 | 26 | 100.00 | 2 | 93998 | 2 | 0.55 |
| 8 | pBac | 200/500 | V19 | (3630) 7163 | 257 | 18.29 | 63.83 | 9 | 100.00 | 2 | 5185 | 2 | 6.67 |
| 9 | pBac | 300/500 | V19 | (3630) 7163 | 192 | 33.85 | 61.54 | 11 | 63.64 | 0 | 2679 | 0 | 0.00 |
| 10 | pBac | 200/500 | V19 | (3630) 7163 | 399 | 13.53 | 61.11 | 13 | 46.15 | 0 | 1313 | 0 | 0.00 |
| 11 | pBac | 200/500 | V19 | (3630) 7163 | 462 | 14.72 | 48.53 | 7 | 100.00 | 2 | 1389 | 2 | 6.06 |
| 12 | pBac | 300/300 | V368 | (5835) 11097 | 257 | 5.84 | 60.00 | 7 | 42.86 | 0 | 502 | 0 | 0.00 |
| 13 | pBac | 100/300 | V368 | (5835) 11097 | 148 | 5.41 | 62.50 | 4 | 75.00 | 0 | 382 | 0 | 0.00 |
| avg | 1.46 | ||||||||||||
| 14 | HypB | 100/200 | V286 | (5600) 9102 | 594 | 8.08 | 75.00 | 12 | 75.00 | 0 | 9382 | 0 | 0.00 |
| 15 | HypB | 200/200 | V286 | (5600) 9102 | 730 | 8.63 | 79.37 | 17 | 70.59 | 0 | 7604 | 0 | 0.00 |
| 16 | HypB | 200/200 | V258 | (6190) 9690 | 895 | 6.82 | 65.57 | 13 | 69.23 | 0 | 5199 | 0 | 0.00 |
| avg | 0.00 |
| Exp. no. | helper template | [helper/ donor] (ng/µl) | donor construct | donor plasmid (insert) size (bp) | no. injected eggs | no. hatched larvae | hatch rate (%) | no. fertile adults | fertile eclosion rate (%) | no. transg. lines | transf. eff. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ds 1 | pBac | 200/500 | AH443 | (9191) 12576 | 75 | n. d. | n. d. | 25 | n.d. | 4 | 16.00 |
| Ds 2 | pBac | 200/500 | V220 | (7865) 11249 | 1601 | 231 | 14.43 | 65 | 28.14 | 1 | 1.54 |
| Ds 3 | pBac | 200/500 | V221 | (7867) 11252 | 443 | 102 | 23.02 | 9 | 8.82 | 0 | 0.00 |
| Ds 4 | pBac | 200/500 | V146 | (7118) 10503 | 481 | 173 | 35.97 | 20 | 11.56 | 2 | 10.00 |
| Ds 5 | pBac | 200/500 | V183 | (7583) 10968 | 753 | 305 | 40.50 | 43 | 14.10 | 5 | 11.63 |
| Ds 6 | pBac | 200/500 | V184 | (9438) 12823 | 640 | 167 | 26.09 | 17 | 10.18 | 0 | 0.00 |
| Ds 7 | pBac | 200/500 | V185 | (8493) 11878 | 802 | 346 | 43.14 | 53 | 15.32 | 6 | 11.32 |
| Ds 8 | pBac | 200/500 | V188 | (10347) 13732 | 631 | 285 | 45.17 | 55 | 19.30 | 1 | 1.82 |
| Ds 9 | pBac | 200/500 | V213 | (10059) 13443 | 538 | 107 | 19.89 | 27 | 25.23 | 0 | 0.00 |
| Ds 10 | pBac | 200/500 | V215 | (8204) 11589 | 626 | 173 | 27.64 | 29 | 16.76 | 1 | 3.45 |
| Ds 11 | pBac | 200/500 | V226 | (8163) 12054 | 410 | 51 | 12.44 | 9 | 17.65 | 1 | 11.11 |
| Ds 12 | pBac | 200/500 | V227 | (10018) 13909 | 493 | 122 | 24.75 | 12 | 9.84 | 1 | 8.33 |
| Ds 13 | pBac | 200/500 | V228 | (8607) 12498 | 310 | 97 | 31.29 | 13 | 13.40 | 0 | 0.00 |
| Ds 14 | pBac | 200/500 | V250 | (9072) 12963 | 378 | 129 | 34.13 | 28 | 21.71 | 0 | 0.00 |
| Ds 15 | pBac | 200/500 | V229 | (10434) 14325 | 339 | 92 | 27.14 | 26 | 28.26 | 1 | 3.85 |
| Ds 16 | pBac | 200/500 | V251 | (10927) 14818 | 413 | 70 | 16.95 | 10 | 14.29 | 0 | 0.00 |
| Ds 17 | pBac | 200/500 | V265 | (9952) 13337 | 520 | 124 | 23.85 | 32 | 25.81 | 0 | 0.00 |
| avg | 4.65 | ||||||||||
| Ds 18 | pBac | 300/700 | V265 | (9952) 13337 | 582 | 238 | 40.89 | 77 | 32.35 | 5 | 6.49 |
