Submitted:
31 August 2023
Posted:
04 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Experimental Materials
2.2. Experimental Method
2.2.1. Arthrospira Platensis Culture
2.2.2. Sodium selenite treatment concentrations
2.2.3. Sodium Selenite Addition Method
One-Time Addition
Additions in Batches
2.2.4. Arthrospira Biomass Determination
2.2.5. Determination of the Soluble Protein and Phycocyanin Contents of the Arthrospira
2.2.6. Determination of Chlorophyll a Content in the Arthrospira
2.2.7. Determination of the Antioxidant Enzyme Activity in the Arthrospira
Determination of Superoxide Dismutase (SOD)
Measurement of Catalase (CAT)
Determination of Malondialdehyde (MDA)
2.2.8. Measurement of the Chlorophyll a Fluorescence Parameters
2.3. Data Processing
3. Results and Discussion
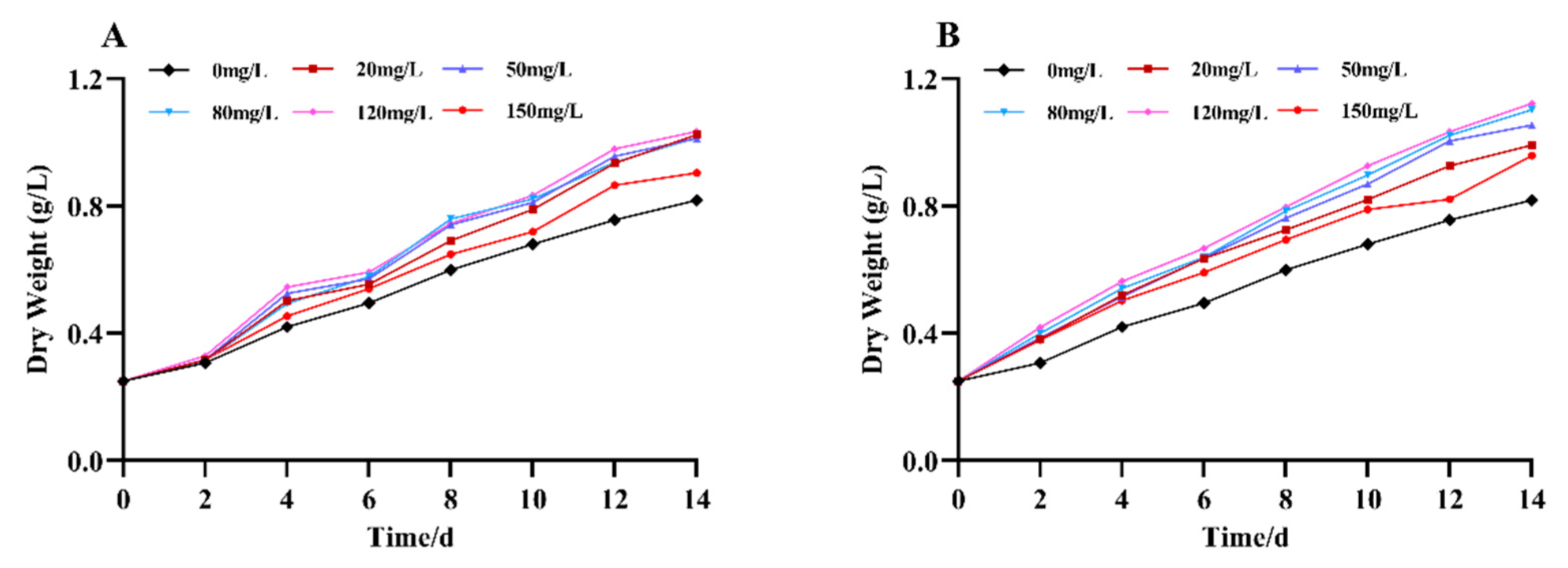
3.1. Effect of different Na2SeO3 Concentration Treatments on the Growth of Arthrospira
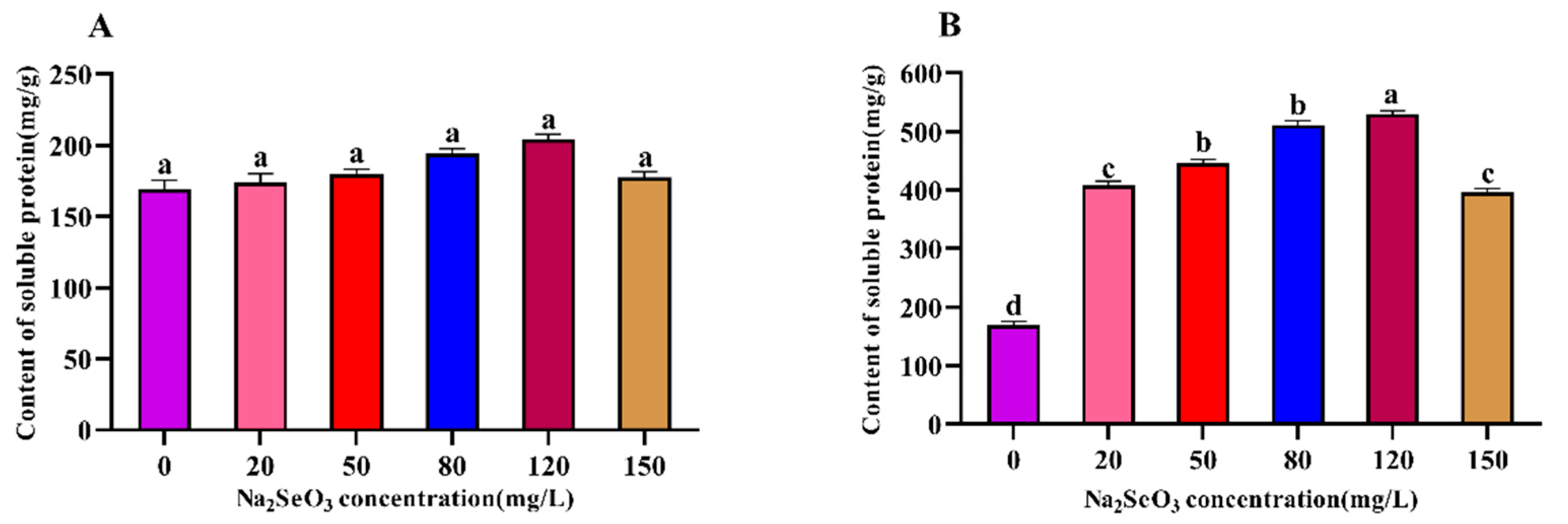
3.2. Effect of different Na2SeO3 Concentration Treatments on the Soluble Protein Content of the Arthrospira
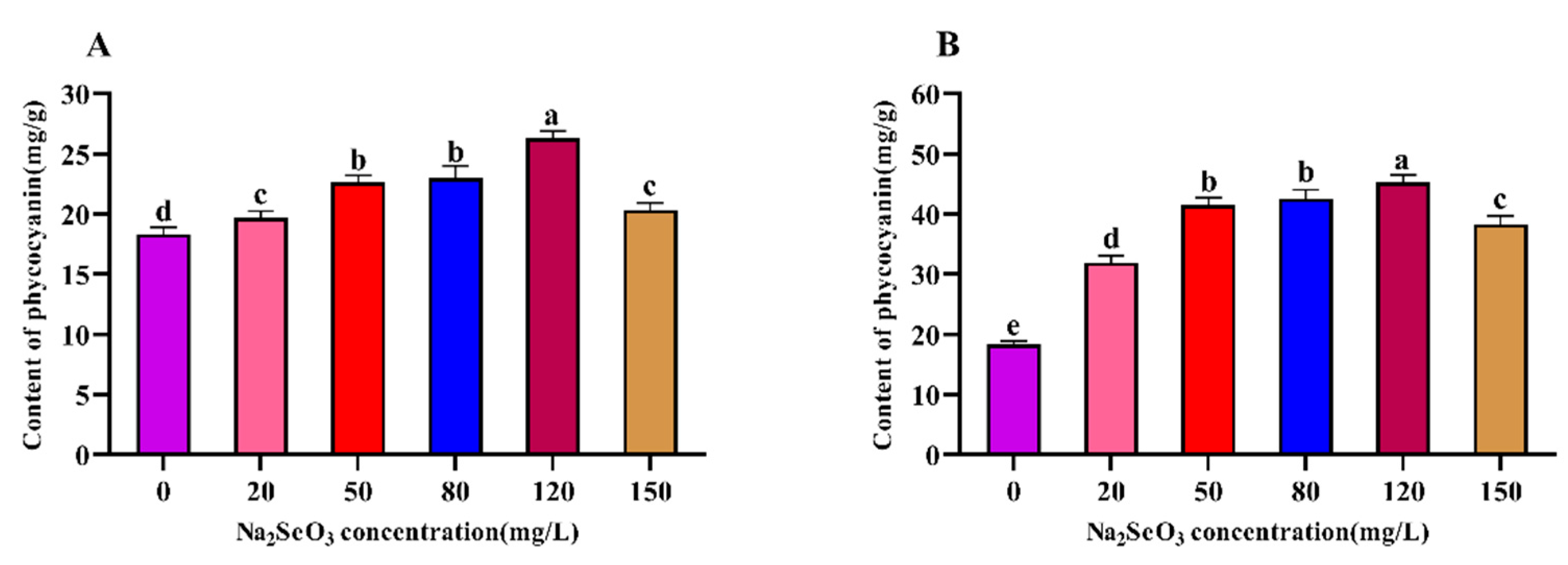
3.3. Effect of Different Na2SeO3 Concentrations on the Phycocyanin Content of the Arthrospira
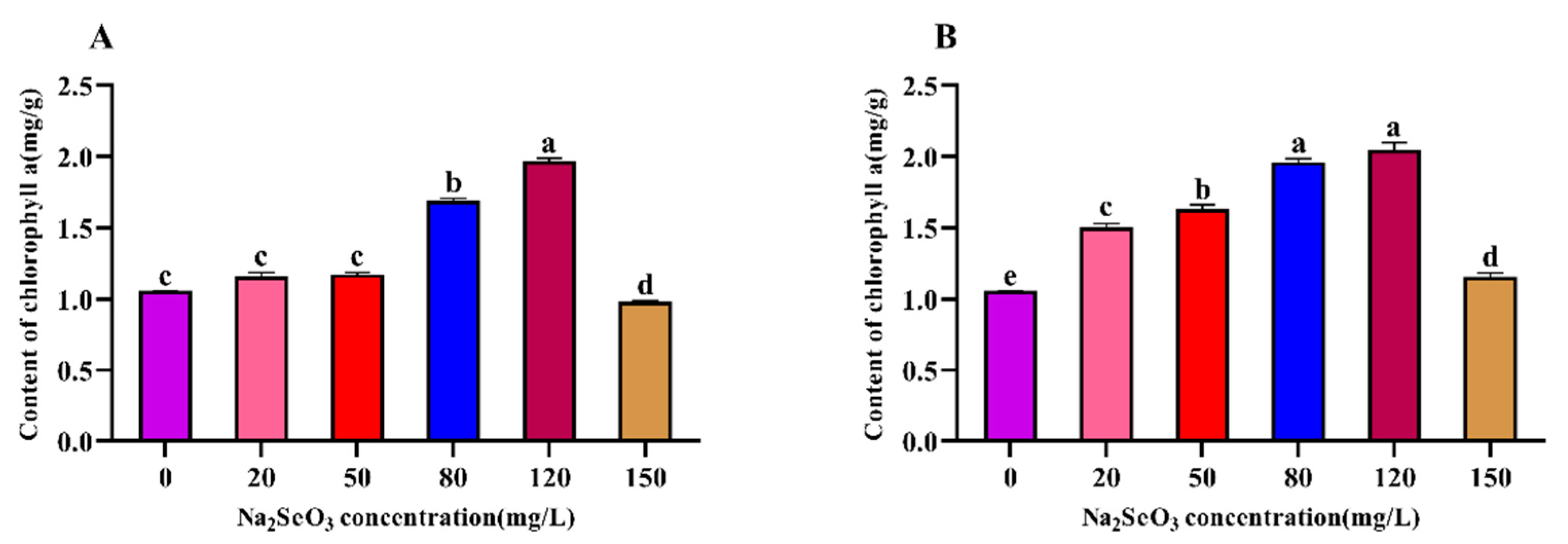
