Submitted:
01 September 2023
Posted:
04 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
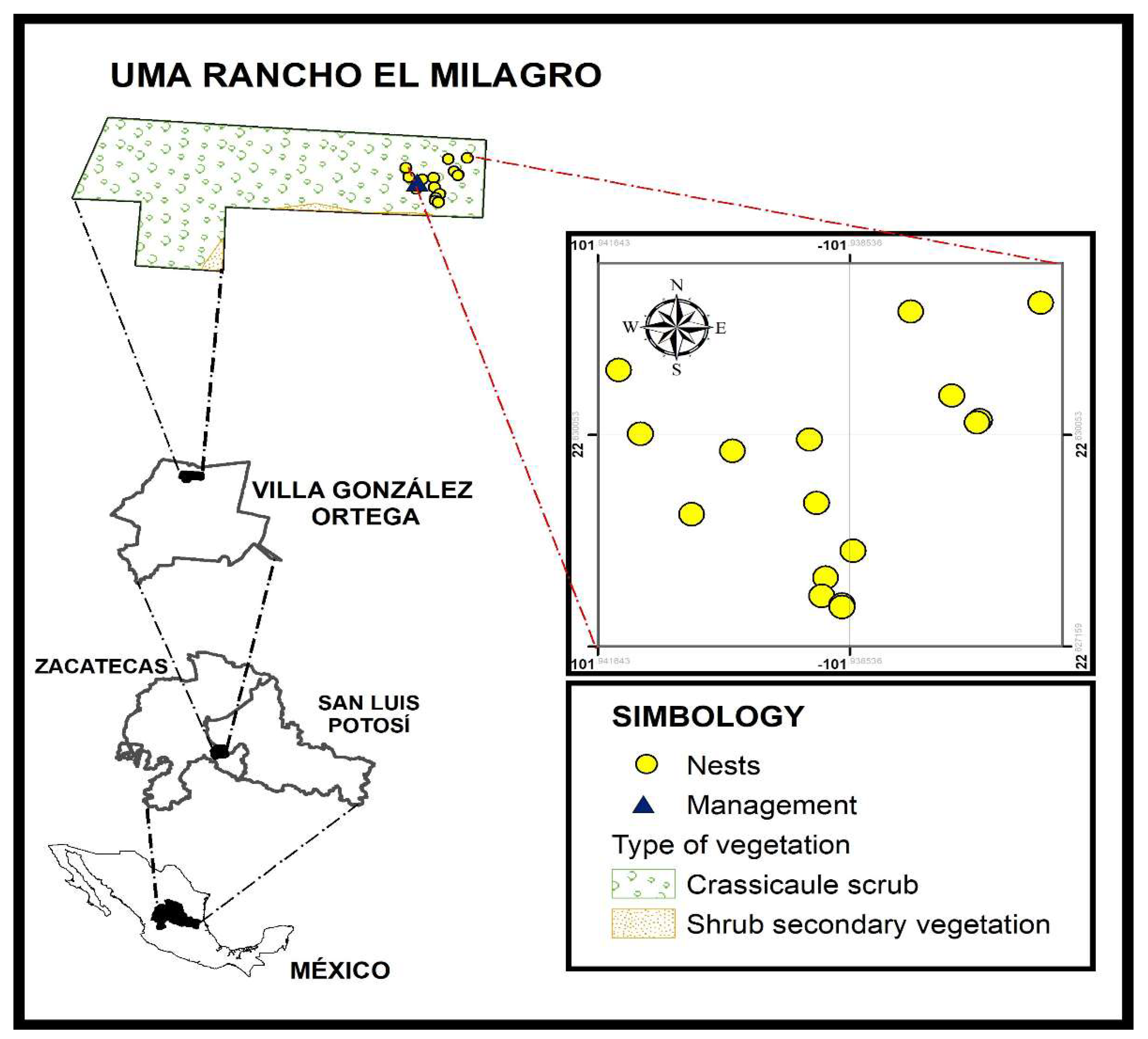
2.1. Air sampling inside the nests
2.2. Sampling on foraging trails and ant counts
2.3. Ant trapping
2.4. Laboratory analysis
2.4.1. Determination of semiochemicals with the electronic nose (e-nose)
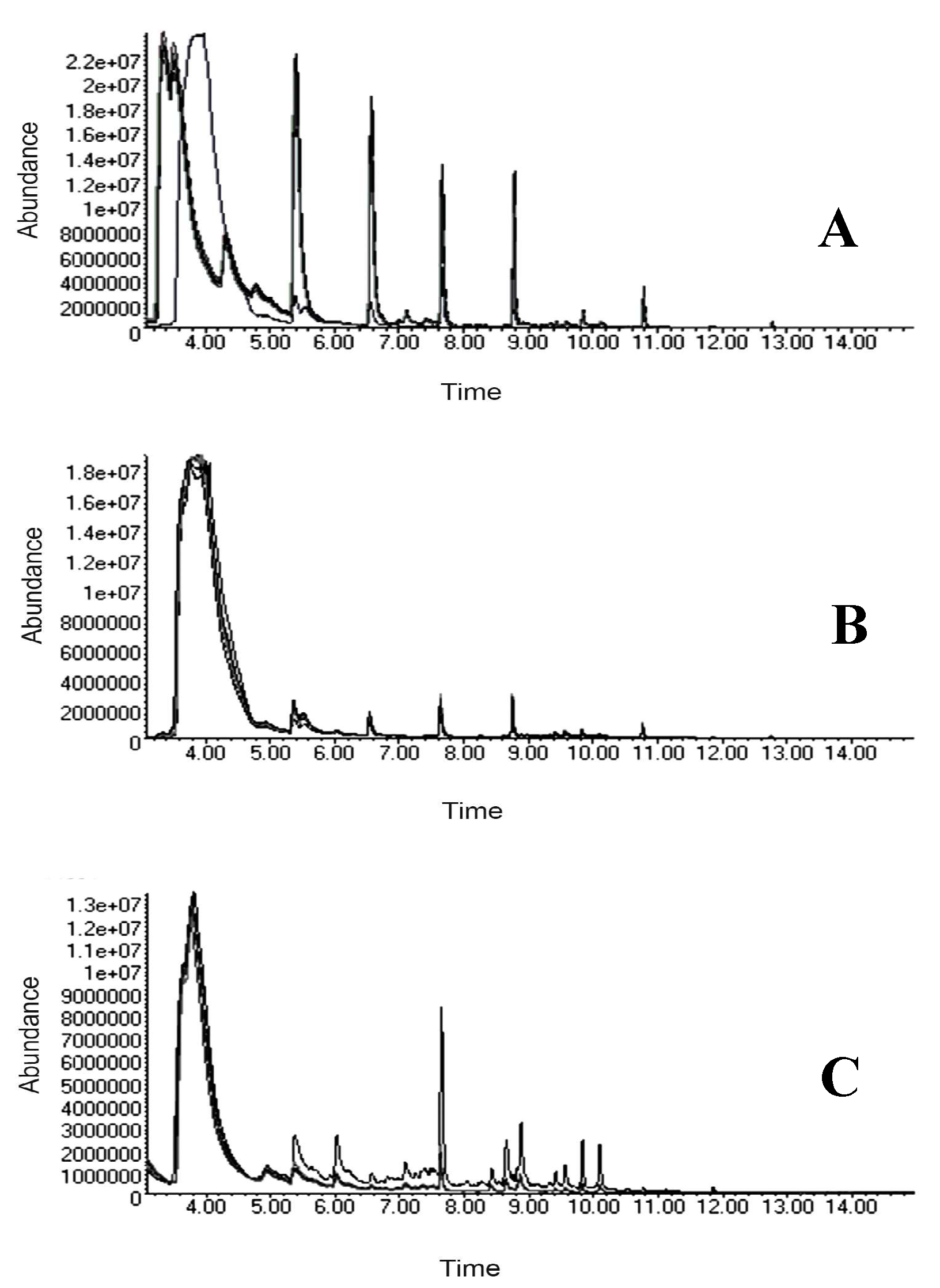
2.4.2. Identification of semiochemicals by gas chromatography coupled to mass spectrometry (GC-MS) on activated carbon cloths
2.4.3. Identification of semiochemicals by GC-MS inside nests
2.4.4. Identification of semiochemicals by GC-MS of ant gasters
2.5. Statistical analysis
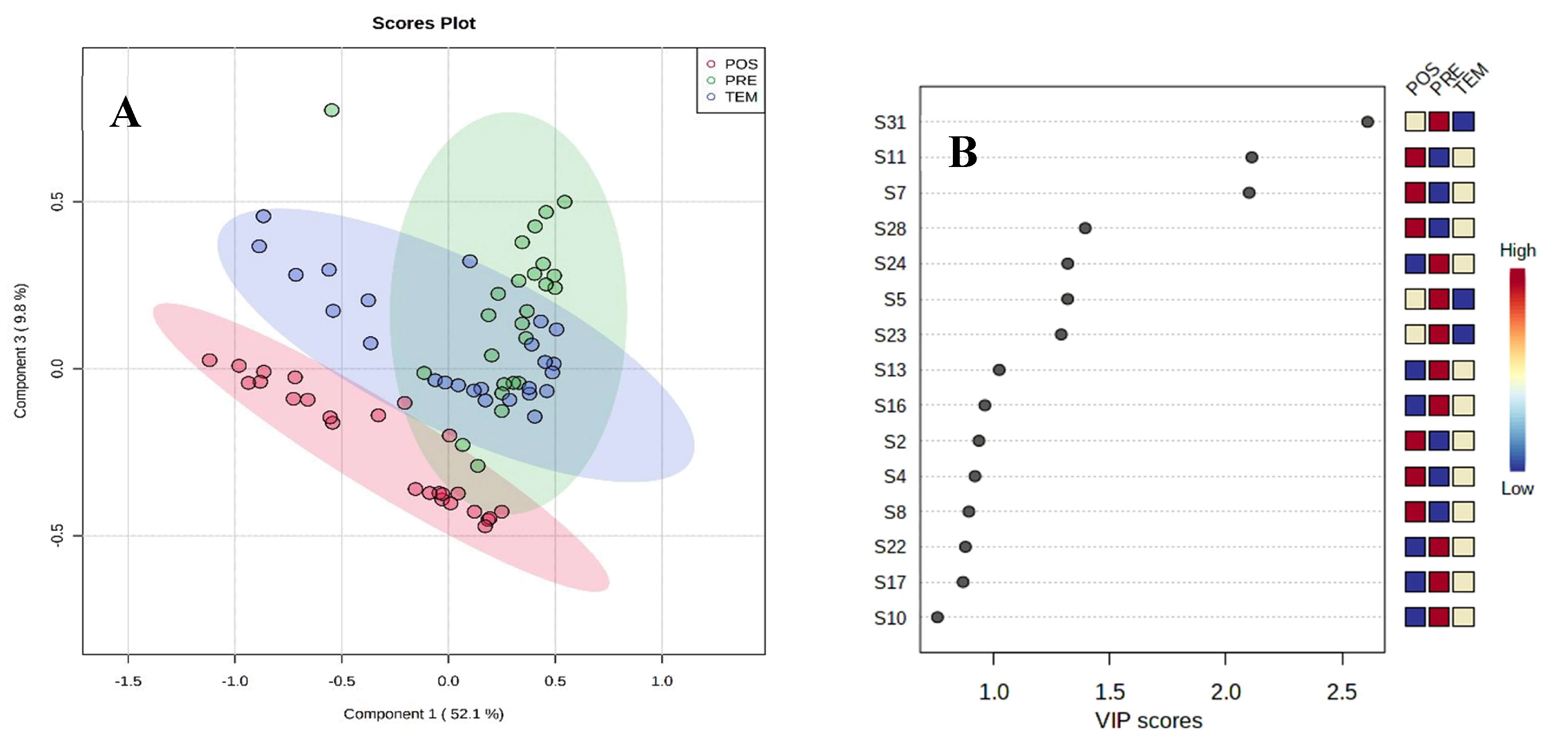
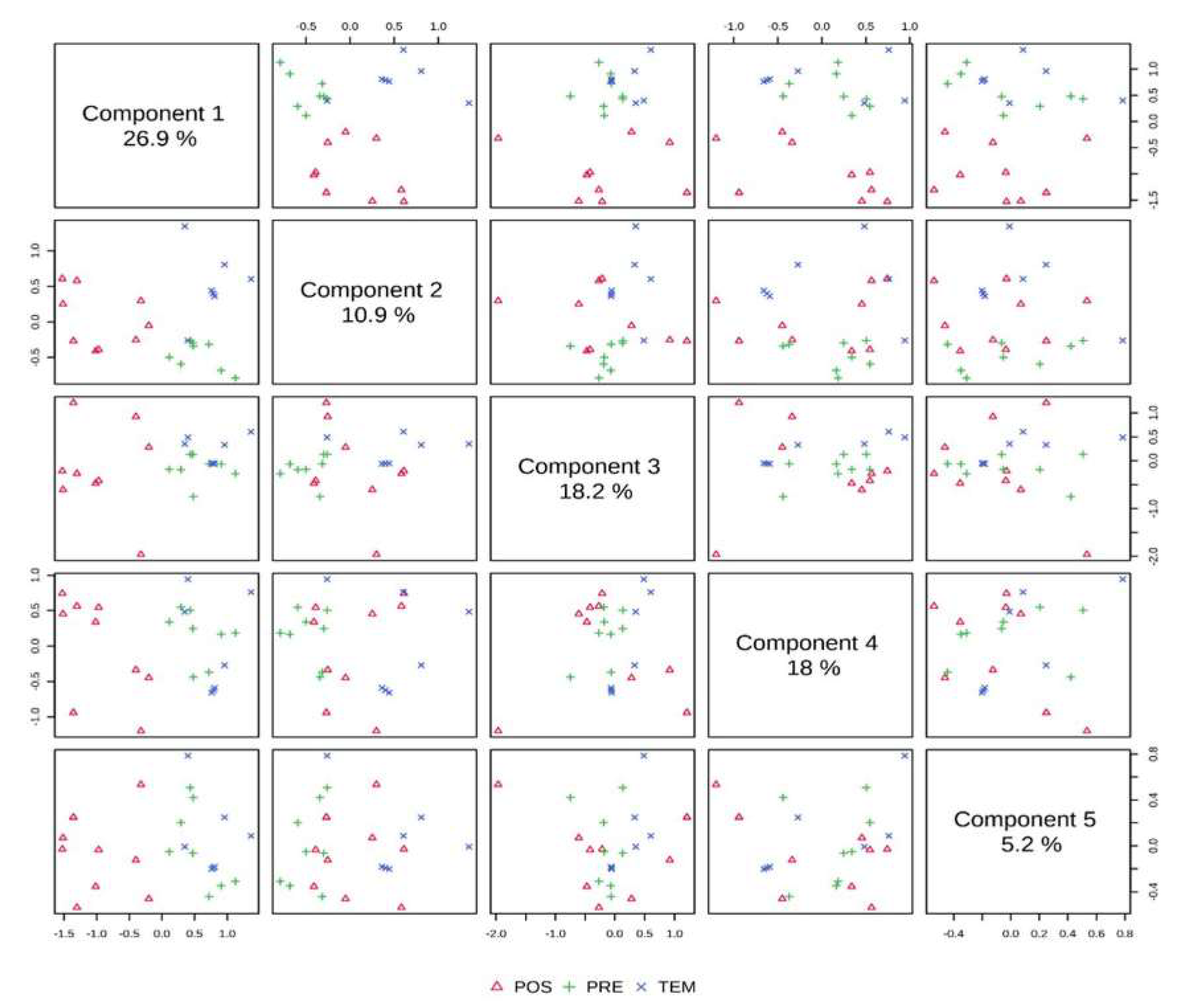
2.5.1. Analysis of data obtained from the e-nose
2.5.2. Analysis of data obtained with CG-SM on nests
2.5.3. Ant counts, effect of semiochemicals on observed frequencies
3. Results
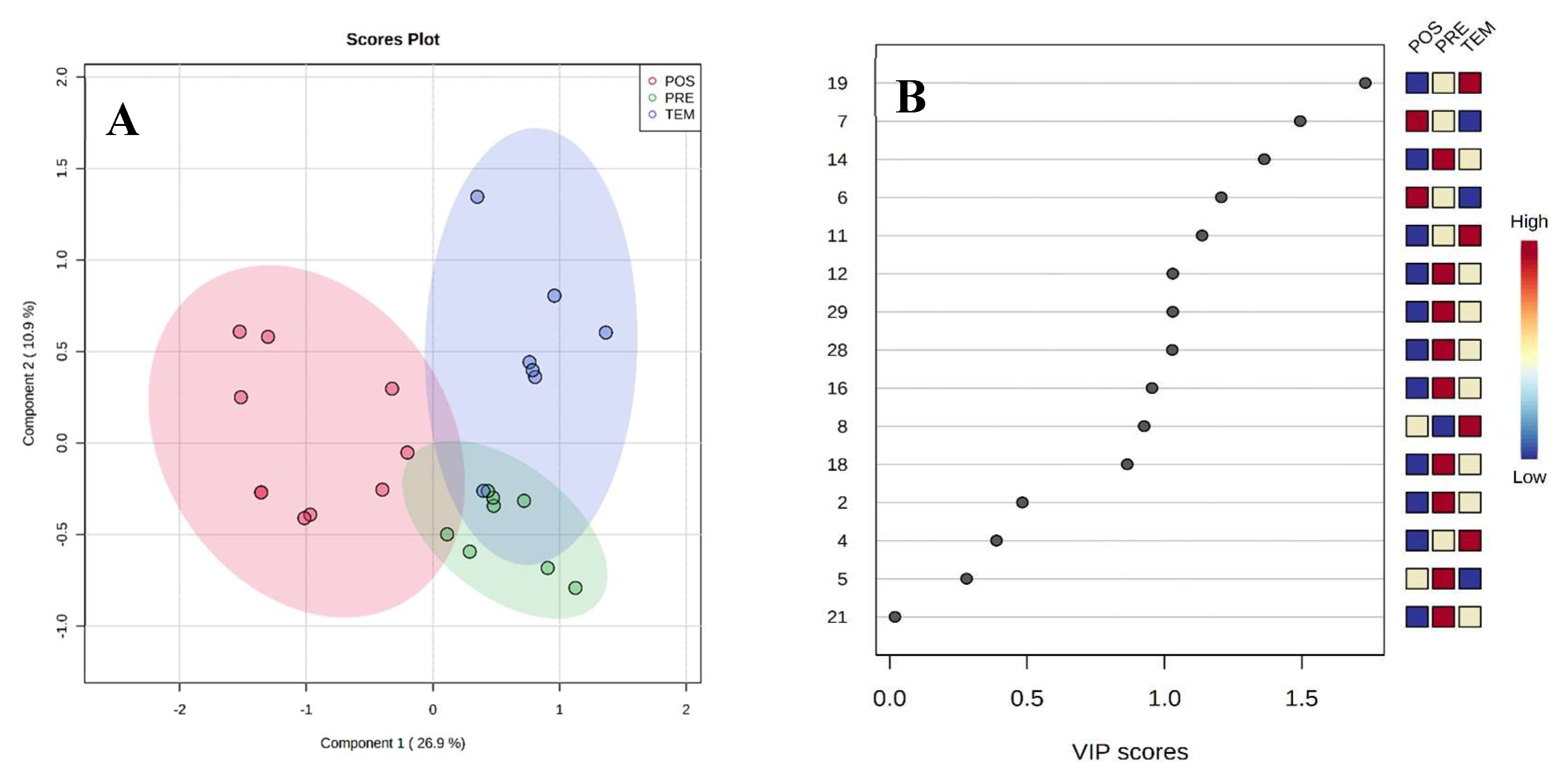
3.1. E-nose
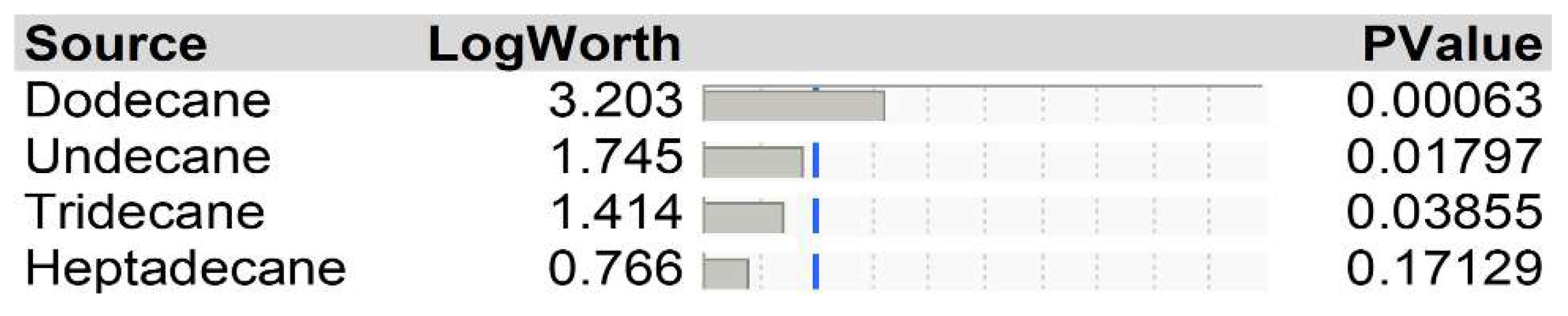
3.2. Gas chromatograph
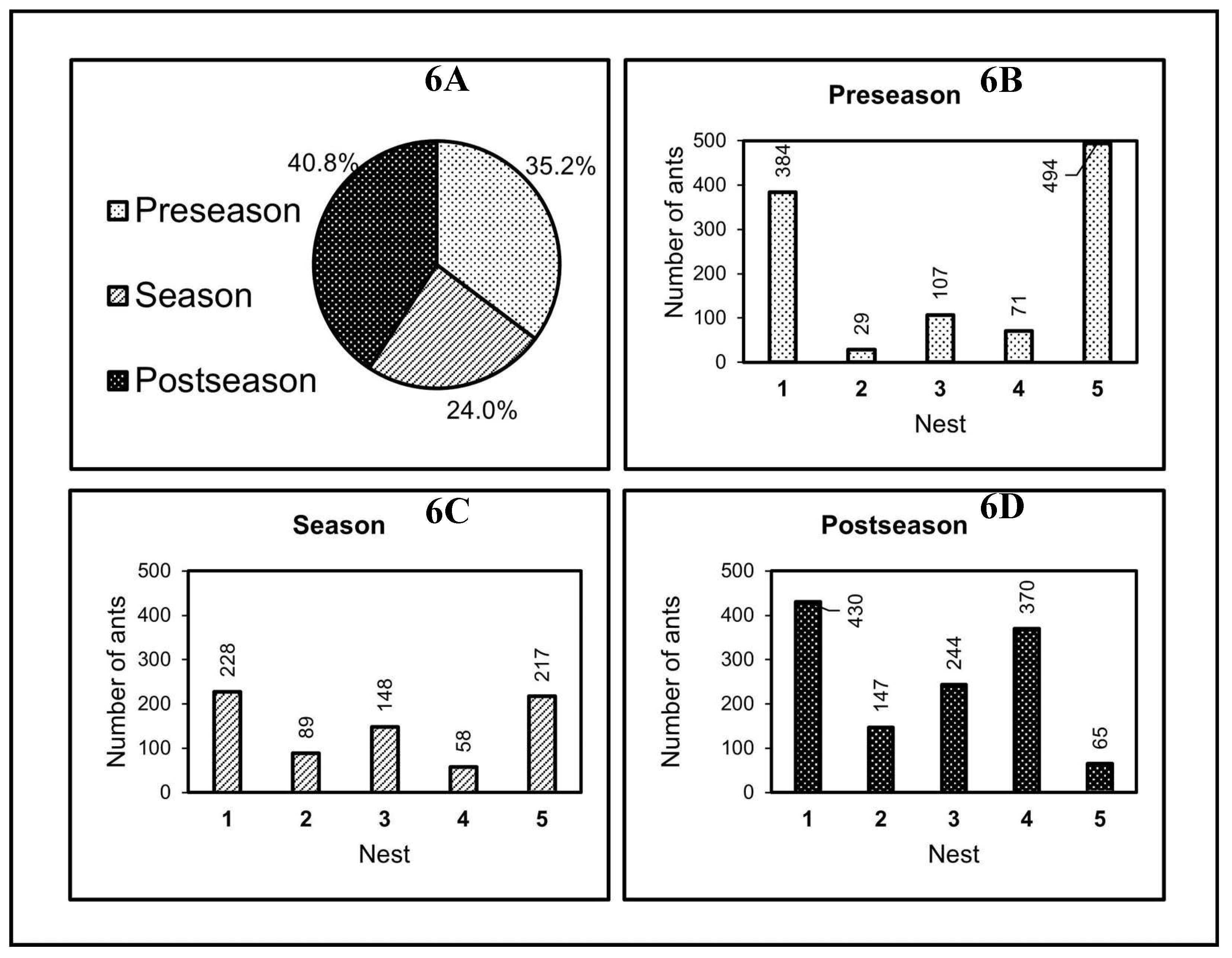
3.3. Ant counts, effect of semiochemicals on observed frequencies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escárraga, M.; Guerrero, R. Hormigas. Un Mundo de Meñiques Gigantes. INFOZOA Boletín de Zoología 2014, 4, 1–16. [Google Scholar]
- Guénard, B.; Weiser, M.D.; Gómez, K.; Narula, N.; Economo, E.P. The Global Ant Biodiversity Informatics (GABI) Database: Synthesizing Data on the Geographic Distribution of Ant Species (Hymenoptera: Formicidae). Myrmecological News 2017, 24, 83–89. [Google Scholar] [CrossRef]
- Stork, N.E. How Many Species of Insects and Other Terrestrial Arthropods Are There on Earth? Annual Review of Entomology 2018, 63, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Hakala, S.M.; Seppä, P.; Helanterä, H. Evolution of Dispersal in Ants (Hymenoptera: Formicidae): A Review on the Dispersal Strategies of Sessile Superorganisms. Myrmecological News 2019, 29, 35–55. [Google Scholar] [CrossRef]
