1. Introduction
Mammalian fertilization involves a series of coordinated cellular and molecular events, that ultimately lead to the fusion of sperm and egg [
1,
2,
3].
During a natural fertilization process, sperm must pass through the cumulus cell layer, consisting of several thousand cells, tightly glued together by the extracellular matrix (ECM) that surrounds the oocyte. There is a significant amount of literature that confirms the importance of both cumulus cells (CCs) and ECM in the fertilization process. First of all, the sperm head of mature spermatozoa has a hyaluronan-specific receptor that allows them to bind with hyaluronan; studies in mice demonstrated that they cannot penetrate the oocyte without the presence of CCs [
4]. In addition, the interaction between CCs and male gametes results in physiological changes in the sperm, which ultimately make them capable of fertilizing [
5,
6,
7]. First of all, CCs secrete progesterone, which is a crucial hormone that acts as a chemoattractant and stimulates sperm hyper-activated flagellar movement and acrosome reaction (AR). It has been found that even small concentrations of progesterone can effectively stimulate calcium ion channels localized in the human sperm flagellum [
8]. Noteworthy, the processes of sperm motility hyperactivation and AR both involve calcium mobilization.
Moreover, it was reported that progesterone speeds up the process of tyrosine phosphorylation, which is a significant sign of sperm capacitation in sperm, determining the preparation for the AR event [
9]. Additionally, numerous studies have demonstrated that CCs provide factors able to impact sperm functions, such as prostaglandins (PGE1, PGE2, and PFG2a). These latter were detected in the incubation medium of
cumulus oophorus complexes (COCs); when indomethacin was used to block their biosynthesis, the fertilization rate decreased [
10].
Therefore, all these pieces of evidence have brought to light the communication between sperm and the CCs and matrix. These discoveries not only prompt a rethinking of the role of CCs in sperm penetration and fertilization but also open the possibility of their involvement in sperm selection for in vitro fertilization.
Several studies have explored the efficacy of COCs as a more natural approach to select sperm for
intracytoplasmic sperm injection (ICSI). In a study conducted by Wang and colleagues [
11], oocytes injected with COC-selected spermatozoa showed a higher rate of fertilization compared to the conventional ICSI (85.31% vs. 74.77%). Another study found that spermatozoa successfully penetrating through the cumulus oophorus exhibited higher rates of normal morphology, AR and had improved motility patterns [
12]. Interestingly, hyaluronic acid was found to regulate sperm motility without any impact on AR; on the other hand, CCs extract did not affect sperm motility, but it did induce AR. As far as we know, it appears that only one study has been conducted to examine the impact of cumulus oophorus complexes on sperm selection on sperm DNA fragmentation. The authors demonstrated a significant decrease in the level of sperm DNA fragmentation in the COCs selected sperm compared to the control [
13]
Therefore, sperm selection by using CCs for ICSI seems to be effective, even in terms of blastocyst development and quality [
11]. Anyway, very little is known with regard to how cumulus cells play a direct and critical role in fertilization, or whether the CCs components and secreted molecules/vesicles can promote fertilization.
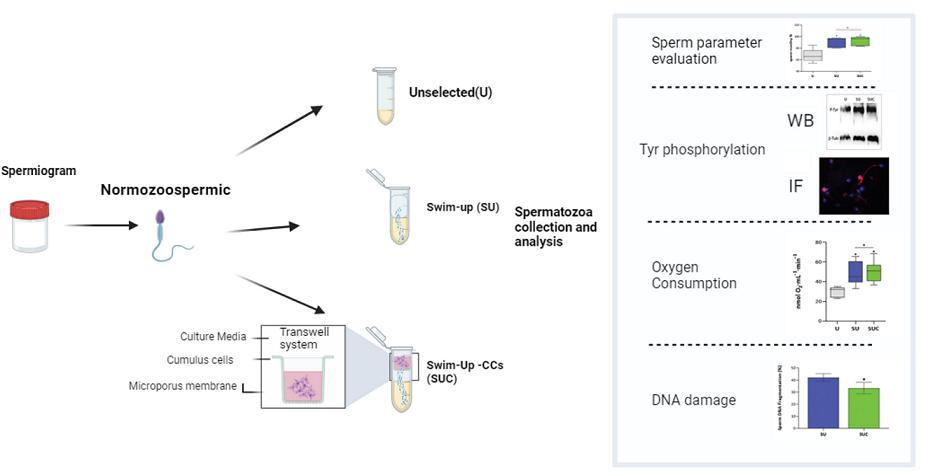
On this basis, this study was designed to investigate the efficacy of sperm exposure to cumulus cell secretome during swim-up treatment, compared to the routinely used swim-up method. The effectiveness of this method was assessed by examining biological factors that are critical for the ability of sperm to fertilize an oocyte; including capacitation, AR, tyrosine phosphorylation signature, DNA integrity, and mitochondrial functionality.
2. Materials and Methods
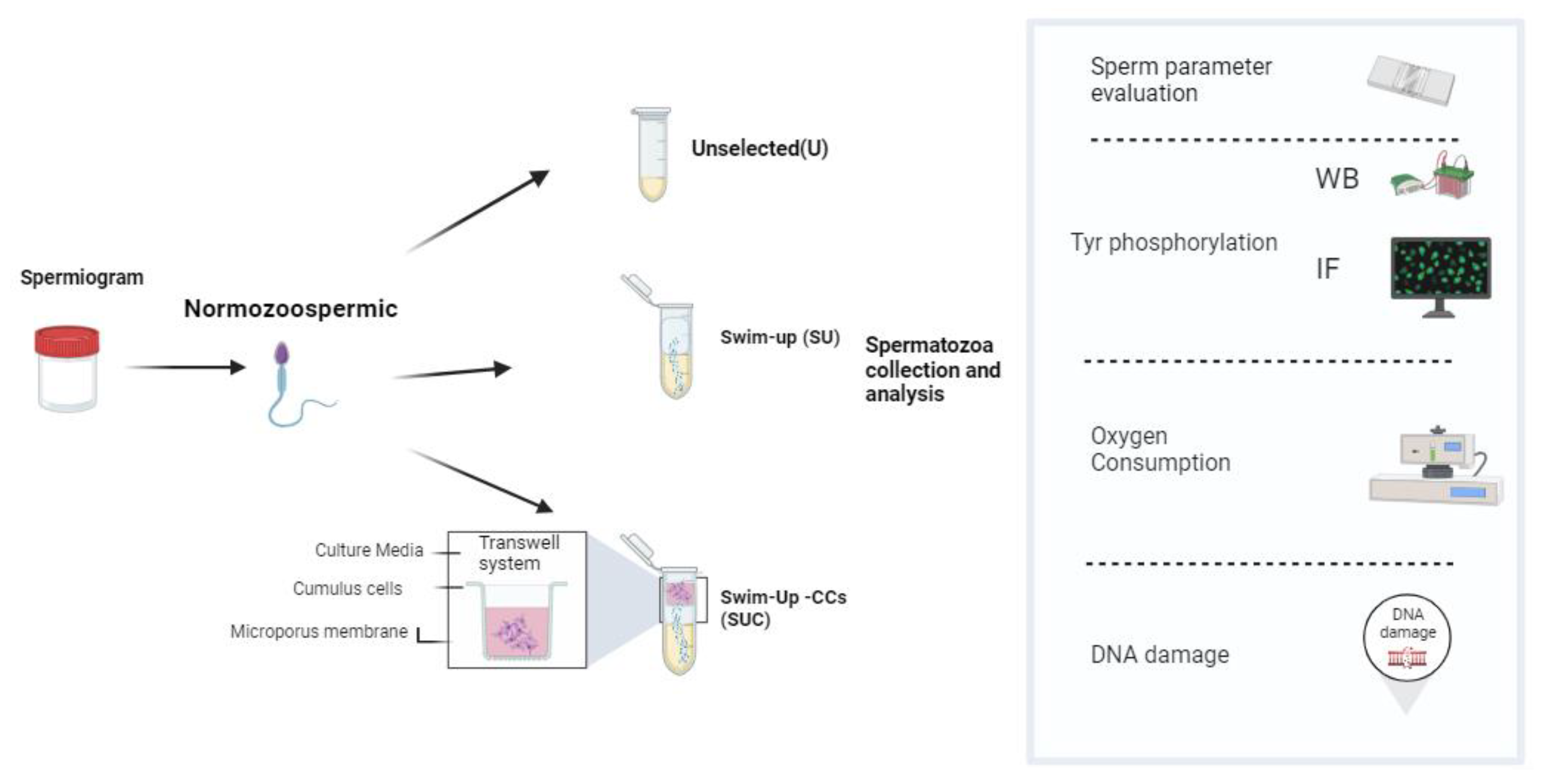
The flowchart of this study is summarized in the Scheme 1.