3.4. Effect of different Na2SeO3 Concentration Treatments on the Chlorophyll a Content of the Arthrospira
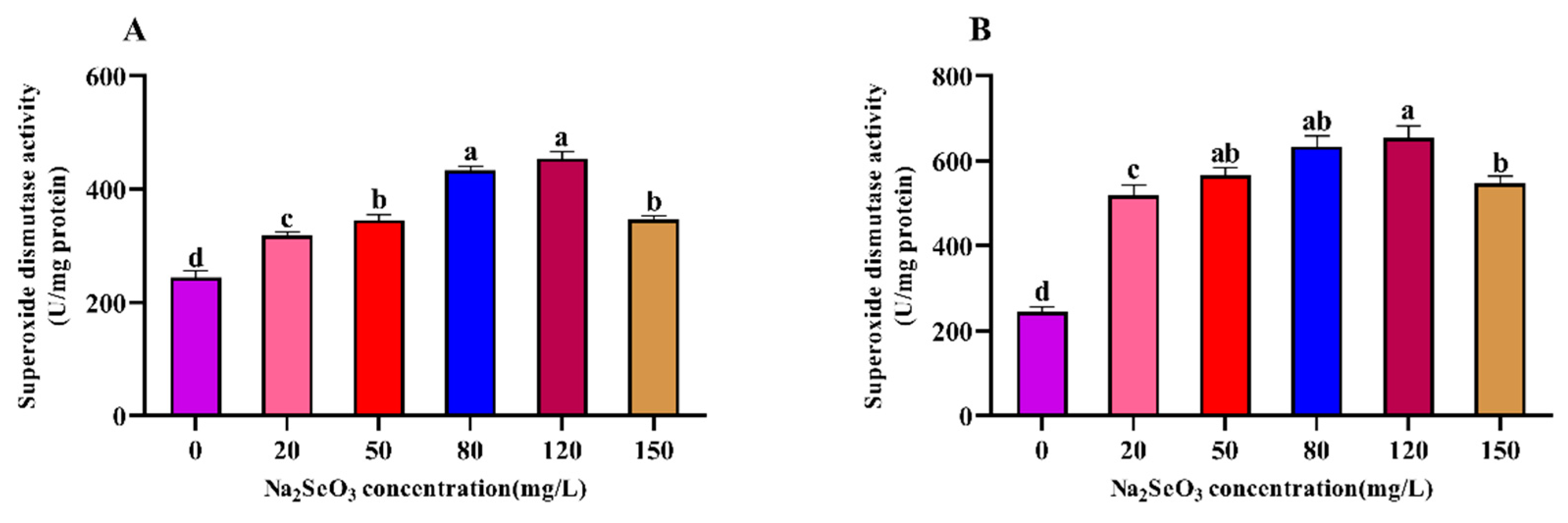
3.5. Effect of different Na2SeO3 Concentration Treatments on the Superoxide Dismutase (SOD) Activity in the Arthrospira
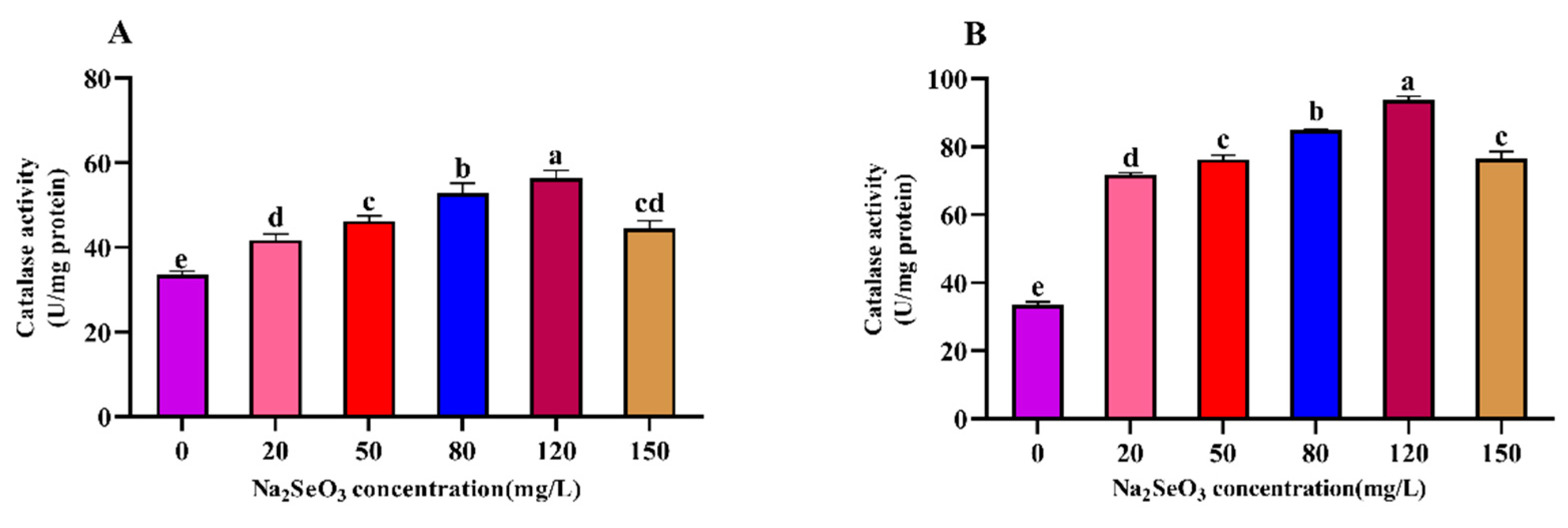
3.6. Effect of Different Na2SeO3 Concentration Treatments on the Catalase (CAT) Activity of the Arthrospira
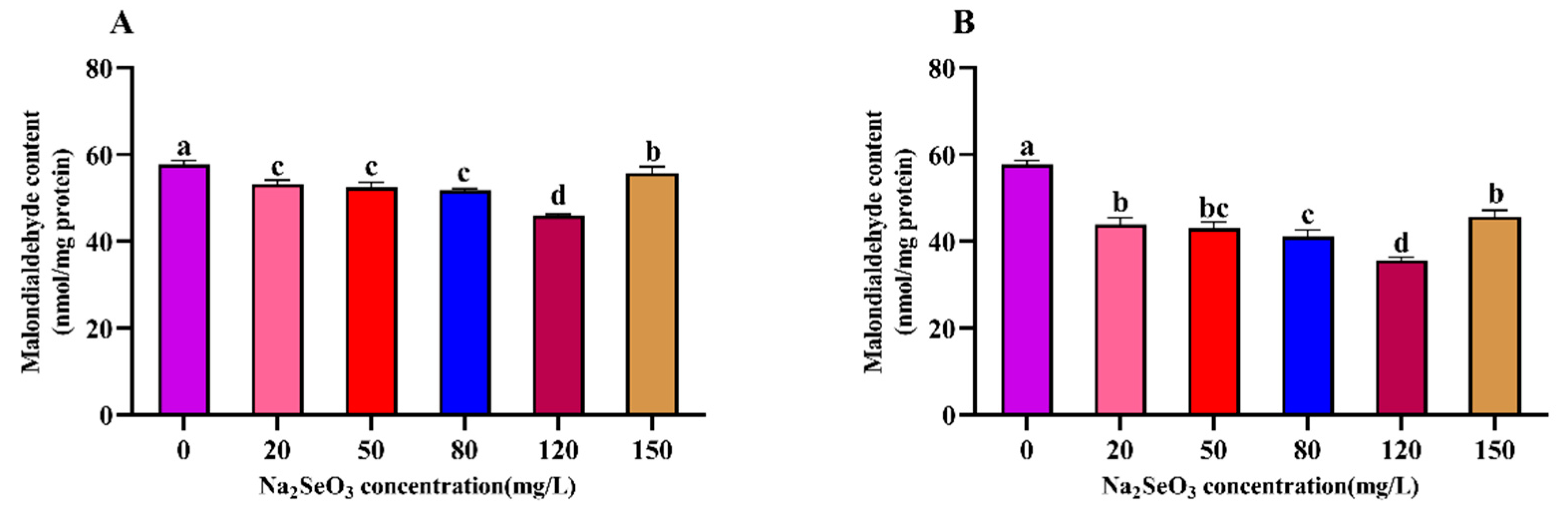
3.7. Effect of Different Na2SeO3 Concentrations on the Malondialdehyde Content (MDA) in the Arthrospira
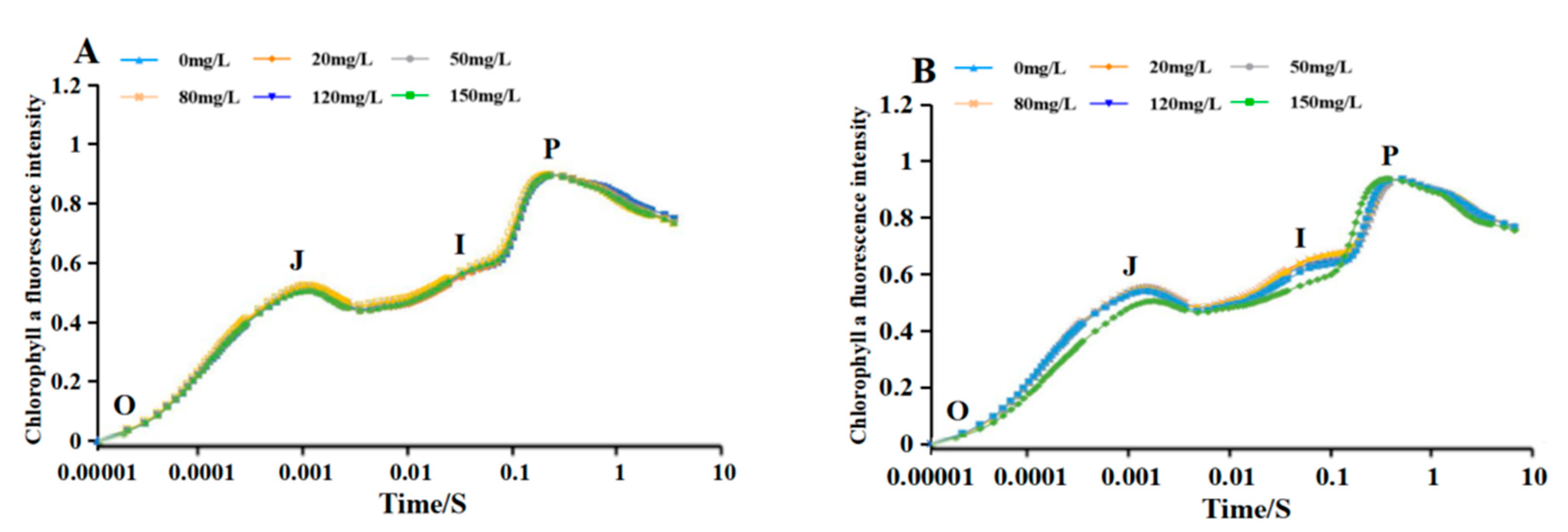
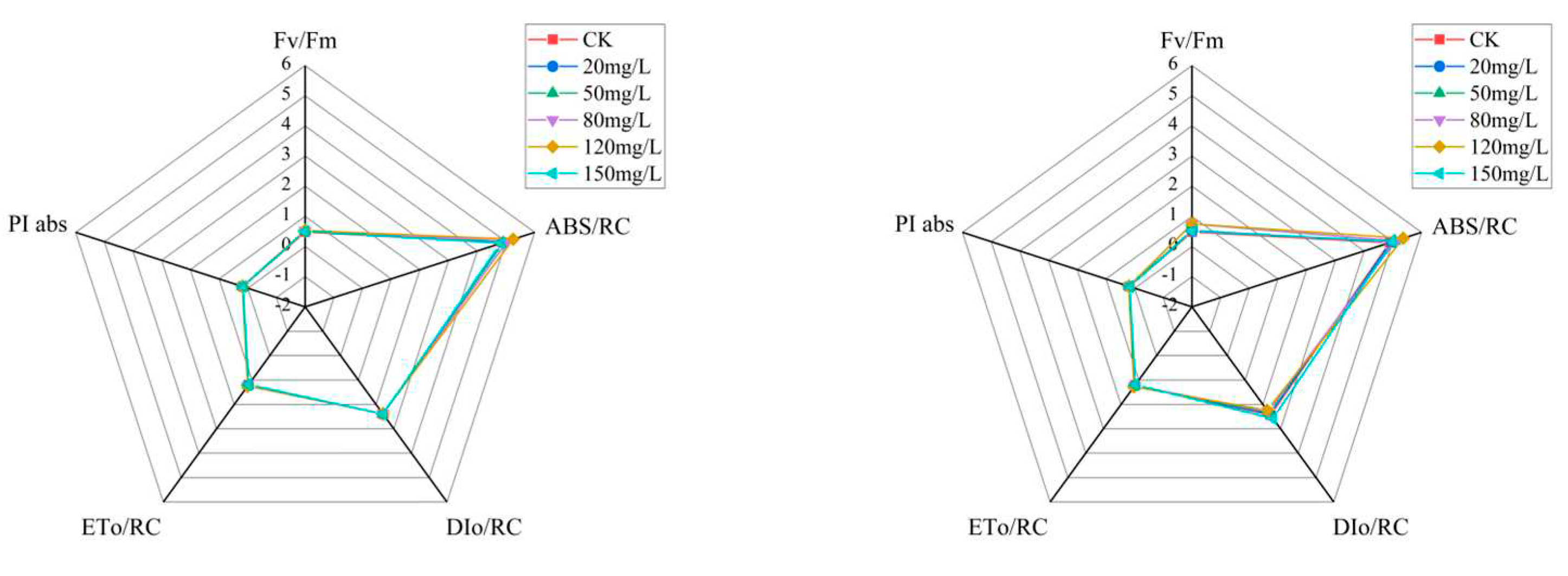