- Lessard, J.P. Ant Community Response to Disturbance: A Global Synthesis. Journal of Animal Ecology 2019, 88, 346–349. [Google Scholar] [CrossRef]
- Fernandez-Bou, A.S.; Dierick, D.; Swanson, A.C.; Allen, M.F.; Alvarado, A.G.F.; Artavia-León, A.; Carrasquillo-Quintana, O.; Lachman, D.A.; Oberbauer, S.; Pinto-Tomás, A.A.; et al. The Role of the Ecosystem Engineer, the Leaf-Cutter Ant Atta Cephalotes, on Soil CO2 Dynamics in a Wet Tropical Rainforest. Journal of Geophysical Research: Biogeosciences 2019, 124, 260–273. [Google Scholar] [CrossRef]
- De Almeida, T.; Mesléard, F.; Santonja, M.; Gros, R.; Dutoit, T.; Blight, O. Above- and below-Ground Effects of an Ecosystem Engineer Ant in Mediterranean Dry Grasslands. Proceedings of the Royal Society B: Biological Sciences 2020, 287, 20201840. [Google Scholar] [CrossRef]
- Del Toro, I.; Pacheco, J.A.; Mackay, W.P. Revision of the Ant Genus Liometopum (Hymenoptera: Formicidae). Sociobiology 2009, 53, 309–316. [Google Scholar]
- Dáttilo, W.; Vásquez-Bolaños, M.; Ahuatzin, D.A.; Antoniazzi, R.; Chávez-González, E.; Corro, E.; Luna, P.; Guevara, R.; Villalobos, F.; Madrigal-Chavero, R.; et al. Mexico Ants: Incidence and Abundance along the Nearctic–Neotropical Interface. Ecology 2020, 101, e02944–1. [Google Scholar] [CrossRef]
- Ramos Rostro, B.; Salazar, B.Q.; Ramos-Elorduy, J.; Moreno, J.M.P.; Campos, S.C.A.; Pérez, A.G.; García, V.D.B. Análisis Químico y Nutricional de Tres Insectos Comestibles de Interés Comercial en la Zona Arqueologica del Municipio de San Juan Teotihuacan y en Otumba, en el Estado de México. Interciencia 2012, 37, 914–920. [Google Scholar]
- Miller, T.E.X. Does Having Multiple Partners Weaken the Benefits of Facultative Mutualism? A Test with Cacti and Cactus-Tending Ants. Oikos 2007, 116, 500–512. [Google Scholar] [CrossRef]