2.1. Collection of semen samples and semen analysis.
This study was conducted on a cohort of 30 males (mean age 34.2 years, 16-51), undergoing semen analisys for fertility evaluation at the Unit of Medically Assisted Reproduction, Siena University Hospital and at New Fertility Group – European Hospital (NFG) in Rome. The study protocol received approval from the Ethical Committee of the Siena University Hospital (approval ID: CEAVSE protocol number 18370, 2/10/2020); before participating, all subjects gave their written informed consent. A comprehensive clinical history was obtained for all participants, and subjects with possible preexisting causes of male infertility, such as varicocele, cryptorchidism, or endocrine disorders, were excluded. The sperm samples were collected via masturbation into sterile containers after a period of abstinence of 2 -5 days. After complete liquefaction at room temperature, semen analysis was performed according to World Health Organization criteria [
14]. Seminal parameters were evaluated by a blinded observer and independently repeated by another blinded observer to ensure quality control. The data reported are the mean value of the two observations. For this study, only normozoospermic samples were included.
2.2. COCs collection and cumulus cells culture
After follicular aspiration, COCs were retrieved from follicular fluid and oocyte were isolated from patients attending the Unit of Medically Assisted Reproduction, Siena University Hospital and at New Fertility Group –European Hospital (NFG) in Rome for ICSI treatment. CCs were isolated as previously reported [
15] and then cultured at 37ºC with 5% CO
2 in DMEM supplemented with 10% fetal bovine serum, 1% L-glutamine, 1% penicillin/streptomycin and 1% non-essential amino acids in a transwell chamber (size 6.5 mm, pore 0.4 µm, Corning).
2.3. Sperm selection with Swim-Up(SU) and Swim-Up -CC (SUC)
For this study, each ejaculate was divided into three aliquots: two aliquots underwent sperm capacitation through either a swim-up process (referred as SU) or a modified procedure that involved both swim-up and CCs interaction (referred as SUC); one aliquot was evaluated in basal conditions (referred as unselected, U). To perform SU selection, 1 mL of sperm washing medium (FUJIFILM Irvine Scientific, California, US) was gently layered on an equal volume of the semen sample. After 45 min of incubation at 37°C, 400 μL of the upper layer of the sample, containing selected sperm, was carefully collected.
In the case of the SUC, the transwell chamber with the CCs was placed on the top of the sperm washing medium. Proteins and soluble or incapsulated factors secreted by CCs can pass through the microporous filter membrane into the lower chamber, while the sperm are retained in the tube by the filter membrane. As in the SU procedure, at the end of the incubation 400 μL of the medium was carefully aspirated from the upper layer of the sample. Sperm analysis according to WHO 2021 was repeated by using 10 µl of each selected sample, while the remaining sperm selected sample was stored for the subsequent experiments.
2.4. Acrosomal staining
The acrosomal status was determined by using the acrosome marker fluorescein isothiocyanate-labeled Pisum sativum agglutinin (FITC-PSA). Briefly, sperm samples (SUC, SU and U) were incubated with 30 µg/mL FITC–PSA in PBS for 30 min and washed with distilled water for 10 min. Sperm nuclei were then counterstained with DAPI (6-diamino-2phenylindole) for 10 min at RT. After washing in PBS, the sperm were smeared on glass slides and mounted with DABCO mounting solution. Fluorescence images were acquired by the Leica AF6500 Integrated System for Imaging and Analysis (Leica Microsystems, Germany), equipped with the LAS software. Then, the fluorescence intensity was measured with Image-J software (U. S. National Institutes of Health, Bethesda, Maryland, USA). The percentages of acrosome-reacted sperm/sperm with intact acrosome were determined.
2.5. Detection of Tyrosine phosphorylation level
Tyrosine phosphorylation level was measured by using both immunofluorescence and western blot techniques. Immunofluorescence analysis was carried out following a previously published protocol [
16]. Briefly, 50µl of each sperm sample was washed with PBS and fixed for 15 min in 4% PFA. Subsequently, the sperm were permeabilized with 0.5% Triton X-100 in PBS for 5 minutes. After blocking in 5% bovine serum albumin (BSA)/1% normal goat serum (NGS) in PBS for 30 min, spermatozoa were incubated with the primary antibody diluted in 1% NGS, 1% BSA in PBS, overnight at 4°C. After three washes with PBS, the cells were then incubated for 1 hour at RT with the secondary antibody (
Supplementary Table S1). The nuclei were counterstained with DAPI and mounted with DABCO antifade solution. The sperm samples were examined using a Leica AF6500 Integrated System for Imaging and Analysis (Leica Microsystems, Germany). equipped with the LAS software.
For western blot analysis, sperm samples SUC, SU and U were washed with ice-cold PBS, and then lysed using RIPA buffer supplemented with Proteases Inhibitor (1:100) and phenylmethylsulphonyl fluoride (PMSF; 1:200). The samples were centrifuged at 12000g for 20 min at 4°C, the soluble protein-containing supernatant was carefully collected for further analysis. Protein concentration was determined by BSA Bradford assay. Western blotting was carried out according to an in-house developed protocol [
17]. 25µg of protein of each sample were separated by electrophoresis and then transferred onto nitrocellulose membrane. After blocking, the membrane was incubated overnight at 4°C with primary antibodies (see
Supplementary Table S1), then washed 3 times and finally incubated with the appropriate HRP-conjugated secondary antibody (see
Supplementary Table S1) for 1 hour at room temperature. After three washing in PBS-Tween 20 0.1%, immunostained bands were visualized by chemiluminescence with ImageQuant LAS 4000 (GE Healthcare, Chicago, IL, USA).
2.6. Mitochondrial Membrane potential assay
MitoTracker staining was used to assess the mitochondrial membrane potential (MMP) of sperm in the three experimental groups, according to a validated protocol [
16]. 4′,6-diamidino-2-phenylindole (DAPI) was used to counterstain the nuclei. Sperm were categorized according to the intensity and the patterns of staining of the midpiece. For each sample, at least 200 sperm in at least ten different fields were observed.
