1. Introduction
The European Parliament voted in favor of proposed amendments [
1] to Regulation (EU) 2019/631, which suggest a 100% reduction of CO
2 emission in the automotive sector by 2035 through banning the sale of new passenger cars and new light-duty vehicles with internal combustion engines. In addition to the immense efforts put into the electrification of the European mobility and transportation sector, care should also be taken to investigate such technologies, that will allow us to decrease emissions of used internal combustion engine vehicles.
The average passenger car age in the EU as of 2020 [
2] is 11.8 years – with the two extremes of 6.7 years on average in Luxembourg and 17 years in Lithuania. Considering light-duty and heavy-duty vehicles, the averages age increases to 11.9 and 13.8 years respectively [
2]. Given the post-COVID economic environment burdened with the impact of the Russian-Ukrainian conflict and a looming energy crisis, the average age of the European vehicle fleet is expected to stagnate – if not rise – in the upcoming 5–10-year period. These factors also affect raw material prices – i.e., cobalt, nickel, and lithium – necessary for EV battery production [
3]. In other words, the internal combustion engine is expected to overstay its welcome.
The perception of biofuels and synthetic fuels ranges on a broad spectrum [
4], [
5][
6], [
7], [
8], [
9]since compared to battery electric and fuel cell electric vehicles they only offer a minor reduction in local harmful emissions. Nonetheless, considering a lifecycle point of view, implementing these technologies on used vehicles still contributes to a reduction of greenhouse gas (GHG) emission and end-of-life waste production. The key of utilizing alternative fuels lies in compatibility, which must be proved before widespread adoption. In addition to part compatibility – e.g., seals, fuel lines, pumps, etc. –, a vital aspect of correct internal combustion engine operation is its possible impact on lubrication. Since engine oil dilution with fuel and the resulting degradation of the lubricant is a known and well-researched phenomenon [
10,
11,
12,
13,
14], engine oil compatibility with alternative fuels must be regarded with great care during the introduction of new fuel formulations.
Highly detailed qualitative and quantitative information on the chemical composition of lubricants and their additives can be obtained using mass spectrometry (MS). To separate the complex mixture of base oil components and additives, mass spectrometry is frequently combined with chromatographic techniques, such as gas chromatography (GC) [
15,
16] or liquid chromatography (LC) [
17,
18], which typically involves increased time and more elaborate sample preparation. For direct comprehensive chemical analysis of lubricants by MS, ambient ionization techniques such as desorption electrospray ionization (DESI) [
19,
20], matrix assisted laser desorption ionization (MALDI) [
21], direct analysis in real time (DART) [
22], and atmospheric solids analysis probe (ASAP) [
23] are becoming more widespread and popular. Instruments with high resolution and mass accuracy allow additives to be identified without complex sample preparation or the use of standards, as demonstrated by Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) [
24] or dielectric barrier discharge ionization mass spectrometry (DBDI-MS) [
25].
The presented study concludes a series of preliminary works, which aim to establish the groundwork of alternative fuels research in modern internal combustion engines through investigating engine oil dilution and degradation in the field, with currently available EN 228 [
26] compatible gasoline and EN 590 diesel [
27] fuels. In addition to already published conventional oil analysis results [
28] and results of principal component analysis of FTIR spectra [
29], this article provides a detailed analysis of additive degradation and degradation products on the molecular level through mass spectrometry. A comparison with the corresponding conventional results is given, with the aim of uncovering the chemical structures responsible for commonly established degradation parameters. This could help understanding the differences in reaction pathways of fuel induced oil degradation patterns and offers insights for formulating engines oils that can withstand alternative fuels.
2. Materials and Methods
A field test with various petrol- and diesel fueled passenger cars were conducted utilizing 12 vehicles under real, on-road driving conditions, where oil samples through the dipstick pipes were taken at regular intervals. All participating vehicles were using the same fully synthetic SAE 0W-30 fresh oil (properties are displayed in
Table 1).
The methodology and various oil analysis results are presented in detail in [
28] and [
29], accordingly, here only a brief summary of the relevant points is given.
2.1. Field test under real driving conditions
In detail, 9 petrol and 3 diesel drivetrains were studied, both utilizing an engine with 2.0-liter total displacement and direct injection. The vehicles were equipped with a GPS tracking system, which enabled a detailed analysis of utilization profiles. Based on the average speed [
30] and average trip length [
31], “short range” and “long range” groups were selected [
28,
29].
Table 2 gives an overview of the vehicles, drivetrains, utilization profiles and total covered milage. From the available vehicle pool, 3 vehicles were selected for in-depth oil characterization (indicated in bold). Here, a medium and high mileage sample from each vehicle were analyzed according to the methodology described in detail in 2.2.
2.2. Oil Condition Monitoring
Within this study, MS results were compared and correlated to conventional oil parameters. Fourier-transform infrared spectroscopy was performed (FTIR, Tensor 27 spectrometer, Bruker, Ettlingen, Germany), with subsequent evaluation of the following parameters [
28,
29,
32,
33]:
Additionally, boron content of the used engine oil samples was determined via inductively coupled plasma optical emission spectroscopy (ICP-OES, iCAP 7400 ICP-OES Duo, ThermoFisher, Waltham, Massachusetts, USA) after microwave-assisted nitric acid digestion.
High resolution mass spectrometry (MS) was utilized as an advanced analytical method for lubricants to gain information on additive depletion and degradation products on a molecular level. The analysis was carried out according to the method described in [
32,
33,
34,
35] via an LTQ Orbitrap XL hybrid tandem high-resolution mass spectrometer (Orbitrap-MS) (ThermoFisher Scientific, Bremen, Germany). After sample preparation, solutions were infused into the ion-source where the analyte molecules undergo ionization. Subsequently, fragmentation of the analyte ions was performed for accurate identification of the chemical structures. Generated fragment ions were detected a high-resolution Orbitrap-MS detector.
Table 3 gives an overview of the relevant measurement parameters.
Data processing and evaluation were done using Xcalibur version 2.0.7 and Mass Frontier version 6.0 software (ThermoFisher Scientific, Bremen, Germany). The presented m/z values describe the mass-to-charge ratio of the detected species. Since all analyzed ions were single-charged species, m/z also describes the monoisotopic molecular masses of the detected compounds. It is noted that the ionization capability of chemical compounds is dependent on multiple parameters, such as the chemical structure, solvent, or matrix effects. Hence, a comparison of the obtained intensities of the individual ions is only possible between the same species found in the analyzed oil samples. Furthermore, the exact position of alkyl sidechains might vary within the depicted molecules, although their presence can be detected accurately through the mass-to-charge ratio,. The reported molecules are proposed structures in all cases, e.g., in the case of substituted aromatic molecules ortho-, meta- and para-isomers are all possible.
3. Results and discussion
3.1. Characterization of the fresh oil
The respective degradation products of ZDDP and boron-ester antiwear additives were of particular interest since wear protection is one of the most important indicators of the tribological performance of any given lubricant. With the depletion of antiwear additives the tribofilm composition also changes [
35] which usually results in higher wear rates [
35]. Complementarily, organic, and inorganic acids as well as selected oxidation and nitration products are presented.
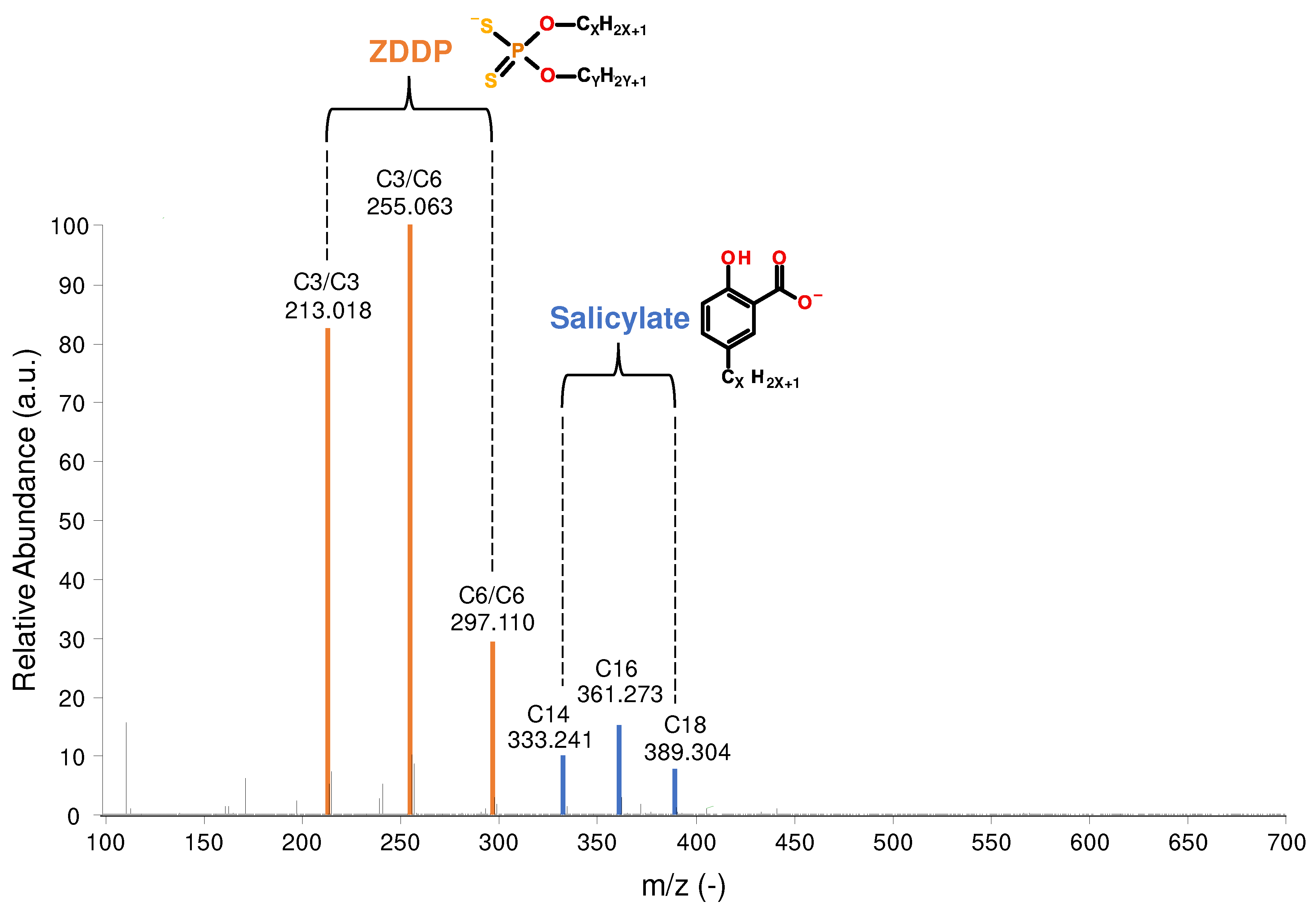
Figure 1 shows the identified ZDDP species in the fresh engine oil by MS, namely dipropyl dithiophosphate (C3/C3; m/z 213.018) and propyl-hexyl dithiophosphate (C3/C6; m/z 255.064) as major compounds and dihexyl dithiophosphate (C6/C6; m/z 297.111) in lower concentrations. Furthermore, 3 differently alkylated salicylate detergents were identified, in detail with tetradecyl (C14; m/z 333.241), hexadecyl (C16; m/z 361.273) and octadecyl (C18; m/z 389.304) sidechains.
