Submitted:
31 August 2023
Posted:
04 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and methods
2.1. Materials
2.2. Methods
2.2.1. Antimycobacterial drug susceptibility assay
2.2.2. Biofilm growth assay
2.3. Proteomic analysis
3. Results
3.1. HA compounds present anti-mycobacterial activities
3.2. HA10Fe2, HA12Fe2 and HA12FeCl can also reduce pre-formed P. aeruginosa biofilm.
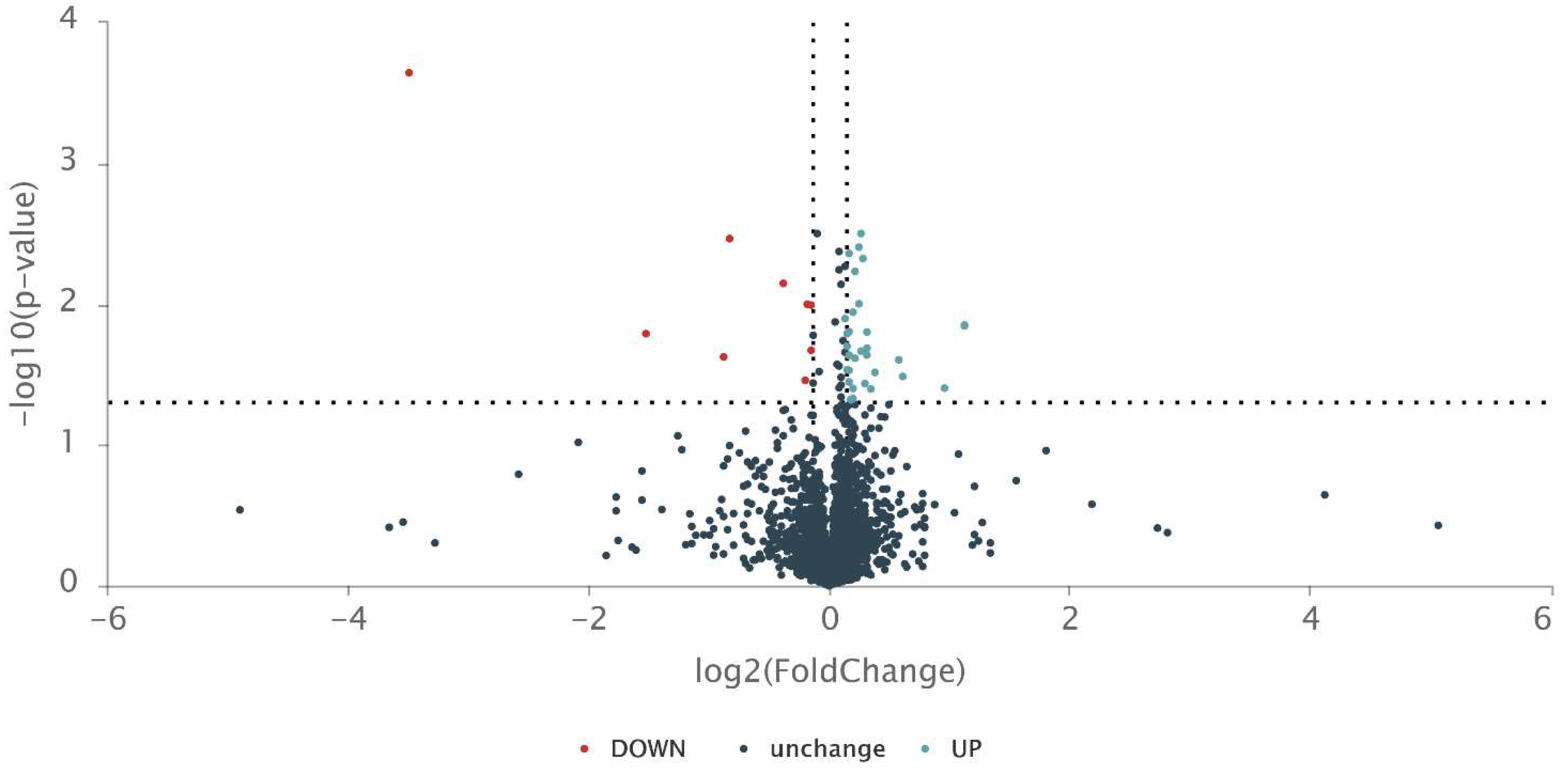
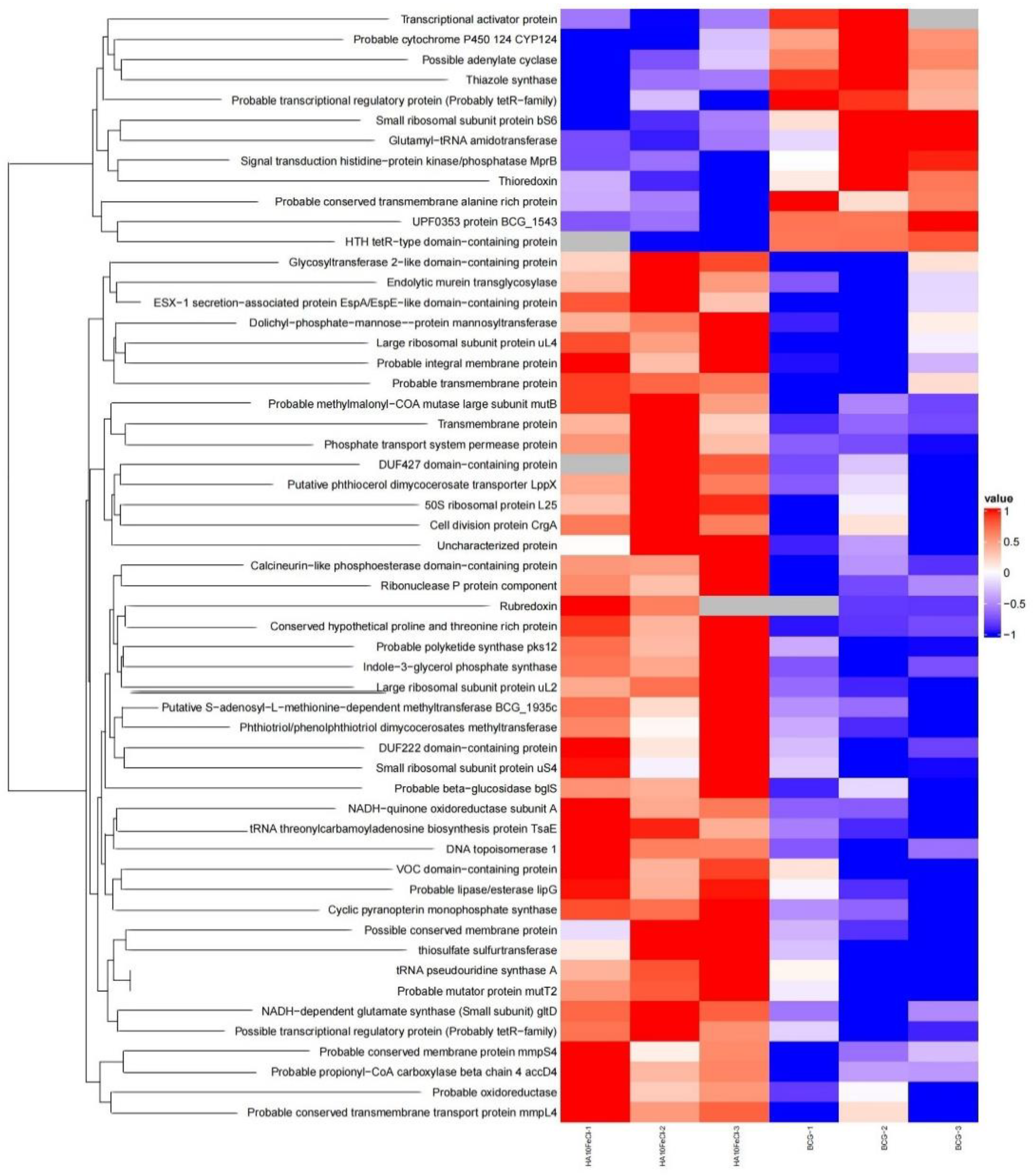
3.3. Proteomic profile of the HA10FeCl-treated bacilli
4. Discussion
5. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- W.H. Organization, Global tuberculosis report 2022, (2022).