2.7. Hypotonic Swelling and Oxygraphic Assay
The sperm samples SUC, SU and U were centrifuged at 800 g for 10 minutes, the pellet was resuspended in an isotonic salt medium and subjected to hypotonic treatment according to the published protocol [
18]
. Briefly, sperm cells were incubated in an ice-chilled hypotonic medium consisting of 2 mmol/L of KH2PO4 and 8 mmol/L of K2HPO4 supplemented with 2 g/L of bovine serum albumin (BSA) adjusted to pH 7.4, for 1.5 hours. Following hypotonic treatment, sperm were washed three times using the isotonic medium and the sperm concentration was adjusted to approximately 1-4 million for all samples in each oxygraphic experiment. The measurement of oxygen uptake by spermatozoa was performed at 36°C using a Clark-type oxygen probe (Hansatech Oxygraph; Pentney, King's Lynn, UK). Demembranated sperm cells were vigorously stirred in a reaction chamber containing an isotonic salt medium without EDTA and equilibrated to the experimental temperature. The rate of oxygen uptake by the spermatozoa was quantified as nmol O
2·mL
−1·min
−1. The oxygen consumed by the sperm cells was determined by calculating the difference between the initial oxygen level at the time of spermatozoa addition and the oxygen level after 1 minute.
2.8. Assessment of sperm DNA fragmentation.
Sperm DNA fragmentation was assessed using the sperm chromatin dispersion test (SCD test; Halosperm G2® assay, Halotech DNA SL, Madrid, Spain). In brief, samples were mixed with an agarose gel before being applied to a precoated slide, refrigerated, and then exposed to a denaturing agent and lysis solution. Slides were then stained and assessed by counting 300 sperm per sample, to determine the variability in DNA fragmentation levels (DFLs) [
19]
2.10. Statistical analysis
Statistical analysis was performed using the GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). All variables were checked for normal distribution using the one-sample Kolmogorov–Smirnov test. One-way ANOVA was used to compare Acrosome staining, oxygen consumption and DNA fragmentation in the three groups of samples and post-hoc analyses were done using Tukey's test. Independent samples T-test was used to compare the different sperm subpopulations (MitoTracker positive versus MitoTracker negative) for all numerical variables. Pearson Chi-square was used to compare the results of sperm mitochondrial staining. P<0.05 was considered significant.
3. Results
3.1. Sperm parameters in Unselected, SU and SUC-selected sperm
The baseline characteristics of semen samples evaluated in this study are summarized in
Supplementary Table S2.
The mean sperm concentration was 75 x106/mL (range 20-240). The mean percentage of sperm motility was 65% (47-68) and the normal morphology was 9% (4-16).
In order to assess if and how CCs may impact the quality of isolated sperm, each semen sample was separated into three aliquots and processed as follow: 1) Unselected (U), 2) swim-up only (SU) and 3) swim-up combined with exposure to CCs secretome (SUC).
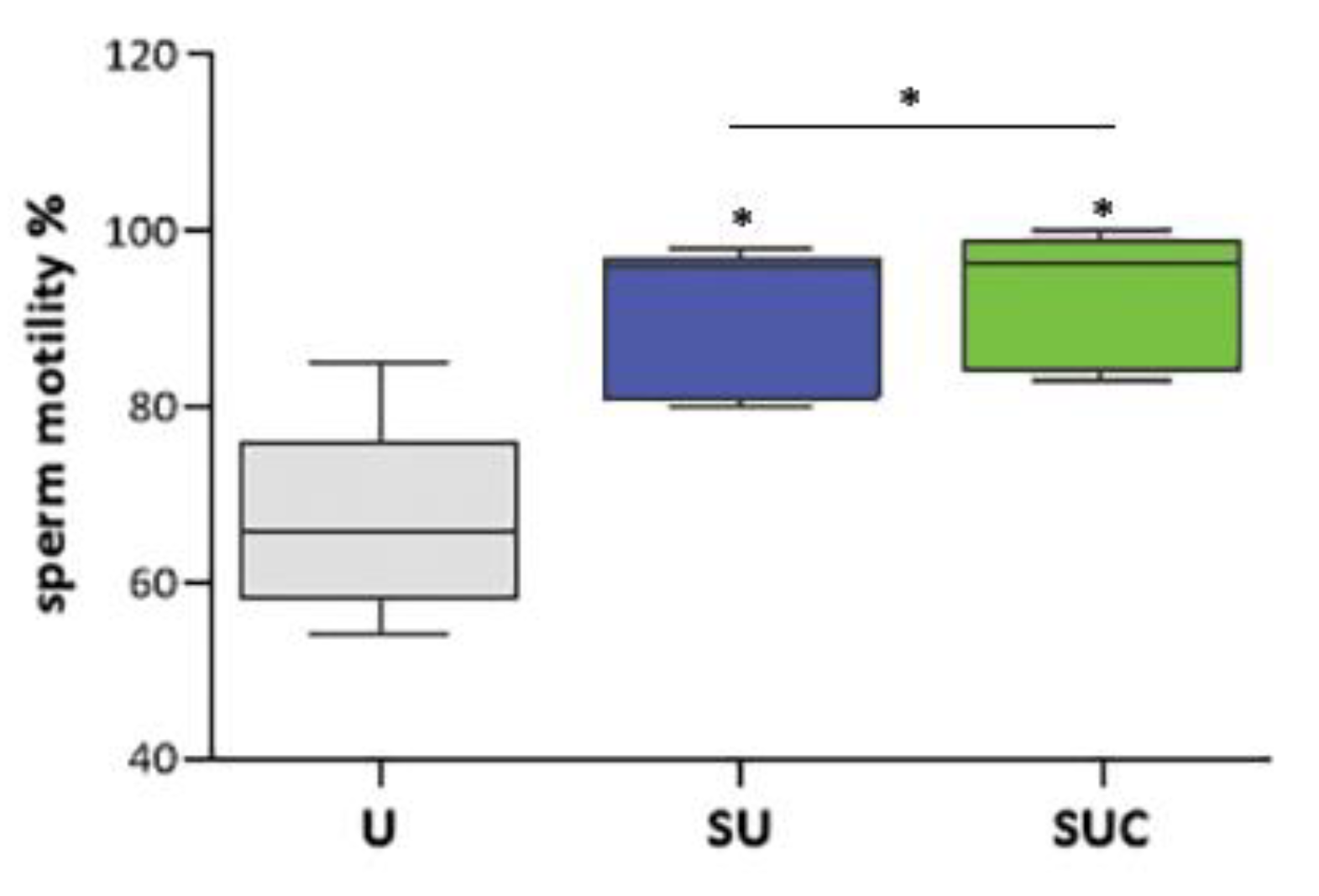
The number of sperm collected by SU and SUC were comparable (data not shown). The effects of the type of selection on sperm motility are shown in
Figure 1. Both procedures allow isolating sperm with higher motility, but the sperm collected by SUC method exhibited a significantly higher motility percentage when compared to those isolated by SU protocol (P < 0.05).
