Submitted:
31 August 2023
Posted:
01 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
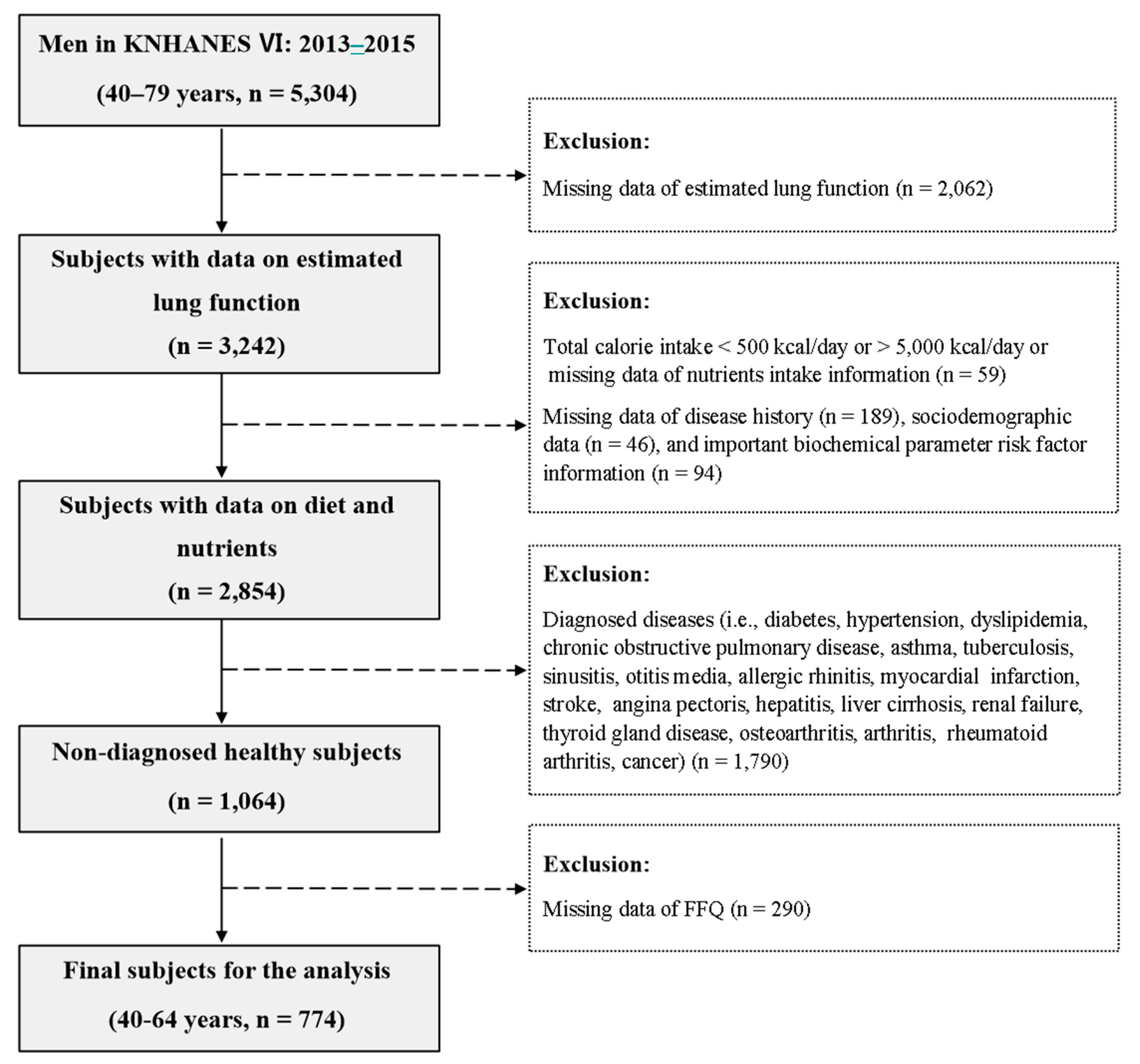
2.1. Subjects
2.2. Assessment of Dietary Patterns
2.3. Assessment of COPD
2.4. Basic Information
2.5. Anthropometric and Biochemical Parameters
2.6. Statistical Analysis
3. Results
3.1. General Characteristics of Participants According to Smoking Status
3.2. Anthropometric and Biochemical Parameters and Lung Function Measurements of Participants According to Smoking Status