- Daffé, M.; Marrakchi, H. Unraveling the Structure of the Mycobacterial Envelope. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Astarie-Dequeker, C.; Le Guyader, L.; Malaga, W.; Seaphanh, F.-K.; Chalut, C.; Lopez, A.; Guilhot, C. Phthiocerol Dimycocerosates of M. tuberculosis Participate in Macrophage Invasion by Inducing Changes in the Organization of Plasma Membrane Lipids. PLOS Pathog. 2009, 5, e1000289–e1000289. [Google Scholar] [CrossRef]
- Soetaert, K.; Rens, C.; Wang, X.-M.; De Bruyn, J.; Lanéelle, M.-A.; Laval, F.; Lemassu, A.; Daffé, M.; Bifani, P.; Fontaine, V.; et al. Increased Vancomycin Susceptibility in Mycobacteria: a New Approach To Identify Synergistic Activity against Multidrug-Resistant Mycobacteria. Antimicrob. Agents Chemother. 2015, 59, 5057–5060. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, R.; Thakur, S.; Nehra, K. ct-DNA Binding and Antibacterial Activity of Octahedral Titanium (IV) Heteroleptic (Benzoylacetone and Hydroxamic Acids) Complexes. Int. J. Med. Chem. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Bakulina, O.; Bannykh, A.; Levashova, E.; Krasavin, M. Conjugates of Iron-Transporting N-Hydroxylactams with Ciprofloxacin. Molecules 2022, 27, 3910. [Google Scholar] [CrossRef] [PubMed]
- L.A. McAllister, J.I. L.A. McAllister, J.I. Montgomery, J.A. Abramite, U. Reilly, M.F. Brown, J.M. Chen, R.A. Barham, Y. Che, S.W. Chung, C.A. Menard, M. Mitton-Fry, L.M. Mullins, M.C. Noe, J.P. O'Donnell, R.M. Oliver, 3rd, J.B. Penzien, M. Plummer, L.M. Price, V. Shanmugasundaram, A.P. Tomaras, D.P. Uccello, Heterocyclic methylsulfone hydroxamic acid LpxC inhibitors as Gram-negative antibacterial agents, Bioorg Med Chem Lett 22(22) (2012) 6832-8.
