Submitted:
30 August 2023
Posted:
01 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
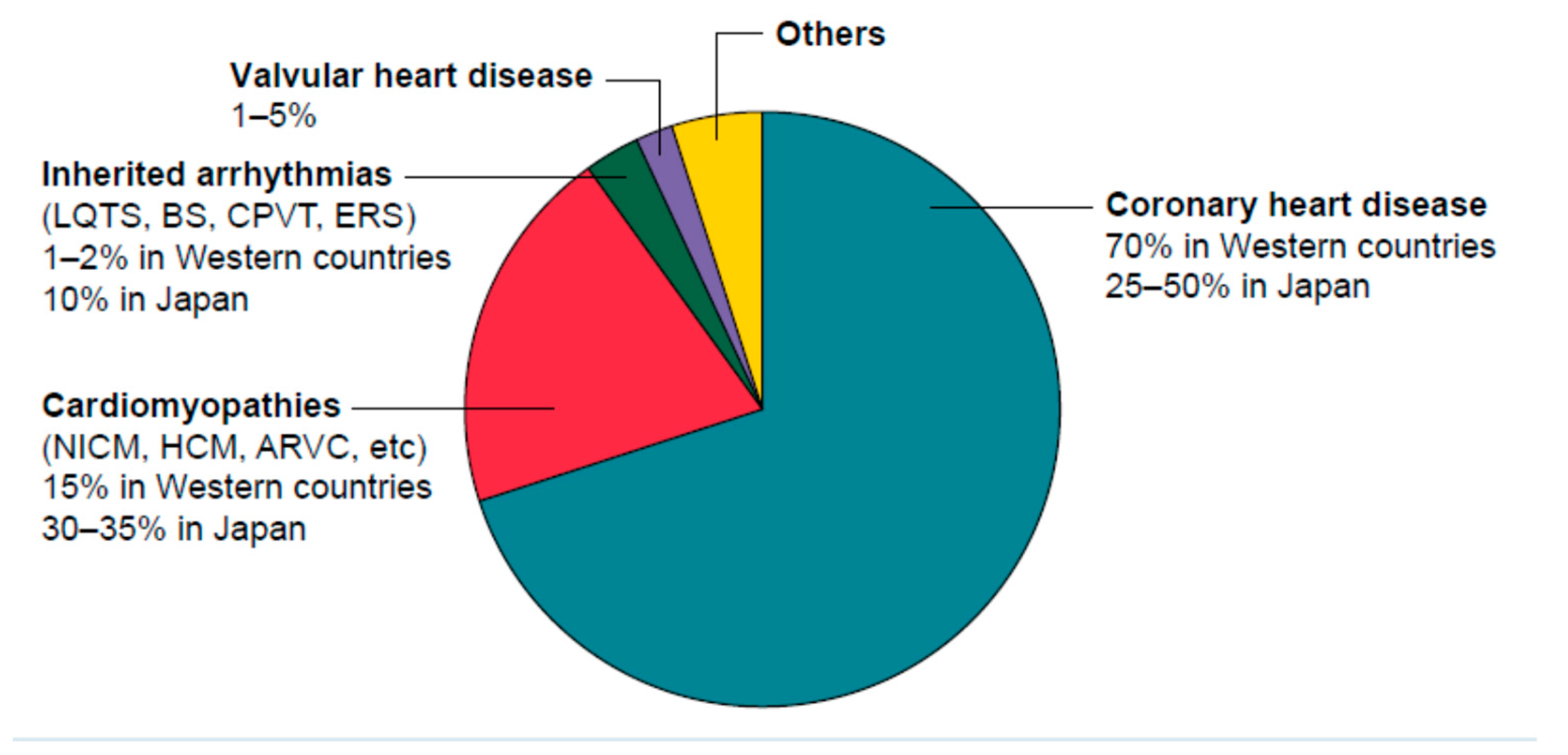
2. Myocardial fibrosis: the common arrhythmia initiation substrate in organic heart disease.
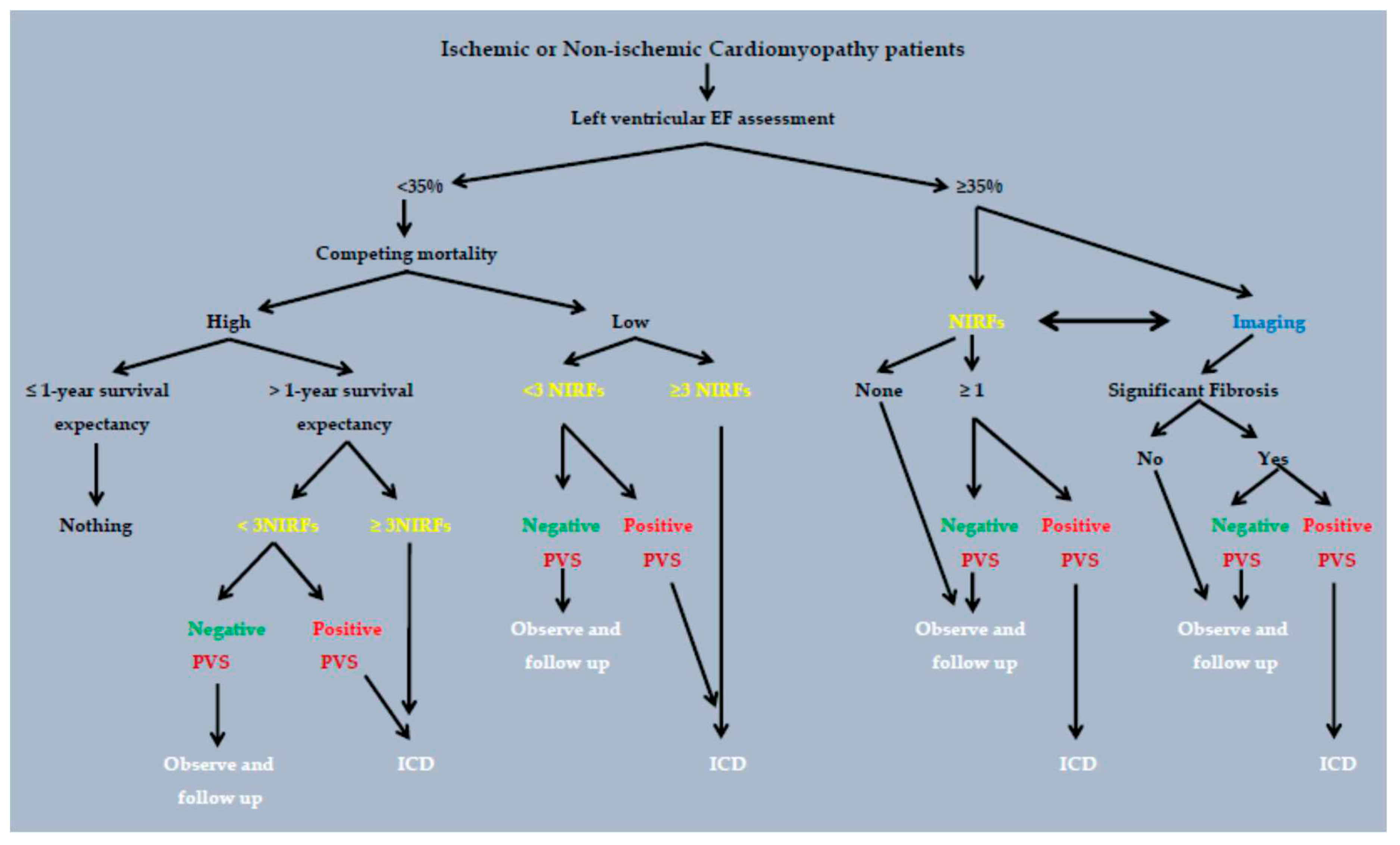
3. The two step approach: Non-invasive risk factors (NIRFs) guiding to programmed ventricular stimulation in electrophysiology study.
| Study | n | EF | Therapy | NIRFs | NIRFS+ | EPS(n) | EPS + | Follow up (m) |
End Points (n) |
Sens/Spec/PPV/NPV |
|---|---|---|---|---|---|---|---|---|---|---|
| Pedretti, [17], 1993 |
303 Post- AMI |
<40% | Thromb | EF<40% SAECG VPCs |
(≥2)* 67/303 |
47/67 | 20/47 | 17 | 19 | Sens = 81% Spec = 97% PPV = 65% NPV= 99% |
