Submitted:
31 August 2023
Posted:
04 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Galleria mellonella Survival Assays
2.3. Determination of fungal burden during the survival curve
2.4. Determination of hemocyte density in hemolymph
2.5. Analysis of gene expression
2.5.1. RNA extraction and cDNA sythesis
2.5.2. Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Statistical Analyses
3. Results
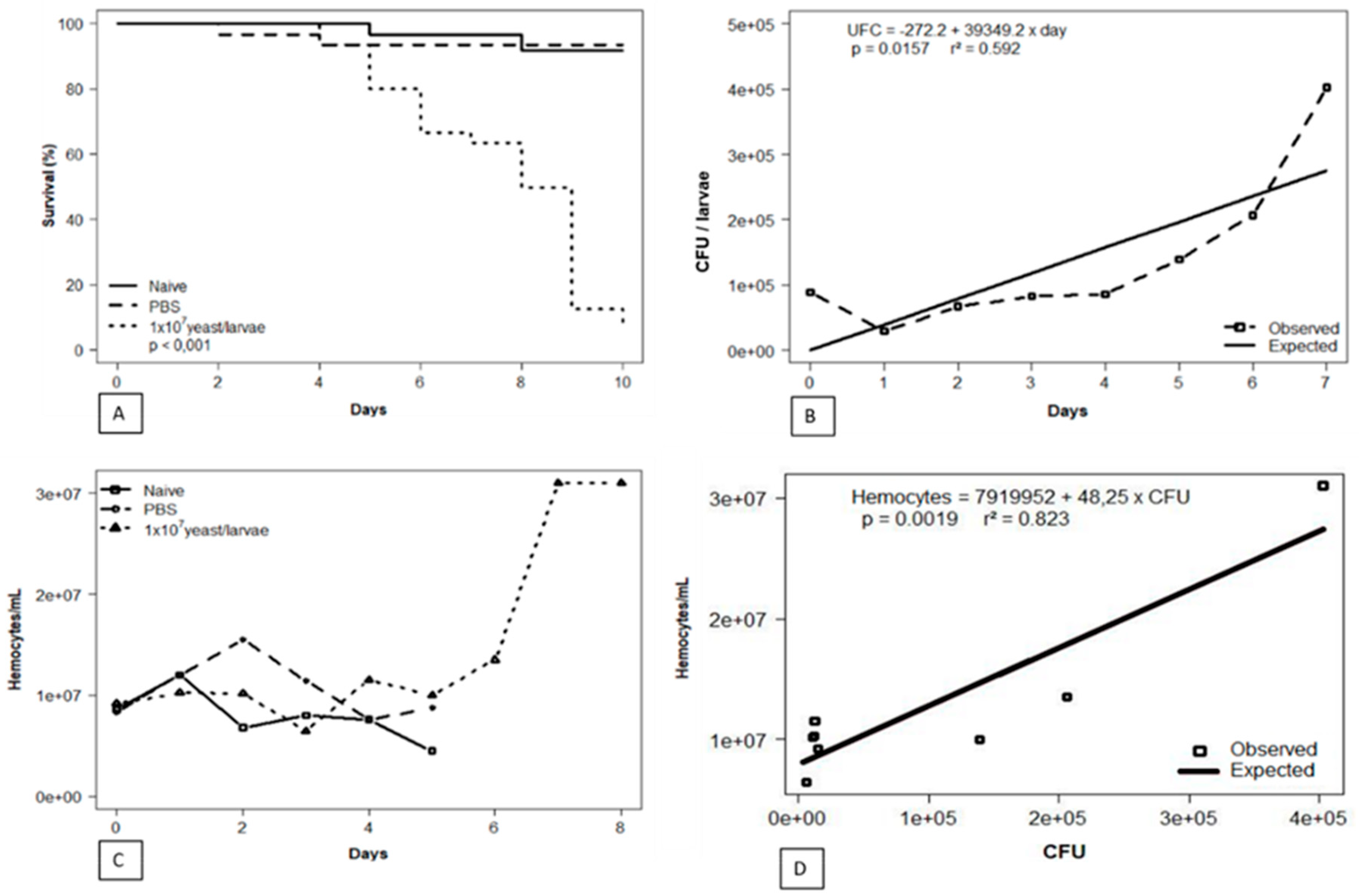
3.1. Galleria mellonella Survival Assays
3.2. Fungal load during the infection
3.3. Sporothrix brasiliensis-Galleria mellonella Interaction
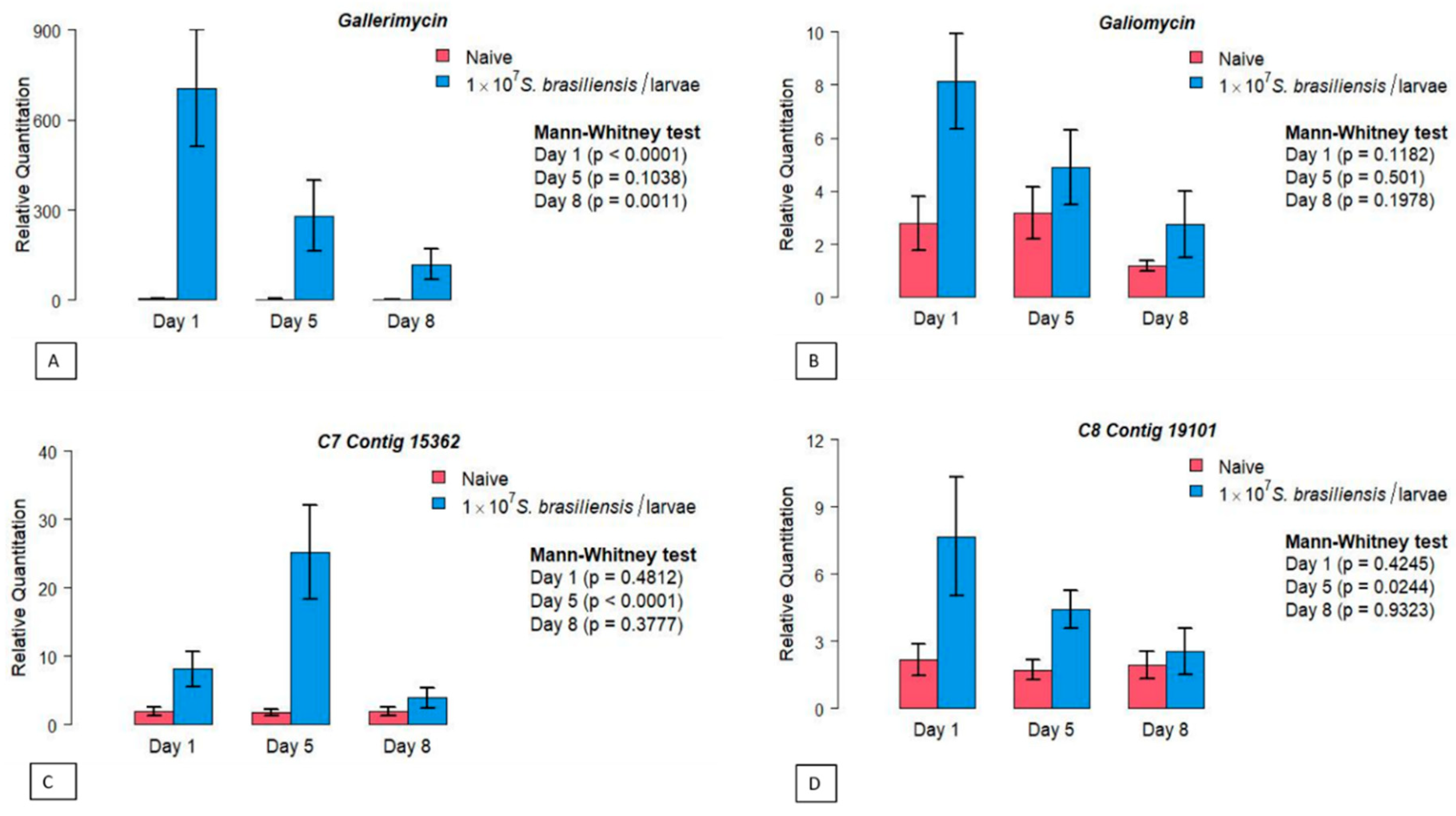
3.4. Gene Expression
3.4.1. Antimicrobial peptides
3.4.2. Stress managing genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gremião, I. D. F.; Miranda, L. H. M.; Reis, E. G.; Rodrigues, A. M.; Pereira, S. A. Zoonotic Epidemic of Sporotrichosis: Cat to Human Transmission. PLoS Pathog 2017, 13, e1006077. [Google Scholar] [CrossRef]
- Rodrigues, A. M.; Della Terra, P. P.; Gremião, I. D.; Pereira, S. A.; Orofino-Costa, R.; de Camargo, Z. P. The Threat of Emerging and Re-Emerging Pathogenic Sporothrix Species. Mycopathologia 2020, 185, 813–842. [Google Scholar] [CrossRef]
- Della Terra, P. P.; Rodrigues, A. M.; Fernandes, G. F.; Nishikaku, A. S.; Burger, E.; de Camargo, Z. P. Exploring Virulence and Immunogenicity in the Emerging Pathogen Sporothrix brasiliensis. PLoS Negl Trop Dis 2017, 11, e0005903. [Google Scholar] [CrossRef]
- Nakasu, C. C. T.; Waller, S. B.; Ripoll, M. K.; Ferreira, M. R. A.; Conceição, F. R.; Gomes, A. dos R.; Osório, L. da G.; de Faria, R. O.; Cleff, M. B. Feline Sporotrichosis: A Case Series of Itraconazole-Resistant Sporothrix brasiliensis Infection. Braz J Microbiol 2021, 52, 163–171. [Google Scholar] [CrossRef]
- Gremião, I. D. F.; Oliveira, M. M. E.; Monteiro de Miranda, L. H.; Saraiva Freitas, D. F.; Pereira, S. A. Geographic Expansion of Sporotrichosis, Brazil. Emerg. Infect. Dis. 2020, 26, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Schechtman, R. C.; Falcão, E. M. M.; Carard, M.; García, M. S. C.; Mercado, D. S.; Hay, R. J. Sporotrichosis: Hyperendemic by Zoonotic Transmission, with Atypical Presentations, Hypersensitivity Reactions and Greater Severity. Anais Brasileiros de Dermatologia 2022, 97, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Etchecopaz, A.; Toscanini, M. A.; Gisbert, A.; Mas, J.; Scarpa, M.; Iovannitti, C. A.; Bendezú, K.; Nusblat, A. D.; Iachini, R.; Cuestas, M. L. Sporothrix brasiliensis: A Review of an Emerging South American Fungal Pathogen, Its Related Disease, Presentation and Spread in Argentina. JoF 2021, 7, 170. [Google Scholar] [CrossRef]
- Thomson, P.; González, C.; Blank, O.; Ramírez, V.; Río, C. del; Santibáñez, S.; Pena, P. Sporotrichosis Outbreak Due to Sporothrix brasiliensis in Domestic Cats in Magallanes, Chile: A One-Health-Approach Study. JoF 2023, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Barnacle, J. R.; Chow, Y. J.; Borman, A. M.; Wyllie, S.; Dominguez, V.; Russell, K.; Roberts, H.; Armstrong-James, D.; Whittington, A. M. The First Three Reported Cases of Sporothrix brasiliensis Cat-Transmitted Sporotrichosis Outside South America. Medical Mycology Case Reports 2023, 39, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Orofino-Costa, R.; Macedo, P. M. de; Rodrigues, A. M.; Bernardes-Engemann, A. R. Sporotrichosis: An Update on Epidemiology, Etiopathogenesis, Laboratory and Clinical Therapeutics. An. Bras. Dermatol. 2017, 92, 606–620. [Google Scholar] [CrossRef]
- Gremião, I. D. F.; Martins da Silva da Rocha, E.; Montenegro, H.; Carneiro, A. J. B.; Xavier, M. O.; de Farias, M. R.; Monti, F.; Mansho, W.; de Macedo Assunção Pereira, R. H.; Pereira, S. A.; Lopes-Bezerra, L. M. Guideline for the Management of Feline Sporotrichosis Caused by Sporothrix brasiliensis and Literature Revision. Braz J Microbiol 2021, 52, 107–124. [Google Scholar] [CrossRef]
- Dekkerová-Chupáčová, J.; Borghi, E.; Morace, G.; Bujdáková, H. Up-Regulation of Antimicrobial Peptides Gallerimycin and Galiomicin in Galleria mellonella Infected with Candida Yeasts Displaying Different Virulence Traits. Mycopathologia 2018, 183, 935–940. [Google Scholar] [CrossRef] [PubMed]
- García-Carnero, L. C.; Clavijo-Giraldo, D. M.; Gómez-Gaviria, M.; Lozoya-Pérez, N. E.; Tamez-Castrellón, A. K.; López-Ramírez, L. A.; Mora-Montes, H. M. Early Virulence Predictors during the Candida Species–Galleria mellonella Interaction. JoF 2020, 6, 152. [Google Scholar] [CrossRef]