- Sow, I.S.; Gelbcke, M.; Meyer, F.; Vandeput, M.; Marloye, M.; Basov, S.; Van Bael, M.J.; Berger, G.; Robeyns, K.; Hermans, S.; et al. Synthesis and biological activity of iron(II), iron(III), nickel(II), copper(II) and zinc(II) complexes of aliphatic hydroxamic acids. J. Co-ord. Chem. 2023, 76, 76–105. [Google Scholar] [CrossRef]
- Coelho, T.S.; Halicki, P.C.B.; Silva, L.; Vicenti, J.R.d.M.; Gonçalves, B.L.; da Silva, P.E.A.; Ramos, D.F. Metal-based antimicrobial strategies against intramacrophageMycobacterium tuberculosis. Lett. Appl. Microbiol. 2020, 71, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, P.; Crumbliss, A.L.; Miller, M.J.; Möllmann, U. Synthesis and biological activity of saccharide based lipophilic siderophore mimetics as potential growth promoters for mycobacteria. BioMetals 2007, 21, 41–51. [Google Scholar] [CrossRef]
- Mavrikaki, V.; Pagonis, A.; Poncin, I.; Mallick, I.; Canaan, S.; Magrioti, V.; Cavalier, J.-F. Design, synthesis and antibacterial activity against pathogenic mycobacteria of conjugated hydroxamic acids, hydrazides and O-alkyl/O-acyl protected hydroxamic derivatives. Bioorganic Med. Chem. Lett. 2022, 64, 128692. [Google Scholar] [CrossRef]
- Majewski, M.W.; Cho, S.; Miller, P.A.; Franzblau, S.G.; Miller, M.J. Syntheses and evaluation of substituted aromatic hydroxamates and hydroxamic acids that target Mycobacterium tuberculosis. Bioorganic Med. Chem. Lett. 2015, 25, 4933–4936. [Google Scholar] [CrossRef]
- Carvalho, E.M.; Paulo, T.d.F.; Saquet, A.S.; Abbadi, B.L.; Macchi, F.S.; Bizarro, C.V.; Campos, R.d.M.; Ferreira, T.L.A.; Nascimento, N.R.F.D.; Lopes, L.G.F.; et al. Pentacyanoferrate(II) complex of pyridine-4- and pyrazine-2-hydroxamic acid as source of HNO: investigation of anti-tubercular and vasodilation activities. JBIC J. Biol. Inorg. Chem. 2020, 25, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Valentini, D.; Zumla, A.; Maeurer, M. Evaluation of the efficacy of valproic acid and suberoylanilide hydroxamic acid (vorinostat) in enhancing the effects of first-line tuberculosis drugs against intracellular Mycobacterium tuberculosis. Int. J. Infect. Dis. 2018, 69, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Rens, C.; Laval, F.; Daffé, M.; Denis, O.; Frita, R.; Baulard, A.; Wattiez, R.; Lefèvre, P.; Fontaine, V. Effects of Lipid-Lowering Drugs on Vancomycin Susceptibility of Mycobacteria. Antimicrob. Agents Chemother. 2016, 60, 6193–6199. [Google Scholar] [CrossRef] [PubMed]
- D. Yang, G. D. Yang, G. Vandenbussche, D. Vertommen, D. Evrard, R. Abskharon, J.F. Cavalier, G. Berger, S. Canaan, M.S. Khan, S. Zeng, A. Wohlkonig, M. Prevost, P. Soumillion, V. Fontaine, Methyl arachidonyl fluorophosphonate inhibits Mycobacterium tuberculosis thioesterase TesA and globally affects vancomycin susceptibility, FEBS Lett 594(1) (2020) 79-93.
- Yang, D.; Klebl, D.P.; Zeng, S.; Sobott, F.; Prévost, M.; Soumillion, P.; Vandenbussche, G.; Fontaine, V. Interplays between copper and Mycobacterium tuberculosis GroEL1. Metallomics 2020, 12, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Constant, P.; Yang, D.; Baulard, A.; Lefèvre, P.; Daffé, M.; Wattiez, R.; Fontaine, V. Cpn60.1 (GroEL1) Contributes to Mycobacterial Crabtree Effect: Implications for Biofilm Formation. Front. Microbiol. 2019, 10, 1149. [Google Scholar] [CrossRef] [PubMed]
- M. Tre-Hardy, C. M. Tre-Hardy, C. Nagant, N. El Manssouri, F. Vanderbist, H. Traore, M. Vaneechoutte, J.P. Dehaye, Efficacy of the combination of tobramycin and a macrolide in an in vitro Pseudomonas aeruginosa mature biofilm model, Antimicrob Agents Chemother 54(10) (2010) 4409-15.