- Lara, P.; Aguirre, J.R.; Castillo, P.; Reyes, J.A. Biología y Aprovechamiento de la Hormiga de Escamoles, Liometopum Apiculatum Mayr (Hymenoptera: Formicidae). Acta zool. mexicana 2015, 31, 251–264. [Google Scholar]
- Ramos-Elorduy, J.; Delage Darchen, B.; Cuadriello Aguilar, J.I.; Galindo Miranda, N.; Pino Moreno, J.M. Ciclo de Vida y Fundación de las Sociedades de Liometopum apiculatum M. (Hymenoptera, Formicidae). Anales del Instituto de Biología. UNAM. Ser Zool. 184, 54, 161–176. [Google Scholar]
- Ramos-Elorduy, J.; Delage-Darchen, B.; Miranda, N.G.; Moreno, J.M.P. Observaciones Bioecotologicas de Liometopum Apiculatum M. y Liometopum Occidentale Var. Luctuosum W. (Hymenoptera-Formicidae). An. Inst. Biol. Univ. Nac. Auton. Mexico Ser. Zool. 1988, 58, 341–354. [Google Scholar]
- Ángeles Tovar, N.; Estrada Yescas, M.T.; Sandoval García, M. del P.; Vega Serrano, F.V.; Onofre Sánchez, J.E. Recolección y Temporalidad de Liometopum apiculatum M. (Escamoles) En El Municipio de Nantzha, Hidalgo México. Punto de vista 2022, 12, 72–87. [Google Scholar] [CrossRef]
- Escamilla, F.; Ariza, J. Nutrient and Oil Profile of Escamol, an Edible Larva of Ants (Liometopum Apiculatum Mayr). Future of Food: Journal on Food, Agriculture and Society 2021, 9, 1–9. [Google Scholar] [CrossRef]
- Velasco, C.; Corona-Vargas, M.; Peña-Martinez, R. Liometopum apiculatum (Formicidae: Dolichoderinae) y su Relacion Trofobiotica con Hemiptera Sternorrhyncha en Tlaxco, Tlaxcala, México. Acta Zoológica Mexicana (n.s) 2007, 23, 31–42. [Google Scholar] [CrossRef]
- Hoey-Chamberlain, R.; Rust, M.K.; Klotz, J.H. A Review of the Biology, Ecology and Behavior of Velvety Tree Ants of North America. Sociobiology 2013, 60, 1–10. [Google Scholar] [CrossRef]
- Mackay, W.; Mackay, E. The Ants of New Mexico (Hymenoptera: Fromicidae), 1st ed.; The Edwin Mellen Press: Lewiston, Ny, 2002; pp. 231–234. [Google Scholar]