- Trevijano-Contador, N.; Herrero-Fernández, I.; García-Barbazán, I.; Scorzoni, L.; Rueda, C.; Rossi, S. A.; García-Rodas, R.; Zaragoza, O. Cryptococcus neoformans Induces Antimicrobial Responses and Behaves as a Facultative Intracellular Pathogen in the Non Mammalian Model Galleria mellonella. Virulence 2015, 6, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, I. Animal Models to Study Mucormycosis. JoF 2019, 5, 27. [Google Scholar] [CrossRef]
- Maurer, E.; Hörtnagl, C.; Lackner, M.; Grässle, D.; Naschberger, V.; Moser, P.; Segal, E.; Semis, M.; Lass-Flörl, C.; Binder, U. Galleria mellonella as a Model System to Study Virulence Potential of Mucormycetes and Evaluation of Antifungal Treatment. Medical Mycology 2019, 57, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Stączek, S.; Zdybicka-Barabas, A.; Wojda, I.; Wiater, A.; Mak, P.; Suder, P.; Skrzypiec, K.; Cytryńska, M. Fungal α-1,3-Glucan as a New Pathogen-Associated Molecular Pattern in the Insect Model Host Galleria mellonella. Molecules 2021, 26, 5097. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, L.; García-Rodas, R.; Guimarães, A. J.; Taborda, C. P.; Zaragoza, O.; Nosanchuk, J. D. Galleria mellonella as a Model Host to Study Paracoccidioides lutzii and Histoplasma capsulatum. Virulence 2013, 4, 139–146. [Google Scholar] [CrossRef]
- Singulani, J. L.; Scorzoni, L.; de Oliveira, H. C.; Marcos, C. M.; Assato, P. A.; Fusco-Almeida, A.; Mendes-Giannini, M. Applications of Invertebrate Animal Models to Dimorphic Fungal Infections. JoF 2018, 4, 118. [Google Scholar] [CrossRef]
- Freitas, D. F.; Santos, S. S.; Almeida-Paes, R.; de Oliveira, M. M.; do Valle, A. C.; Gutierrez-Galhardo, M. C.; Zancopé-Oliveira, R. M.; Nosanchuk, J. d. Increase in Virulence of Sporothrix brasiliensis over Five Years in a Patient with Chronic Disseminated Sporotrichosis. Virulence 2015, 6, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Clavijo-Giraldo, D. M.; Matínez-Alvarez, J. A.; Lopes-Bezerra, L. M.; Ponce-Noyola, P.; Franco, B.; Almeida, R. S.; Mora-Montes, H. M. Analysis of Sporothrix schenckii sensu stricto and Sporothrix brasiliensis Virulence in Galleria mellonella. Journal of Microbiological Methods 2016, 122, 73–77. [Google Scholar] [CrossRef]
- Lozoya-Pérez, N. E.; Casas-Flores, S.; Martínez-Álvarez, J. A.; López-Ramírez, L. A.; Lopes-Bezerra, L. M.; Franco, B.; Mora-Montes, H. M. Generation of Sporothrix schenckii Mutants Expressing the Green Fluorescent Protein Suitable for the Study of Host-Fungus Interactions. Fungal Biology 2018, 122, 1023–1030. [Google Scholar] [CrossRef]