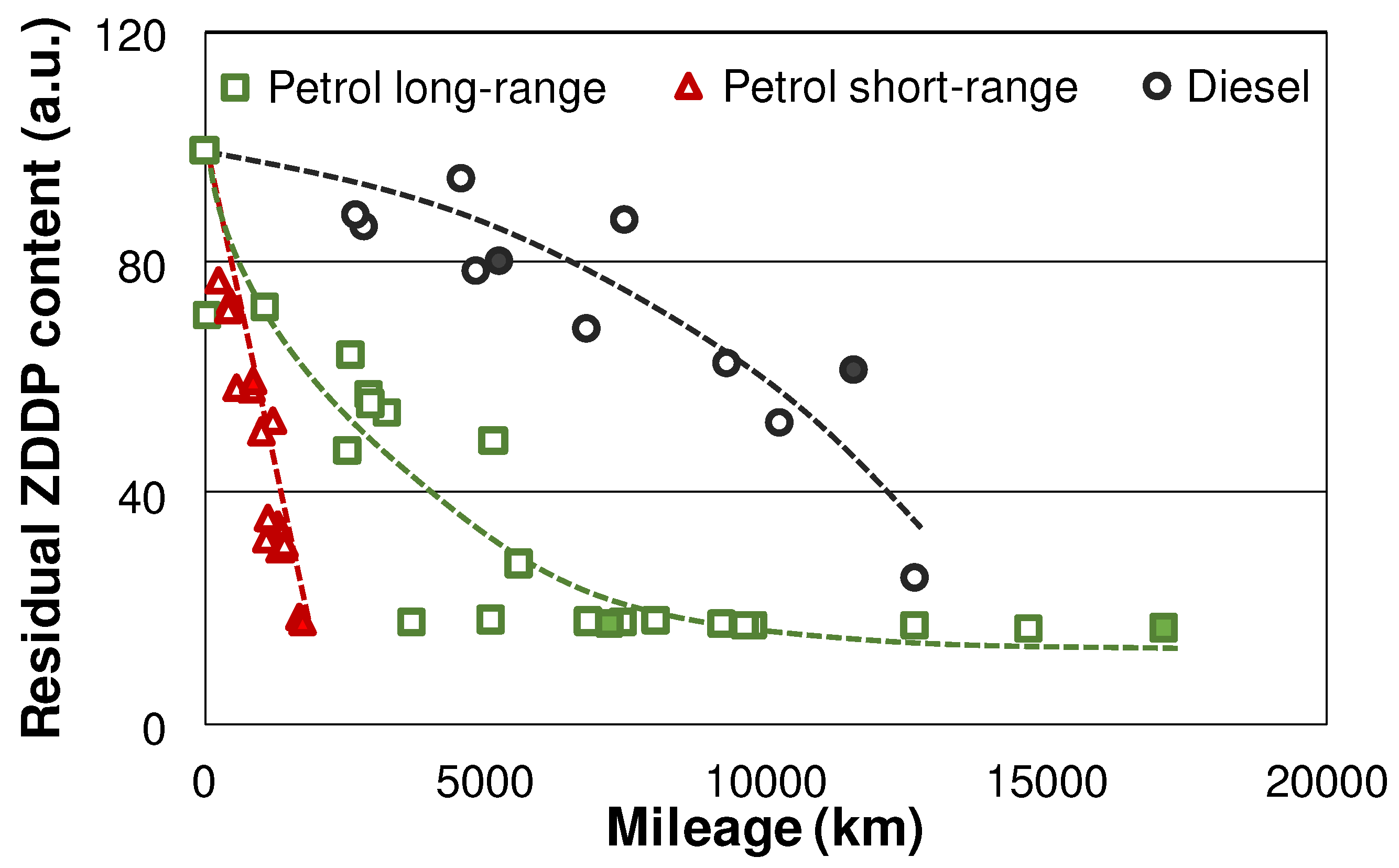
3.2. ZDDP degradation in the used oils
Figure 2 gives an overview of the ZDDP degradation in the petrol and diesel vehicles, as observed in the field test via FTIR. The petrol vehicles generally exhibit a faster ZDDP degradation, which is especially accelerated in the short-range samples. This was attributed to the lower air-to-fuel ratio (AFR) in petrol engines compared to diesel counterparts, which results in a higher abundance of reactive, not completely combusted species, hence faster additive degradation [
28]. The AFR is even lower in case of a short-range utilization profile, where the vehicles run predominantly colder, hence the engine control unit (ECU) has to inject extra fuel to compensate for the condensation on the cylinder walls [
28].
All ZDDP species showed well comparable degradation, hence the respective degradation pathways are being demonstrated on the example of dipropyl dithiophosphate.
Figure 3 a) displays the abundance of dipropyl dithiophosphate (m/z 213.018), one of the main ZDDP components in the fresh and the used oils. The abundance of this molecule is diminishing rapidly, it is detectable only in the first diesel used oil sample (5,300 km mileage). In this context, it is notable that even the short-range petrol engine oils show a close to complete depletion of the original ZDDP structure despite the relatively low mileage.
Figure 3 b) presents the abundance of dipropyl thiophosphate (m/z 197.041), where a sulfur atom of the dipropyl dithiophosphate is substituted with oxygen, as well as dipropyl phosphate (m/z 181.063) where both sulfur atoms are substituted with oxygen. The long-range petrol and diesel engine oils show different patterns. In the diesel engine, the first sample (5,300 km mileage) contains mostly dipropyl thiophosphate and some dipropyl phosphate, the subsequent sample (11,600 km) contains both dipropyl thiophosphate and dipropyl phosphate with higher abundance. Comparatively, the long-range petrol engine oil samples contain practically no dipropyl thiophosphate, only in traces after 7,200 km, while dipropyl phosphate is characterized by high abundance at both mileages. These findings suggest that ZDDP degradation is significantly faster in the long-range petrol engine, which relates to a faster formation of dipropyl phosphate. This corresponds well with the results of the conventional oil analysis (see
Figure 2), where a significantly faster ZDDP degradation in case of the petrol engines was found. The short-range petrol vehicle also shows expedited ZDDP degradation compared to the diesel engine, when the significantly lower mileage is considered. The initial short-range petrol engine oil sample with only 900 km mileage contains both dipropyl thiophosphate and dipropyl phosphate in a higher abundance, similarly to the diesel engine oil after 11,600 km. This demonstrates how much faster the degradation process propagates. The subsequent short-range petrol sample (1,750 km) contains only traces of dipropyl thiophosphate but significant amounts of dipropyl phosphate, similarly to the long-range petrol engine oils.
Figure 3 c) shows the abundance of decyl sulfate (m/z 237.117) and decyl phosphate (m/z 237.126), both considered to be further ZDDP degradation products. The release of alkyl side chains (as olefins) during the later stages of ZDDP degradation was previously described in [
36] as well. Decyl sulfate and decyl phosphate exhibit comparable trends in abundance to already discussed ZDDP degradation products. The molecules show an increasing abundance with the mileage and are less pronounced in the diesel engine oil samples. It is also noteworthy, that at this point the alkyl chains of the original ZDDP molecules change during utilization, as no decyl species were found in the original ZDDP composition. The short-range petrol samples display a high abundance of these degradation products, especially considering their low mileage. Banerji et al. proposed that hydroxyl groups contained in ethanol molecules could facilitate ZDDP degradation, especially alkyl ligand exchange between the oxygen and sulfur atoms [
37]. As petrol contains 5-10 v% ethanol depending on the product, this might give a possible explanation for the emergence of decyl sidechains in petrol vehicles.
Figure 3 d) compares the abundance of sulfuric acid (m/z 96.960) in the fresh and used oil samples. Phosphoric acid (m/z 96.970) (not shown) was also identified in the used oil samples in trace amounts, with comparable trends in the relative abundance as sulfuric acid. Sulfuric acid exhibits comparable behavior in all used oil samples, although the abundance seems to be somewhat higher in the long-range petrol samples, where the ZDDP degradation is most pronounced. It is noteworthy that the short-range petrol engine oil samples show a relatively high abundance of sulfuric acid despite their low total mileage.
It can be stated that the degradation of ZDDP is similar to prior field studies. In [
32], it was also found that the ZDDP depletes rapidly in the first 5,000 – 6,000 km and the emergence of dialkyl thiophosphates followed by their rapid depletion was observed. Other degradation products were found in increasing amounts with mileage, especially dialkyl phosphates as well as phosphoric and sulfuric acid. In detail, the formation of sulfuric acid was visible after 8,000 – 9000 km and the abundance of the species then remained at high level [
32]. The presented results are comparable with this observation, where sulfuric acid can be detected in the used oil samples subsequent to the degradation of ZDDP but does not show a significant increase with the mileage. Follow-up tribological studies [
35] revealed, that once both dialkyl dithiophosphates and dialkyl thiophosphates are depleted, the tribofilms formed by the used oils contain less phosphorus and zinc, and the wear rate increases.