- Rafael-Valdez, J.; Tarango-Arámbula, L.A.; Ugalde-Lezama, S.; Cruz-Labana, J.D.; Clemente-Sánchez, F.; Cadena-Iñiguez, J. Foraging Amplitude of the Escamolera Ant (Liometopum Apiculatum Mayr, Hymenoptera: Formicidae) in a Semi-Arid Area of the Zacatecan Highlands. Revista Chapingo Serie Zonas Áridas 2019, 18, 5–19. [Google Scholar] [CrossRef]
- Xu, T.; Xu, M.; Lu, Y.; Zhang, W.; Sun, J.; Zeng, R.; Turlings, T.C.J.; Chen, L. A Trail Pheromone Mediates the Mutualism between Ants and Aphids. Current Biology 2021, 31, 4738–4747. [Google Scholar] [CrossRef]
- Xu, T.; Chen, L. Chemical Communication in Ant-Hemipteran Mutualism: Potential Implications for Ant Invasions. Current Opinion in Insect Science 2021, 45, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kleeberg, I.; Menzel, F.; Foitzik, S. The Influence of Slavemaking Lifestyle, Caste and Sex on Chemical Profiles in Temnothorax Ants: Insights into the Evolution of Cuticular Hydrocarbons. Proceedings of the Royal Society B: Biological Sciences 2017, 284, 20162249. [Google Scholar] [CrossRef]
- Wyatt, T.D. Pheromones. Current Biology 2017, 27, R739–R743. [Google Scholar] [CrossRef] [PubMed]
- Welzel, K.F.; Lee, S.H.; Dossey, A.T.; Chauhan, K.R.; Choe, D.H. Verification of Argentine Ant Defensive Compounds and Their Behavioral Effects on Heterospecific Competitors and Conspecific Nestmates. Scientific Reports 2018, 8, 1477. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Grodowitz, M.J.; Chen, J. Electrophysiological Responses of Eighteen Species of Insects to Fire Ant Alarm Pheromone. Insects 2019, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Billen, J.; Morgan, E.D. Pheromone Communication in Social Insects: Sources and Secretions. In Pheromone Communication in Social Insects: Ants, Wasps, Bees, and Termites, 1st ed; Vander Meer, R.K., Breed, M.D., Winston, M., Espelie, K.E, Eds.; Taylor & Francis: New York, NY, 2019; Volume 2, pp. 1–31. [Google Scholar]