- Borba-Santos, L. P.; Barreto, T. L.; Vila, T.; Chi, K. D.; dos Santos Monti, F.; de Farias, M. R.; Alviano, D. S.; Alviano, C. S.; Futuro, D. O.; Ferreira, V.; de Souza, W.; Ishida, K.; Rozental, S. In Vitro and In Vivo Antifungal Activity of Buparvaquone against Sporothrix brasiliensis. Antimicrob Agents Chemother 2021, 65, e00699–21. [Google Scholar] [CrossRef]
- Browne, N.; Heelan, M.; Kavanagh, K. An Analysis of the Structural and Functional Similarities of Insect Hemocytes and Mammalian Phagocytes. Virulence 2013, 4, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, G.; Garvey, A.; Croke, M.; Kavanagh, K. Innate Humoral Immune Defences in Mammals and Insects: The Same, with Differences ? Virulence 2018, 9, 1625–1639. [Google Scholar] [CrossRef]
- Wojda, I. Immunity of the Greater Wax Moth Galleria mellonella: Galleria mellonella Immunity. Insect Science 2017, 24, 342–357. [Google Scholar] [CrossRef]
- Ratcliffe, N. A.; Gagen, S. J. Studies on the in Vivo Cellular Reactions of Insects: An Ultrastructural Analysis of Nodule Formation in Galleria mellonella. Tissue and Cell 1977, 9, 73–85. [Google Scholar] [CrossRef]
- Lavine, M. D.; Strand, M. R. Insect Hemocytes and Their Role in Immunity. Insect Biochemistry and Molecular Biology 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Pereira, T.; de Barros, P.; Fugisaki, L.; Rossoni, R.; Ribeiro, F.; de Menezes, R.; Junqueira, J.; Scorzoni, L. Recent Advances in the Use of Galleria mellonella Model to Study Immune Responses against Human Pathogens. JoF 2018, 4, 128. [Google Scholar] [CrossRef] [PubMed]
- Melo, N. R. de; Abdrahman, A.; Greig, C.; Mukherjee, K.; Thornton, C.; Ratcliffe, N. A.; Vilcinskas, A.; Butt, T. M. Myriocin Significantly Increases the Mortality of a Non-Mammalian Model Host during Candida Pathogenesis. PLoS ONE 2013, 8, e78905. [Google Scholar] [CrossRef] [PubMed]