| Ds 19 | pBac + hyPB | 200+200/500 | V221 | (7867) 11252 | 1457 | 271 | 18.60 | 42 | 15.50 | 1 | 2.38 |
| Ds 20 | pBac + hyPB | 200+200/500 | V222 | (7861) 11246 | 1898 | 401 | 21.13 | 34 | 8.48 | 0 | 0.00 |
| Ds 21 | pBac + hyPB | 200+200/500 | V223 | (7865) 11249 | 488 | 82 | 16.80 | 30 | 36.59 | 2 | 6.67 |
| avg | 3.02 | ||||||||||
| Ds 22 | hyPB | 200/500 | V209 | (5538) 8922 | 1081 | 252 | 23.31 | 37 | 14.68 | 2 | 5.41 |
| Ds 23 | hyPB | 200/500 | V265 | (9952) 13337 | 98 | 32 | 32.65 | 8 | 25.00 | 0 | 0.00 |
| avg | 2.70 |
| Exp. no | helper template | [helper/ donor] (ng/µl) | donor construct | donor plasmid (insert) size (bp) | no. injected embryos | hatch rate (%) | eclosion rate (%) | total no. G0 families | no. fertile G0 families | no. transg. G0 families | total no. G1 screened | no. transg. events * | no. transg. events/ G0 | transf. eff. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | plasmid | 300/150 | AH452 *** | (5267) 8649 | 623 | 29.21 | 78.57 | 8 | 8 | 4 | n.d. | 4 | 1 | 2.80 |
| 2 | plasmid | 200/500 | V3 | (3545) 6911 | 234 | 31.20 | 86.30 | 25 | 13 | 1 | 8830 | 1 | 1 | 1.59 |
| 3 | plasmid | 400/600 | V285 | (5180) 8682 | 705 | 25.82 | 64.29 | 18 | 16 | 2 | 14966 | 2 | 1 | 1.71 |
| 4 | plasmid | 300/500 | V286 | (5600) 9102 | 680 | 13.38 | 41.76 | 15 | 9 | 0 | 8772 | 0 | n.a. | 0.00 |
| 5 | plasmid | 160/185 | V258 | (6190) 9690 | 922 | 10.41 | 67.71 | 18 | 15 | 0 | 6631 | 0 | n.a. | 0.00 |
| 6 | plasmid | 400/600; 228/342 | V258 | (6190) 9690 | 599 | 24.21 | 58.62 | 11 | 11 | 0 | 18721 | 0 | n.a. | 0.00 |
| 7 | plasmid | 300/150 | V257 | (7088) 10587 | 1113 | 42.59 | 76.37 | 26 | 26 | 2 | 93998 | 2 | n.d. | 0.55 |
| 8 | plasmid | 200/500 | V19 | (3630) 7163 | 257 | 18.29 | 63.83 | 9 | 9 | 2 | 5185 | 2 | 1 | 6.67 |
| 9 | plasmid | 300/500 | V19 | (3630) 7163 | 192 | 33.85 | 61.54 | 11 | 7 | 0 | 2679 | 0 | n.a. | 0.00 |
| 10 | plasmid | 200/500 | V19 | (3630) 7163 | 399 | 13.53 | 61.11 | 13 | 6 | 0 | 1313 | 0 | n.a. | 0.00 |
| 11 | plasmid | 200/500 | V19 | (3630) 7163 | 462 | 14.72 | 48.53 | 7 | 7 | 2 | 1389 | 2 | 1 | 6.06 |
| 12 | plasmid | 300/300 | V368 | (5835) 11097 | 257 | 5.84 | 60.00 | 7 | 3 | 0 | 502 | 0 | n.a. | 0.00 |
| 13 | plasmid | 100/300 | V368 | (5835) 11097 | 148 | 5.41 | 62.50 | 4 | 3 | 0 | 382 | 0 | n.a. | 0.00 |
| avg | 1.49 | |||||||||||||
| 14 | mRNA | 182/300 | V96 | (5278) 8813 | 113 | 11.50 | 46.15 | 6 | 3 | 1 | 370 | 4 | 4 | 66.67 |
| 15 | mRNA | 182/300 | V97 | (4307) 7841 | 298 | 5.03 | 73.33 | 11 | 4 | 2 | 514 | 7 | ≥ 2 - ≥5 | 63.64 |
| 16 | mRNA | 300/300 | V370 | (5870) 11131 | 576 | 31.25 | 86.67 | 12 | 12 | 11 | 4388 | > 19 | ≥1 | 12.18 |
| 17 | mRNA | 300/300 | V369 | (6420) 11684 | 520 | 16.35 | 80.00 | 6 | 6 | 5 | 2173 | > 8 | ≥1 | 11.76 |
| 18 | mRNA | 300/300 | V19 | (3630) 7163 | 527 | 8.92 | 48.94 | 28 | 17 | 10 | 1813 | ≥ 24 | 1 - ≥5 | 104.35 ** |
| 19 | mRNA | 100/300 | V19 | (3630) 7163 | 310 | 5.48 | 70.59 | 9 | 6 | 2 | 1195 | ≥ 7 | ≥3 - ≥4 | 58.33 |
