Submitted:
30 August 2023
Posted:
01 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Theoretical Background and Computational Details
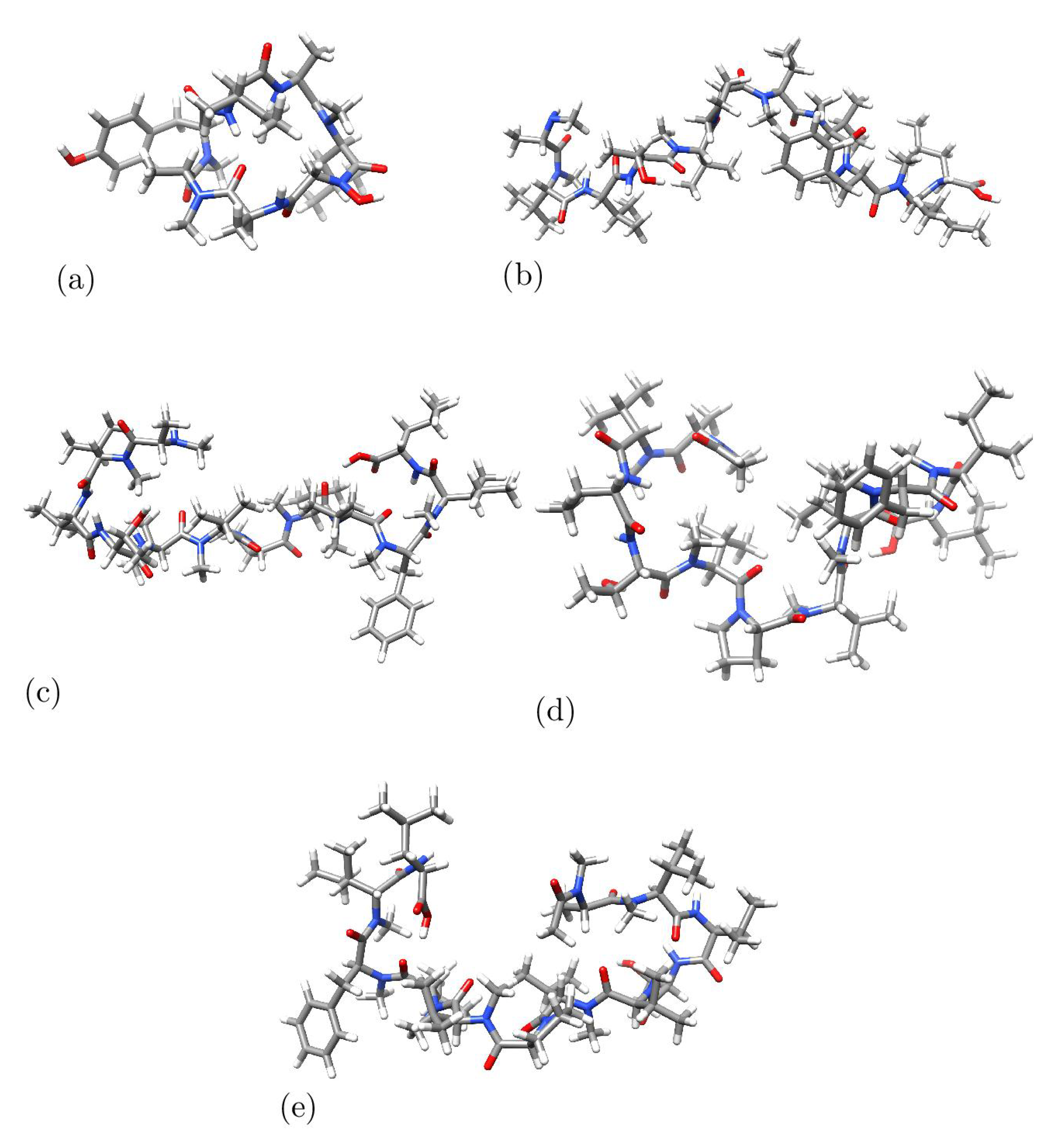
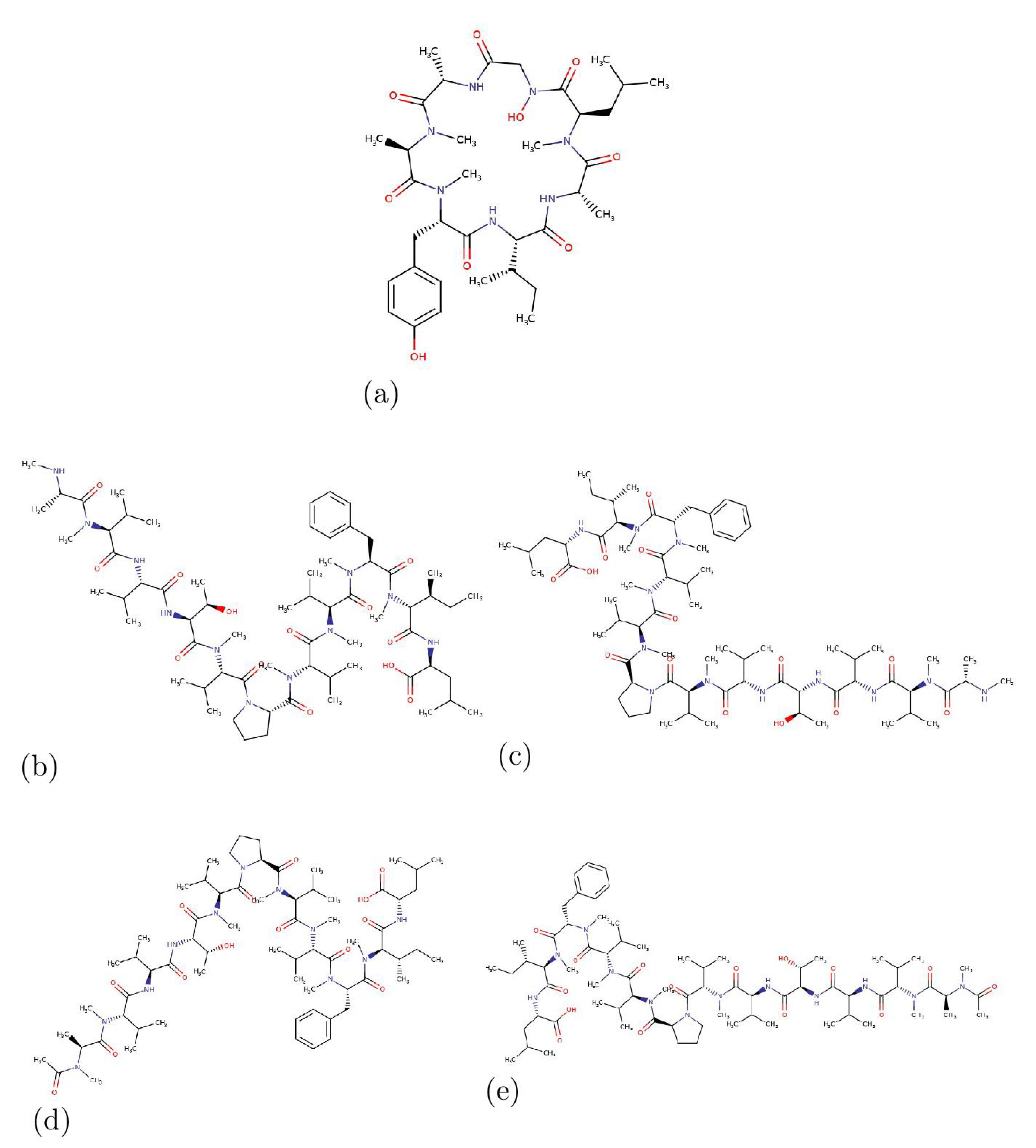
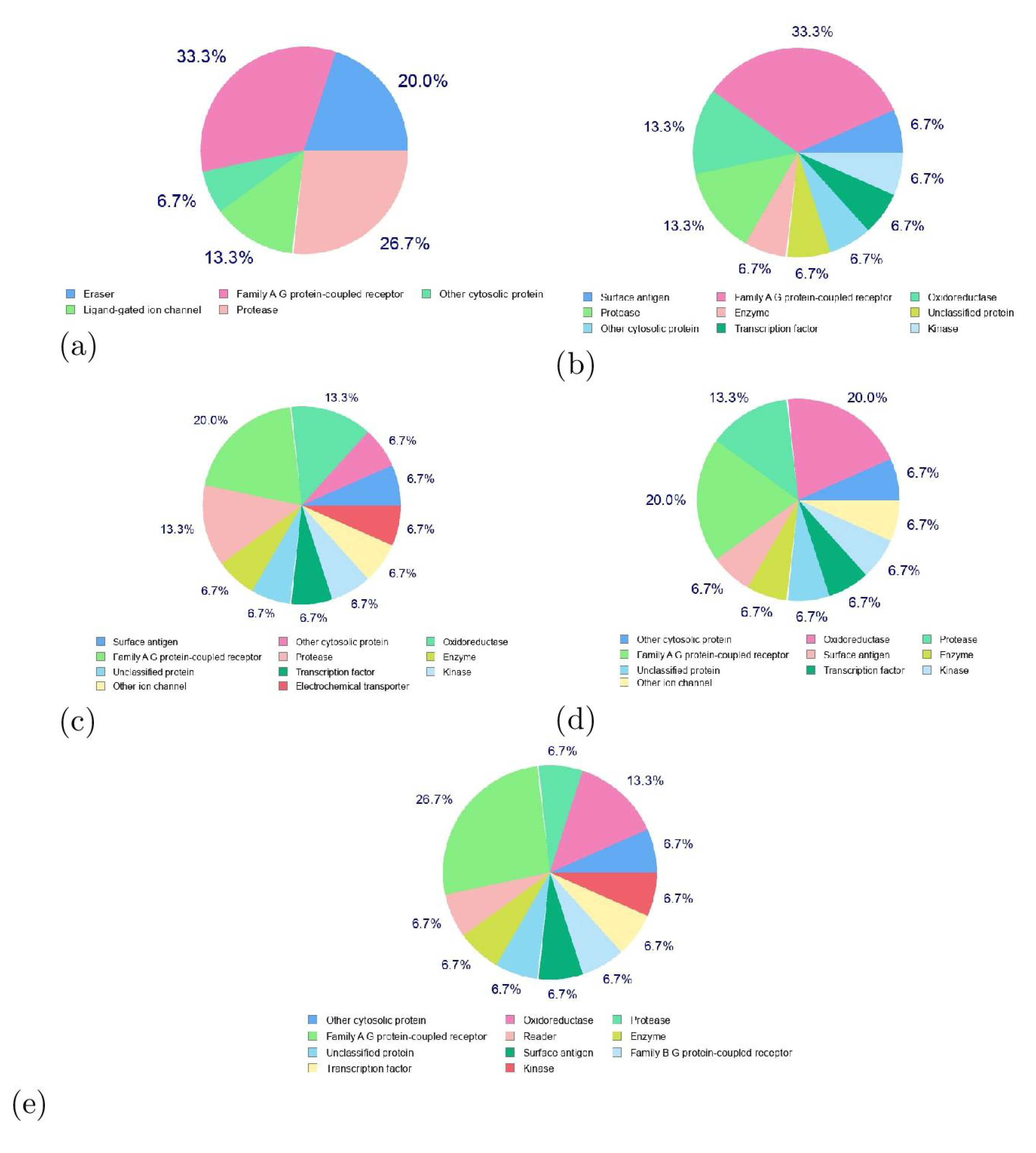
3. Results and Discussion
| Electronegativity | |
| Global Hardness | |
| Electrophilicity | = |
| Electrodonating Power | = |
| Electroaccepting Power | = |
| Net Electrophilicity | |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varijakzhan, D.; Loh, J.Y.; Yap, W.S.; Yusoff, K.; Seboussi, R.; Lim, S.H.E.; Lai, K.S.; Chong, C.M. Bioactive Compounds from Marine Sponges: Fundamentals and Applications. Marine Drugs 2021, 19, 246. [Google Scholar] [CrossRef] [PubMed]
- Erwin, P.M.; López-Legentil, S.; Schuhmann, P.W. The Pharmaceutical Value of Marine Biodiversity for anti-Cancer Drug Discovery. Ecological Economics 2010, 70, 445–451. [Google Scholar] [CrossRef]
- Macedo, M.W.F.S.; da Cunha, N.B.; Carneiro, J.A.; da Costa, R.A.; de Alencar, S.A.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine Organisms as a Rich Source of Biologically Active Peptides. Frontiers in Marine Science 2021, 8. [Google Scholar] [CrossRef]