| Zoni Berisso [18], 1996 |
286 Post-AMI |
≤40% | Thromb (46%) | EF<40% SAECG VPCs NSVT |
(≥1) * | 103/286 | 16 /103 | 12 | 10 | Sens = 55% Spec = 99% PPV = 67% |
| Andersen [19], 1999 |
657 Post- AMI |
47% | Thromb | EF<40% VPCs NSVT | (≥1)* 304/657 | 146/304 | 22/146 | 37 | 24 | Sens = 44% Spec = 86% PPV = 18% NPV= 96% |
| Schmidt [20], 2001 |
1436 Post- AMI |
32% | ThrombAngiopl | EF SAECG VPCs HRV |
(≥3) * 248/1436 |
98/248 | 21/98 | 19 | 7/21 | Sens = 78% Spec = 84% PPV = 33% NPV= 97% |
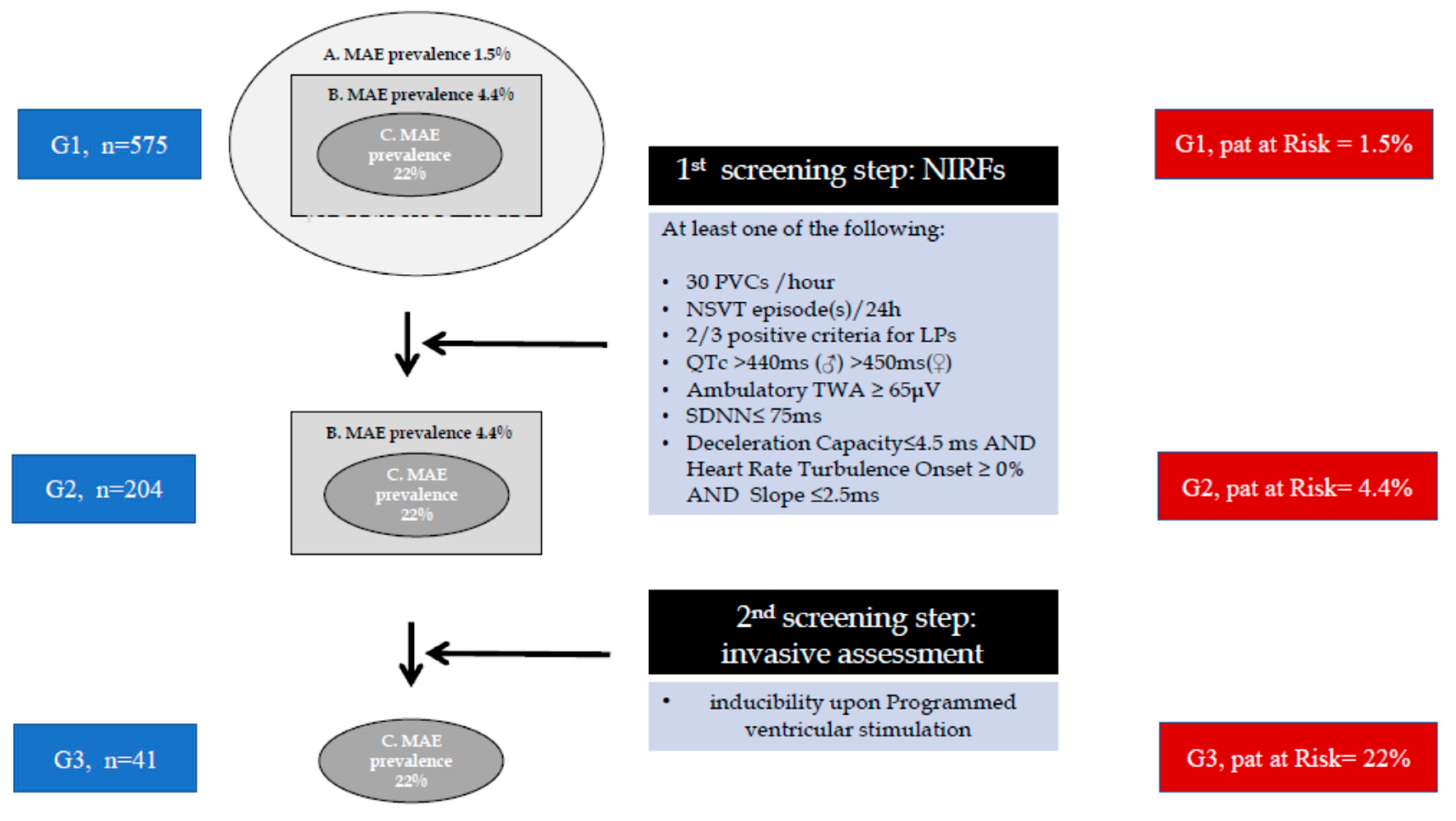
| Gatzoulis PRESERVE-EF [10], 2019 |
575 Post- AMI |
≥40% | Angiopl CABG |
VPCs NSVT SAECG QTc TWA DC+HRT HRV |
(≥1) * 204/575 |
152/204 | 41/152 | 32 | 9 | Sens = 100% Spec = 93% PPV = 22% NPV= 100% |
4. PRESERVE EF study, in 2019, restores the two-step arrhythmic risk stratification approach in clinical practice.
| NIRF | Prevalence in the total Preserve-EF (n=577) | Prevalence in the High arrhythmic risk group (n=41) | Prevalence in 9 MAE/SCD patients |
|---|---|---|---|
| LPs (%) | 13.8 | 51.2 | 78 (7/9) |
| NSVT (%) | 8.6 | 46.3 | 66 (6/9) |
| QTc (%) | 13.6 | 36.6 | 55 (5/9 |
| VPBs (%) | 10.8 | 39 | 33 (3/9) |
| TWA (%) | 6.8 | 24.4 | 11 (1/9) |
| SDNN (%) | 2.8 | 9.8 | 0 (0/9) |
| HRT&DC (%) | 2.8 | 9.8 | 0 (0/9) |
5. Two-step approach, stratifies the SCD risk, in heart failure with mid range ejection fraction, in non-ischemic and in hypertrophic cardiomyopathy patients.