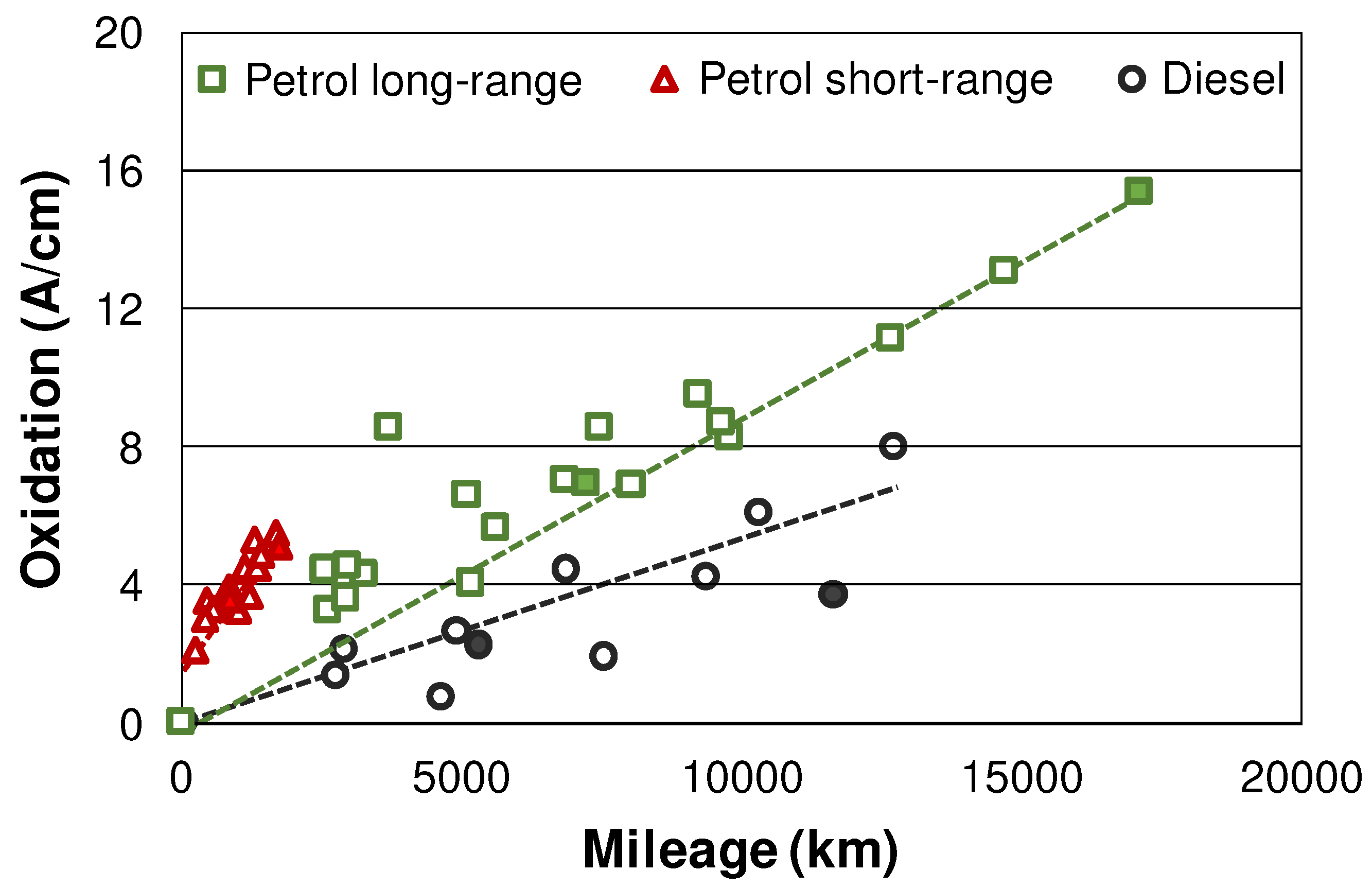
3.3. Combustion related degradation in the used oils (Oxidation, nitration, and AO depletion)
Figure 4 displays the oxidation measured by FTIR in the whole field data matrix. The increase of oxidation is slightly slower in diesel vehicles compared to long-range petrol cars. In the case of the short-range petrol drivetrains, oxidation is further elevated. These differences once again can be attributed to the lower AFR in petrol engines [
28].
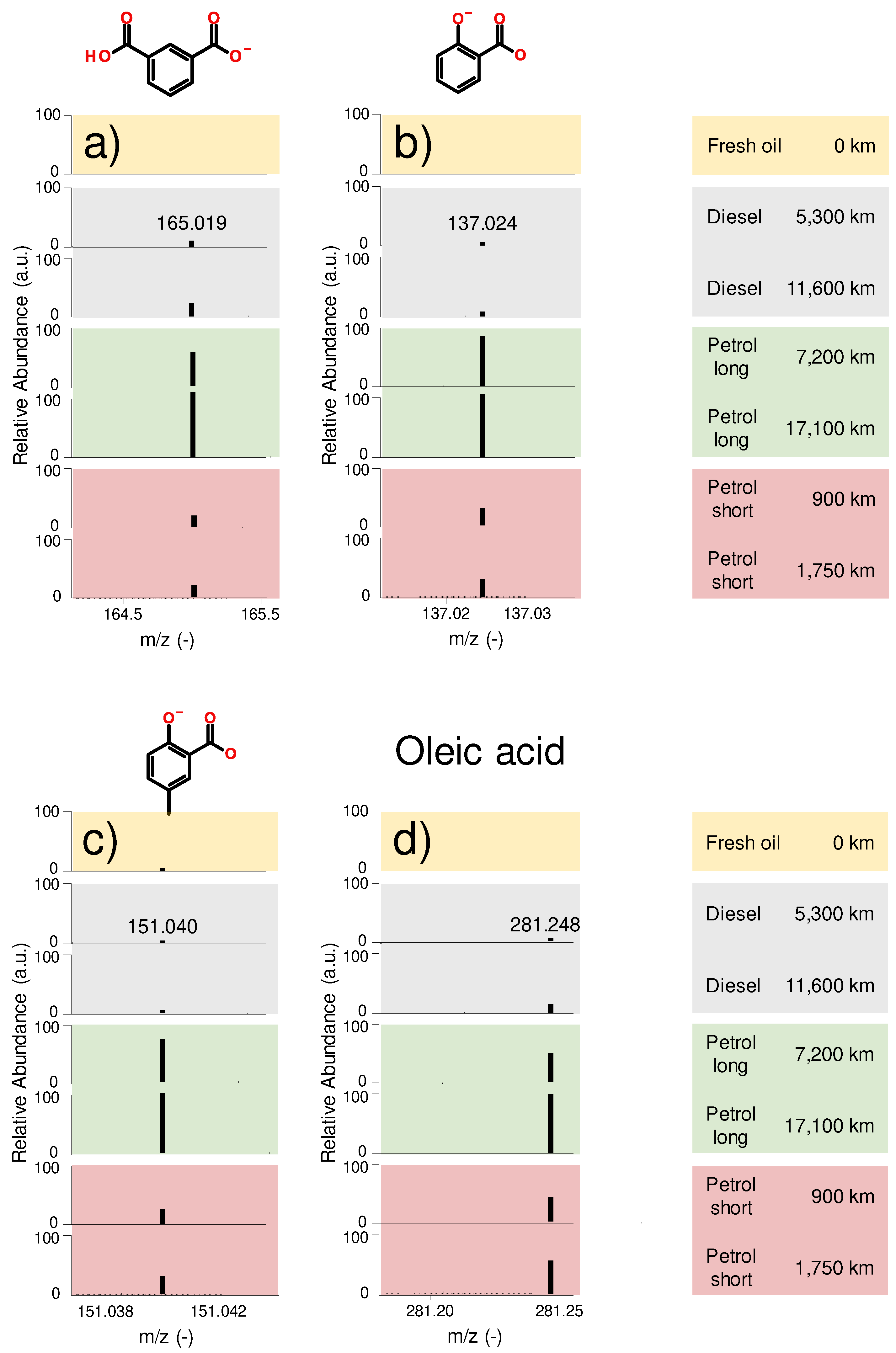
Figure 5 a) – d) gives an overview of the identified oxidation products in the engine oil samples. Aromatic dicarboxylic diacids, e.g., m/z 165.019 (
Figure 5 a)) as well as aromatic carboxylic acids e.g., salicylic acid (m/z 137.024) and methylated salicylic acid (m/z 151.040) were identified (
Figure 5 b) and
Figure 5 c) respectively). Additionally, oleic acid, an aliphatic carboxylic acid, was found (
Figure 5 d)). All 4 mentioned organic acids are considered oxidation products, as they were not detected in the fresh oil. The abundance of all these degradation products seems to increase over mileage.
Short-range petrol and long-range diesel samples display comparable low abundance of all oxidation products, which is in agreement with the FTIR evaluation which indicated similar oxidation values of the oils (see
Figure 4). This is especially remarkable in regard of their considerably different mileages.
Long-range petrol vehicles are showing a significantly higher abundance. This is again corroborated by FTIR results (see
Figure 4), where a faster oxidation in case of the petrol vehicles was highlighted. As discussed in detail in [
28], the faster oxidation in petrol drivetrains can once again be attributed to the lower AFR compared to diesel vehicles.
Figure 6 gives an overview of the nitration of engine oils determined by FTIR. Similarly to the oxidation, nitration is also slower in diesel engines compared to petrol ones, and the short-range utilization profile once again results in a more rapid nitration product accumulation.