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Boopathi, S.; Ramasamy, S.; Haridevamuthu, B.; Murugan, R.; Veerabadhran, M.; Jia, A.-Q.; Arockiaraj, J. Intercellular communication and social behaviors in mycobacteria. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Niño-Padilla, E.I.; Velazquez, C.; Garibay-Escobar, A. Mycobacterial biofilms as players in human infections: a review. Biofouling 2021, 37, 410–432. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Dufrêne, Y.F.; Nigou, J. Mycobacterial Adhesion: From Hydrophobic to Receptor-Ligand Interactions. Microorganisms 2022, 10, 454. [Google Scholar] [CrossRef]
- Belardinelli, J.M.; Stevens, C.M.; Li, W.; Tan, Y.Z.; Jones, V.; Mancia, F.; Zgurskaya, H.I.; Jackson, M. The MmpL3 interactome reveals a complex crosstalk between cell envelope biosynthesis and cell elongation and division in mycobacteria. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Bailo, R.; Bhatt, A.; Aínsa, J.A. Lipid transport in Mycobacterium tuberculosis and its implications in virulence and drug development. Biochem. Pharmacol. 2015, 96, 159–167. [Google Scholar] [CrossRef]
- Kurthkoti, K.; Amin, H.; Marakalala, M.J.; Ghanny, S.; Subbian, S.; Sakatos, A.; Livny, J.; Fortune, S.M.; Berney, M.; Rodriguez, G.M. The Capacity of Mycobacterium tuberculosis To Survive Iron Starvation Might Enable It To Persist in Iron-Deprived Microenvironments of Human Granulomas. Mbio 2017, 8. [Google Scholar] [CrossRef]
- Sushko, T.; Kavaleuski, A.; Grabovec, I.; Kavaleuskaya, A.; Vakhrameev, D.; Bukhdruker, S.; Marin, E.; Kuzikov, A.; Masamrekh, R.; Shumyantseva, V.; et al. A new twist of rubredoxin function in M. tuberculosis. Bioorganic Chem. 2021, 109, 104721. [Google Scholar] [CrossRef]
- B. Ramos, S.V. B. Ramos, S.V. Gordon, M.V. Cunha, Revisiting the expression signature of pks15/1 unveils regulatory patterns controlling phenolphtiocerol and phenolglycolipid production in pathogenic mycobacteria, PLoS One 15(5) (2020) e0229700.
- Sirakova, T.D.; Dubey, V.S.; Kim, H.-J.; Cynamon, M.H.; Kolattukudy, P.E. The Largest Open Reading Frame ( pks12 ) in the Mycobacterium tuberculosis Genome Is Involved in Pathogenesis and Dimycocerosyl Phthiocerol Synthesis. Infect. Immun. 2003, 71, 3794–3801. [Google Scholar] [CrossRef] [PubMed]
- Siméone, R.; Constant, P.; Malaga, W.; Guilhot, C.; Daffé, M.; Chalut, C. Molecular dissection of the biosynthetic relationship between phthiocerol and phthiodiolone dimycocerosates and their critical role in the virulence and permeability of Mycobacterium tuberculosis. FEBS J. 2007, 274, 1957–1969. [Google Scholar] [CrossRef] [PubMed]
- V. Boradia, A. V. Boradia, A. Frando, C. Grundner, The Mycobacterium tuberculosis PE15/PPE20 complex transports calcium across the outer membrane, PLoS Biol 20(11) (2022) e3001906.