- Gandra, R. M.; McCarron, P.; Viganor, L.; Fernandes, M. F.; Kavanagh, K.; McCann, M.; Branquinha, M. H.; Santos, A. L. S.; Howe, O.; Devereux, M. In Vivo Activity of Copper(II), Manganese(II), and Silver(I) 1,10-Phenanthroline Chelates Against Candida Haemulonii Using the Galleria mellonella Model. Front. Microbiol. 2020, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.; Altincicek, B.; Glöckner, G.; Vilcinskas, A. A Comprehensive Transcriptome and Immune-Gene Repertoire of the Lepidopteran Model Host Galleria mellonella. BMC Genomics 2011, 12, 308. [Google Scholar] [CrossRef]
- Nale, J. Y.; Chutia, M.; Cheng, J. K. J.; Clokie, M. R. J. Refining the Galleria mellonella Model by Using Stress Marker Genes to Assess Clostridioides difficile Infection and Recuperation during Phage Therapy. Microorganisms 2020, 8, 1306. [Google Scholar] [CrossRef]
- Champion, O.; Titball, R.; Bates, S. Standardization of G. mellonella Larvae to Provide Reliable and Reproducible Results in the Study of Fungal Pathogens. JoF 2018, 4, 108. [Google Scholar] [CrossRef]
- Senior, N. J.; Titball, R. W. Isolation and Primary Culture of Galleria mellonella Hemocytes for Infection Studies. F1000Res 2021, 9, 1392. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; de Lucas, M. P.; Mesa-Arango, A. C.; Fusco-Almeida, A. M.; Lozano, E.; Cuenca-Estrella, M.; Mendes-Giannini, M. J.; Zaragoza, O. Antifungal Efficacy during Candida krusei Infection in Non-Conventional Models Correlates with the Yeast In Vitro Susceptibility Profile. PLoS ONE 2013, 8, e60047. [Google Scholar] [CrossRef] [PubMed]
- Brown, S. E.; Howard, A.; Kasprzak, A. B.; Gordon, K. H.; East, P. D. A Peptidomics Study Reveals the Impressive Antimicrobial Peptide Arsenal of the Wax Moth Galleria mellonella. Insect Biochemistry and Molecular Biology 2009, 39, 792–800. [Google Scholar] [CrossRef]
- Zhao, H.-X.; Xiao, W.-Y.; Ji, C.-H.; Ren, Q.; Xia, X.-S.; Zhang, X.-F.; Huang, W.-Z. Candidate Chemosensory Genes Identified from the Greater Wax Moth, Galleria mellonella, through a Transcriptomic Analysis. Sci Rep 2019, 9, 10032. [Google Scholar] [CrossRef]
- Miranda, L. H. M.; Santiago, M. de A.; Schubach, T. M. P.; Morgado, F. N.; Pereira, S. A.; Oliveira, R. de V. C. de; Conceição-Silva, F. Severe Feline Sporotrichosis Associated with an Increased Population of CD8 low Cells and a Decrease in CD4 + Cells. Med. Myco. 2015, myv079. [Google Scholar] [CrossRef]
- Legrand, N.; Weijer, K.; Spits, H. Experimental Models to Study Development and Function of the Human Immune System In Vivo. The Journal of Immunology 2006, 176, 2053–2058. [Google Scholar] [CrossRef]