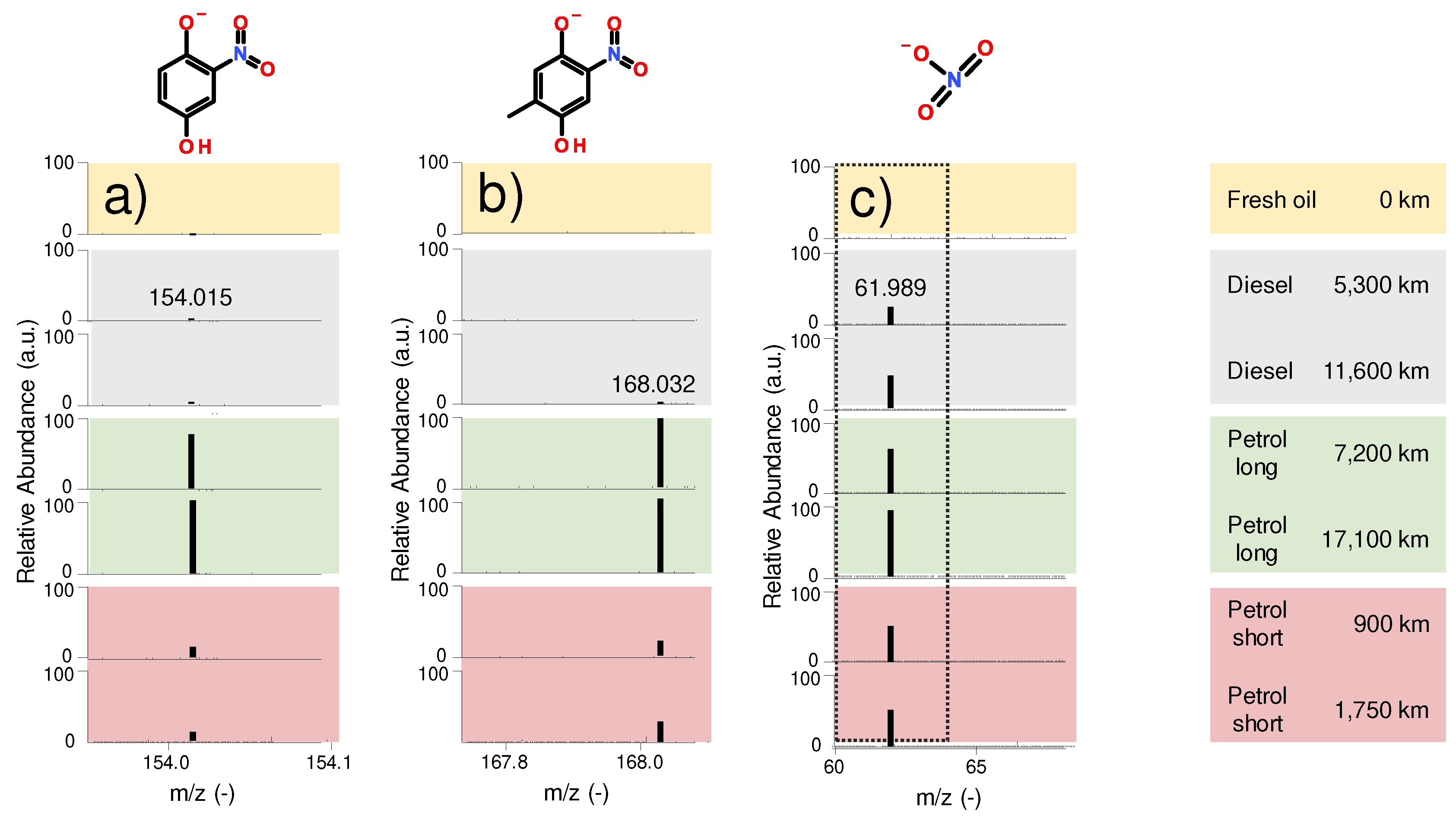
Figure 7 a-c) displays the abundance of various identified nitration products (m/z 168.032; m/z 154.015) and nitric acid (m/z 61.989) in the oil samples. The organic nitration products are aromatic, also containing oxo and hydroxyl groups. Previous investigations of used petrol engine oils [
33] suggested similar aromatic structures as well. The diesel engine oil only contains trace amounts of organic nitration products, whereas the petrol engine oils exhibit a higher abundance, most notably in the long-range petrol engine oil. This corresponds to the results obtained by conventional oil analysis (see
Figure 6), where a considerably higher nitration in case of the long-range petrol engines was found.
Figure 7 c) shows the abundance of nitric acid as combustion by-product [
32]. Similarly to the discussed oxidation and nitration products, nitric acid also exhibits a lower abundance in the diesel engine oil than in the long-range petrol samples. The amount of nitric acid is comparable, even slightly lower than that of the short-range petrol engine oil, although the mileage of the diesel vehicle is almost 7 times higher.
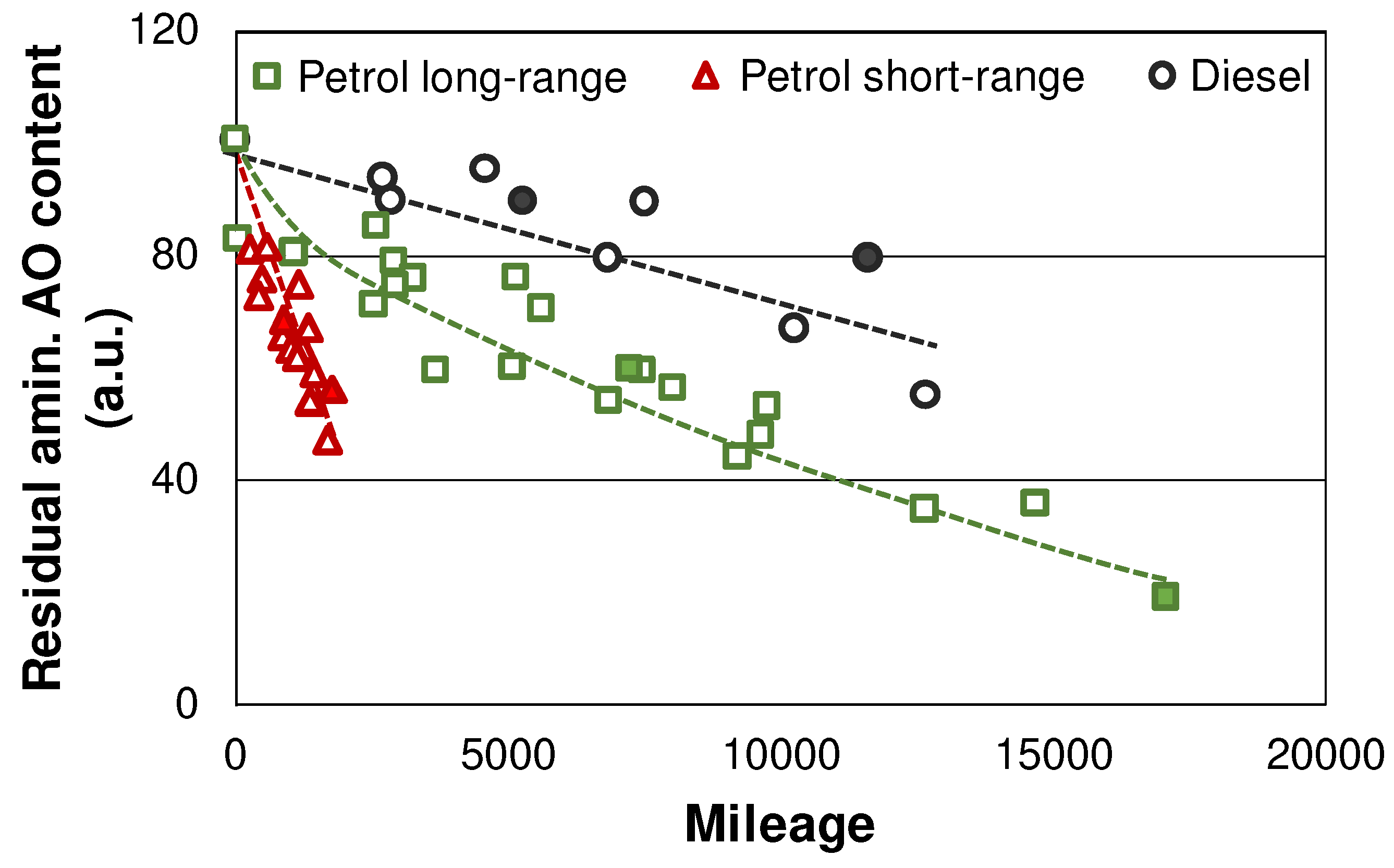
Figure 8 displays the depletion of aminic AOs during the field test. The degradation of the AOs is analogous to the oxidation values: Petrol vehicles show a faster oxidative degradation, characterized by more pronounced oxidation (see
Figure 4) and, hence, AO depletion, while diesels seem to be slower in this aspect. Short-range utilization in case of petrol engines accelerates the AO degradation further, which can again be attributed to a lower AFR caused by the cold operation of those engines [
28]. However, aminic AOs are not completely depleted in any of the investigated vehicles.
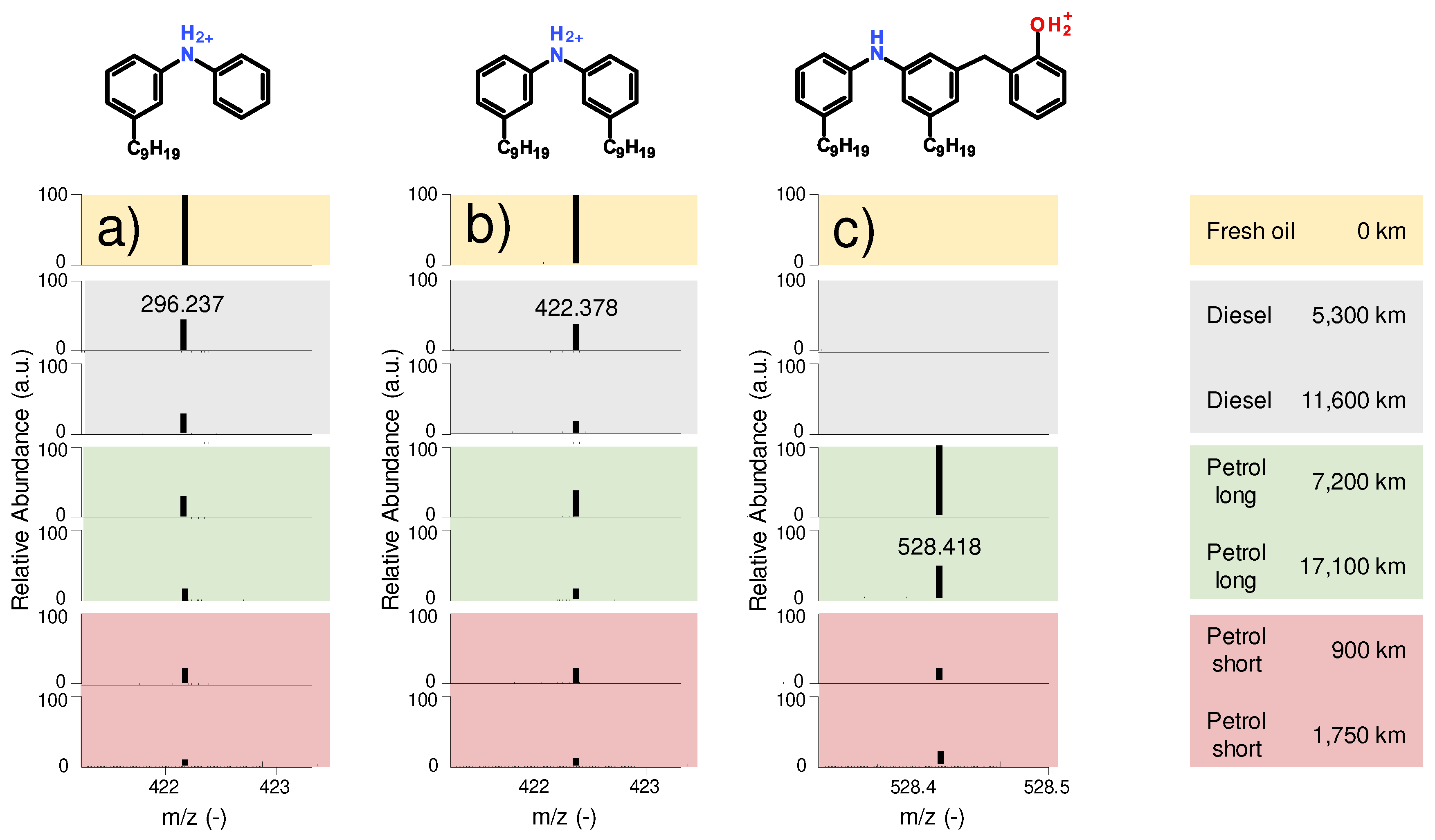
Figure 9 a) shows the antioxidant diphenylamine with a nonyl sidechain substituent on one of the phenyl groups (m/z 296.237). A chemically similar substance containing a nonyl sidechain on both phenyl groups (m/z 422.378), which showed comparable tendencies regarding abundance was also found and is depicted
Figure 9 b). The amount of aminic antioxidant is considerably reduced in all oil samples, but not completely depleted. The reduction in abundance is more pronounced with higher mileages, by trend. This confirms the findings of the FTIR (see
Figure 8), which revealed the faster depletion of the aminic antioxidants over the mileage, but detectable residual amounts at the end of the field test. The short-range petrol engine oils show a comparable abundance with the other oil samples, despite their mileage is significantly lower, which indicates increased chemical stress due to the short-range utilization profile (lower AFR [
28]).