3.2. Acrosomal reaction rate and tyrosine phosphorylation in selected sperm
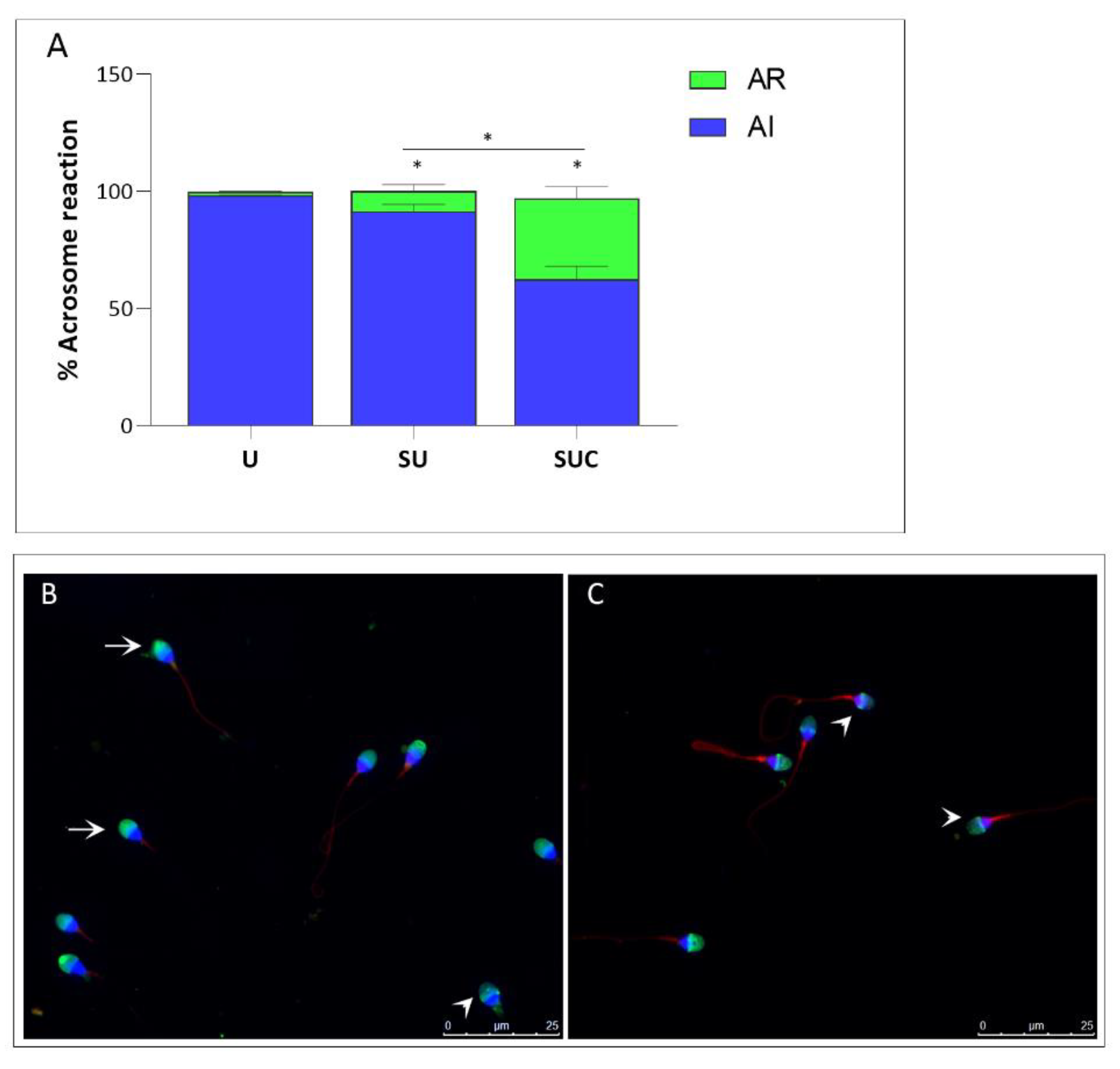
After fluorescent staining with FITC-PSA and image acquisition, the acrosome reaction rate of sperm was calculated by two blinded operators. In
Figure 2, the mean percentage of AR in the Unselected, SU and SUC samples are represented. As expected, the percentage of reacted acrosome increased in both SU and SUC compared to the unselected sperm samples. In the SUC group, the percentage of reacted acrosome is 34,53% vs 8,53% of SU group (p<0.05).
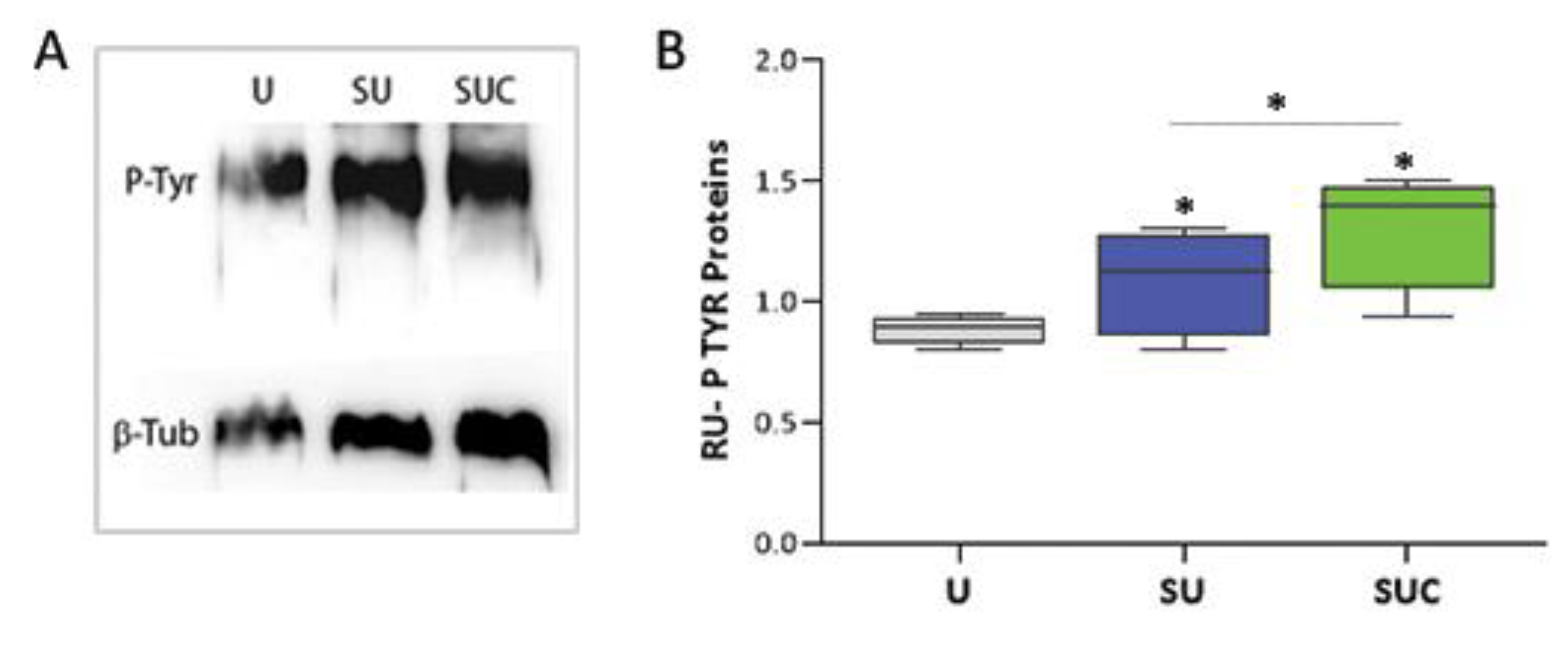
Then, we analyzed the phosphorylation level of proteins containing tyrosine residues in both unselected and selected sperm, as the progression of capacitation is linked to the Tyr-P level [
20]. To achieve this, we utilized both western blot and immunofluorescence techniques. Western blot comparison of tyrosine-phosphorylated protein levels between the three study groups is shown in
Figure 3A; all samples show the band of about 200kD corresponding to the family of proteins with Tyrosine-phosphorylated residues. The relative quantification of the spots, shown in
Figure 3B, demonstrated that SU and SUC selection procedures lead to an increase of sperm Tyr phosphorylation; this increase was about 25% in the case of SU and about 45% in the case of SUC when compared to unselected sperm (p<0.05).