- Zhang, J.N.; Xia, Y.X.; Zhang, H.J. Natural Cyclopeptides as Anticancer Agents in the Last 20 Years. International Journal of Molecular Sciences 2021, 22, 3973. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Natural Product Reports 2020, 37, 175–223. [Google Scholar] [CrossRef]
- Abdalla, M.; McGaw, L. Natural Cyclic Peptides as an Attractive Modality for Therapeutics: A Mini Review. Molecules 2018, 23, 2080. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ashour, M.L.; Singab, A.N.B.; Wink, M. A Comprehensive Review of Bioactive Peptides from Marine Fungi and Their Biological Significance. Marine Drugs 2019, 17, 559. [Google Scholar] [CrossRef]
- Kallen, A. Computational Pharmacokinetics; CRC Press: London, England, 2019. [Google Scholar]
- Parr, R.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, 1989. [Google Scholar]
- Chermette, H. Chemical Reactivity Indexes in Density Functional Theory. Journal of Computational Chemistry 1999, 20, 129–154. [Google Scholar] [CrossRef]
- Geerlings, P.; Proft, F.D.; Langenaeker, W. Conceptual Density Functional Theory. Chemical Reviews 2003, 103, 1793–1874. [Google Scholar] [CrossRef]
- Geerlings, P.; Chamorro, E.; Chattaraj, P.K.; Proft, F.D.; Gázquez, J.L.; Liu, S.; Morell, C.; Toro-Labbé, A.; Vela, A.; Ayers, P. Conceptual Density Functional Theory: Status, Prospects, Issues. Theoretical Chemistry Accounts 2020, 139. [Google Scholar] [CrossRef]
- Toro-Labbé, A. (Ed.) Theoretical Aspects of Chemical Reactivity; Elsevier Science: Amsterdam, 2007. [Google Scholar]
- Chattaraj, P.K. (Ed.) Chemical Reactivity Theory - A Density Functional View; CRC Press. Taylor & Francis Group: Boca Raton, FL, 2009. [Google Scholar]
- Chakraborty, D.; Chattaraj, P.K. Conceptual Density Functional Theory Based Electronic Structure Principles. Chemical Science 2021, 12, 6264–6279. [Google Scholar] [CrossRef] [PubMed]
- Frau, J.; Glossman-Mitnik, D. Molecular Reactivity and Absorption Properties of Melanoidin Blue-G1 through Conceptual DFT. Molecules 2018, 23, 559–15. [Google Scholar] [CrossRef] [PubMed]
- Frau, J.; Glossman-Mitnik, D. Conceptual DFT Study of the Local Chemical Reactivity of the Dilysyldipyrrolones A and B Intermediate Melanoidins. Theoretical Chemistry Accounts 2018, 137, 1210. [Google Scholar] [CrossRef]