6. Current status.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Straus, S.M.; Bleumink, G.S.; Dieleman, J.P.; van der Lei, J.; Stricker, B.H.; Sturkenboom, M.C. The incidence of sudden cardiac death in the general population. J Clin Epidemiol. 2004, 57, 98–102. [Google Scholar] [CrossRef]
- Chugh, S.S.; Reinier, K.; Teodorescu, C.; Evanado, A.; Kehr, E.; Al Samara, M.; Mariani, R.; Gunson, K.; Jui, J. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008, 51, 213–228. [Google Scholar] [CrossRef]
- Wong, C.X.; Brown, A.; Lau, D.H.; Chugh, S.S.; Albert, C.M.; Kalman, J.M.; Sanders, P. Epidemiology of Sudden Cardiac Death: Global and Regional Perspectives. Heart Lung Circ. 2019, 28, 6–14. [Google Scholar] [CrossRef]
- Ikeda, T.; Yoshino, H.; Sugi, K.; Tanno, K.; Shimizu, H.; Watanabe, J.; Kasamaki, Y.; Yoshida, A.; Kato, T. Predictive value of microvolt T-wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction: results of a collaborative cohort study. J Am Coll Cardiol. 2006, 48, 2268–2274. [Google Scholar] [CrossRef]
- Moss, A.J.; Zareba, W.; Hall, W.J.; Klein, H.; Wilber, D.; J. ; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Brown, M.W.; Andrews, M.L. Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002, 346, 877–883. [Google Scholar] [CrossRef]
- Kadish, A.; Dyer, A.; Daubert, J.P.; Quigg, R.; Estes, N.A.; Anderson, K.P.; Calkins, H.; Hoch, D.; Goldberger, J.; Shalaby, A.; Sanders, W.E.; Schaechter, A.; Levine, J.H. Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004, 350, 2151–2158. [Google Scholar] [CrossRef]
- Elliott, P.M.; Gimeno, J.R.; Thaman, R.; Shah, J.; Ward, D.; Dickie, S.; Tome Esteban, M.T.; McKenna, W.J. Historical trends in reported survival rates in patients with hypertrophic cardiomyopathy. Heart 2006, 92, 785–791. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; Eckardt, L.; Friede, T.; Haugaa, K.H.; Hocini, M.; Lambiase, P.D.; Marijon, E.; Merino, J.L.; Peichl, P.; Priori, S.G.; Reichlin, T.; Schulz-Menger, J.; Sticherling, C.; Tzeis, S.; Verstrael, A.; Volterrani, M. ESC Scientific Document Group. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart, J. 2022, ahead of print. [CrossRef]
- Arsenos, P.; Gatzoulis, K.A.; Tsiachris, D.; Dilaveris, P.; Sideris, S.; Sotiropoulos, I.; Archontakis, S.; Antoniou, C.K.; Kordalis, A.; Skiadas, I.; Toutouzas, K.; Vlachopoulos, C.; Tousoulis, D.; Tsioufis, K. Arrhythmic risk stratification in ischemic, non-ischemic and hypertrophic cardiomyopathy: A two-step multifactorial, electrophysiology study inclusive approach. World J Cardiol. 2022, 14, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Gatzoulis, K.A.; Tsiachris, D.; Arsenos, P.; Antoniou, C.K.; Dilaveris, P.; Sideris, S.; Kanoupakis, E.; Simantirakis, E.; Korantzopoulos, P.; Goudevenos, I.; Flevari, P.; Iliodromitis, E.; Sideris, A.; Vassilikos, V.; Fragakis, N.; Trachanas, K.; Vernardos, M.; Konstantinou, I.; Tsimos, K.; Xenogiannis, I.; Vlachos, K.; Saplaouras, A.; Triantafyllou, K.; Kallikazaros, I.; Tousoulis, D. Arrhythmic risk stratification in post-myocardial infarction patients with preserved ejection fraction: the PRESERVE EF study. Eur Heart, J. 2019, 40, 2940–2949. [Google Scholar] [CrossRef] [PubMed]