Figure 9 c) shows a degradation product of the aminic antioxidant (m/z 528.418). This and further chemically analogous species are only present in the petrol engine oils, especially abundant in the long-range petrol engine oils. Since these structures were not present in the diesel engine oils with a similar abundance, it can be assumed that different reaction pathways are pronounced in the various engine types and that this specific degradation product is characteristic for petrol engines.
Phenolic AOs were also detected in the fresh engine oil, e.g., di-tert-butyl-hydroquinone (m/z 221.153; not shown). The detailed analysis of phenolic AOs was not possible via the applied Orbitrap-MS method, as these species are impacted by ionization effects, especially when ZDDP is present in the engine oils. For an accurate determination of phenolic AOs other techniques such as GC-MS would be required, as described in [
38], which were not in the focus of the current investigation.
3.4. Boron ester antiwear additive degradation in the used oils
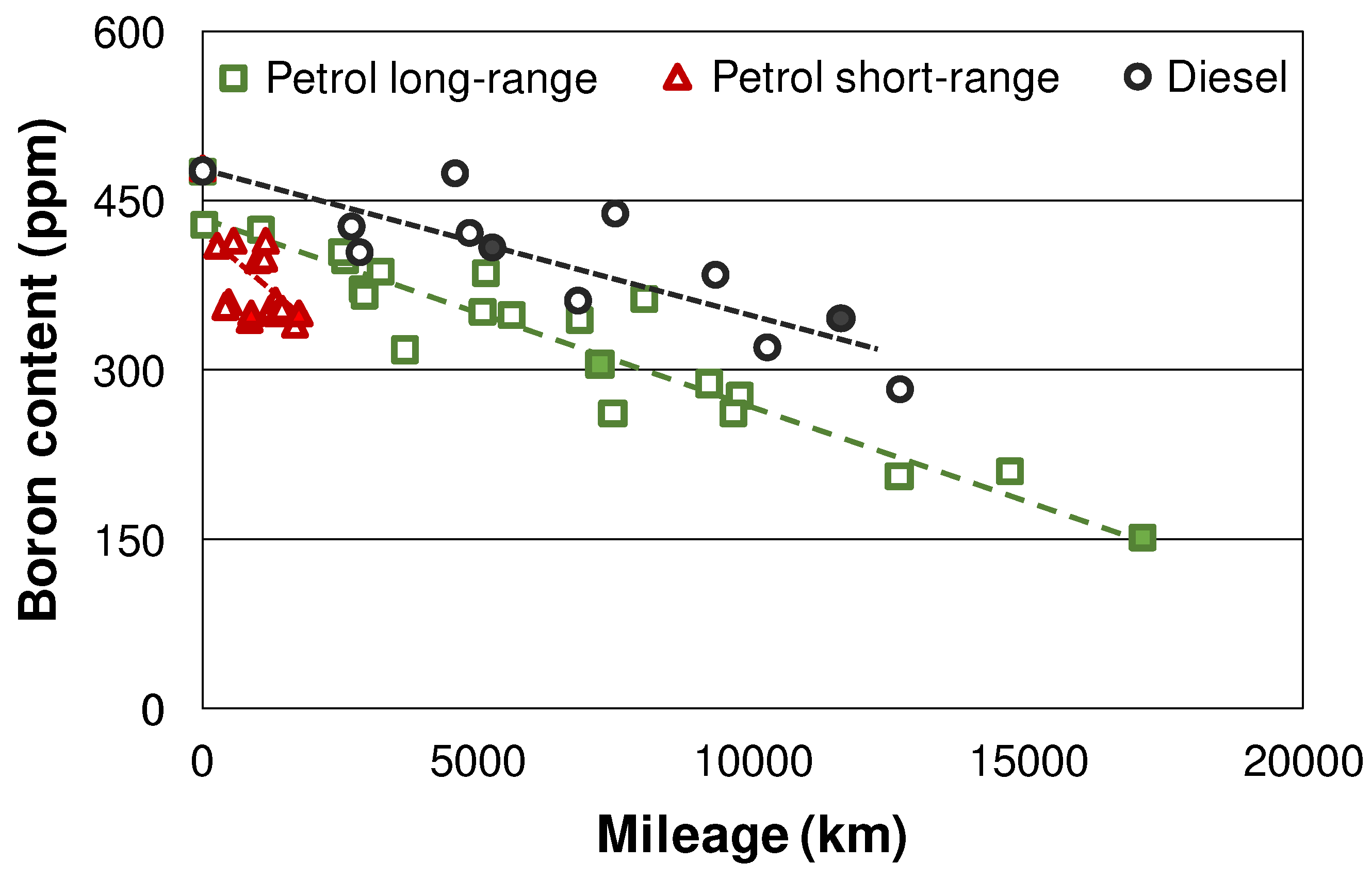
Figure 10 displays the boron content in the engine oils during the field study determined by ICP-OES. Boron shows a similar behavior as the previously discussed additives: The depletion is slower in diesel vehicles, and especially accelerated in the short-range petrol engines. Among other processes, Boron depletion is caused by hydrolysis of the borate ester additives, with subsequent formation of volatile boric acid which is removed with the exhaust gases [
39].
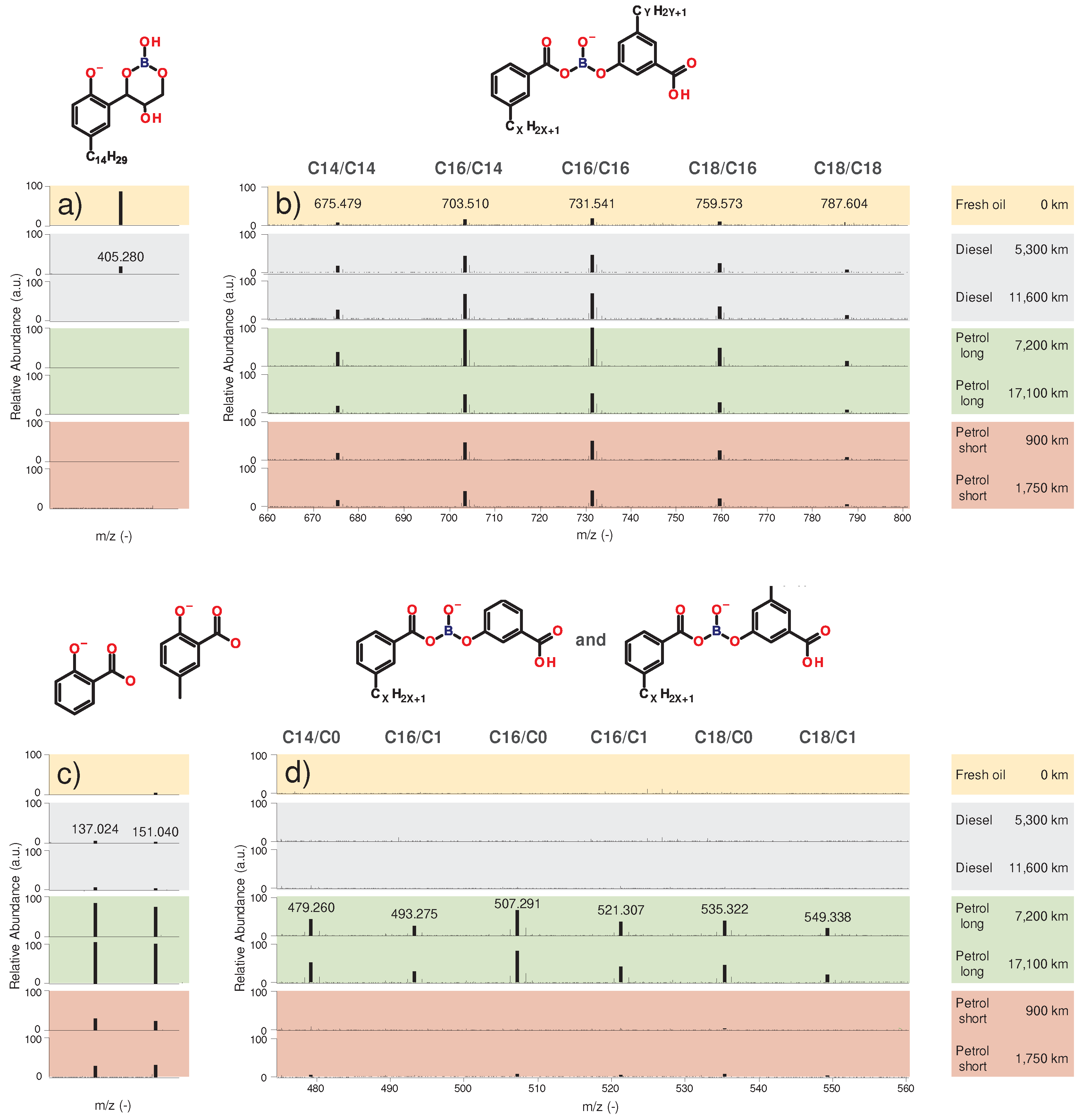
Figure 11 a) shows an identified borate ester species which is considered an antiwear additive. Multiple sidechains are detectable, additionally to the displayed tetradecyl group (C14; m/z 405.281), hexadecyl (C16; m/z 433.31) and octadecyl (C18; m/z 461.340) groups are present (one sidechain per aromatic ring is present, the exact position of the alkyl sidechain on the aromatic ring is not known, see 2.2). All identified species exhibit similar trends in abundance regardless of the length of the specific alkyl sidechain. As displayed, the original borate-ester additive depletes rapidly during utilization, as it is not detectable in the used engine oils, except the diesel samples with 5,300 km mileage.