- Frau, J.; Glossman-Mitnik, D. Conceptual DFT Study of the Local Chemical Reactivity of the Colored BISARG Melanoidin and Its Protonated Derivative. Frontiers in Chemistry 2018, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Frau, J.; Glossman-Mitnik, D. Computational Study of the Chemical Reactivity of the Blue-M1 Intermediate Melanoidin. Computational and Theoretical Chemistry 2018, 1134, 22–29. [Google Scholar] [CrossRef]
- Frau, J.; Glossman-Mitnik, D. Chemical Reactivity Theory Applied to the Calculation of the Local Reactivity Descriptors of a Colored Maillard Reaction Product. Chemical Science International Journal 2018, 22, 1–14. [Google Scholar] [CrossRef]
- Frau, J.; Glossman-Mitnik, D. Blue M2: An Intermediate Melanoidin Studied via Conceptual DFT. Journal of Molecular Modeling 2018, 24, 1–13. [Google Scholar] [CrossRef]
- Frau, J.; Flores-Holguín, N.; Glossman-Mitnik, D. Chemical Reactivity Properties, pKa Values, AGEs Inhibitor Abilities and Bioactivity Scores of the Mirabamides A–H Peptides of Marine Origin Studied by Means of Conceptual DFT. Marine Drugs 2018, 16, 302–19. [Google Scholar] [CrossRef]
- Costa, L.; Sousa, E.; Fernandes, C. Cyclic Peptides in Pipeline: What Future for These Great Molecules? Pharmaceuticals 2023, 16, 996. [Google Scholar] [CrossRef]
- Glossman-Mitnik, D. (Ed.) Density Functional Theory - Recent Advances, New Perspectives and Applications; IntechOpen: London, UK, 2022. [Google Scholar]
- Halgren, T.A. Merck Molecular Force Field. I. Basis, Form, Scope, Parameterization, and Performance of MMFF94. Journal of Computational Chemistry 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck Molecular Force Field. II. MMFF94 van der Waals and Electrostatic Parameters for Intermolecular Interactions. Journal of Computational Chemistry 1996, 17, 520–552. [Google Scholar] [CrossRef]
- Halgren, T.A. MMFF VI. MMFF94s Option for Energy Minimization Studies. Journal of Computational Chemistry 1999, 20, 720–729. [Google Scholar] [CrossRef]
- Halgren, T.A.; Nachbar, R.B. Merck Molecular Force Field. IV. Conformational Energies and Geometries for MMFF94. Journal of Computational Chemistry 1996, 17, 587–615. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck Molecular Force Field. V. Extension of MMFF94 Using Experimental Data, Additional Computational Data, and Empirical Rules. Journal of Computational Chemistry 1996, 17, 616–641. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision C.01, 2016. Gaussian Inc. Wallingford CT.