3.3. Nutrient Intake Information and DAL Levels of Participants According to Smoking Status
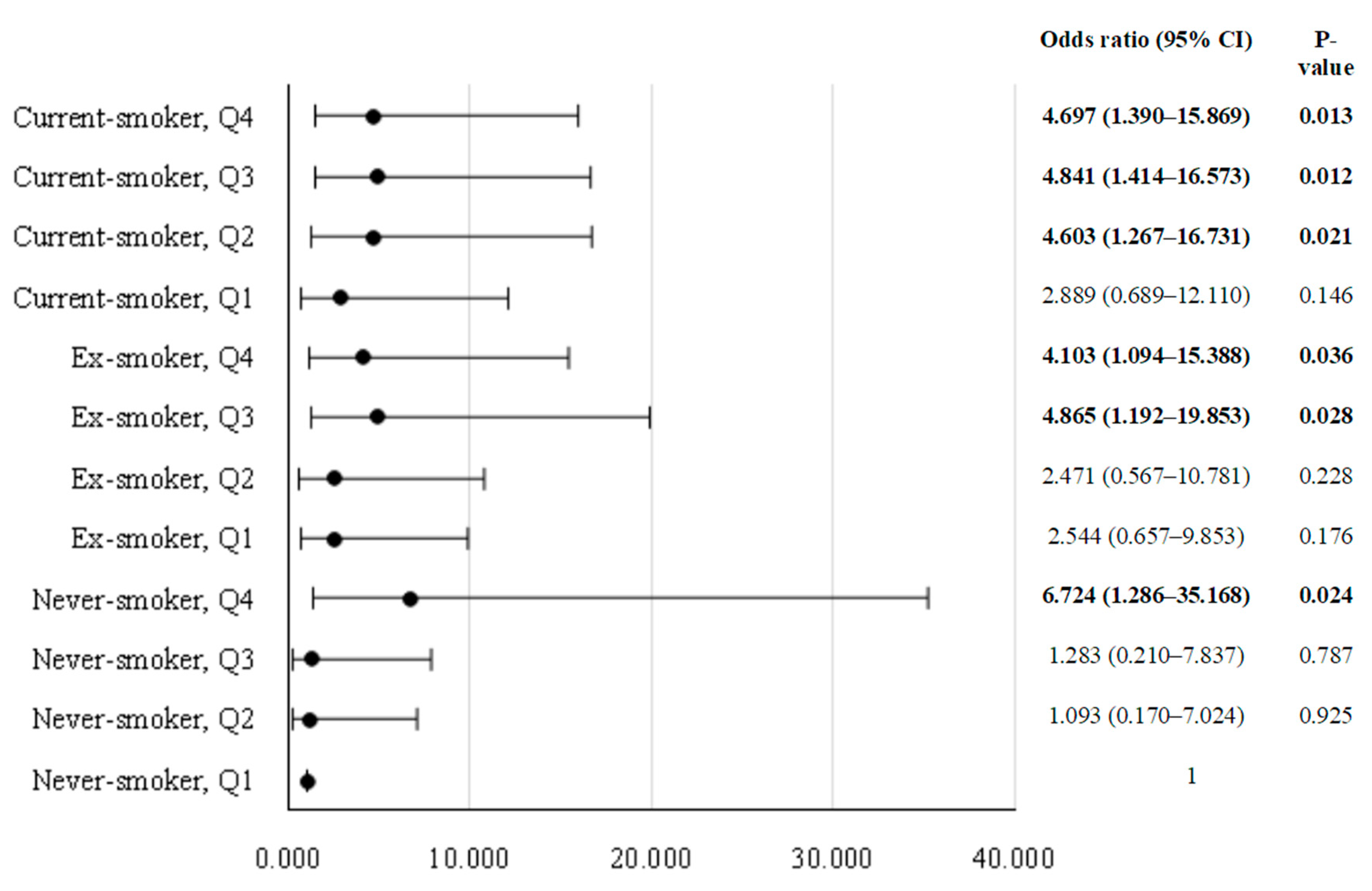
3.4. Association between Smoking Status and Risk of COPD
3.5. Association between NEAP Scores and Risk of COPD
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar] [CrossRef]
- World Health Organization (WHO) facts about Chronic obstructive pulmonary disease (COPD). https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) Web site. Updated 2022. Accessed November 20, 2022. 20 November.