Figure 11 b) shows the identified reaction products, where various combinations of the alkyl sidechains are present, namely C14-C14 (m/z 675.479), C14-C16 (m/z 703.510), C16-C16 (m/z 731.542), C16-C18 (m/z 759.573) and C18-C18 (m/z 787.604), collectively referred to as “long-sidechain” reaction products. These species are present in the fresh oil only in a low amount, then accumulate in the used oil samples with comparable abundance in all 6 oils, regardless of engine fueling or utilization profile. During the utilization in all engines, a new borate ester was formed with another aromatic compound. Since this species also contains a C14, C16 and C18 sidechains it can be assumed that these reaction products originate either through the “dimerization” of the original additive molecules, or through a reaction with the salicylate detergents, which also contain C14, C16 and C18 alkyl sidechains (see
Figure 1). An accurate description of the reaction pathway would be possible using isotope tracing [
40,
41] but was not considered for this study.
As shown in
Figure 11 d), further similar species were identified, where a notable difference is that the alkyl sidechain on the second aromatic ring (depicted on the right side) is either a methyl group (C1) or missing completely (C0). The discovered species with a short or missing sidechain on the second aromatic ring are: C14-C0 (m/z 479.260), C14-C1 (m/z 493.276), C16-C0 (m/z 507.291), C16-C1 (m/z 521.307), C18-C0 (m/z 535.322) and C18-C1 (m/z 549.388), referred to as “short-sidechain” reaction products. The degradation products presented in
Figure 11 d) are mostly present in the petrol engine oils in a higher concentration, especially abundant in the long-range oil samples, but also detectable in the short-range oil samples in traces. Compared to the abundance of the structure presented in
Figure 11 b), containing 2 long alkyl side chains, it is visible that different reaction partners have to be involved, as the relative abundances are showing completely different trends despite the similarity of the structures.
Figure 11 c) displays two identified oxidation products, (salicylic acid and methylated salicylic acid). Regardless, whether the reaction products originate from “dimerization” of two borate esters, or reaction with salicylates, it seems likely that a side-reaction with the presented oxidation products can also occur. The relative abundances of the oxidation products also support this theory, as salicylic acid and 5-methyl salicylic acid are most abundant in the long-range petrol engines oil, corresponding to the highest oxidation. These reaction products display a smaller abundance in the short-range petrol engines and only traces in the diesel samples, going along with the low abundances of the degraded borate esters with C0 and C1 sidechains. Hence, it is most likely that recombination with oxidation products is responsible for the origin of the structures presented in
Figure 11 d).
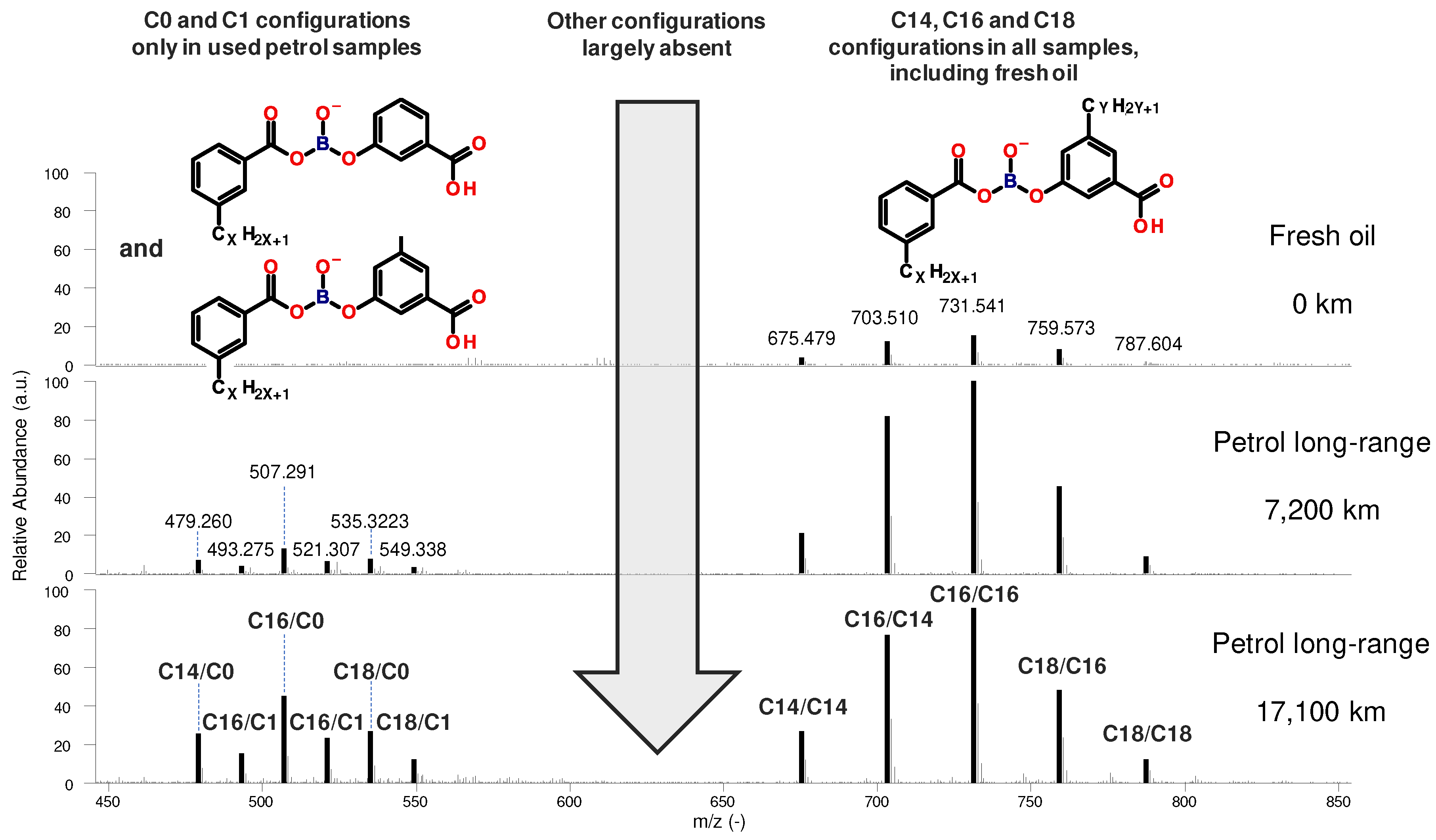
Figure 12 gives an overview of all boron-ester species in the long-range petrol oil samples. In the position on the second (“right”) aromatic ring either the original alkyl sidechains (C14, C16, C18) or the two displayed variants (C0, C1) were found, no other species could be identified It also has to be mentioned that the measured counts of any given degradation product with short sidechains are lower compared to the long sidechain counterparts, as shown in
Figure 12. The individual counts are influenced by the abundance and ionization capability of the respective species. In this case, the ionization capability can be assumed to be comparable since the structures are very similar. However, for clear evidence, ionization studies would have to be performed. Nevertheless, it can be assumed that the long sidechain species represents the main reaction pathway, which occurs in both diesel and petrol engines in comparable abundance, while the C0 and C1 species are the result of a side reaction unique to the petrol samples.
3.5. Summary
A general overview of the obtained results is presented in
Table 4.
4. Conclusions
The effect of fueling (petrol vs. diesel) and utilization profile (short-range vs. long range) on engine oil degradation was investigated. The MS analysis was focused on commonly utilized oil additives, namely antioxidants, antiwear additives and detergents. Furthermore, established degradation products, such as oxidation and nitration species were analyzed.
Petrol engine oils generally showed higher oxidative degradation compared to diesel engine oils, which was further accelerated in case of a short-range utilization profile. This was visible in the higher abundance of both aliphatic and aromatic organic acids as well as nitration products. Additionally, antioxidant and antiwear additive depletion was faster in the petrol engine oils, especially in case of the short-range utilization profile considering the low mileage. Regarding ZDDP degradation, differences between petrol and diesel engine oils were highlighted, where petrol engines displayed a more advanced depletion of the original additives as well as higher abundance of various degradation products. The short-range petrol engines showed expedited additive degradation in multiple cases, e.g., borate ester and ZDDP. These additives exhibited close to complete depletion as well as a high abundance of various corresponding degradation products, such as alkyl phosphates and organic acids was found, despite the relatively short mileage.
For petrol engines, also differences in the borate ester antiwear additive degradation were detected possibly related to the mentioned higher abundance of various organic acids. In case of petrol engine oils, a side-reaction between borate esters and aromatic carboxylic acids (oxidation products) was indicated, which was not present in diesel engine oils. This highlights the high interrelation of chemical degradation in the complex systems of internal combustion engine oils and how differences in one degradation process can influence another reaction on the molecular level, simply by supplying further compatible reactants. Similar variations in the degradation of aminic antioxidants were also shown.
Concludingly, it can be stated the presented study provides a valuable input in understanding oil degradation in internal combustion engines, especially when it comes to variations in fueling or utilization profile. Based on obtained results, appropriate countermeasures can be established enabling prolonged oil service life, hence, more economic, and sustainable automotive lubrication.
5. Acknowledgments
Presented results were realized in research projects with financial support from the participating project partners and the Austrian COMET program (Project InTribology, No. 872176).
The COMET program is funded by the Austrian Federal Government and concerning InTribology by the provinces of Lower Austria and Vorarlberg.
The authors would like to thank the general support of AUDI HUNGARIA Zrt. in carrying out the field study.
Supplementary Materials
None.
Author Contributions
Conceptualization, A.A., A.L.N. and C.B.; methodology, A.A., A.L.N., A.R.; validation, C.B. and M.F.; formal analysis, A.A., A.R. and M.F.; investigation, A.A, A.L.N. and ZS.T..; resources, J.R.B and M.F..; data curation, A.A.; writing—original draft preparation, A.A., A.L.N. and ZS.T.; writing—review and editing, P.R., C.B. and M.F.; visualization, A.A.; supervision, P.R., C.B. and M.F.; project administration, P.R., C.B. and M.F. funding acquisition, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the AUSTRIAN RESEARCH PROMOTIN AGENCY, grant number 872176.
Data Availability Statement
Data is contained within the article.
