Submitted:
29 August 2023
Posted:
01 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Analytical Probe and Analytical Response of Nanozyme-Based Sensors
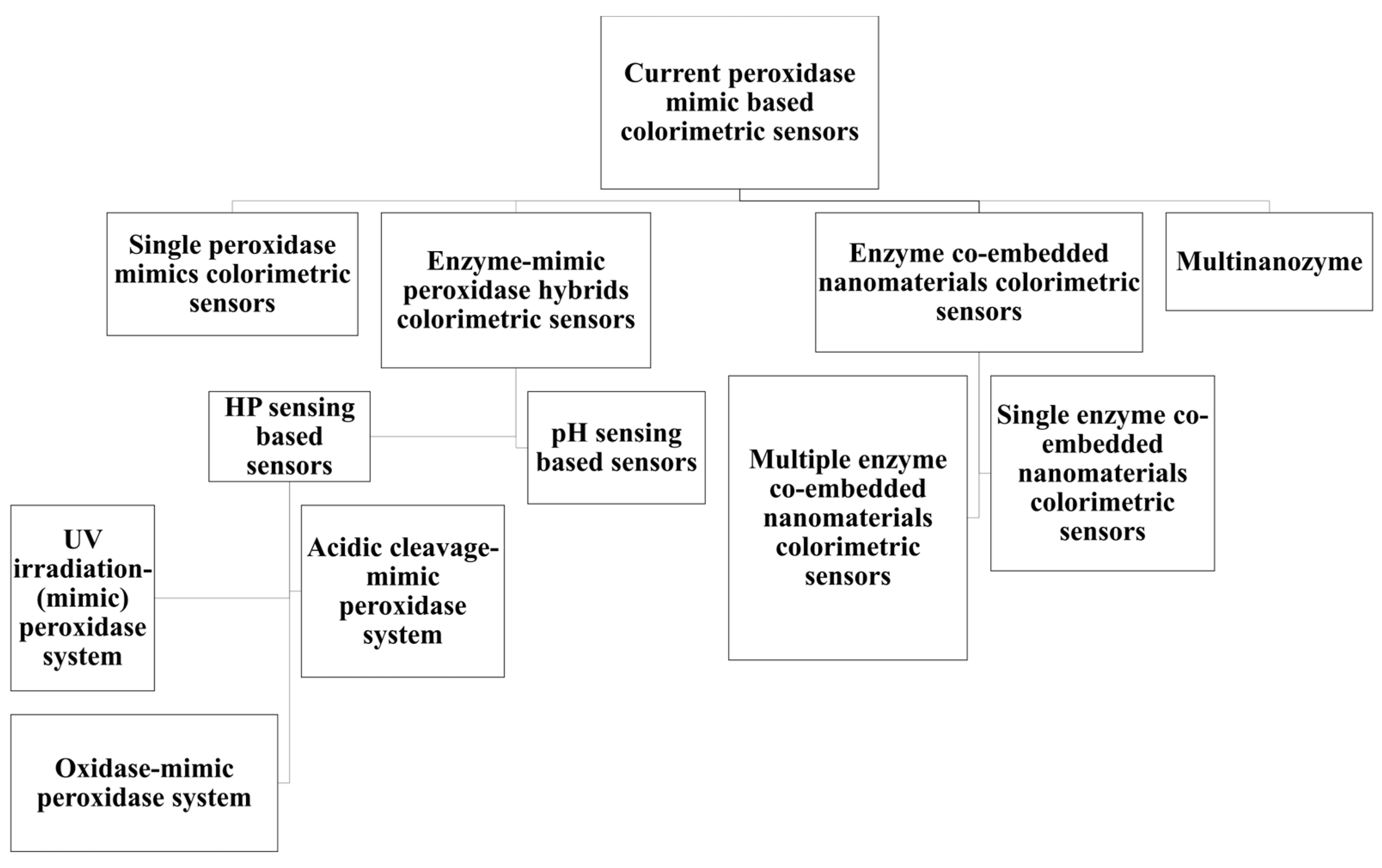
3. Current Classes of Nanozymatic Sensors Based on Sensor Design
4. Multinanozyme Systems
4. Multinanozyme System vs. Single Nanozymatic Systems
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Hormozi Jangi, S. R. (2023). Evaluation of Biochemical Behavior and Stability of Gold Nanoparticles with High Intrinsic Peroxidase-Like Activity. Petro Chem Indus Intern, 6(4), 234-239.