- Kyung Sook, C. Current Status of Chronic Obstructive Pulmonary Disease (COPD) in the Republic of Korea. Division of Chronic Disease Control, Bureau of Chronic Disease Control, Korea Disease Control and Prevention Agency (KDCA). 2021; 949-951.
- Riley, C.M.; Sciurba, F.C. Diagnosis and Outpatient Management of Chronic Obstructive Pulmonary Disease: A Review. JAMA 2019, 321, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S. Tobacco smoking and environmental risk factors for chronic obstructive pulmonary disease. Clin Chest Med. 2014, 35, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.D. COPD and the response of the lung to tobacco smoke exposure. Pulm Pharmacol Ther. 2010, 23, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Godtfredsen, N.S.; Lam, T.H.; Hansel, T.T.; et al. COPD-related morbidity and mortality after smoking cessation: status of the evidence. Eur Respir J. 2008, 32, 844–853. [Google Scholar] [CrossRef]
- Tonnesen, P. Smoking cessation and COPD. Eur Respir Rev. 2013, 22, 37–43. [Google Scholar] [CrossRef]
- Temitayo Orisasami, I.; Ojo, O. Evaluating the effectiveness of smoking cessation in the management of COPD. Br J Nurs. 2016, 25, 786–791. [Google Scholar] [CrossRef]
- Barnes, P.J.; Burney, P.G.J.; Silverman, E.K.; et al. Chronic obstructive pulmonary disease. Nat Rev Dis Primers. 2015, 1, 15076. [Google Scholar] [CrossRef]
- Hu, G.; Zhou, Y.; Tian, J.; et al. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest. 2010, 138, 20–31. [Google Scholar] [CrossRef]
- Brandsma, C.; de Vries, M.; Costa, R.; Woldhuis, R.R.; Konigshoff, M.; Timens, W. Lung ageing and COPD: is there a role for ageing in abnormal tissue repair? Eur Respir Rev. 2017, 26, 170073. [Google Scholar] [CrossRef]
- Kim, E.; Yoon, S.; Kim, Y.; Go, D.; Jung, Y. Effects of Aging and Smoking Duration on Cigarette Smoke-Induced COPD Severity. J Korean Med Sci. 2018, 34 (Suppl 1). [Google Scholar] [CrossRef]
- Scoditti, E.; Massaro, M.; Garbarino, S.; Toraldo, D.M. Role of Diet in Chronic Obstructive Pulmonary Disease Prevention and Treatment. Nutrients. 2019, 11, E1357. [Google Scholar] [CrossRef] [PubMed]
- Varraso, R.; Chiuve, S.E.; Fung, T.T.; et al. Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: prospective study. BMJ. 2015, 350, h286. [Google Scholar] [CrossRef] [PubMed]
- Voortman, T.; Kiefte-de Jong, J.C.; Ikram, M.A.; et al. Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam Study. Eur J Epidemiol. 2017, 32, 993–1005. [Google Scholar] [CrossRef]