- Disertori, M.; Masè, M.; Ravelli, F. Myocardial fibrosis predicts ventricular tachyarrhythmias. Trends Cardiovasc Med. 2017, 27, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Kariki, O.; Antoniou, C.K.; Mavrogeni, S.; Gatzoulis, K.A. Updating the Risk Stratification for Sudden Cardiac Death in Cardiomyopathies: The Evolving Role of Cardiac Magnetic Resonance Imaging. An Approach for the Electrophysiologist. Diagnostics (Basel). 2020, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Disertori, M.; Rigoni, M.; Pace, N.; Casolo, G.; Masè, M.; Gonzini, L.; Lucci, D.; Nollo, G.; Ravelli, F. Myocardial fibrosis assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction. A meta-analysis. J Am Coll Cardiol Img. 2016, 9, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Gatzoulis, K.A.; Carlson, M.D.; Biblo, L.A.; Rizos, I.; Gialafos, J.; Toutouzas, P.; Waldo, A.L. ; Time domain analysis of the signal averaged electrocardiogram in patients with a conduction defect or a bundle branch block. Eur Heart, J. 1995, 16, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Gatzoulis, K.A.; Arsenos, P.; Trachanas, K.; Dilaveris, P.; Antoniou, C.; Tsiachris, D.; Sideris, S.; Kolettis, T.M.; Tousoulis, D. Signal-averaged electrocardiography: Past, present, and future. J Arrhythm. 2018, 34, 222–229. [Google Scholar] [CrossRef]
- Arsenos, P.; Gatzoulis, K.; Dilaveris, P.; Manis, G.; Tsiachris, D.; Archontakis, S.; Vouliotis, AI.; Sideris, S.; Stefanadis, C. Arrhythmic sudden cardiac death: substrate, mechanisms and current risk stratification strategies for the post-myocardial infarction patient. Hellenic J Cardiol. 2015, 54, 301–315. [Google Scholar]
- Pedretti, R.; Etro, M.D.; Laporta, A.; Sarzi Braga, S.; Carù, B. Prediction of late arrhythmic events after acute myocardial infarction from combined use of noninvasive prognostic variables and inducibility of sustained monomorphic ventricular tachycardia. Am J Cardiol. 1993, 71, 1131–1141. [Google Scholar] [CrossRef]
- Zoni-Berisso, M.; Molini, D.; Mela, G.S.; Vecchio, C. Value of programmed ventricular stimulation in predicting sudden death and sustained ventricular tachycardia in survivors of acute myocardial infarction. Am J Cardiol. 1996, 77, 673–680. [Google Scholar] [CrossRef]
- Andresen, D.; Steinbeck, G.; Brüggemann, T.; Müller, D.; Haberl, R.; Behrens, S.; Hoffmann, E.; Wegscheider, K.; Dissmann, R.; Ehlers, H.C. Risk stratification following myocardial infarction in the thrombolytic era: a two-step strategy using noninvasive and invasive methods. J Am Coll Cardiol. 1999, 33, 131–138. [Google Scholar] [CrossRef]
- Schmitt, C.; Barthel, P.; Ndrepepa, G.; Schreieck, J.; Plewan, A.; Schömig, A.; Schmidt, G. Value of programmed ventricular stimulation for prophylactic internal cardioverter-defibrillator implantation in postinfarction patients preselected by noninvasive risk stratifiers. J Am Coll Cardiol. 2001, 37, 1901–1907. [Google Scholar] [CrossRef]
- Arsenos, P.; Gatzoulis, K.A.; Dilaveris, P.; Gialernios, T.; Sideris, S.; Lazaros, G.; Archontakis, S.; Tsiachris, D.; Kartsagoulis, E.; Stefanadis, C. The rate-corrected QT interval calculated from 24-hour Holter recordings may serve as a significant arrhythmia risk stratifier in heart failure patients. Int J Cardiol. 2011, 147, 321–323. [Google Scholar] [CrossRef]
- Verrier, R.L.; Nearing, B.D.; La Rovere, M.T.; Pinna, G.D.; Mittleman, M.A.; Bigger, J.T. Jr; Schwartz, P.J. ATRAMI Investigators. Ambulatory electrocardiogram-based tracking of T wave alternans in post myocardial infarction patients to assess risk of cardiac arrest or arrhythmic death. J Cardiovasc Electrophysiol. 2003, 14, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, A.P.; Zuanetti, G.; Franzosi, M.G.; Rovelli, F.; Santoro, E.; Staszewsky, L.; Tavazzi, L.; Tognoni, G. Prevalence and prognostic significance of ventricular arrhythmias after acute myocardial infarction in the fibrinolytic era. GISSI-2 results. Circulation. 1993, 87, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Hashimoto, K.; Yoshioka, K.; Miwa, Y.; Yodogawa, K.; Watanabe, E.; Nakamura, K.; Nakagawa, M.; Nakamura, K.; Watanabe, T.; Yusu, S.; Tachibana, M.; Nakahara, S.; Mizumaki, K.; Ikeda, T. Risk stratification for cardiac mortality using electrocardiographic markers based on 24-hour Holter recordings: the JANIES-SHD study. J Cardiol. 2020, 75, 155–163. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Bigger, J. T.Jr; Marcus, F.I.; Mortara, A.; Schwartz, P.J. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998, 351, 478–484. [Google Scholar] [CrossRef]