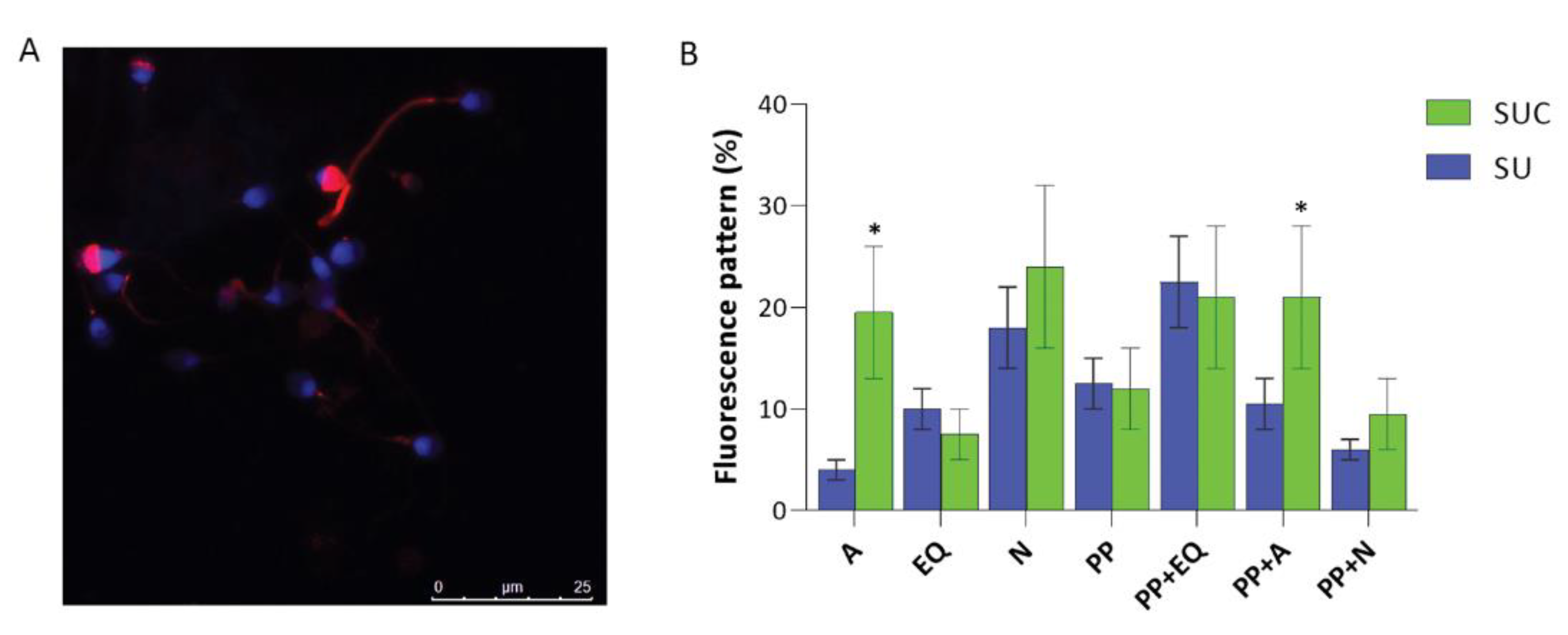
The immunofluorescence analysis (
Figure 4) confirmed the increase of Tyr phosphorylation in sperm selected with the procedure that use both swim-up and CCs incubation. Indeed, in SUC-selected sperm, a larger proportion of sperm contained tyrosine phosphorylated proteins (83%), while this proportion decreases to 75% in SU selected group (p<0.05). Interestingly, this approach detected variations in the regional localization of phosphotyrosine in SU versus SUC-selected sperm. Phospho-tyrosine residues have been identified in various compartments of spermatozoa: the acrosomal cap; the equatorial segment; the neck, the principal piece, as well as the combined principal piece and acrosomal region, principal piece and equatorial segment., principal piece and neck region (
Figure 4B).
When we compared the phosphotyrosine staining in SU and SUC samples, we observed the presence of different staining patterns, occurring at different percentage. It is of interest that in SUC-selected sperm there is a higher prevalence of sperm with tyrosine phosphorylation in the acrosomal cap and in the combined principal piece plus acrosomal region.
3.3. Assessment of Mitochondrial Functionality
MitoTracker is a reliable tool for monitoring the mitochondrial membrane potential (MMP) of a sperm sample [
21]. It works by staining the midpiece of a variable percentage of sperm. To determine if the SUC procedure is more effective in isolating functional sperm than the standard swim-up technique, we evaluated the number of mitotracker-positive sperm in each sample. The mean percentage of sperm stained with mitotracker (assessed by fluorescence microscopy;
Figure 4a-d) was higher for the SUC group and lower for the SU samples (86% and 76% respectively, OR 0.53, CI 0.31-0.90, p<0.01).
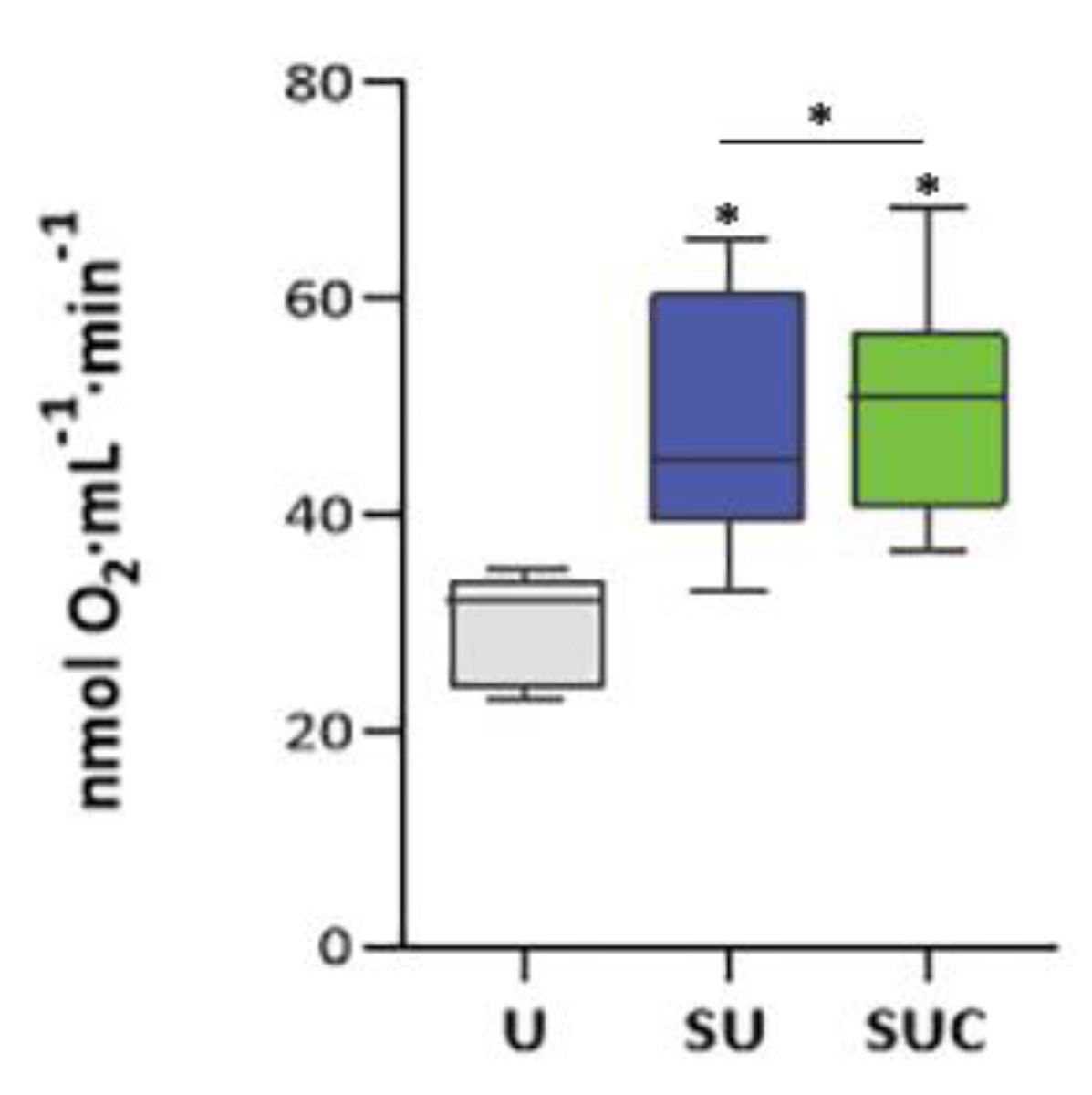
To further confirm the effectiveness of SUC procedure in isolating high-functional sperm, we analyzed their oxygen consumption by using oxygraphic analysis; this serves as an index of oxidative phosphorylation and, consequently, ATP production. As shown in
Figure 5, the SU procedure determines a significant increase in oxygen consumption of 60% (p < 0.01) whereas the modified SUC procedure assures an increase of up to 75% in oxygen consumption when compared with unselected sperm samples (p < 0.01).