- Fischer, A.; Johansson, I.; Blomberg, A.; Sundstrom, B. Adherence to a Mediterranean-like Diet as a Protective Factor Against COPD: A Nested Case-Control Study. COPD. 2019, 16, 272–277. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients. 2020, 12, E334. [Google Scholar] [CrossRef]
- Ostrowska, J.; Janiszewska, J.; Szostak-Wegierek, D. Dietary Acid Load and Cardiometabolic Risk Factors-A Narrative Review. Nutrients. 2020, 12, E3419. [Google Scholar] [CrossRef]
- Osuna-Padilla, I.A.; Leal-Escobar, G.; Garza-Garcia, C.A.; Rodriguez-Castellanos, F.E. Dietary Acid Load: mechanisms and evidence of its health repercussions. Nefrologia (Engl Ed). 2019, 39, 343–354. [Google Scholar] [CrossRef]
- Carnauba, R.A.; Baptistella, A.B.; Paschoal, V.; Hubscher, G.H. Diet-Induced Low-Grade Metabolic Acidosis and Clinical Outcomes: A Review. Nutrients. 2017, 9, E538. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. 1994, 59, 1356–1361. [Google Scholar] [CrossRef]
- Frassetto, L.A.; Todd, K.M.; Morris, R.C.J.; Sebastian, A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. 1998, 68, 576–583. [Google Scholar] [CrossRef]
- Dehghan, P.; Abbasalizad Farhangi, M. Dietary acid load, blood pressure, fasting blood sugar and biomarkers of insulin resistance among adults: Findings from an updated systematic review and meta-analysis. Int J Clin Pract. 2020, 74, e13471. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, Z.; Liang, Y.; Wang, P.; Peng, J. Elevated hypertension risk associated with higher dietary acid load: A systematic review and meta-analysis. Clin Nutr ESPEN. 2019, 33, 171–177. [Google Scholar] [CrossRef]
- Abbasalizad Farhangi, M.; Nikniaz, L.; Nikniaz, Z. Higher dietary acid load potentially increases serum triglyceride and obesity prevalence in adults: An updated systematic review and meta-analysis. PLoS One. 2019, 14, e0216547. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.; Moreira, A.; Moreira, P.; Delgado, L. Dietary diversity and childhood asthma-Dietary acid load, an additional nutritional variable to consider. Allergy. 2020, 75, 2418–2420. [Google Scholar] [CrossRef] [PubMed]
- Malmir, H.; Onvani, S.; Ardestani, M.E.; Feizi, A.; Azadbakht, L.; Esmaillzadeh, A. Adherence to Low Carbohydrate Diet in Relation to Chronic Obstructive Pulmonary Disease. Front Nutr. 2021, 8, 690880. [Google Scholar] [CrossRef]
- Kane, K.K. The role of the lungs in the adjustment of acid-base balance. Ann Clin Lab Sci. 1973, 3, 323–328. [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease, (GOLD). GLOBAL STRATEGY FOR PREVENTION, DIAGNOSIS AND MANAGEMENT OF COPD: 2023 Report. 2022, 22-29.