- Ge, J.; Ge, Z.; Zhu, D.; Wang, X. Pheromonal Regulation of the Reproductive Division of Labor in Social Insects. Frontiers in Cell and Developmental Biology 2020, 8. [Google Scholar] [CrossRef]
- Pekár, S. Ant-Mimicking Spider Actively Selects Its Mimetic Model (Araneae: Gnaphosidae; Hymenoptera: Formicidae). Myrmecological News 2020, 30, 131–137. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.M. Semiochemicals for Controlling Insect Pests. Journal of Plant Protection Research 2019, 59, 1–11. [Google Scholar] [CrossRef]
- Oliver, T.H.; Mashanova, A.; Leather, S.R.; Cook, J.M.; Jansen, V.A.A. Ant Semiochemicals Limit Apterous Aphid Dispersal. Proceedings of the Royal Society B: Biological Sciences 2007, 274, 3127–3131. [Google Scholar] [CrossRef]
- Appiah, E.F.; Ekesi, S.; Afreh-Nuamah, K.; Obeng-Ofori, D.; Mohamed, S.A. African Weaver Ant-Produced Semiochemicals Impact on Foraging Behaviour and Parasitism by the Opiine Parasitoid, Fopius Arisanus on Bactrocera Invadens (Diptera: Tephritidae). Biological Control 2014, 79, 49–57. [Google Scholar] [CrossRef]
- Welzel, K.F.; Choe, D.H. Development of a Pheromone-Assisted Baiting Technique for Argentine Ants (Hymenoptera: Formicidae). Journal of Economic Entomology 2016, 109, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Chalissery, J.M.; Renyard, A.; Gries, R.; Hoefele, D.; Alamsetti, S.K.; Gries, G. Ants Sense, and Follow, Trail Pheromones of Ant Community Members. Insects 2019, 10, 383. [Google Scholar] [CrossRef]
- Renyard, A.; Alamsetti, S.K.; Gries, R.; Munoz, A.; Gries, G. Identification of the Trail Pheromone of the Carpenter Ant Camponotus Modoc. Journal of Chemical Ecology 2019, 45, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Angulo, E.; Hoffmann, B.D.; Ballesteros-Mejia, L.; Taheri, A.; Balzani, P.; Bang, A.; Renault, D.; Cordonnier, M.; Bellard, C.; Diagne, C.; et al. Economic Costs of Invasive Alien Ants Worldwide. Biological Invasions 2022, 24, 2041–2060. [Google Scholar] [CrossRef]