3.8. Effect of different Na2SeO3 concentrations on the chlorophyll fluorescence induction kinetic curves of the Arthrospira
3.9. Comparison of the Parameters of the Fluorescence Induction Kinetic Curves of the Arthrospira at Different Concentrations of Na2SeO3
4. Discussion
4.1. Effect of Na2SeO3 on the Growth, Chlorophyll a and Protein Contents of the Arthrospira
4.2. Effect of Na2SeO3 on the Antioxidant Enzyme Activity of Arthrospira
4.3. Effect of Na2SeO3 on the Chlorophyll Fluorescence of the Arthrospira
5. Conclusions
References
- Xu,W.T.; Wang,Y.; Luo,Y.B.; Li,Y.F.; Zhang,F.F.;Huang,K.L. Bioaccumulation of Cr (III) in Spirulina platensis and its effects on cultivation of Spirulina platensis. Food Sci. 2009, 30, 153–157.
- Chen,T.F. Mixotrophic culture of high selenium-enrich Spirulina platensis and the speciation of selenium. Jinan University: Guangzhou, China, 2005.
- Li,X.F.;Wang,C.H.;Wen,S.H. Study on culture conditions of Spirulina. Food Ferment Ind. 1999, 25, 15–19.
- Li,X.;Huang,W.; Huang,B. Effect of Selenium-Enriched Spirulina platensis on Serum lipid levels of Acute Hyperlipidemic Mice. Food Sci. 2016, 37, 194–197.