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Duffy, S.P.; Criner, G.J. Chronic Obstructive Pulmonary Disease: Evaluation and Management. Med Clin North Am. 2019, 103, 453–461. [Google Scholar] [CrossRef]
- Nacul, L.C.; Soljak, M.; Meade, T. Model for estimating the population prevalence of chronic obstructive pulmonary disease: cross sectional data from the Health Survey for England. Popul Health Metr. 2007, 5, 8–8. [Google Scholar] [CrossRef]
- Safiri, S.; Carson-Chahhoud, K.; Noori, M.; et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990-2019: results from the Global Burden of Disease Study 2019. BMJ. 2022, 378, e069679–e069679. [Google Scholar] [CrossRef]
- Hikichi, M.; Mizumura, K.; Maruoka, S.; Gon, Y. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J Thorac Dis. 2019, 11 (Suppl 17), S2129–S2140. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pleasants, R.A.; Croft, J.B.; et al. Smoking duration, respiratory symptoms, and COPD in adults aged >/=45 years with a smoking history. Int J Chron Obstruct Pulmon Dis. 2015, 10, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.J.; Lee, M.S.; Jang, K.W.; Ahn, J.B.; Hurh, K.; Park, E. Association between smoking cessation and obstructive spirometry pattern among Korean adults aged 40-79 years. Sci Rep. 2021, 11, 18667–9. [Google Scholar] [CrossRef] [PubMed]
- Szmidt, M.K.; Kaluza, J.; Harris, H.R.; Linden, A.; Wolk, A. Long-term dietary fiber intake and risk of chronic obstructive pulmonary disease: a prospective cohort study of women. Eur J Nutr. 2020, 59, 1869–1879. [Google Scholar] [CrossRef]
- Kaluza, J.; Larsson, S.C.; Orsini, N.; Linden, A.; Wolk, A. Fruit and vegetable consumption and risk of COPD: a prospective cohort study of men. Thorax. 2017, 72, 500–509. [Google Scholar] [CrossRef]
- Yang, I.A.; Jenkins, C.R.; Salvi, S.S. Chronic obstructive pulmonary disease in never-smokers: risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir Med. 2022, 10, 497–511. [Google Scholar] [CrossRef]
- Remer, T.; Dimitriou, T.; Manz, F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am J Clin Nutr. 2003, 77, 1255–1260. [Google Scholar] [CrossRef]
- Hamidianshirazi, M.; Ekramzadeh, M. Dietary Acid Load and Chronic Kidney Disease. Saudi J Kidney Dis Transpl. 2021, 32, 1511–1522. [Google Scholar] [CrossRef]
- Toba, K.; Hosojima, M.; Kabasawa, H.; et al. Higher estimated net endogenous acid production with lower intake of fruits and vegetables based on a dietary survey is associated with the progression of chronic kidney disease. BMC Nephrol. 2019, 20, 421–8. [Google Scholar] [CrossRef]
- López, M.; Moreno, G.; Lugo, G.; Marcano, G. Dietary acid load in children with chronic kidney disease. Eur J Clin Nutr. 2020, 74 (Suppl 1), 57–62. [Google Scholar] [CrossRef] [PubMed]