- Diamond, A.; Schmuker, M.; Berna, A.Z.; Trowell, S.; Nowotny, T. Classifying Continuous, Real-Time e-Nose Sensor Data Using a Bio-Inspired Spiking Network Modelled on the Insect Olfactory System. Bioinspiration & Biomimetics 2016, 11, 26002. [Google Scholar] [CrossRef]
- Díaz de León-Martínez, L.; Flores-Ramírez, R.; López-Mendoza, C.M.; Rodríguez-Aguilar, M.; Metha, G.; Zúñiga-Martínez, L.; Ornelas-Rebolledo, O.; Alcántara-Quintana, L.E. Identification of Volatile Organic Compounds in the Urine of Patients with Cervical Cancer. Test Concept for Timely Screening. Clinica Chimica Acta 2021, 522, 132–140. [Google Scholar] [CrossRef]
- Dinwiddie, M.L.; Jones, R.W.; Roitman-Genoud, P.; Tarango-Arámbula, L.A.; Malda-Barrera, G.X. Estudio Etnoentomologico de la Hormiga Escamolera (Liometopum Apiculatum) en dos Localidades del estado de Querétaro. AGROProductividad 2013, 6, 27–34. [Google Scholar]
- Berumen Jiménez, M.; Valdez Cepeda, R.D.; Méndez Gallegos, S. de J.; Cadena Íñiguez, J.; Esparza Orozco, A.; Tarango Arámbula, L.A. Determination of the Conservation Status of the “Escamolera” Ant (Liometopum apiculatum Mayr) in Mexico by the Species Risk Assessement Methodology – MER. Agrociencia 2021, 55, 539–555. [Google Scholar] [CrossRef]
- Briones-Santoyo, J.A.; Tarango-Arámbula, L.A.; Velázquez-Martínez, A.; Reyes-Hernández, V.J.; Salazar-Borunda, M.A. Edible Insect Harvest in Pinos, Zacatecas, Mexico. Agro Productividad 2022, 15, 37–49. [Google Scholar] [CrossRef]
- García, E.; Comisión Nacional Para El Conocimiento y Uso de la Biodiversidad (CONABIO, 2001). “Climas” (Clasificación de Koppen, Modificado Por García). Available online: http://www.conabio.gob.mx/informacion/gis/?vns=gis_root/clima/climas/clima1mgw (accessed on 11 April 2023).