- Peverati, R.; Truhlar, D.G. Screened-Exchange Density Functionals with Broad Accuracy for Chemistry and Solid-State Physics. Physical Chemistry Chemical Physics 2012, 14, 16187. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Physical Chemistry Chemical Physics 2005, 7, 3297. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting Basis Sets for H to Rn. Physical Chemistry Chemical Physics 2006, 8, 1057. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. The Journal of Physical Chemistry B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Gázquez, J.; Cedillo, A.; Vela, A. Electrodonating and Electroaccepting Powers. Journal of Physical Chemistry A 2007, 111, 1966–1970. [Google Scholar] [CrossRef]
- Chattaraj, P.; Chakraborty, A.; Giri, S. Net Electrophilicity. Journal of Physical Chemistry A 2009, 113, 10068–10074. [Google Scholar] [CrossRef]
- Parr, R.; Szentpaly, L.; Liu, S. Electrophilicity Index. Journal of the American Chemical Society 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Chattaraj, P.; Sarkar, U.; Roy, D. Electrophilicity Index. Chemical Reviews 2006, 106, 2065–2091. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Electrophilicity. In Chemical Reactivity Theory - A Density Functional View; Chattaraj, P., Ed.; CRC Press. Taylor & Francis Group: Boca Raton, 2009; chapter 13. [Google Scholar]
- Domingo, L.R.; Chamorro, E.; Perez, P. Understanding the Reactivity of Captodative Ethylenes in Polar Cycloaddition Reactions. A Theoretical Study. The Journal of Organic Chemistry 2008, 73, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, P.; Domingo, L.R.; Chamorro, E.; Pérez, P. A Further Exploration of a Nucleophilicity Index Based on the Gas-Phase Ionization Potentials. Journal of Molecular Structure: THEOCHEM 2008, 865, 68–72. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A. Understanding the Mechanism of Polar Diels-Alder Reactions. Organic and Biomolecular Chemistry 2009, 7, 3576–3583. [Google Scholar] [CrossRef]