- Nagami, G.T.; Hamm, L.L. Regulation of Acid-Base Balance in Chronic Kidney Disease. Adv Chronic Kidney Dis. 2017, 24, 274–279. [Google Scholar] [CrossRef]
- Cunha, P.; Paciencia, I.; Cavaleiro Rufo, J.; et al. Dietary Acid Load: A Novel Nutritional Target in Overweight/Obese Children with Asthma? Nutrients. 2019, 11, E2255. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.S.; Kozan, P.; Samocha-Bonet, D. The role of dietary acid load and mild metabolic acidosis in insulin resistance in humans. Biochimie. 2016, 124, 171–177. [Google Scholar] [CrossRef]
- Remer, T. Influence of diet on acid-base balance. Semin Dial. 2000, 13, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Leme, A.S.; Shapiro, S.D. Emerging genetics of COPD. EMBO Mol Med. 2012, 4, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Silverman, E.K. Genetics of COPD. Annu Rev Physiol. 2020, 82, 413–431. [Google Scholar] [CrossRef]
- Agusti, A.; Melen, E.; DeMeo, D.L.; Breyer-Kohansal, R.; Faner, R. Pathogenesis of chronic obstructive pulmonary disease: understanding the contributions of gene-environment interactions across the lifespan. Lancet Respir Med. 2022, 10, 512–524. [Google Scholar] [CrossRef]
| Variables | Non-smokers (n = 168) |
Ex-smokers (n = 272) |
Current-smokers (n = 334) |
Total (n = 774) |
P-value |
|---|---|---|---|---|---|
| Age (y) | 49.05 ± 0.49 | 50.75 ± 0.40 | 48.95 ± 0.36 | 49.59 ± 0.24 | 0.001 |
| Education period (y) | |||||
| ≤ 12 | 81 (47.9%) | 158 (59.9%) | 206 (63.6%) | 445 (58.9%) | 0.009 |
| > 12 | 87 (52.1%) | 114 (40.1%) | 128 (36.4%) | 329 (41.1%) | |
| Household income status | |||||
| Lowest | 7 (4.2%) | 18 (5.9%) | 27 (7.4%) | 52 (6.2%) | 0.208 |
| Lower middle | 40 (25.2%) | 59 (24.9%) | 74 (23.5%) | 173 (24.3%) | |
| Upper middle | 44 (27.4%) | 84 (30.9%) | 118 (37.2%) | 246 (32.9%) | |
| Highest | 77 (43.2%) | 111 (38.3%) | 115 (31.9%) | 303 (36.5%) | |
| Current drinker | 104 (64.1%) | 203 (73.2%) | 280 (85.0%) | 587 (76.5%) | < 0.001 |
| COPD status, n (%) | 11 (6.4%) | 33 (12.0%) | 49 (12.8%) | 93 (11.1%) | 0.146 |
| Variables | Non-smokers (n = 168) |
Ex-smokers (n = 272) |
Current-smokers (n = 334) |
Total (n = 774) |
P0 | P1 | P2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 24.24 | ± | 0.23 | 24.58 | ± | 0.19 | 24.21 | ± | 0.16 | 24.34 | ± | 0.11 | 0.319 | ||
| Height (cm) | 169.99 | ± | 0.51 | 170.44 | ± | 0.38 | 170.58 | ± | 0.38 | 170.34 | ± | 0.25 | 0.654 | 0.208 | 0.207 |
| Weight (kg) | 70.11 | ± | 0.73 | 71.53 | ± | 0.68 | 70.56 | ± | 0.57 | 70.73 | ± | 0.38 | 0.334 | 0.174 | 0.174 |
| Waist circumference (cm) | 83.86 | ± | 0.62 | 85.57 | ± | 0.52 | 84.34 | ± | 0.44 | 84.59 | ± | 0.31 | 0.079 | 0.206 | 0.209 |
| SBP (mmHg) | 116.44 | ± | 0.97 | 119.20 | ± | 0.81 | 117.34 | ± | 0.82 | 117.66 | ± | 0.51 | 0.065 | 0.228 | 0.183 |