- García, E.; Comisión Nacional Para El Conocimiento y Uso de La Biodiversidad (CONABIO, 2008). Climatología, Precipitación. Available online: http://www.conabio.gob.mx/informacion/gis/?vns=gis_root/clima/precip/isoyt1mgw (accessed on 11 April 2023).
- INEGI Conjunto de Datos Vectoriales de Uso de Suelo y Vegetación. Escala 1:250 000, Serie VII. Conjunto Nacional.’, Escala: 1:250 000. Edición: 1. Instituto Nacional de Estadística y Geografía, México Available online:. Available online: http://www.conabio.gob.mx/informacion/gis/?vns=gis_root/clima/precip/isoyt1mgw (accessed on 11 April 2023).
- Hernández-Roldan, E.; Tarango-Arámbula, L.A.; Ugalde-Lezama, S.; Hernández-Juárez, A.; Cortez-Romero, C.; Cruz-Miranda, Y.; Morales-Flores, F.J. Hábitat y Densidad de Nidos de la Hormiga Escamolera (Liometopum apiculatum Mayr) En Una UMA de Zacatecas, México. Agroproductividad 2017, 10, 10–17. [Google Scholar]
- Oberhauser, F.B.; Schlemm, A.; Wendt, S.; Czaczkes, T.J. Private Information Conflict: Lasius niger Ants Prefer Olfactory Cues to Route Memory. Animal Cognition 2019, 22, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Oberhauser, F.B.; Wendt, S.; Czaczkes, T.J. Trail Pheromone Does Not Modulate Subjective Reward Evaluation in Lasius niger Ants. Ants. Front. Psychol. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nishisue, K.; Koyama, S.; Satoh, T. Identification of the Argentine Ant Linepithema humile (Hymenoptera: Formicidae) Using an Artificially Synthesized Trail Pheromone and Its Effects on Native Japanese Ants. Applied Entomology and Zoology 2020, 55, 141–147. [Google Scholar] [CrossRef]
- Bustamante, S.; Amarillo-Suárez, A.R. AntCounter Software: Counting Leaf-Cutting Ants Was Never so Precise, Fast and Easy. Journal of Insect Behavior 2016, 29, 262–272. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, M.; Díaz de León-Martínez, L.; Gorocica-Rosete, P.; Pérez-Padilla, R.; Domínguez-Reyes, C.A.; Tenorio-Torres, J.A.; Ornelas-Rebolledo, O.; Mehta, G.; Zamora-Mendoza, B.N.; Flores-Ramírez, R. Application of Chemoresistive Gas Sensors and Chemometric Analysis to Differentiate the Fingerprints of Global Volatile Organic Compounds from Diseases. Preliminary Results of COPD, Lung Cancer and Breast Cancer. Clinica Chimica Acta 2021, 518, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lee-Rangel, H.A.; Mendoza-Martinez, G.D.; Díaz de León-Martínez, L.; Relling, A.E.; Vazquez-Valladolid, A.; Palacios-Martínez, M.; Hernández-García, P.A.; Chay-Canul, A.J.; Flores-Ramirez, R.; Roque-Jiménez, J.A. Application of an Electronic Nose and HS-SPME/GC-MS to Determine Volatile Organic Compounds in Fresh Mexican Cheese. Foods 2022, 11, 1887. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; Morais, D.A. de L.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Research 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Becher, P.G.; Hagman, A.; Verschut, V.; Chakraborty, A.; Rozpędowska, E.; Lebreton, S.; Bengtsson, M.; Flick, G.; Witzgall, P.; Piškur, J. Chemical Signaling and Insect Attraction Is a Conserved Trait in Yeasts. Ecology and Evolution 2018, 8, 2962–2974. [Google Scholar] [CrossRef]
- Hölldobler, B.; Oldham, N.J.; Liebig, J.; Liu, Y.; David Morgan, E. Dufour Gland Secretion in the Harvester Ant Genus Pogonomyrmex. Chemoecology 2004, 14, 101–106. [Google Scholar] [CrossRef]
- Morgan, D.E. Trail Pheromones of Ants. Physiological Entomology 2009, 34, 1–17. [Google Scholar] [CrossRef]
- Igwe, O.U.; Offiong, S. Chemistry of Semiochemicals Used as Trail Pheromones in Tropical Fire Ant (Solenopsis geminata) 2015, 7, 34–40.