- Arrillaga-Moncrieff, I.; Capilla, J.; Mayayo, E.; Marimon, R.; Marine, M.; Genis, J.; Cano, J.; Guarro, J. Different Virulence Levels of the Species of Sporothrix in a Murine Model. Clinical Microbiology and Infection 2009, 15, 651–655. [Google Scholar] [CrossRef]
- Borman, A. M. Of Mice and Men and Larvae: Galleria mellonella to Model the Early Host-Pathogen Interactions after Fungal Infection. Virulence 2018, 9, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gaviria, M.; Martínez-Álvarez, J. A.; Mora-Montes, H. M. Current Progress in Sporothrix brasiliensis Basic Aspects. JoF 2023, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Piatek, M.; Sheehan, G.; Kavanagh, K. Utilising Galleria mellonella Larvae for Studying in vivo Activity of Conventional and Novel Antimicrobial Agents. Pathogens and Disease 2020, 78, ftaa059. [Google Scholar] [CrossRef] [PubMed]
- Dinh, H.; Semenec, L.; Kumar, S. S.; Short, F. L.; Cain, A. K. Microbiology’s next Top Model: Galleria in the Molecular Age. Pathogens and Disease 2021, ftab006. [Google Scholar] [CrossRef] [PubMed]
- Binder, U.; Maurer, E.; Lass-Flörl, C. Galleria mellonella: An Invertebrate Model to Study Pathogenicity in Correctly Defined Fungal Species. Fungal Biology 2016, 120, 288–295. [Google Scholar] [CrossRef]
- Fakhim, H.; Vaezi, A.; Dannaoui, E.; Chowdhary, A.; Nasiry, D.; Faeli, L.; Meis, J. F.; Badali, H. Comparative Virulence of Candida Auris with Candida haemulonii, Candida glabrata and Candida albicans in a Murine Model. Mycoses 2018, 61, 377–382. [Google Scholar] [CrossRef]
- Corrêa-Moreira, D.; Menezes, R. C.; Romeo, O.; Borba, C. M.; Oliveira, M. M. E. Clinical and Anatomopathological Evaluation of BALB/c Murine Models Infected with Isolates of Seven Pathogenic Sporothrix Species. Pathogens 2021, 10, 1647. [Google Scholar] [CrossRef]
- Trevijano-Contador, N.; Zaragoza, O. Immune Response of Galleria mellonella against Human Fungal Pathogens. JoF 2018, 5, 3. [Google Scholar] [CrossRef]
- Tamez-Castrellón, A. K.; van der Beek, S. L.; López-Ramírez, L. A.; Martínez-Duncker, I.; Lozoya-Pérez, N. E.; van Sorge, N. M.; Mora-Montes, H. M. Disruption of Protein Rhamnosylation Affects the Sporothrix schenckii-Host Interaction. The Cell Surface 2021, 7, 100058. [Google Scholar] [CrossRef]