- Domingo, L.R.; Perez, P. The Nucleophilicity N Index in Organic Chemistry. Organic and Biomolecular Chemistry 2011, 9, 7168–7175. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Morell, C.; Grand, A.; Toro-Labbé, A. New Dual Descriptor for Chemical Reactivity. The Journal of Physical Chemistry A 2004, 109, 205–212. [Google Scholar] [CrossRef]
- Morell, C.; Grand, A.; Toro-Labbé, A. Theoretical Support for Using the Δf(r) Descriptor. Chemical Physics Letters 2006, 425, 342–346. [Google Scholar] [CrossRef]
- Martínez-Araya, J.I. Explaining Reaction Mechanisms Using the Dual Descriptor: A Complementary Tool to the Molecular Electrostatic Potential. Journal of Molecular Modeling 2012, 19, 2715–2722. [Google Scholar] [CrossRef]
- Martínez-Araya, J.I. Why is the Dual Descriptor a More Accurate Local Reactivity Descriptor than Fukui Functions? Journal of Mathematical Chemistry 2015, 53, 451–465. [Google Scholar] [CrossRef]
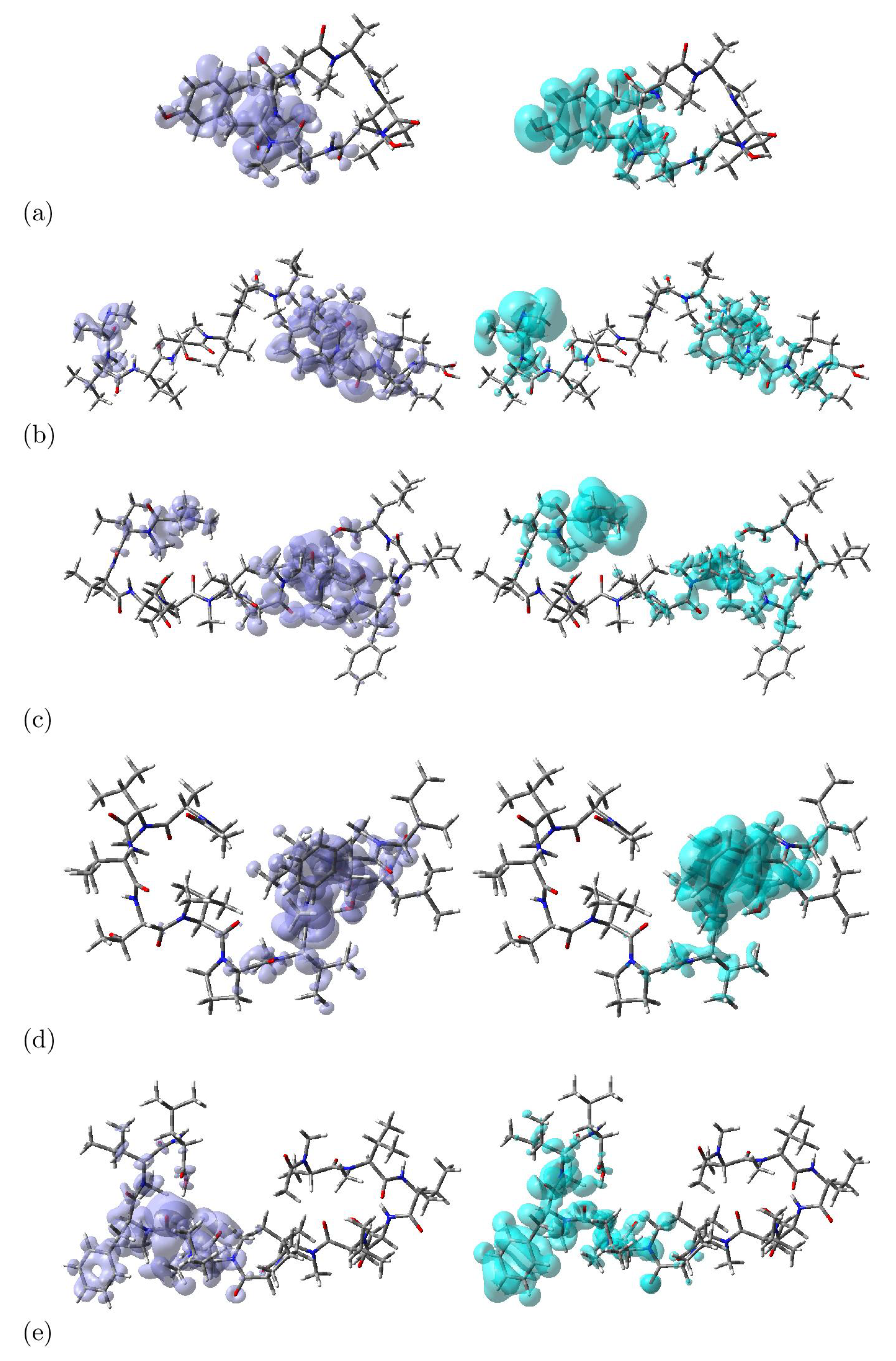
| HOMO | LUMO | SOMO | H-L Gap | J(I) | J(A) | J(HL) | SL | |
|---|---|---|---|---|---|---|---|---|
| Talarolide A | -6.14 | -0.93 | -0.96 | 5.21 | 0.04 | 0.02 | 0.05 | 0.02 |
| Talaropeptide A | -6.22 | -0.85 | -0.87 | 5.37 | 0.02 | 0.00 | 0.02 | 0.01 |
| Talaropeptide B | -6.16 | -0.92 | -0.92 | 5.24 | 0.00 | 0.00 | 0.00 | 0.00 |
| Talaropeptide C | -6.54 | -1.00 | -1.03 | 5.54 | 0.01 | 0.01 | 0.02 | 0.02 |
| Talaropeptide D | -6.56 | -0.92 | -0.91 | 5.63 | 0.01 | 0.00 | 0.01 | 0.01 |
| S | N | |||||||
|---|---|---|---|---|---|---|---|---|
| Talarolide A | 3.54 | 5.21 | 1.20 | 0.19 | 2.65 | 4.50 | 0.96 | 5.46 |
| Talaropeptide A | 3.54 | 5.37 | 1.17 | 0.19 | 2.57 | 4.44 | 0.90 | 5.34 |
| Talaropeptide B | 3.54 | 5.24 | 1.20 | 0.19 | 2.63 | 4.50 | 0.95 | 5.45 |
| Talaropeptide C | 3.77 | 5.54 | 1.29 | 0.18 | 2.25 | 4.80 | 1.03 | 5.83 |
| Talaropeptide D | 3.74 | 5.63 | 1.24 | 0.18 | 2.24 | 4.70 | 0.96 | 5.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