Acknowledgments
Presented results were realized in research projects with financial support from the participating project partners and the Austrian COMET program (Project InTribology, No. 872176). The COMET program is funded by the Austrian Federal Government and concerning InTribology by the provinces of Lower Austria and Vorarlberg. The authors would like to thank the general support of AUDI HUNGARIA Zrt. in carrying out the field study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- European Parliament European Parliament. CO2 Emission Standards for Cars and Vans. Legislative Observatory 2022. Available online: https://oeil.secure.europarl.europa.eu/oeil/popups/summary.do?id=1706841&t=d&l=en (accessed on 3 August 2023).
- European Automobile Manufacturers’ Association European Automobile Manufacturers’ Association. Average Age of the EU Vehicle Fleet, by Country. Available online: https://www.acea.auto/figure/average-age-of-eu-vehicle-fleet-by-country/ (accessed on 3 August 2023).
- International Energy Agency Global Electric Car Sales Have Continued Their Strong Growth in 2022 after Breaking Records Last Year. Available online: https://www.iea.org/news/global-electric-car-sales-have-continued-their-strong-growth-in-2022-after-breaking-records-last-year (accessed on 4 August 2023).
- Tozlu, A. Techno-Economic Assessment of a Synthetic Fuel Production Facility by Hydrogenation of CO2 Captured from Biogas. Int J Hydrogen Energy 2022, 47, 3306–3315. [Google Scholar] [CrossRef]
- Hübner, T.; von Roon, S. Synthetic Fuels in the German Industry Sector Depending on Climate Protection Level. Smart Energy 2021, 3, 100042. [Google Scholar] [CrossRef]
- Wahl, J.; Kallo, J. Carbon Abatement Cost of Hydrogen Based Synthetic Fuels – A General Framework Exemplarily Applied to the Maritime Sector. Int J Hydrogen Energy 2022, 47, 3515–3531. [Google Scholar] [CrossRef]
- Ben Hnich, K.; Martín-Gamboa, M.; Khila, Z.; Hajjaji, N.; Dufour, J.; Iribarren, D. Life Cycle Sustainability Assessment of Synthetic Fuels from Date Palm Waste. Science of The Total Environment 2021, 796, 148961. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Monsalve-Serrano, J.; Lago Sari, R.; Martinez-Boggio, S. Energy Sustainability in the Transport Sector Using Synthetic Fuels in Series Hybrid Trucks with RCCI Dual-Fuel Engine. Fuel 2022, 308, 122024. [Google Scholar] [CrossRef]
- Hänggi, S.; Elbert, P.; Bütler, T.; Cabalzar, U.; Teske, S.; Bach, C.; Onder, C. A Review of Synthetic Fuels for Passenger Vehicles. Energy Reports 2019, 5, 555–569. [Google Scholar] [CrossRef]
- Hakeem, M.; Anderson, J.; Surnilla, G.; Yamada, S.S. Characterization and Speciation of Fuel Oil Dilution in Gasoline Direct Injection (DI) Engines. In Proceedings of the Volume 1: Large Bore Engines; Fuels; Advanced Combustion; American Society of Mechanical Engineers, November 8 2015. [Google Scholar]
- Kleiner, F.; Kaspar, M.; Artmann, C.; Rabl, H.-P. Online Oil Dilution Measurement at GDI Engines.; 2014. 13 October.
- Hu, T.; Teng, H.; Luo, X.; Chen, B. Impact of Fuel Injection on Dilution of Engine Crankcase Oil for Turbocharged Gasoline Direct-Injection Engines. SAE Int J Engines 2015, 8, 1107–1116. [Google Scholar] [CrossRef]
- Haenel, P.; de Bruijn, R.; Tomazic, D.; Kleeberg, H. Analysis of the Impact of Production Lubricant Composition and Fuel Dilution on Stochastic Pre-Ignition in Turbocharged, Direct-Injection Gasoline Engines.; 2019. 2 April.
- Hulkkonen, T.; Tilli, A.; Kaario, O.; Ranta, O.; Sarjovaara, T.; Vuorinen, V.; Larmi, M.; Lehto, K. Late Post-Injection of Biofuel Blends in an Optical Diesel Engine: Experimental and Theoretical Discussion on the Inevitable Wall-Wetting Effects on Oil Dilution. International Journal of Engine Research 2017, 18, 645–656. [Google Scholar] [CrossRef]
- Del Nogal Sánchez, M.; Glanzer, P.; Pérez Pavón, J.L.; García Pinto, C.; Moreno Cordero, B. Determination of Antioxidants in New and Used Lubricant Oils by Headspace-Programmed Temperature Vaporization-Gas Chromatography-Mass Spectrometry. Anal Bioanal Chem 2010, 398, 3215–3224. [Google Scholar] [CrossRef]
- Levermore, D.M.; Josowicz, M.; Rees, J.S.; Janata, J. Headspace Analysis of Engine Oil by Gas Chromatography/Mass Spectrometry. Anal Chem 2001, 73, 1361–1365. [Google Scholar] [CrossRef]
- Kreisberger, G.; Klampfl, C.W.; Buchberger, W.W. Determination of Antioxidants and Corresponding Degradation Products in Fresh and Used Engine Oils. Energy and Fuels 2016, 30, 7638–7645. [Google Scholar] [CrossRef]
- Kassler, A.; Pittenauer, E.; Doerr, N.; Allmaier, G. Ultrahigh-Performance Liquid Chromatography/Electrospray Ionization Linear Ion Trap Orbitrap Mass Spectrometry of Antioxidants (Amines and Phenols) Applied in Lubricant Engineering. Rapid Communications in Mass Spectrometry 2014, 28, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, C.; Turner, M.; Reynolds, J.C.; Whitmarsh, S.; Lynch, T.; Creaser, C.S. Direct Analysis of Oil Additives by High-Field Asymmetric Waveform Ion Mobility Spectrometry-Mass Spectrometry Combined with Electrospray Ionization and Desorption Electrospray Ionization. Anal Chem 2016, 88, 2453–2458. [Google Scholar] [CrossRef]
- Da Costa, C.; Reynolds, J.C.; Whitmarsh, S.; Lynch, T.; Creaser, C.S. The Quantitative Surface Analysis of an Antioxidant Additive in a Lubricant Oil Matrix by Desorption Electrospray Ionization Mass Spectrometry. Rapid Communications in Mass Spectrometry 2013, 27, 2420–2424. [Google Scholar] [CrossRef] [PubMed]
- Ramopoulou, L.; Widder, L.; Brenner, J.; Ristic, A.; Allmaier, G. Atmospheric Pressure Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry of Engine Oil Additive Components. Rapid Communications in Mass Spectrometry 2022, 36. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, C.; Whitmarsh, S.; Lynch, T.; Creaser, C.S. The Qualitative and Quantitative Analysis of Lubricant Oil Additives by Direct Analysis in Real Time-Mass Spectrometry. Int J Mass Spectrom 2016, 405, 24–31. [Google Scholar] [CrossRef]
- Barrère, C.; Hubert-Roux, M.; Afonso, C.; Racaud, A. Rapid Analysis of Lubricants by Atmospheric Solid Analysis Probe-Ion Mobility Mass Spectrometry. Journal of Mass Spectrometry 2014, 49, 709–715. [Google Scholar] [CrossRef]
- Lacroix-Andrivet, O.; Moualdi, S.; Hubert-Roux, M.; Loutelier Bourhis, C.; Mendes Siqueira, A.L.; Afonso, C. Molecular Characterization of Formulated Lubricants and Additive Packages Using Kendrick Mass Defect Determined by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. J Am Soc Mass Spectrom 2022, 33, 1194–1203. [Google Scholar] [CrossRef]
- Basham, V.; Hancock, T.; McKendrick, J.; Tessarolo, N.; Wicking, C. Detailed Chemical Analysis of a Fully Formulated Oil Using Dielectric Barrier Discharge Ionisation–Mass Spectrometry. Rapid Communications in Mass Spectrometry 2022, 36. [Google Scholar] [CrossRef]
- European Committee for Standardization EN 228:2008 Automotive Fuels - Unleaded Petrol - Requirements and Test Methods 2008.
- European Committee for Standardization DIN EN 590:2022 Automotive Fuels - Diesel - Requirements and Test Methods 2022.
- Agocs, A.; Nagy, A.L.; Tabakov, Z.; Perger, J.; Rohde-Brandenburger, J.; Schandl, M.; Besser, C.; Dörr, N. Comprehensive Assessment of Oil Degradation Patterns in Petrol and Diesel Engines Observed in a Field Test with Passenger Cars – Conventional Oil Analysis and Fuel Dilution. Tribol Int 2021, 161, 107079. [Google Scholar] [CrossRef]
- Nagy, A.L.; Agocs, A.; Ronai, B.; Raffai, P.; Rohde-Brandenburger, J.; Besser, C.; Dörr, N. Rapid Fleet Condition Analysis through Correlating Basic Vehicle Tracking Data with Engine Oil FT-IR Spectra. Lubricants 2021, 9, 114. [Google Scholar] [CrossRef]
- Allgemeiner Deutscher Automobil-Club, e.V. (ADAC) Studie: Tempo in Großstädten Sinkt. Available online: https://www.adac.de/der-adac/aktuelles/studie-verkehrsfluss-in-staedten/ (accessed on 4 August 2023).