- Bauer, A.; Kantelhardt, J.W.; Barthel, P.; Schneider, R.; Mäkikallio, T.; Ulm, K.; Hnatkova, K.; Schömig, A.; Huikuri, H.; Bunde, A.; Malik, M.; Schmidt, G. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: cohort study. Lancet. 2006, 367, 1674–1681. [Google Scholar] [CrossRef]
- Bauer, A.; Barthel, P.; Schneider, R.; Ulm, K.; Müller, A.; Joeinig, A.; Stich, R.; Kiviniemi, A.; Hnatkova, K.; Huikuri, H.; Schömig, A.; Malik, M.; Schmidt, G. Improved Stratification of Autonomic Regulation for risk prediction in post-infarction patients with preserved left ventricular function (ISAR-Risk). Eur Heart, J. 2009, 30, 576–583. [Google Scholar] [CrossRef]
- Køber, L.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbæk, L.; Korup, E.; Jensen, G.; Hildebrandt, P.; Steffensen, F.H.; Bruun, N.E.; Eiskjær, H.; Brandes, A.; Thøgersen, A.M.; Gustafsson, F.; Egstrup, K.; Videbæk, R.; Hassager, C.; Svendsen, J.H.; Høfsten, D.E.; Torp-Pedersen, C.; Pehrson, S. DANISH Investigators. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med. 2016, 375, 1221–1230. [Google Scholar] [CrossRef]
- Maron, B.J.; Casey, S.A.; Chan, R.H.; Garberich, R.F.; Rowin, E.J.; Maron, M.S. Independent Assessment of the European Society of Cardiology Sudden Death Risk Model for Hypertrophic Cardiomyopathy. Am J Cardiol. 2015, 116, 757–764. [Google Scholar] [CrossRef]
- Halliday, B.P.; Baksi, A.J.; Gulati, A.; Ali, A.; Newsome, S.; Izgi, C.; Arzanauskaite, M.; Lota, A.; Tayal, U.; Vassiliou, V.S.; Gregson, J.; Alpendurada, F.; Frenneaux, M.P.; Cook, S.A.; Cleland, J.G.F.; Pennell, D.J.; Prasad, S.K. Outcome in Dilated Cardiomyopathy Related to the Extent, Location, and Pattern of Late Gadolinium Enhancement. JACC Cardiovasc Imaging. 2019, 12 Pt 2, 1645–1655. [Google Scholar] [CrossRef]
- Adabag, A.S.; Maron, B.J.; Appelbaum, E.; Harrigan, C.J.; Buros, J.L.; Gibson, C.M.; Lesser, J.R.; Hanna, C.A.; Udelson, J.E.; Manning, W.J.; Maron, M.S. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008, 51, 1369–1374. [Google Scholar] [CrossRef]
- Selvanayagam, J.B.; Hartshorne, T.; Billot, L.; Grover, S.; Hillis, G.S.; Jung, W.; Krum, H.; Prasad, S.; McGavigan, A.D. Cardiovascular magnetic resonance-GUIDEd management of mild to moderate left ventricular systolic dysfunction (CMR GUIDE): Study protocol for a randomized controlled trial. Ann Noninvasive Electrocardiol. 2017, 22, e12420. [Google Scholar] [CrossRef] [PubMed]
- Arsenos, P.; Gatzoulis, K.A.; Doundoulakis, I.; Dilaveris, P.; Antoniou, C.K.; Soulaidopoulos, S.; Sideris, S.; Sotiropoulos, I.; Tousoulis, D. Arrhythmic risk stratification in heart failure mid-range ejection fraction patients with a non-invasive guiding to programmed ventricular stimulation two-step approach. J Arrhythm. 2020, 36, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Gatzoulis, K.A.; Vouliotis, A.I.; Tsiachris, D.; Salourou, M.; Archontakis, S.; Dilaveris, P.; Gialernios, T.; Arsenos, P.; Karystinos, G.; Sideris, S.; Kallikazaros, I.; Stefanadis, C. Primary prevention of sudden cardiac death in a nonischemic dilated cardiomyopathy population: reappraisal of the role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol. 2013, 6, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Gatzoulis, K.A.; Georgopoulos, S.; Antoniou, C.K.; Anastasakis, A.; Dilaveris, P.; Arsenos, P.; Sideris, S.; Tsiachris, D.; Archontakis, S.; Sotiropoulos, E.; Theopistou, A.; Skiadas, I.; Kallikazaros, I.; Stefanadis, C.; Tousoulis, D. Programmed ventricular stimulation predicts arrhythmic events and survival in hypertrophic cardiomyopathy. Int J Cardiol. 