| DBP (mmHg) | 79.86 | ± | 0.71 | 81.02 | ± | 0.66 | 78.69 | ± | 0.56 | 79.86 | ± | 0.37 | 0.024 | 0.006 | 0.004 |
| Fasting glucose (mg/dL) * | 97.94 | ± | 1.23 | 101.49 | ± | 1.18 | 103.09 | ± | 1.35 | 100.84 | ± | 0.72 | 0.008 | 0.053 | 0.094 |
| HbA1c (%)*† | 5.59 | ± | 0.04 | 5.73 | ± | 0.04 | 5.85 | ± | 0.04 | 5.72 | ± | 0.02 | < 0.001 | < 0.001 | < 0.001 |
| Total cholesterol (mg/dL) | 196.55 | ± | 2.58 | 199.45 | ± | 2.38 | 197.54 | ± | 1.91 | 197.85 | ± | 1.29 | 0.704 | 0.766 | 0.762 |
| Triglyceride (mg/dL) * | 142.30 | ± | 8.58 | 170.75 | ± | 10.20 | 201.97 | ± | 9.47 | 171.67 | ± | 5.36 | < 0.001 | < 0.001 | < 0.001 |
| HDL-cholesterol (mg/dL) | 46.03 | ± | 0.73 | 48.04 | ± | 0.74 | 45.82 | ± | 0.67 | 46.63 | ± | 0.42 | 0.051 | 0.002 | 0.003 |
| LDL-cholesterol (mg/dL) | 124.32 | ± | 2.37 | 121.46 | ± | 2.24 | 118.22 | ± | 1.86 | 121.33 | ± | 1.23 | 0.121 | 0.412 | 0.410 |
| AST (IU/L) * | 24.42 | ± | 0.82 | 24.83 | ± | 0.75 | 22.96 | ± | 0.44 | 24.07 | ± | 0.42 | 0.059 | 0.039 | 0.029 |
| ALT (IU/L) * | 27.46 | ± | 1.49 | 27.36 | ± | 1.55 | 24.75 | ± | 0.81 | 26.52 | ± | 0.78 | 0.270 | 0.284 | 0.193 |
| BUN (mg/dL) | 15.70 | ± | 0.32 | 15.71 | ± | 0.23 | 14.20 | ± | 0.21 | 15.20 | ± | 0.15 | < 0.001 | < 0.001 | < 0.001 |
| Creatinine (mg/dL) | 0.99 | ± | 0.01 | 0.97 | ± | 0.01 | 0.95 | ± | 0.01 | 0.97 | ± | 0.00 | 0.003 | 0.010 | 0.014 |
| Urine pH† | 5.82 | ± | 0.07 | 5.58 | ± | 0.05 | 5.54 | ± | 0.05 | 5.65 | ± | 0.03 | 0.007 | 0.004 | 0.005 |
| Urine creatinine (mg/dL)† | 180.94 | ± | 6.94 | 169.22 | ± | 5.09 | 184.84 | ± | 5.11 | 178.34 | ± | 3.38 | 0.061 | 0.146 | 0.155 |
| eGFR (mL/min/1.73m2) | 93.06 | ± | 0.88 | 93.95 | ± | 0.71 | 96.82 | ± | 0.67 | 94.61 | ± | 0.43 | 0.001 | 0.011 | 0.012 |
| FEV1 (% of predicted) | 93.62 | ± | 0.97 | 92.14 | ± | 0.78 | 91.22 | ± | 0.70 | 92.33 | ± | 0.48 | 0.140 | 0.088 | 0.099 |
| FVC (% of predicted) | 94.82 | ± | 0.91 | 94.70 | ± | 0.71 | 94.25 | ± | 0.65 | 94.59 | ± | 0.44 | 0.848 | 0.513 | 0.513 |
| FEV1/FVC | 0.79 | ± | 0.00 | 0.77 | ± | 0.00 | 0.77 | ± | 0.00 | 0.78 | ± | 0.00 | 0.009 | 0.040 | 0.053 |
| Nutrient intake (per day) | Non-smoker (n = 168) |
Ex-smoker /(n = 272) |
Current-smoker (n = 334) |
Total (n = 774) |
P0 | P1 | P2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCI (kcal) | 2390.44 | ± | 71.74 | 2499.39 | ± | 55.18 | 2481.05 | ± | 52.75 | 2456.96 | ± | 34.78 | 0.480 | ||
| Carbohydrate (% of TCI) | 64.38 | ± | 1.05 | 62.68 | ± | 0.85 | 58.30 | ± | 0.94 | 61.79 | ± | 0.55 | < 0.001 | < 0.001 | 0.022 |
| Protein (% of TCI) | 13.97 | ± | 0.36 | 13.75 | ± | 0.22 | 13.67 | ± | 0.23 | 13.80 | ± | 0.16 | 0.800 | 0.850 | 0.063 |
| Fat (% of TCI) | 17.95 | ± | 0.63 | 17.87 | ± | 0.48 | 18.15 | ± | 0.47 | 17.99 | ± | 0.30 | 0.917 | 0.793 | 0.928 |