- Hormozi Jangi, S. R., & Dehghani, Z. (2023). Kinetics and biochemical characterization of silver nanozymes and investigating impact of storage conditions on their activity and shelf-life. Chemical Research and Nanomaterials, 1(4), 25-33.
- Hormozi Jangi, S. R. (2023). Biochemical characterization of enzyme-like silver nanoparticles toward nanozyme-catalysed oxidation reactions, Micromaterials and Interfaces 1 (1), 2170.
- Hormozi Jangi, S. R. (2023). A Comparative Study on Kinetics Performances of BSA-gold Nanozymes for Nanozyme-mediated Oxidation of 3,3',5,5'-Tetramethylbenzidine and 3,3'-Diaminobenzidine. Biochemistry & Molecular Biology Journal 9(3), 21.
- Jangi, S. R. H., & Akhond, M. (2021). High throughput urease immobilization onto a new metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) for water treatment and safe blood cleaning. Process Biochemistry, 105, 79-90. [CrossRef]
- Jangi, S. R. H., & Akhond, M. (2022). Introducing a covalent thiol-based protected immobilized acetylcholinesterase with enhanced enzymatic performances for biosynthesis of esters. Process Biochemistry, 120, 138-155. [CrossRef]
- Jangi, S. R. H., Akhond, M., & Dehghani, Z. (2020). High throughput covalent immobilization process for improvement of shelf-life, operational cycles, relative activity in organic media and enzymatic kinetics of urease and its application for urea removal from water samples. Process Biochemistry, 90, 102-112. [CrossRef]
- Hormozi Jangi, S. R. (2023). Synthesis and characterization of magnesium-based metal-organic frameworks and investigating the effect of coordination solvent on their biocompatibility. Chemical Research and Nanomaterials, 1(4), 1-9.
- Chaturvedi, S., Dave, P. N., & Shah, N. K. (2012). Applications of nano-catalyst in new era. Journal of Saudi Chemical Society, 16(3), 307-325. [CrossRef]
- HORMOZI JANGI, S. R., & Akhond, M. (2020). High throughput green reduction of tris (p-nitrophenyl) amine at ambient temperature over homogenous AgNPs as H-transfer catalyst. Journal of Chemical Sciences, 132, 1-8.
- Hormozi Jangi, S. R. (2023). Low-temperature destructive hydrodechlorination of long-chain chlorinated paraffins to diesel and gasoline range hydrocarbons over a novel low-cost reusable ZSM-5@ Al-MCM nanocatalyst: a new approach toward reuse instead of common mineralization. Chemical Papers, 1-15. [CrossRef]
- Freeman, R., & Willner, I. (2012). Optical molecular sensing with semiconductor quantum dots (QDs). Chemical Society Reviews, 41(10), 4067-4085. [CrossRef]
- Dehghani, Z., Akhond, M., Jangi, S. R. H., & Absalan, G. (2024). Highly sensitive enantioselective spectrofluorimetric determination of R-/S-mandelic acid using l-tryptophan-modified amino-functional silica-coated N-doped carbon dots as novel high-throughput chiral nanoprobes. Talanta, 266, 124977.
- Jangi, S. R. H., & Akhond, M. (2021). Ultrasensitive label-free enantioselective quantification of d-/l-leucine enantiomers with a novel detection mechanism using an ultra-small high-quantum yield N-doped CDs prepared by a novel highly fast solvent-free method. Sensors and Actuators B: Chemical, 339, 129901.