2018, 254, 175–181. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Sideris, A.; Kanoupakis, E.; Sideris, S.; Nikolaou, N.; Antoniou, C.K.; Kolettis, T.M. Arrhythmic risk stratification in heart failure: Time for the next step? Ann Noninvasive Electrocardiol. 2017, 22, e12430. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Dilaveris, P.; Arsenos, P.; Tsiachris, D.; Antoniou, C.K.; Sideris, S.; Kolettis, T.; Kanoupakis, E.; Sideris, A.; Flevari, P.; Vassilikos, V.; Kappos, K.; Maounis, T.; Katsivas, A.; Kotsakis, A.; Karvounis, H.; Kossyvakis, C.; Leventopoulos, G.; Kalpakos, D.; Tousoulis, D. ReCONSIDER study Investigators. Arrhythmic risk stratification in nonischemic dilated cardiomyopathy: The ReCONSIDER study design - A two-step, multifactorial, electrophysiology-inclusive approach. Hellenic J Cardiol. 2021, 62, 169–172. [Google Scholar] [CrossRef]
- Hohnloser, S.H.; Kuck, K.H.; Dorian, P.; Roberts, R.S.; Hampton, J.R.; Hatala, R.; Fain, E.; Gent, M.; Connolly, S.J. DINAMIT Investigators. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004, 351, 2481–2488. [Google Scholar] [CrossRef]
- Steinbeck, G.; Andresen, D.; Seidl, K.; Brachmann, J.; Hoffmann, E.; Wojciechowski, D.; Kornacewicz-Jach, Z.; Sredniawa, B.; Lupkovics, G.; Hofgärtner, F.; Lubinski, A.; Rosenqvist, M.; Habets, A.; Wegscheider, K.; Senges, J. IRIS Investigators. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009, 361, 1427–1436. [Google Scholar] [CrossRef]
- Bigger, J.T. Jr. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. N Engl J Med. 1997, 337, 1569–1575. [Google Scholar] [CrossRef]
- Goldberger, J.J. Evidence-based analysis of risk factors for sudden cardiac death. Heart Rhythm. 2009, 6 (Suppl. S3), S2–S7. [Google Scholar] [CrossRef]
- Xenogiannis, I.; Gatzoulis, K.A.; Flevari, P.; Ikonomidis, I.; Iliodromitis, E.; Trachanas, K.; Vlachos, K.; Arsenos, P.; Tsiachris, D.; Tousoulis, D.; Brilakis, E.S.; Alexopoulos, D. Temporal changes of noninvasive electrocardiographic risk factors for sudden cardiac death in post-myocardial infarction patients with preserved ejection fraction: Insights from the PRESERVE-EF study. Ann Noninvasive Electrocardiol. 2020, 25, e12701. [Google Scholar] [CrossRef] [PubMed]
- Tsimos, K.P.; Korantzopoulos, P.; Arsenos, P.; Doundoulakis, I.; Tsiachris, D.; Antoniou, C.K.; Krikonis, K.; Sideris, S.; Dilaveris, P.; Triantafyllou, K.; Soulaidopoulos, S.; Kanoupakis, E.; Fragakis, N.; Sideris, A.; Trachanas, K.; Iliodromitis, E.; Tousoulis, D.; Tsioufis, K.; Kolettis, T.M.; Gatzoulis, K.A. Association of non-invasive electrocardiographic risk factors with left ventricular systolic function in post-myocardial infarction patients with mildly reduced or preserved ejection fraction: Insights from the PRESERVE-EF study. Ann Noninvasive Electrocardiol. 2022, 27, e12946. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Pinna, G.D.; Maestri, R.; Mortara, A.; Capomolla, S.; Febo, O.; Ferrari, R.; Franchini, M.; Gnemmi, M.; Opasich, C.; Riccardi, P.G.; Traversi, E.; Cobelli, F. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003, 107, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Arsenos, P.; Gatzoulis, K.A.; Dilaveris, P.; Sideris, S.; Tousoulis, D. T wave alternans extracted from 30-minute short resting Holter ECG recordings predicts mortality in heart failure. J Electrocardiol. 2018, 51, 588–591. [Google Scholar] [CrossRef]