3.4. DNA fragmentation in selected sperm
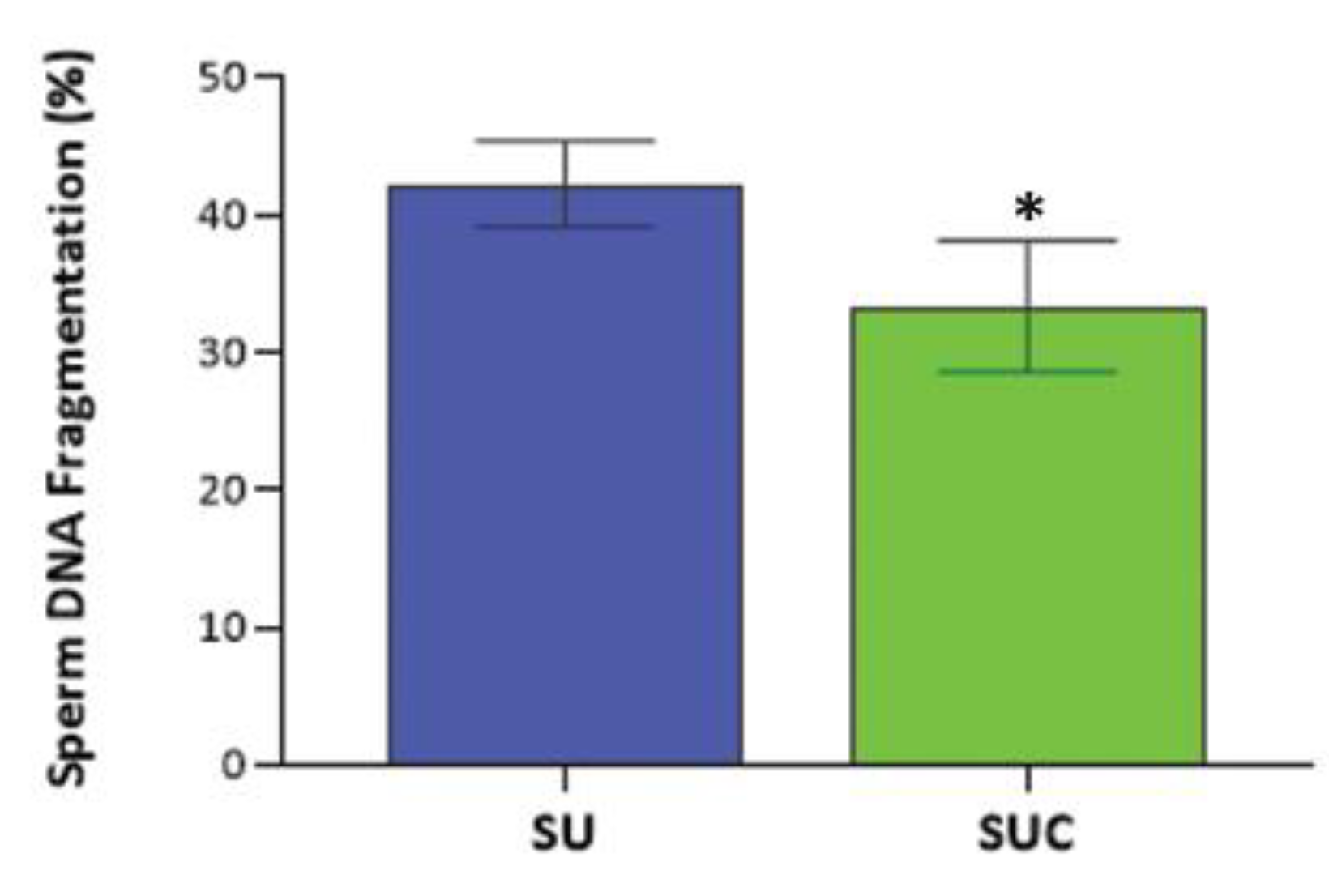
To assess the effect of selection methods on sperm DNA fragmentation (SDF), we compared the SDF of swim-up-only prepared sperm, with CCs and swim-up combined selection, by using the Halo sperm procedure. The mean SDF in SU selected sperm was 42% (
s.d. 15%, range: 28-57%); the exposure to the CCs secretome allows the selection of sperm with a significantly lower SDF (33%,
s.d. 11%, range: 20-45%; p<0.05) (
Figure 6). Therefore, we further provide evidence that the SUC procedure is able to select sperm with lower DNA damage.
4. Discussion
The present study shows that the sperm selection procedure taking advantage of the cumulus cells secretome allow recovering sperm of higher quality, in term of acrosome reaction, DNA integrity and mitochondrial functionality. Therefore, our results suggest that SUC is a good method to select sperm in IVF laboratory.