- O’Kelly, M.E.; Niedzielski, M.A. Efficient Spatial Interaction: Attainable Reductions in Metropolitan Average Trip Length. J Transp Geogr 2008, 16, 313–323. [Google Scholar] [CrossRef]
- Dörr, N.; Agocs, A.; Besser, C.; Ristić, A.; Frauscher, M. Engine Oils in the Field: A Comprehensive Chemical Assessment of Engine Oil Degradation in a Passenger Car. Tribol Lett 2019, 67, 68. [Google Scholar] [CrossRef]
- Besser, C.; Agocs, A.; Ristic, A.; Frauscher, M. Implementation of Nitration Processes in Artificial Ageing for Closer-to-Reality Simulation of Engine Oil Degradation. Lubricants 2022, 10, 298. [Google Scholar] [CrossRef]
- Agocs, A.; Budnyk, S.; Besser, C.; Ristic, A.; Frauscher, M.; Ronai, B.; Dörr, N. Production of Used Engine Oils with Defined Degree of Degradation in a Large-Scale Device. Acta Technica Jaurinensis 2020, 13, 131–150. [Google Scholar] [CrossRef]
- Agocs, A.; Besser, C.; Brenner, J.; Budnyk, S.; Frauscher, M.; Dörr, N. Engine Oils in the Field: A Comprehensive Tribological Assessment of Engine Oil Degradation in a Passenger Car. Tribol Lett 2022, 70. [Google Scholar] [CrossRef]
- Dörr, N.; Brenner, J.; Ristić, A.; Ronai, B.; Besser, C.; Pejaković, V.; Frauscher, M. Correlation Between Engine Oil Degradation, Tribochemistry, and Tribological Behavior with Focus on ZDDP Deterioration. Tribol Lett 2019, 67. [Google Scholar] [CrossRef]
- Banerji, A.; Edrisy, A.; Francis, V.; Alpas, A.T. Effect of Bio-Fuel (E85) Addition on Lubricated Sliding Wear Mechanisms of a Eutectic Al–Si Alloy. Wear 2014, 311, 1–13. [Google Scholar] [CrossRef]
- Frauscher, M.; Agocs, A.; Besser, C.; Rögner, A.; Allmaier, G.; Dörr, N. Time-Resolved Quantification of Phenolic Antioxidants and Oxidation Products in a Model Fuel by GC-EI-MS/MS. Energy and Fuels 2020, 34, 2674–2682. [Google Scholar] [CrossRef]
- Animashaun, L.A. Tribochemistry of Boron-Containing Lubricant Additives on Ferrous Surfaces for Improved Internal Combustion Engine Performance; Leeds, 2017.
- Frauscher, M.; Besser, C.; Allmaier, G.; Dörr, N. Elucidation of Oxidation and Degradation Products of Oxygen Containing Fuel Components by Combined Use of a Stable Isotopic Tracer and Mass Spectrometry. Anal Chim Acta 2017, 993, 47–54. [Google Scholar] [CrossRef]
- Frauscher, M.; Besser, C.; Allmaier, G.; Dörr, N. Oxidation Products of Ester-Based Oils with and without Antioxidants Identified by Stable Isotope Labelling and Mass Spectrometry. Applied Sciences 2017, 7, 396. [Google Scholar] [CrossRef]
Figure 1.
Overview of the additives present in the fresh oil.
Figure 1.
Overview of the additives present in the fresh oil.
Figure 2.
ZDDP evaluated by FTIR. Samples selected for MS indicated by full markers.
Figure 2.
ZDDP evaluated by FTIR. Samples selected for MS indicated by full markers.
Figure 3.
ZDDP and its respective degradation products, a) abundance of dipropyl dithiophosphate, b) abundance of dipropyl thiophosphate and dipropyl phosphate, c) abundance of decyl sulfate and decyl phosphate, d) abundance of sulfuric acid.
Figure 3.
ZDDP and its respective degradation products, a) abundance of dipropyl dithiophosphate, b) abundance of dipropyl thiophosphate and dipropyl phosphate, c) abundance of decyl sulfate and decyl phosphate, d) abundance of sulfuric acid.
Figure 4.
Oxidation evaluated by FTIR. Samples selected for MS indicated by full markers.
Figure 4.
Oxidation evaluated by FTIR. Samples selected for MS indicated by full markers.
Figure 5.
Oxidation products. a) abundance of an aromatic dicarboxylic acid, b) abundance of salicylic acid (aromatic carboxylic acid), c) abundance of methylated salicylic acid (aromatic carboxylic acid), d) abundance of oleic acid (aliphatic carboxylic acid).
Figure 5.
Oxidation products. a) abundance of an aromatic dicarboxylic acid, b) abundance of salicylic acid (aromatic carboxylic acid), c) abundance of methylated salicylic acid (aromatic carboxylic acid), d) abundance of oleic acid (aliphatic carboxylic acid).
Figure 6.
Nitration evaluated by FTIR. Samples selected for MS indicated by full markers.
Figure 6.
Nitration evaluated by FTIR. Samples selected for MS indicated by full markers.
Figure 7.
Nitration products a)-b) abundance of aromatic nitration products, c) abundance of nitric acid.
Figure 7.
Nitration products a)-b) abundance of aromatic nitration products, c) abundance of nitric acid.
Figure 8.
Residual amine AO. evaluated by FTIR. Samples selected for MS indicated by full markers.
Figure 8.
Residual amine AO. evaluated by FTIR. Samples selected for MS indicated by full markers.
Figure 9.
a) – b) abundance of aminic antioxidants, c) abundance of corresponding oxidation products.
Figure 9.
a) – b) abundance of aminic antioxidants, c) abundance of corresponding oxidation products.
Figure 10.
Boron content evaluated by ICP-OES. Samples selected for MS indicated by full markers.
Figure 10.
Boron content evaluated by ICP-OES. Samples selected for MS indicated by full markers.
Figure 11.
Borate-ester and respective degradation products. a) abundance of a fresh borate ester, b) abundance of a degraded borate esters, long sidechains c) abundance of oxidation products, d) abundance of a degraded borate esters, short sidechains.
Figure 11.
Borate-ester and respective degradation products. a) abundance of a fresh borate ester, b) abundance of a degraded borate esters, long sidechains c) abundance of oxidation products, d) abundance of a degraded borate esters, short sidechains.
Figure 12.
Borate-ester degradation products in the long-range petrol samples - All detected configurations: Only C0, C1, C14, C16 and C18 sidechains are present, other configurations are largely absent.
Figure 12.
Borate-ester degradation products in the long-range petrol samples - All detected configurations: Only C0, C1, C14, C16 and C18 sidechains are present, other configurations are largely absent.
Table 1.
Fresh oil parameters.
Table 1.
Fresh oil parameters.
| |
Oil Property |
Value |
Chemical
properties
|
Neutralization number (mg KOH/g) |
1.6 |
| Total base number (mg KOH/g) |
8.4 |
Elemental
composition
|
Boron (B) (mg/kg) |
480 |
| Calcium (Ca) (mg/kg) |
2020 |
| Phosphor (P) (mg/kg) |
750 |
| Sulfur (S) (mg/kg) |
1620 |
| Zinc (Zn) (mg/kg) |
830 |
Physical
properties
|
SAE viscosity classification |
0W-30 |
| Kinematic viscosity at 40 °C (mm²/s) |
57.6 |
| Kinematic viscosity at 100 °C (mm²/s) |
11.7 |
| Viscosity index (-) |
205 |
Table 2.
Vehicle parameters of the conducted field test. Vehicles selected for mass spectrometry (MS) analysis are indicated in bold.
Table 2.
Vehicle parameters of the conducted field test. Vehicles selected for mass spectrometry (MS) analysis are indicated in bold.
| Engine power (kW) |
130 |
140 |
140 |
155 |
155 |
155 |
185 |
185 |
185 |
221 |
221 |
221 |
| Engine type |
Diesel |
Diesel |
Diesel |
Petrol |
Petrol |
Petrol |
Petrol |
Petrol |
Petrol |
Petrol |
Petrol |
Petrol |
| Range |
Long |
Long |
Long |
Short |
Short |
Short |
Long |
Long |
Long |
Long |
Long |
Long |
| Transmission |
MT |
AT |
AT |
AT |
AT |
AT |
AT |
AT |
AT |
AT |
AT |
AT |
| Drivetrain |
AWD |
AWD |
FWD |
AWD |
AWD |
FWD |
AWD |
AWD |
AWD |
AWD |
AWD |
AWD |
| Model year |
2014 |
2017 |
2016 |
2013 |
2012 |
2011 |
2018 |
2018 |
2018 |
2014 |
2014 |
2016 |
| Mileage (km) |
12,700 |
11,600 |
9,300 |
1,670 |
1,150 |
1,750 |
14,700 |
17,100 |
9,200 |
8,000 |
5,100 |
7,500 |
| Engine power (kW) |
130 |
140 |
140 |
155 |
155 |
155 |
185 |
185 |
185 |
221 |
221 |
221 |
Table 3.
Orbitrap-MS measurement parameters.
Table 3.
Orbitrap-MS measurement parameters.
| |
|
Description and parameters |
| Sample preparation |
Solvent |
Methanol-chloroform mixture (volumetric ratio 3:7) |
| Dilution factor |
1:000 |
| Infusion |
Infusion |
Automatized direct infusion |
| Injection volume |
20 µl |
| Flow rate |
100 µl/min |
| Ionization |
Ion source |
Electrospray ionization (ESI) |
| Modes |
Negative and positive ion mode |
| Sheat gas |
Nitrogen |
| Fragmentation |
Type |
Low-energy collision-induced dissociation (CID) |
| Equipment |
Linear ion-trap |
| Buffer gas |
Helium |
| Collision gas |
Helium |
| Detection |
Detector |
Orbitrap-MS |
| Resolution |
60,000 (full width at half maximum, FWHM) |
| Accuracy |
5 ppm or better |
Table 4.
Summary of MS results.
Table 4.
Summary of MS results.
| |
|
Fresh oil
0 km |
Diesel
5,300 km |
Diesel
11,600 km |
Petrol long-range
7,200 km |
Petrol long-range
17,100 km |
Petrol short-range
900 km |
Petrol short-range
1,750 km |
| ZDDP |
Dialkyl dithiophosphates
Original additive |
+++ |
+ |
0 |
0 |
0 |
0 |
0 |
Dialkyl thiophosphates
1st degradation step |
0 |
+++ |
+++ |
+ |
0 |
+++ |
+ |
Dialkyl phosphates
2nd degradation step |
0 |
+ |
+++ |
+++ |
+++ |
+++ |
+++ |
Alkyl phosphates
3rd degradation step |
0 |
+ |
++ |
+++ |
+++ |
+++ |
+++ |
Sulfuric acid
4th degradation step |
0 |
++ |
++ |
++ |
++ |
++ |
++ |
| Combustion-related degradation products |
Oxidation
Aromatic carboxylic acids |
0 |
+ |
+ |
+++ |
+++ |
++ |
++ |
Oxidation
Aliphatic carboxylic acids |
0 |
+ |
+ |
+++ |
+++ |
++ |
++ |
Nitration
Aromatic nitration products |
0 |
0 |
0 |
+++ |
+++ |
+ |
+ |
| Nitric acid |
0 |
+ |
+ |
+++ |
+++ |
++ |
++ |
Anti-
oxidants |
Aminic antioxidants |
+++ |
++ |
+ |
+ |
+ |
+ |
0 |
Boron
ester
antiwear |
Boron ester antiwear
Original additive |
+++ |
+ |
0 |
0 |
0 |
0 |
0 |
| Long-sidechain reaction products |
+ |
+ |
+++ |
+++ |
+++ |
++ |
++ |
| Short-sidechain reaction products |
0 |
0 |
0 |
+++ |
+++ |
0 |
0 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).