| 20 | mRNA | 100/300 | V19 | (3630) 7163 | 646 | 1.61 | 80.00 | 7 | 7 | 3 | 1030 | 4 | 1 - 2 | 50.00 |
| 21 | mRNA | 300/300 | V368 | (5835) 11097 | 449 | 7.35 | 66.67 | 18 | 10 | 5 | 1014 | ≥ 12 | ≥2 - 4 | 54.55 |
| 22 | mRNA | 100/300 | V368 | (5835) 11097 | 281 | 4.98 | 85.71 | 7 | 6 | 5 | 870 | ≥ 9 | 1 - ≥3 | 75.00 |
| avg | 49.02 |
| Exp. no | helper template | [helper/ donor] (ng/µl) | donor construct | donor plasmid (insert) size (bp | no. injected embryos | hatch rate (%) | eclosion rate (%) | total no. G0 families | no. fertile G0 families | no. transg. G0 families | total G1 screened | no. transg events | no. transg. events/ G0 | transf. eff. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | mRNA | 300/150 | AH452 modified * | (5205) 7845 | 721 | 39.53 | 83.51 | 19 | 19 | 15 | 7049 | ≥ 15 | ≥ 1 | 6.3 % |
| Exp. no | helper template | [helper/ donor] (ng/µl) | donor construct | donor plasmid (insert) size (bp | no. injected embryos | hatch rate (%) | eclosion rate (%) | total no. G0 families | no. fertile G0 families | no. transg. G0 families | total G1 screened | no. transg. events | recomb. eff. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | phsp-phiC31 | 150/300 | V101 | (4404) 8216 | 510 | 3.14 | 75.00 | 9 | 6 | 0 | 1690 | 0 | 0 |
| 2 | phsp-phiC31 | 150/300 | V101 | (4404) 8216 | 237 | 7.59 | 61.11 | 5 | 5 | 0 | 2851 | 0 | 0 |
| 3 | mRNA | 150/300 | V101 | (4404) 8216 | 502 | 4.38 | 59.09 | 11 | 4 | 0 | 534 | 0 | 0 |
| 4 | mRNA | 150/300 | V101 | (4404) 8216 | 335 | 3.28 | 54.55 | 4 | 2 | 0 | 458 | 0 | 0 |
| 5 | mRNA | 150/300 | V101 | (4404) 8216 | 361 | 3.32 | 83.33 | 7 | 4 | 1 * | 318 | 1 | 10 |
| 6 | phsp-phiC31 | 300/500 | V101 | (4404) 8216 | 443 | 2.71 | 91.67 | 9 | 4 | 0 | 1059 | 0 | 0 |
| 7 | phsp-phiC31 | 300/500 | V101 | (4404) 8216 | 302 | 13.25 | 77.50 | 11 | 9 | 2 | 6068 | 2 | 6.45 |
| 8 | mRNA | 300/500 | V101 | (4404) 8216 | 412 | 2.18 | 100.00 | 9 | 9 | 0 | 6250 | 0 | 0 |
| 9 | mRNA | 300/500 | V101 | (4404) 8216 | 248 | 13.71 | 73.53 | 10 | 6 | 0 ** | 3650 | 0 | 0 |
| Exp. no | helper template | [helper/ donor] (ng/µl) | donor construct | donor plasmid (insert) size (bp | no. injected embryos | hatch rate (%) | eclosion rate (%) | total no. G0 families | no. fertile G0 families | no. transg. G0 families | total G1 screened | no. transg. events | recomb. eff. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | phsp-Cre | 450/350 | V20 | (1297) 4997 | 765 | 18.69 | 57.34 | 13 | 12 | 2 * | 9283 | 2 | 2.44 |
| 2 | phsp-Cre | 450/350 | V20 | (1297) 4997 | 165 | 6.67 | 72.73 | 4 | 1 | 0 | 390 | 0 | 0 |
| 3 | phsp-Cre | 450/350 | V20 | (1297) 4997 | 397 | 1.26 | 80.00 | 3 | 1 | 0 | 137 | 0 | 0 |
| 4 | mRNA | 450/350 | V20 | (1297) 4997 | 413 | 10.90 | 73.33 | 4 | 1 | 1 ** | 2419 | 1 | 3.03 |
| 5 | mRNA | 450/350 | V20 | (1297) 4997 | 230 | 5.22 | 58.33 | 3 | 3 | 0 | 996 | 0 | 0 |
| 6 | mRNA | 450/350 | V20 | (1297) 4997 | 348 | 5.75 | 80.00 | 12 | 9 | 0 | 4926 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