Our study demonstrated that the interaction of sperm with the CCs secretome occurring using the SUC procedure allows isolating sperm with a higher percentage of reacted acrosomes. This is consistent with other studies in the literature, showing that sperm that penetrated through the cumulus oophorus had higher percentages of reacted acrosome [
11,
12,
22]. It has been reported that the pre-ovulatory human cumulus oophorus and mural granulosa cells were associated with activity capable of initiating the human sperm acrosome reaction in vitro [
23]. Stock and colleagues [
24] have demonstrated that the co-culture of spermatozoa with COC increased the percentage of acrosome-reacted spermatozoa from 15% to 31% when compared with spermatozoa not incubated with COC. The mechanism responsible for this activity of cumulus cells is still under debate. Some studies have pointed to progesterone as an important factor that can induce the acrosome reaction (Meizel et al., 1997), while others have indicated that the hyaluronic acid in the extracellular matrix of COC plays a critical role in this process [
12]
Based on our findings related to the tyrosine phosphorylation footprint, we confirmed that the cumulus cell secretome is effective in promoting sperm capacitation. Indeed, the level of P-Tyr is increased by about 45% in sperm selected by SUC method, while an increase of only 25% is detectable in sperm selected by standard swim-up. The increased proportion of sperm showing tyrosine phosphorylation staining is consistent with the acquisition of hyperactivated motility, which is required for zona pellucida penetration [
25]. Noteworthy, we detected that sperm exposed to the CCs secretome shows a higher level of P-Tyr in the acrosome. It has been reported that the phosphorylation of tyrosine residues in the head of sperm is a subsurface event occurring early during capacitation and is closely related to the acquisition of the ability to display P-stimulated ARs [
26,
27]
Sperm function and quality are closely linked to proper mitochondrial activity [
28]. In humans, the mitochondrial membrane potential (MMP) is a valid indicator of mitochondrial functions. MMP has been found to be associated with sperm viability [
29] and the ability to undergo acrosome reaction [
30] as well as the capacity to fertilize [
31]. In fact, it has been reported that sperm with high MMP levels are generally more competent [
21]. In the present study, MMP has been characterized by staining with MitoTracker, whose accumulation is MMP-dependent [
32].
When sperm selection was accomplished by using swim-up associated with CCs exposure, the mean percentage of mitotracker-stained sperm was higher compared to conventional swim-up. This lets us hypothesize that the interaction with the molecules secreted by cumulus cells allows the recovery of sperm with better motility and higher fertilization capability. This is confirmed by the oxygraphic analysis, showing a significant increase in oxygen consumption in sperm recovered by the SUC method. This implies an increased production of ATP, which is positively correlated with sperm motility. Indeed, Ruiz-Pesini and colleagues described that the mitochondrial membrane potential and the Oxygen Consumption Rate (OCR) are positively associated with ATP content, the proportion of motile sperm and sperm velocity [
33]. Therefore, we propose that exposure to the CCs secretome during swim-up enhances the selection of sperm with better mitochondrial functionality, both in terms of MMP and OCR.
Conventional swim-up and density gradient centrifugation are gold standard techniques for sperm preparation in IVF laboratories, however numerous studies pointed to an increase of the DNA fragmentation level in selected sperm due to the excessive amounts of ROS produced during centrifugation [
34]. In the present study, sperm DNA fragmentation resulted significantly lower in sperm selected by swim-up combined with CCs exposure than in the swim-up-only group. The results were similar to those of previous studies. Franken et al. reported a decline in the percentage of chromomycin A3 positive sperm (an indicator for abnormal chromatin packaging) in COCs-penetrated spermatozoa compared to control spermatozoa. This data suggested that the sperm chromatin quality was dramatically enhanced during pass through COCs [
11]. This is not surprising, since there is evidence that cumulus cells undergo metabolic events to reduce oxidative stress in fertilization media [
6,
35].
5. Conclusions
The exposure of sperm to CCs secretome during swim-up selection assures recovery of better male gametes. This activity of cumulus cells is mediated via the secretory products of these cells. This interaction allows improved acrosome reaction, sperm capacitation and mitochondrial activity. Despite the main limitations of this study, represented by the limited sample size, our data suggest SUC as a more physiological and integrated method of sperm selection. Therefore, optimizing sperm preparation before IVF to improve sperm capacitation and minimize the DNA fragmentation effect would be definitely beneficial for ART outcomes.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Supplementary Table S1: List of antibodies used in this study; Supplementary Table S2: List of patients' (n=27) characteristics and seminal values.
Author Contributions
Conceptualization, PP, AL, CS; methodology, FPL.; validation, P.P..; formal analysis, FPL, SPC, AH, M.S., F.B. and L.G..; data curation, FPL, AL; writing—original draft preparation, FPL and AL; writing—review and editing, PP and CS; supervision, AL; funding acquisition, PP and AL. All authors have read and agreed to the published version of the manuscript.
Funding
Internal funds of the University of Siena.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the University of Siena (CEAVSE protocol number 18370, 2/10/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Acknowledgments
We thank all the patients for their participation in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Georgadaki, K.; Khoury, N.; Spandidos, D.A.; Zoumpourlis, V. The Molecular Basis of Fertilization (Review). Int J Mol Med 2016, 38, 979–986. [Google Scholar] [CrossRef]
- Trebichalská, Z.; Holubcová, Z. Perfect Date—the Review of Current Research into Molecular Bases of Mammalian Fertilization. J Assist Reprod Genet 2020, 37, 243–256. [Google Scholar] [CrossRef]
- Evans, J.P.; Florman, H.M. The State of the Union: The Cell Biology of Fertilization. Nat Cell Biol 2002, 4 Suppl, s57–s63. [Google Scholar] [CrossRef]
- Zhuo, L.; Yoneda, M.; Zhao, M.; Yingsung, W.; Yoshida, N.; Kitagawa, Y.; Kawamura, K.; Suzuki, T.; Kimata, K. Defect in SHAP-Hyaluronan Complex Causes Severe Female Infertility. A Study by Inactivation of the Bikunin Gene in Mice. J Biol Chem 2001, 276, 7693–7696. [Google Scholar] [CrossRef]
- Van Soom, A.; Tanghe, S.; De Pauw, I.; Maes, D.; De Kruif, A. Function of the Cumulus Oophorus Before and During Mammalian Fertilization. Reproduction in Domestic Animals 2002, 37, 144–151. [Google Scholar] [CrossRef]
- Tanghe, S.; Van Soom, A.; Nauwynck, H.; Coryn, M.; de Kruif, A. Minireview: Functions of the Cumulus Oophorus during Oocyte Maturation, Ovulation, and Fertilization. Molecular Reproduction and Development 2002, 61, 414–424. [Google Scholar] [CrossRef]
- Keeble, S.; Firman, R.C.; Sarver, B.A.J.; Clark, N.L.; Simmons, L.W.; Dean, M.D. Evolutionary, Proteomic, and Experimental Investigations Suggest the Extracellular Matrix of Cumulus Cells Mediates Fertilization Outcomes†. Biol Reprod 2021, 105, 1043–1055. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone Activates the Principal Ca2+ Channel of Human Sperm. Nature 2011, 471, 387–391. [Google Scholar] [CrossRef]
- Sumigama, S.; Mansell, S.; Miller, M.; Lishko, P.V.; Cherr, G.N.; Meyers, S.A.; Tollner, T. Progesterone Accelerates the Completion of Sperm Capacitation and Activates CatSper Channel in Spermatozoa from the Rhesus Macaque. Biol Reprod 2015, 93, 130. [Google Scholar] [CrossRef]
- Viggiano, J.M.; Herrero, M.B.; Cebral, E.; Boquet, M.G.; de Gimeno, M.F. Prostaglandin Synthesis by Cumulus-Oocyte Complexes: Effects on in Vitro Fertilization in Mice. Prostaglandins Leukot Essent Fatty Acids 1995, 53, 261–265. [Google Scholar] [CrossRef]
- Wang, C.; Feng, G.; Shu, J.; Zhou, H.; Zhang, B.; Chen, H.; Lin, R.; Gan, X.; Wu, Z.; Wei, T. Cumulus Oophorus Complexes Favor Physiologic Selection of Spermatozoa for Intracytoplasmic Sperm Injection. Fertility and Sterility 2018, 109, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-J.; Chiu, P.C.-N.; Lee, K.-F.; Tse, J.Y.-M.; Ho, P.-C.; Yeung, W.S.-B. Cumulus Cells and Their Extracellular Matrix Affect the Quality of the Spermatozoa Penetrating the Cumulus Mass. Fertility and Sterility 2009, 92, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Naknam, W.; Salang, L.; Sothornwit, J.; Amnatbuddee, S.; Seejorn, K.; Pongsritasana, T.; Sukkasame, S. Effect of Sperm Selection Method by Cumulus Oophorus Complexes and Conventional Sperm Preparation Method on Sperm Quality and DNA Fragmentation for Assisted Reproduction Techonology. European Journal of Obstetrics & Gynecology and Reproductive Biology 2019, 243, 46–50. [Google Scholar] [CrossRef]
- WHO Laboratory Manual for the Examination and Processing of Human Semen. Available online: https://www.who.int/publications-detail-redirect/9789240030787 (accessed on 7 August 2023).