| Dietary cholesterol (mg) | 269.94 | ± | 20.42 | 301.39 | ± | 16.71 | 323.70 | ± | 18.11 | 298.34 | ± | 10.49 | 0.159 | 0.489 | 0.645 |
| Dietary fiber (g) | 30.68 | ± | 1.18 | 30.43 | ± | 0.87 | 25.05 | ± | 0.77 | 28.72 | ± | 0.58 | < 0.001 | < 0.001 | < 0.001 |
| Calcium (mg) | 583.39 | ± | 26.33 | 608.02 | ± | 20.98 | 564.65 | ± | 17.63 | 585.35 | ± | 13.32 | 0.280 | 0.412 | 0.594 |
| Phosphorus (mg) | 1295.98 | ± | 40.89 | 1344.42 | ± | 31.08 | 1278.50 | ± | 31.53 | 1306.30 | ± | 20.76 | 0.281 | 0.332 | 0.235 |
| Iron (mg) | 20.11 | ± | 0.69 | 21.47 | ± | 0.56 | 20.28 | ± | 0.77 | 20.62 | ± | 0.41 | 0.196 | 0.585 | 0.668 |
| Sodium (mg)* | 4830.46 | ± | 250.10 | 4696.02 | ± | 140.66 | 4841.71 | ± | 172.78 | 4789.40 | ± | 111.03 | 0.968 | 0.410 | 0.485 |
| Potassium (mg)* | 3794.60 | ± | 160.81 | 3836.63 | ± | 123.92 | 3301.56 | ± | 86.13 | 3644.27 | ± | 76.52 | < 0.001 | < 0.001 | 0.022 |
| NEAP (mEq/day)* | 39.19 | ± | 1.36 | 41.63 | ± | 1.41 | 46.87 | ± | 1.25 | 42.56 | ± | 0.78 | < 0.001 | 0.001 | |
| NEAP (quartile) | |||||||||||||||
| Q1 | 54 (32.9%) | 80 (29.5%) | 60 (17.0%) | 194 (24.7%) | 0.001 | ||||||||||
| Q2 | 46 (26.0%) | 63 (22.9%) | 85 (23.5%) | 194 (23.8%) | |||||||||||
| Q3 | 36 (21.5%) | 61 (21.5%) | 96 (30.8%) | 193 (25.6%) | |||||||||||
| Q4 | 32 (19.6%) | 68 (26.1%) | 93 (28.8%) | 193 (25.9%) | |||||||||||
| Model | Non-smoker (n = 168)(reference group) | Ex-smoker (n = 272) | Current-smoker (n = 334) | P-value for pattern | ||
|---|---|---|---|---|---|---|
| ORs (95% CIs) | P-value | ORs (95% CIs) | P-value | |||
| Model 1 | 1 | 1.974 (0.869–4.485) | 0.104 | 2.130 (0.975–4.654) | 0.058 | 0.161 |
| Model 2 | 1 | 1.700 (0.738–3.916) | 0.212 | 2.189 (0.992–4.831) | 0.052 | 0.144 |
| Model 3 | 1 | 1.711 (0.740–3.953) | 0.209 | 2.173 (0.989–4.772) | 0.053 | 0.146 |
| Model 4 | 1 | 1.680 (0.723–3.900) | 0.227 | 2.140 (0.969–4.725) | 0.060 | 0.159 |
| Model 5 | 1 | 1.654 (0.700–3.910) | 0.251 | 2.044 (0.904–4.620) | 0.086 | 0.217 |
| Model 6 | 1 | 1.745 (0.722–4.216) | 0.216 | 2.228 (0.931–5.333) | 0.072 | 0.188 |
| Model 7 | 1 | 1.711 (0.706–4.149) | 0.234 | 2.070 (0.852–5.031) | 0.108 | 0.272 |
| Model | ORs (95% CIs) | |||
|---|---|---|---|---|
| Q1 (Reference group)(n=194) | Q2 (n=194) | Q3(n=193) | Q4(n=193) | |
| Model 1 | 1 | 1.055 (0.486-2.292) | 1.443 (0.705-2.955) | 1.689 (0.841-3.389) |
| Model 2 | 1 | 1.287 (0.574-2.885) | 1.831 (0.858-3.907) | 2.171 (1.039-4.535)* |
| Model 3 | 1 | 1.284 (0.568-2.904) | 1.834 (0.843-3.992) | 2.028 (0.951-4.322)† |
| Model 4 | 1 | 1.353 (0.586-3.126) | 1.928 (0.861-4.315) | 2.172 (0.980-4.811) |
| Model 5 | 1 | 1.293 (0.566-2.957) | 1.765 (0.787-3.962) | 2.011 (0.886-4.564) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