- Dong,Q.; Dong,Y.L.; Qian,K.X. Experimental study on the hypoglycemic effect of selenium-rich Spirulina on diabetic mice. Studies of Trace Elements and Health. 1998, 15, 6–7.
- Gao,D.F.; Zhang,Y.B.; Ling,Q.J. Angiotensin Convert Enzyme Inhibition of Peptides Derived from Water Soluble Total Protein of Selenium-enriched Spirulina platensis. Food Sci. 2011, 32, 7–10.
- Li,Z.Y.; Jin,J.G.; Chen,Y.F. Study on the anti-fatigue and immunity-enhancing functions of selenium-rich Spirulina. Proceedings of the China Marine Biochemical Academic Conference. 2005, 5.
- Liu,Y.M.; Zuo,T.; Qing,C. Study on the anti-tumor effect of selenium-rich Spirulina. Henan Tradit Chin Med. 2009, 29, 1072–1073.
- Well, K.; Johnson, G.N. Chloropjyll fluorescence-A practical guide. J EXP BOT. 2000, 51, 659–668. [Google Scholar]
- Li, P.M.; Gao, H.Y.; Strasser, R.J. Application of the chlorophyll fluorescence induction dynamics in photosynthesis study. Mol Plant. 2005, 31, 559–566. [Google Scholar]
- Zhang,Y.H.; Gao,D.P.; Wang,X.L. Effects of Soil Salinity on Photosystem II of Rice Seedlings. J IRRIG DRAIN ENG. 2022, 41, 52–60.
- Ying,Z.P.; Lu,J.Z.; Gao,Z.H.; Response of photosynthesis, PSII electron transport and reactive oxygen species to short-term high temperature stress in tomato seedling leaves. Northern Horticulture. 2019, 5, 1–11.