- Luddi, A.; Gori, M.; Marrocco, C.; Capaldo, A.; Pavone, V.; Bianchi, L.; Boschi, L.; Morgante, G.; Piomboni, P.; de Leo, V. Matrix Metalloproteinases and Their Inhibitors in Human Cumulus and Granulosa Cells as Biomarkers for Oocyte Quality Estimation. Fertil Steril 2018, 109, 930–939.e3. [Google Scholar] [CrossRef] [PubMed]
- Luongo, F.P.; Passaponti, S.; Haxhiu, A.; Raeispour, M.; Belmonte, G.; Governini, L.; Casarini, L.; Piomboni, P.; Luddi, A. Bitter Taste Receptors and Endocrine Disruptors: Cellular and Molecular Insights from an In Vitro Model of Human Granulosa Cells. Int J Mol Sci 2022, 23, 15540. [Google Scholar] [CrossRef] [PubMed]
- Governini, L.; Semplici, B.; Pavone, V.; Crifasi, L.; Marrocco, C.; De Leo, V.; Arlt, E.; Gudermann, T.; Boekhoff, I.; Luddi, A.; et al. Expression of Taste Receptor 2 Subtypes in Human Testis and Sperm. Journal of Clinical Medicine 2020, 9, 264. [Google Scholar] [CrossRef]
- Ponchia, R.; Bruno, A.; Renzi, A.; Landi, C.; Shaba, E.; Luongo, F.P.; Haxhiu, A.; Artini, P.G.; Luddi, A.; Governini, L.; et al. Oxidative Stress Measurement in Frozen/Thawed Human Sperm: The Protective Role of an In Vitro Treatment with Myo-Inositol. Antioxidants 2022, 11, 10. [Google Scholar] [CrossRef]
- McEvoy, A.; Roberts, P.; Yap, K.; Matson, P. Development of a Simplified Method of Human Semen Storage for the Testing of Sperm DNA Fragmentation Using the Halosperm G2 Test Kit. Fertility and Sterility 2014, 102, 981–988. [Google Scholar] [CrossRef]
- Donà, G.; Tibaldi, E.; Andrisani, A.; Ambrosini, G.; Sabbadin, C.; Pagano, M.A.; Brunati, A.M.; Armanini, D.; Ragazzi, E.; Bordin, L. Human Sperm Capacitation Involves the Regulation of the Tyr-Phosphorylation Level of the Anion Exchanger 1 (AE1). International Journal of Molecular Sciences 2020, 21, 4063. [Google Scholar] [CrossRef]
- Amaral, A.; Ramalho-Santos, J. Assessment of Mitochondrial Potential: Implications for the Correct Monitoring of Human Sperm Function. International Journal of Andrology 2010, 33, e180–e186. [Google Scholar] [CrossRef]
- Franken, D.R.; Bastiaan, H.S. Can a Cumulus Cell Complex Be Used to Select Spermatozoa for Assisted Reproduction? Andrologia 2009, 41, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Siiteri, J.E.; Dandekar, P.; Meizel, S. Human Sperm Acrosome Reaction-Initiating Activity Associated with the Human Cumulus Oophorus and Mural Granulosa Cells. J Exp Zool 1988, 246, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Stock, C.E.; Bates, R.; Lindsay, K.S.; Edmonds, D.K.; Fraser, L.R. Human Oocyte-Cumulus Complexes Stimulate the Human Acrosome Reaction. J Reprod Fertil 1989, 86, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Leppens-Luisier, G.; Lucas, H.; Chardonnens, D.; Campana, A.; Franken, D.R.; Urner, F. Localization of Tyrosine Phosphorylated Proteins in Human Sperm and Relation to Capacitation and Zona Pellucida Binding1. Biology of Reproduction 2003, 68, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, F.; Romano, R.; Santucci, R.; Macerola, B.; Ruvolo, G.; Francavilla, S. Effect of Human Sperm Exposure to Progesterone on Sperm-Oocyte Fusion and Sperm-Zona Pellucida Binding under Various Experimental Conditions. Int J Androl 2002, 25, 106–112. [Google Scholar] [CrossRef]
- Barbonetti, A.; Vassallo, M.R.C.; Cordeschi, G.; Venetis, D.; Carboni, A.; Sperandio, A.; Felzani, G.; Francavilla, S.; Francavilla, F. Protein Tyrosine Phosphorylation of the Human Sperm Head during Capacitation: Immunolocalization and Relationship with Acquisition of Sperm-Fertilizing Ability. Asian J Androl 2010, 12, 853–861. [Google Scholar] [CrossRef]
- Costa, J.; Braga, P.C.; Rebelo, I.; Oliveira, P.F.; Alves, M.G. Mitochondria Quality Control and Male Fertility. Biology (Basel) 2023, 12, 827. [Google Scholar] [CrossRef]
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility. J Clin Med 2020, 9, 363. [Google Scholar] [CrossRef]
- Gallon, F.; Marchetti, C.; Jouy, N.; Marchetti, P. The Functionality of Mitochondria Differentiates Human Spermatozoa with High and Low Fertilizing Capability. Fertil Steril 2006, 86, 1526–1530. [Google Scholar] [CrossRef]
- Marchetti, P.; Ballot, C.; Jouy, N.; Thomas, P.; Marchetti, C. Influence of Mitochondrial Membrane Potential of Spermatozoa on in Vitro Fertilisation Outcome. Andrologia 2012, 44, 136–141. [Google Scholar] [CrossRef]
- Moscatelli, N.; Spagnolo, B.; Pisanello, M.; Lemma, E.D.; De Vittorio, M.; Zara, V.; Pisanello, F.; Ferramosca, A. Single-Cell-Based Evaluation of Sperm Progressive Motility via Fluorescent Assessment of Mitochondria Membrane Potential. Sci Rep 2017, 7, 17931. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pesini, E.; Díez-Sánchez, C.; López-Pérez, M.J.; Enríquez, J.A. The Role of the Mitochondrion in Sperm Function: Is There a Place for Oxidative Phosphorylation or Is This a Purely Glycolytic Process? Curr Top Dev Biol 2007, 77, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Muratori, M.; Tarozzi, N.; Carpentiero, F.; Danti, S.; Perrone, F.M.; Cambi, M.; Casini, A.; Azzari, C.; Boni, L.; Maggi, M.; et al. Sperm Selection with Density Gradient Centrifugation and Swim up: Effect on DNA Fragmentation in Viable Spermatozoa. Sci Rep 2019, 9, 7492. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ohshima, Y.; Sasaki, A.; Yoshikawa, E.; Xu, H.; Nagao, Y. The Secretion and Metabolism of Cumulus Cells Support Fertilization in the Bovine Model. Theriogenology 2022, 193, 136–145. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).