- Wu, T., Liu, X., Liu, Y., Cheng, M., Liu, Z., Zeng, G., ... & He, Q. (2020). Application of QD-MOF composites for photocatalysis: Energy production and environmental remediation. Coordination Chemistry Reviews, 403, 213097. [CrossRef]
- Hormozi Jangi, S. R., & Gholamhosseinzadeh, E. (2023). Developing an ultra-reproducible and ultrasensitive label-free nanoassay for L-methionine quantification in biological samples toward application in homocystinuria diagnosis. Chemical Papers, 1-13. [CrossRef]
- Huang, Y., Ren, J., & Qu, X. (2019). Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chemical reviews, 119(6), 4357-4412. [CrossRef]
- Jangi, S. R. H. (2023). Determining kinetics parameters of bovine serum albumin-protected gold nanozymes toward different substrates. Qeios. [CrossRef]
- Wu, J., Wang, X., Wang, Q., Lou, Z., Li, S., Zhu, Y., ... & Wei, H. (2019). Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chemical Society Reviews, 48(4), 1004-1076. [CrossRef]
- Hormozi Jangi, S. R. (2023). Effect of daylight and air oxygen on nanozymatic activity of unmodified silver nanoparticles: Shelf-stability. Qeios. [CrossRef]
- Gao, L., Zhuang, J., Nie, L., Zhang, J., Zhang, Y., Gu, N., ... & Yan, X. (2007). Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature nanotechnology, 2(9), 577-583. [CrossRef]
- Jangi, A. R. H., Jangi, M. R. H., & Jangi, S. R. H. (2020). Detection mechanism and classification of design principles of peroxidase mimic based colorimetric sensors: A brief overview. Chinese Journal of Chemical Engineering, 28(6), 1492-1503. [CrossRef]
- Wang, Q., Wei, H., Zhang, Z., Wang, E., & Dong, S. (2018). Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. TrAC Trends in Analytical Chemistry, 105, 218-224. [CrossRef]
- Shamsipur, M., Safavi, A., & Mohammadpour, Z. (2014). Indirect colorimetric detection of glutathione based on its radical restoration ability using carbon nanodots as nanozymes. Sensors and Actuators B: Chemical, 199, 463-469. [CrossRef]
- Jangi, S. R. H., & Akhond, M. (2020). Synthesis and characterization of a novel metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) and its application for constructing a reusable nanozyme-based sensor for selective and sensitive glutathione quantification. Microchemical Journal, 158, 105328. [CrossRef]
- Dega, N. K., Ganganboina, A. B., Tran, H. L., Kuncoro, E. P., & Doong, R. A. (2022). BSA-stabilized manganese phosphate nanoflower with enhanced nanozyme activity for highly sensitive and rapid detection of glutathione. Talanta, 237, 122957. [CrossRef]
- Pan, J., He, Q., Lao, Z., Zou, Y., Su, J., Li, Q., ... & Zhao, S. (2022). A bifunctional immunosensor based on osmium nano-hydrangeas as a catalytic chromogenic and tinctorial signal output for folic acid detection. Analyst, 147(1), 55-65. [CrossRef]
- Wang, M., Zhang, J., Zhou, X., Sun, H., & Su, X. (2022). Fluorescence sensing strategy for xanthine assay based on gold nanoclusters and nanozyme. Sensors and Actuators B: Chemical, 358, 131488. [CrossRef]
- Akhond, M., Hormozi Jangi, S. R., Barzegar, S., & Absalan, G. (2020). Introducing a nanozyme-based sensor for selective and sensitive detection of mercury (II) using its inhibiting effect on production of an indamine polymer through a stable n-electron irreversible system. Chemical Papers, 74, 1321-1330. [CrossRef]
- Su, L., Cai, Y., Wang, L., Dong, W., Mao, G., Li, Y., ... & Zhang, H. (2020). Hemin@ carbon dot hybrid nanozymes with peroxidase mimicking properties for dual (colorimetric and fluorometric) sensing of hydrogen peroxide, glucose and xanthine. Microchimica Acta, 187, 1-11. [CrossRef]
- Wu, X., Chen, T., Wang, J., & Yang, G. (2018). Few-layered MoSe 2 nanosheets as an efficient peroxidase nanozyme for highly sensitive colorimetric detection of H 2 O 2 and xanthine. Journal of Materials Chemistry B, 6(1), 105-111.
- Hormozi Jangi, S. R., & Dehghani, Z. (2023). Spectrophotometric quantification of hydrogen peroxide utilizing silver nanozyme. Chemical Research and Nanomaterials, 2(1), 15-23.
- Hormozi Jangi, S. R., Akhond, M., & Absalan, G. (2020). A field-applicable colorimetric assay for notorious explosive triacetone triperoxide through nanozyme-catalyzed irreversible oxidation of 3, 3′-diaminobenzidine. Microchimica Acta, 187, 1-10. [CrossRef]
- Lu, W., Chen, J., Kong, L., Zhu, F., Feng, Z., & Zhan, J. (2021). Oxygen vacancies modulation Mn3O4 nanozyme with enhanced oxidase-mimicking performance for l-cysteine detection. Sensors and Actuators B: Chemical, 333, 129560. [CrossRef]
- Ahmadi-Leilakouhi, B., Hormozi Jangi, S. R., & Khorshidi, A. (2023). Introducing a novel photo-induced nanozymatic method for high throughput reusable biodegradation of organic dyes. Chemical Papers, 77(2), 1033-1046. [CrossRef]
- Wang, F., Chen, L., Liu, D., Ma, W., Dramou, P., & He, H. (2020). Nanozymes based on metal-organic frameworks: Construction and prospects. TrAC Trends in Analytical Chemistry, 133, 116080. [CrossRef]
- Jangi, S. R. H. (2023). Introducing a High Throughput Nanozymatic Method for Eco-Friendly Nanozyme-Mediated Degradation of Methylene Blue in Real Water Media. Sustainable Chemical Engineering, 90-99. [CrossRef]
- Hormozi Jangi, S. R. (2023). A Brief Overview on Clinical and Epidemiological Features, Mechanism of Action, and Diagnosis of Novel Global Pandemic Infectious Disease, Covid-19, And its Comparison with Sars, Mers, And H1n1. World J Clin Med Img, 2(1), 45-52.