- Rossoni, R. D.; Fuchs, B. B.; de Barros, P. P.; Velloso, M. dos S.; Jorge, A. O. C.; Junqueira, J. C.; Mylonakis, E. Lactobacillus Paracasei Modulates the Immune System of Galleria mellonella and Protects against Candida albicans Infection. PLoS ONE 2017, 12, e0173332. [Google Scholar] [CrossRef]
- de Beer, Z. W.; Duong, T. A.; Wingfield, M. J. The Divorce of Sporothrix and Ophiostoma : Solution to a Problematic Relationship. Studies in Mycology 2016, 83, 165–191. [Google Scholar] [CrossRef] [PubMed]
- Meister, M.; Lemaitre, B.; Hoffmann, J. A. Antimicrobial Peptide Defense In Drosophila. Bioessays 1997, 19, 1019–1026. [Google Scholar] [CrossRef]
- Bulet, P.; Hetru, C.; Dimarcq, J.-L.; Hoffmann, D. Antimicrobial Peptides in Insects; Structure and Function. Developmental & Comparative Immunology 1999, 23, 329–344. [Google Scholar] [CrossRef]
- Lee, Y. S.; Yun, E. K.; Jang, W. S.; Kim, I.; Lee, J. H.; Park, S. Y.; Ryu, K. S.; Seo, S. J.; Kim, C. H.; Lee, I. H. Purification, CDNA Cloning and Expression of an Insect Defensin from the Great Wax Moth, Galleria mellonella. Insect Mol Biol 2004, 13, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Gabriele Barros Mothé. Estudo Da Interação Entre Fagócitos Do Felis Catus (LINNAEUS,1758) e os Principais Agentes Etiológicos Da Esporotricose. Tese de Doutorado, Universidade Federal Fluminense, Niterói, Rio de Janeiro, Brasil, 2021. https://app.uff.br/riuff/handle/1/24482.
- Złotko, K.; Wiater, A.; Waśko, A.; Pleszczyńska, M.; Paduch, R.; Jaroszuk-Ściseł, J.; Bieganowski, A. A Report on Fungal (1→3)-α-d-Glucans: Properties, Functions and Application. Molecules 2019, 24, 3972. [Google Scholar] [CrossRef]
| Gene | NCBI Genbank References |
Sequence (5’ – 3’) |
Annealing temperature |
|---|---|---|---|
| Galiomycin | AY528421.1 | F- TCCAGTCCGTTTTGTTGTTG | 60ºC |
| [37] | R- CAGAGGTGTAATTCGTCGCA | ||
| Gallerimycin | AF453824.1 | F- GAAGATCGCTTTCATAGTCGC | 60ºC |
| [37] | R- TACTCCTGCAGTTAGCAATGC | ||
| C7 Contig 15362 | Contig 15362 | F- CGAGCTAAAGACAGGCGATT | 58ºC |
| [30] | R- TCACCTGCGGTTGAATCATA | ||
| C8 Contig 19101 | Contig 19101 | F- ATTGCTAGCCAGGTTCAGGA | 60ºC |
| [30] | R- AGCTATTTGGCGGAAACTCA | ||
| β-actin | [38] |
F- GGACTTGTACGCCAACACAG R- CCACATCTGCTGGAATGTCG |
55ºC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