- Sun,L.L.; Geng,Q.W.; Xing,H. Effect of low temperature treatment of grape roots on leaf PSII activity. Chinese Bulletin of Botany. 2017, 52, 159–166.
- Gao,X.; Zhou,W.C.; Zhang,F.G. Effect of Salt Stress on Photosynthetic Activity and Metabolites of Chlamydomonas reinhardtii. Plant Physiology J. 2015, 51, 1887–1894.
- Vaishnav,A.; Shukla,A.K.; Sharma,A. Endophytic Bacteria in Plant Salt Stress Tolerance: Current and Future Prospects. J PLANT GROWTH REGUL, 2019, 38, 650–668. [CrossRef]
- Xu,G.P.;Liu,F.;Wu,X.B. Pigment content correlated to reflectance spectrums in Phyllostachys edulis leaves stressed by low temperature. Journal of Zhejiang A & F University. 2014, 31, 28–36.
- Han,R. Study on the Mechanism of Tolerance, Absorption and Metabolism of Spirulina to Arsenic. Inner Mongolia University Of Science & Technology: Baotou, China, 2020.
- Li,H.S. Principles and techniques of plant physiological and biochemical experiments. Higher Education Press. Beijing, China, 2000.
- Yang,Y.Y. Studies on High-concentration Cultivation and Se-Bioaccumulation of Spirulina maxima. Ocean University of China. Shandong, China, 2011.
- Gong,H.M. Stlldies on effect of salt stress on the photosynthesis of Spirulina platensis. Graduate School of Chinese Academy of Sciences (Institute of Botany).Beijing, China, 2006.
- Wang,Y.Q.X.; Jin,Z.Y.; Liu,L.J. Research advances of superoxide dismutase 1 in metabolic disorders of glucose and lipids. Chinese Bulletin of Life Sciences. 2020, 32, 1185–1197.
- Chen,W.G.; Liu,Y.; Zhou,J.H. Influence of Low Temperature Stress on the Membrane-protected Enzyme System in Tobacco. Journal of Anhui Agricultural Sciences. 2011, 39, 3978–3980.
- Jv,Z.G.; Zhu,G.L.; Cao,Z.X. Relationship between browning and regionalized distribution of polyphenol oxidase and phenolics in pear fruits in Laiyang. PHYSIOL MOL BIOL PLA. 1985, 14, 356–361.
- Li,G.; Li,J.; Hao,R.; Guo,Y. Activation of catalase activity by a peroxisome-localized small heat shock protein Hsp17.6CII. J Genet Genomics. 2017, 44, 395–404. [CrossRef] [PubMed]
- Li,J.; Liu,J.; Wang,G.; Cha,J.Y.; Li,G.; Chen,S.; Li,Z.; Guo,J.; Zhang,C.; Yang,Y.; Kim,W.Y.; Yun, D.J.; Schumaker,K.S.; Chen,Z.; Guo,Y. A chaperone function of NO CATALASE ACTIVITY1 is required to maintain catalase activity and for multiple stress responses in Arabidopsis. Plant Cell. 2015, 27, 908–925.
- Song,C.; Chen,Q.; Wu,X.; Zhang,J.; Huang,C. Heat stress induces apoptotic-like cell death in two Pleurotus species. Curr Microbiol. 2014, 69, 611–616. [CrossRef] [PubMed]
- Choudhary,M.; Jetley,U.K.; Abash,Khan.M.; Zutshi,S.; Fatma,T. Effect of heavy metal stress on proline, malondialdehyde, and superoxide dismutase activity in the cyanobacterium Spirulina platensis-S5. Ecotoxicol Environ Saf. 2007, 66, 204–209. [CrossRef] [PubMed]
- Wang,B.S. Plant Physiology. Science Press. Beijing, China, 2003, 289-292.
| Experimental group | CK | 1 | 2 | 3 | 4 | 5 |
| Na2SeO3 concentration (mg/L) | 0 | 20 | 50 | 80 | 120 | 150 |
| Tube number | 1 | 2 | 3 | 4 | 5 | 6 |
| Standard protein solution (mL) | 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1 |
| Distilled water content (mL) | 1 | 0.8 | 0.6 | 0.4 | 0.2 | 0 |
| Protein content (ug) | 0 | 20 | 40 | 60 | 80 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