- Jangi, S. R. H. (2023). Natural Polyphenols of Pomegranate and Black Tea Juices can Combat COVID-19 through their SARS-CoV-2 3C-like Protease-inhibitory Activity. Qeios.
- He, M., Xu, X., Wang, H., Wu, Q., Zhang, L., Zhou, D., ... & Liu, H. (2023). Nanozyme-Based Colorimetric SARS-CoV-2 Nucleic Acid Detection by Naked Eye. Small, 2208167.
- Wu, L., Wang, X., Wu, X., Xu, S., Liu, M., Cao, X., ... & Huang, H. (2022). MnO2 nanozyme-mediated CRISPR-Cas12a system for the detection of SARS-CoV-2. ACS Applied Materials & Interfaces, 14(45), 50534-50542.
- Jangi, S. R. H., Akhond, M., & Absalan, G. (2020). A novel selective and sensitive multinanozyme colorimetric method for glutathione detection by using an indamine polymer. Analytica Chimica Acta, 1127, 1-8. [CrossRef]
- Wang, X., Qin, L., Zhou, M., Lou, Z., & Wei, H. (2018). Nanozyme sensor arrays for detecting versatile analytes from small molecules to proteins and cells. Analytical chemistry, 90(19), 11696-11702. [CrossRef]
- Zhu, Y., Wu, J., Han, L., Wang, X., Li, W., Guo, H., & Wei, H. (2020). Nanozyme sensor arrays based on heteroatom-doped graphene for detecting pesticides. Analytical chemistry, 92(11), 7444-7452. [CrossRef]
- Lin, J., Wang, Q., Wang, X., Zhu, Y., Zhou, X., & Wei, H. (2020). Gold alloy-based nanozyme sensor arrays for biothiol detection. Analyst, 145(11), 3916-3921. [CrossRef]
- Park, J. S., & Choi, J. S. (2022). Platinum nanozyme-hydrogel composite (PtNZHG)-impregnated cascade sensing system for one-step glucose detection in serum, urine, and saliva. Sensors and Actuators B: Chemical, 359, 131585.
- Wang, J., Su, P., Li, D., Wang, T., & Yang, Y. (2017). Fabrication of CeO2/rGO nanocomposites with oxidase-like activity and their application in colorimetric sensing of ascorbic acid. Chemical Research in Chinese Universities, 33(4), 540-545.
- Wang, Y., Cho, A., Jia, G., Cui, X., Shin, J., Nam, I., ... & Han, J. W. (2023). Tuning Local Coordination Environments of Manganese Single-Atom Nanozymes with Multi-Enzyme Properties for Selective Colorimetric Biosensing. Angewandte Chemie, 135(15), e202300119. [CrossRef]
- Wang, S., Jin, Y., Ai, W., Wang, X., Zhang, Z., Zhou, T., ... & Wang, F. (2023). H2O2 actuated molybdenum oxide nanodots: Multi-enzyme-like activities, leverage of Fenton reaction, and dual-mode sensitive detection of alendronate sodium. Nano Research, 1-10. [CrossRef]
- Tai, S., Wang, J., Sun, F., Pan, Q., Peng, C. F., & Wang, Z. A Colorimetric Sensor Array Based on Nanoceria Crosslinked and Heteroatom-Doped Graphene Oxide Nanoribbons for the Detection and Discrimination of Multiple Pesticides. Available at SSRN 4348586.