- Thoenen, M. The Effect of Iridomyrmecin on Native Ant Foraging Behavior. Spring 2017, 1–15. [Google Scholar]
- Hamilton, N.; Jones, T.H.; Shik, J.Z.; Wall, B.; Schultz, T.R.; Blair, H.A.; Adams, R.M.M. Context Is Everything: Mapping Cyphomyrmex-Derived Compounds to the Fungus-Growing Ant Phylogeny. Chemoecology 2018, 28, 137–144. [Google Scholar] [CrossRef]
- Chalissery, J.M.; Renyard, A.; Gries, R.; Hoefele, D.; Alamsetti, S.K.; Gries, G. Ants Sense, and Follow, Trail Pheromones of Ant Community Members. Insects 2019, 10, 383. [Google Scholar] [CrossRef]
- Lofqvist, J.; Bergstrom, G. Volatile Communication Substances in Dufour’s Gland of Virgin Females and Old Queens of the Ant Formica Polyctena. Journal of Chemical Ecology 1980, 6, 309–320. [Google Scholar] [CrossRef]
- Santos Junior, L.C.; Michelutti, K.B.; Bernardi, R.C.; Silva, E.P.; Cardoso, C.A.L.; Antonialli-Junior, W.F. You Smell Different! Temperature Interferes with Intracolonial Recognition in Odontomachus brunneus. Sociobiology 2022, 69, e6235. [Google Scholar] [CrossRef]
- Mekonnen, B.; Cheseto, X.; Pirk, C.; Yusuf, A.; Ekesi, S.; Deletre, E.; Torto, B. Re-Analysis of Abdominal Gland Volatilome Secretions of the African Weaver Ant, Oecophylla longinoda (Hymenoptera: Formicidae). Molecules 2021, 26, 871. [Google Scholar] [CrossRef]
- Attygalle, A.B.; Mutti, A.; Rohe, W.; Maschwitz, U.; Garbe, W.; Bestmann, H.J. Trail Pheromone from the Pavan Gland of the Ant Dolichoderus thoracicus (Smith) Pheromones, 108 [1]. Naturwissenschaften 1998, 85, 275–277. [Google Scholar] [CrossRef]
- Han, B.; Chen, Z. Behavioral and Electrophysiological Responses of Natural Enemies to Synomones from Tea Shoots and Kairomones from Tea Aphids, Toxoptera aurantii. Journal of Chemical Ecology 2002, 28, 2203–2219. [Google Scholar] [CrossRef] [PubMed]
- Lammers, A.; Zweers, H.; Sandfeld, T.; Bilde, T.; Garbeva, P.; Schramm, A.; Lalk, M. Antimicrobial Compounds in the Volatilome of Social Spider Communities. Frontiers in Microbiology 2021, 12, 700693. [Google Scholar] [CrossRef]
- Ham, J.E.; Raymond Wells, J. Surface Chemistry of Dihydromyrcenol (2,6-Dimethyl-7-Octen-2-Ol) with Ozone on Silanized Glass, Glass, and Vinyl Flooring Tiles. Atmospheric Environment 2009, 43, 4023–4032. [Google Scholar] [CrossRef]
- Politano, V.T.; Lewis, E.M.; Hoberman, A.M.; Christian, M.S.; Diener, R.M.; Api, A.M. Evaluation of the Developmental Toxicity of Dihydromyrcenol in Rats. International Journal of Toxicology 2009, 28, 80–87. [Google Scholar] [CrossRef]
- McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance Material Review on Dihydromyrcenol. Food and Chemical Toxicology 2010, 48, S70–S75. [Google Scholar] [CrossRef] [PubMed]
- Nunes Moreira, F.I.; Lucena de Medeiros, L.; Moreira de Carvalho, L.; Souza Olegario, L.; Sousa Galvão, M.; Alves Monteiro da Franca, S.; Alencar Bezerra, T.K.; Dos Santos Lima, M.; Suely Madruga, M. Quality of Brazilian Stingless Bee Honeys: Cephalotrigona capitata/Mombucão and Melipona scutellaris Latrelle/Uruçu. Food Chemistry 2023, 404, 134306. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, O.; Gurkan, H.; Sahingil, D.; Degirmenci, A.; Er Kemal, M.; Kolayli, S.; Hayaloglu, A.A. Floral Authentication of Some Monofloral Honeys Based on Volatile Composition and Physicochemical Parameters. European Food Research and Technology 2022, 248, 2145–2155. [Google Scholar] [CrossRef]
- Peerzada, N.; Pakkiyaretnam, T.; Renaud, S. Volatile Constitutents of the Green Ant Oecophylla smaragdina. Agricultural and Biological Chemistry 1990, 54, 3335–3336. [Google Scholar] [CrossRef]
- Belsito, D.; Bickers, D.; Bruze, M.; Calow, P.; Greim, H.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.H.; Sipes, I.G.; Tagami, H. A Safety Assessment of Non-Cyclic Alcohols with Unsaturated Branched Chain When Used as Fragrance Ingredients. Food and Chemical Toxicology 2010, 48, S1–S42. [Google Scholar] [CrossRef]
- Fujiwara-Tsujii, N.; Yamagata, N.; Takeda, T.; Mizunami, M.; Yamaoka, R. Behavioral Responses to the Alarm Pheromone of the Ant Camponotus obscuripes (Hymenoptera: Formicidae). Zoological Science 2006, 23, 353–358. [Google Scholar] [CrossRef]
- Lenz, E.L.; Krasnec, M.O.; Breed, M.D. Identification of Undecane as an Alarm Pheromone of the Ant Formica Argentea. Journal of Insect Behavior 2013, 26, 101–108. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, M.; Tang, L.; Ma, R.; He, H. The Chemical Components of Dufour’s and Venom Gland of Camponotus japonicus (Hymenoptera, Formicidae). Research Square 2022, 1–12. [Google Scholar] [CrossRef]
- Wilson, E.O.; Pavan, M. Glandular Sources and Specificity of Some Chemical Releasers of Social Behavior in Dolichoderine Ants. Psyche: A Journal of Entomology 1959, 66, 70–76. [Google Scholar] [CrossRef]
- Do Nascimento, R.R.; Jackson, B.D.; Morgan, E.D.; Clark, W.H.; Blom, P.E. Chemical Secretions of Two Sympatric Harvester Ants, Pogonomyrmex salinus and Messor lobognathus. Journal of Chemical Ecology 1993, 19, 1993–2005. [Google Scholar] [CrossRef]
- Adams, R.M.M.; Wells, R.L.; Yanoviak, S.P.; Frost, C.J.; Fox, E.G.P. Interspecific Eavesdropping on Ant Chemical Communication. Frontiers in Ecology and Evolution 2020, 8. [Google Scholar] [CrossRef]
- Chen, J. Freeze–Thaw Sample Preparation Method Improves Detection of Volatile Compounds in Insects Using Headspace Solid-Phase Microextraction. Analytical Chemistry 2017, 89, 8366–8371. [Google Scholar] [CrossRef] [PubMed]
- Azhagu Raj, R.; Sathish, R.; Prakasam, A.; Krishnamoorthy, D.; Balachandar, M.; Majesh, T. Extraction and Analysis of Cuticular Hydrocarbons in the Weaver Ant Oecophylla smaragdina (Fabricius) (Hymenoptera: Formicidae). Int. J. Fauna Biol. Stud. 2017, 4, 102–107. [Google Scholar]
- Mashaly, A.M.A.; Ahmed, A.M.; Nunes, T.M.; Morgan, E.D. Secretions of Dufour’s Gland in Some Ants (Hymenoptera: Formicidae). African Entomology 2014, 22, 779–782. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