- Arsenos, P.; Gatzoulis, K.A.; Laina, A.; Doundoulakis, I.; Soulaidopoulos, S.; Kordalis, A.; Oikonomou, G.; Triantafyllou, K.; Fragakis, N.; Vasilikos, V.; Tsioufis, K. QT interval extracted from 30-minute short resting Holter ECG recordings predicts mortality in heart failure. J Electrocardiol. 2022, 72, 109–114. [Google Scholar] [CrossRef]
- Triantafyllou, K.; Fragakis, N.; Gatzoulis, K.A.; Antoniadis, A.; Giannopoulos, G.; Arsenos, P.; Tsiachris, D.; Antoniou, C.K.; Trachanas, K.; Tsimos, K.; Vassilikos, V. Risk assessment of post-myocardial infarction patients with preserved ejection fraction using 45-minute short resting Holter electrocardiographic recordings. Annals of Non-Invasive Electrocardiology, 2023; To be published. [Google Scholar]
- Trachanas, K.; . Sideris, S.; Arsenos, P.; Tsiachris, D.; Antoniou, C.-K.; Dilaveris, P.; Triantafyllou, K.; Xenogiannis, I.; Tsimos, K.; Efremidis, M.; Kanoupakis, E.; Flevari, P.; Vassilikos, V.; Sideris, A.; Korantzopoulos, P.; Tousoulis, D.; Tsioufis, K.; Gatzoulis, K. Noninvasive risk factors for the prediction of inducibility on programmed ventricular stimulation in post-myocardial infarction patients with an ejection fraction ≥40% at risk for sudden cardiac arrest: Insights from the PRESERVE-EF study. Ann Noninvasive Electrocardiol. 2022, 27, e12908. [Google Scholar] [CrossRef]
- Xintarakou, A.; Arsenos, P.; Gatzoulis, K.A.; Manis, G.; Trachanas, K.; Soulaidopoulos, S.; Dilaveris, P.; Doundoulakis, I.; Kordalis, A.; Laina, A.; Xydis, P.; Tsimos, K.; Korantzopoulos, P.; Kolettis, T.; Tsioufis, K. Prediction of programmed ventricular stimulation inducibility using machine learning in post-myocardial infarction patients at risk for sudden cardiac arrest with preserved ejection fraction≥40% European Heart Journal, Volume 43, Issue Supplement_2, October 2022, ehac544.681. 20 October. [CrossRef]
- Gatzoulis K,; Frogoudaki, A. ; Brili, S.; Stefanadis, C. Implantable defibrillators: from the adult cardiac to the grown up congenital heart disease patient. Int J Cardiol. 2004, 97 (Suppl 1), 117–122. [Google Scholar] [CrossRef]
- Brili, S.; Aggeli, C.; Gatzoulis, K.; Tzonou, A.; Hatzos, C.; Pitsavos, C.; Stefanadis, C.; Toutouzas, P. Echocardiographic and signal averaged ECG indices associated with non-sustained ventricular tachycardia after repair of tetralogy of fallot. Heart. 2001, 85, 57–60. [Google Scholar] [CrossRef]
| Non-invasive risk factors (NIRFs) | Abnormal cut-off values | Mechanisms |
|---|---|---|
| SAECG LPs [14] | 2/3 possitive criteria | fibrotic areas, slow conduction reentry |
| QTc [21] | ≥440ms(♂), ≥450ms(♀) | prolonged repolarization, EAD, DAD |
| TWA [22] | ≥65μV (2-channels) | APD and Ca2+ alternans, steep APDR and CVR , steep FSRCR |
| VPBs [23] | ≥30/24h | automaticity (Ca2+oscillations), reentry |
| NSVT [24] | ≥1 episode/24h | automaticity (Ca2+oscillations), reentry |
| SDNN [25] | ≤75ms | enhanced sympathetic tone, autonomic imbalance |
| DC / HRT [26,27] | DC≤4.5ms HRTonset≥0%, HRT slope≤2.5ms |
vagal and sympathetic ANS dysfunction |
| Study | n | EF | Therapy | NIRFs searched |
NIRFS+ | EPS(n) | EPS + | Follow up (months) | EndPoints (n) |
Sens/Spe/PPV/NPV |
|---|---|---|---|---|---|---|---|---|---|---|
| Arsenos [33], 2020 |
48 Post-MI NICM |
45% | Thromb Angiopl | SAECG VPCs NSVT |
(≥1)* 32/48 |
32 | 14/32 | 41 | 9 | Sens = 87% Spec = 71% PPV = 50% NPV= 94% |
| Gatzoulis [34], 2013 |
158 NICM |
39 with EF>35%119 with EF≤35% |
ICDs | Syncope NSVT VPCs |
158 | 44/158 | 46 | 39 | Sens = 85% Spec = 91% PPV = 75% NPV= 95% |
|
| Gatzoulis [35], 2018 |
203 HCM |
64% | ICDs | Family Hist Syncope NSVT Hypot. Resp. Wall≥30 |
(≥1) * 203 |
203 | 79/203 | 60 | 20 | Sens = 95% Spec = 67% PPV = 24% NPV= 99% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