- Deng, H. H., Hong, G. L., Lin, F. L., Liu, A. L., Xia, X. H., & Chen, W. (2016). Colorimetric detection of urea, urease, and urease inhibitor based on the peroxidase-like activity of gold nanoparticles. Analytica chimica acta, 915, 74-80. [CrossRef]
- Jangi, S. R. H., Davoudli, H. K., Delshad, Y., Jangi, M. R. H., & Jangi, A. R. H. (2020). A novel and reusable multinanozyme system for sensitive and selective quantification of hydrogen peroxide and highly efficient degradation of organic dye. Surfaces and Interfaces, 21, 100771. [CrossRef]
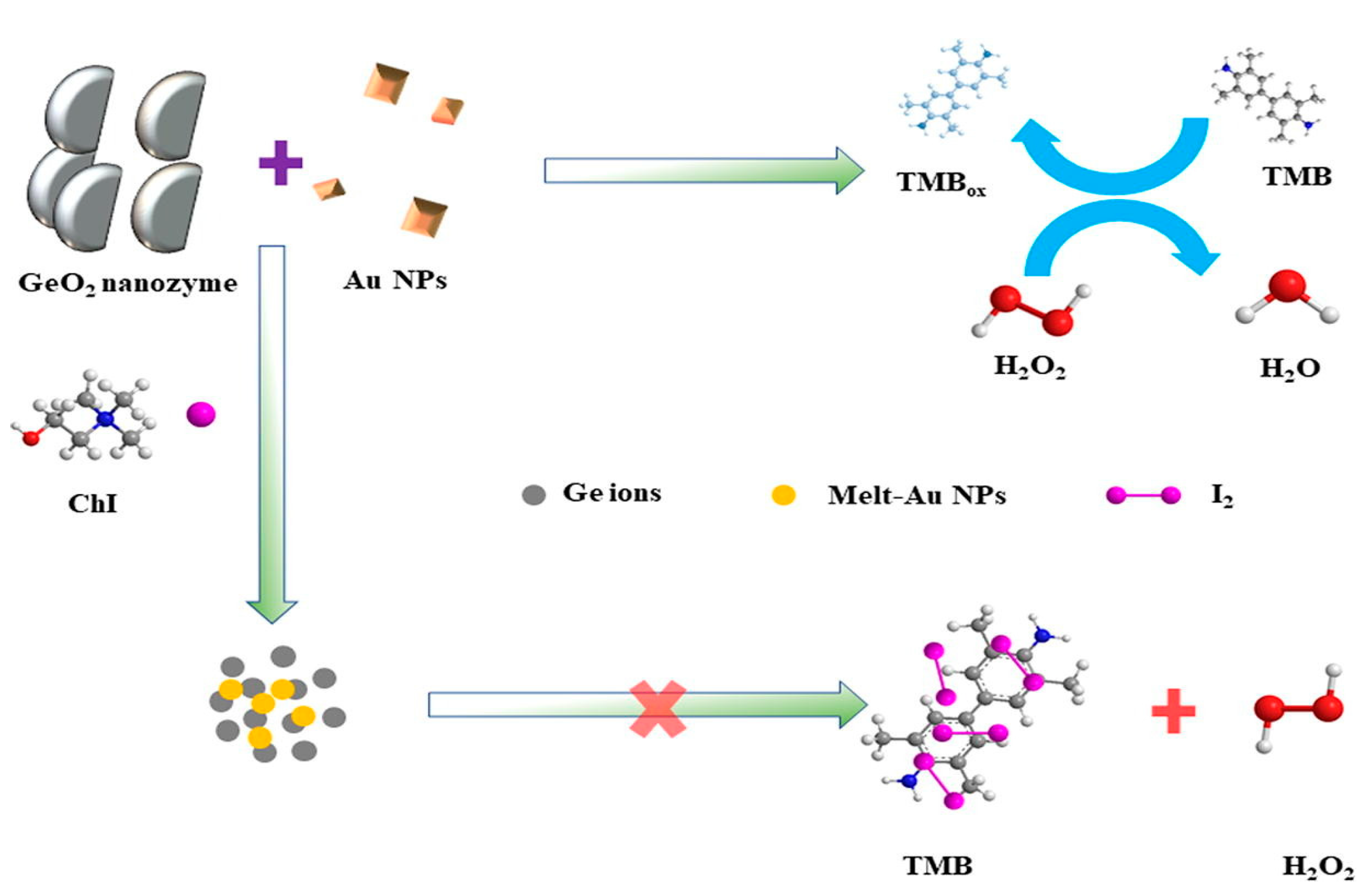
- Tang, R., Xia, X., Zhang, X., Jiang, H., Wang, B., Zhang, P., ... & Zhou, Y. (2022). Synergistic function of Au NPs/GeO2 nanozymes with enhanced peroxidase-like activity and SERS effect to detect choline iodide. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 266, 120467. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