| Compound | M. bovis BCG | M. marinum | M. tuberculosis H37Ra | |||
|---|---|---|---|---|---|---|
| MIC | MBIC | MIC | MBIC | MIC | MBIC | |
| µM | µM | µM | µM | µM | µM | |
| HA2FeCl | > 500 | 500 | > 500 | 250 | > 500 | 250 |
| HA6FeCl | > 500 | 300 | > 500 | ≥ 250 | 500 | 125 |
| HA8FeCl | ≥ 500 | 100 | 200 | 62.5 | 500 | 250 |
| HA10FeCl | 100-200 | 20-100 | > 200 | 62.5 | 125 | 31.25 |
| HA12FeCl | > 200 | 100 | > 200 | 500 | 125 | 125 |
| HA17FeCl | > 500 | > 500 | > 500 | 500 | 250 | > 500 |
| HA2Fe2 | > 500 | > 500 | > 500 | > 500 | > 500 | > 500 |
| HA6Fe2 | > 500 | 100-500 | > 500 | 500 | 500 | 250 |
| HA8Fe2 | > 500 | 100-200 | > 500 | 500 | 500 | 250 |
| HA10Fe2 | > 200 | 20 | > 200 | > 500 | 250 | > 500 |
| HA12Fe2 | > 500 | 100 | > 500 | > 500 | 500 | 500 |
| HA17Fe2 | 250 | > 500 | > 500 | 500 | 125 | 250 |
| HA2Fe3 | 250-500 | 300-500 | > 500 | 250 | > 500 | 125 |
| HA6Fe3 | 500 | 100-500 | > 500 | 250 | ≥ 500 | 62.5-125 |
| HA8Fe3 | 500 | 100 | 250-500 | ≥ 125 | 125 | 31.25 |
| HA10Fe3 | ≥ 200 | 20-40 | > 200 | 62.5 | 125 | 31.25-62.5 |
| HA12Fe3 | ≥ 200 | 100 | > 200 | 200-500 | 125-250 | 125 |
| HA17Fe3 | > 200 | > 500 | > 500 | 200-500 | 125 | 62.5 |
| Compound | M. bovis BCG | M. marinum | M. tuberculosis H37Ra | |||
|---|---|---|---|---|---|---|
| MIC | MBIC | MIC | MBIC | MIC | MBIC | |
| µM | µM | µM | µM | µM | µM | |
| HA2Zn2 | > 500 | > 500 | > 500 | 500 | > 500 | 250 |
| HA6Zn2 | > 500 | 200 | > 500 | 500 | 125 | 125 |
| HA8Zn2 | > 500 | 100-200 | > 500 | 500 | 250 | 250 |
| HA10Zn2 | 250 | 100 | 250 | 250-500 | 250 | 250 |
| HA12Zn2 | > 500 | 100 | > 500 | 250 | 250 | 125 |
| HA17Zn2 | > 500 | > 500 | > 500 | 500 | > 500 | 250 |
| HA2Ni2 | > 500 | 300-500 | > 500 | > 500 | > 500 | 62.5-125 |
| HA6Ni2 | > 500 | 200 | > 500 | 500 | > 500 | 31.25 |
| HA8Ni2 | > 500 | 100 | > 500 | 62.5 | > 500 | 31.25 |
| HA10Ni2 | > 200 | 20 | > 200 | 500 | 500 | 125 |
| HA12Ni2 | > 500 | 100 | > 500 | 125 | > 500 | 250-500 |
| HA17Ni2 | > 500 | > 500 | > 500 | 250 | > 500 | 250 |
| HA2Cu2 | 250-500 | 300 | > 500 | ≥ 250 | ≥ 500 | 250 |
| HA6Cu2 | ≥ 500 | 100 | > 500 | 500 | > 500 | 500 |
| HA8Cu2 | > 200 | 100-200 | > 200 | 250 | > 500 | 250 |
| HA10Cu2 | > 200 | 20 | > 200 | 250 | 250 | 250 |
| HA12Cu2 | > 500 | 100 | > 500 | 125 | > 500 | 250 |
| HA17Cu2 | > 500 | ≥ 500 | > 500 | ≥ 500 | > 500 | 250 |
| Compound | M. bovis BCG | M. marinum | M. tuberculosis H37Ra | |||
|---|---|---|---|---|---|---|
| MIC | MBIC | MIC | MBIC | MIC | MBIC | |
| µM | µM | µM | µM | µM | µM | |
| HA2 | > 500 | > 500 | > 500 | 500 | > 500 | 250 |
| HA6 | > 500 | ~ 100 | > 500 | 500 | 500 | 125 |
| HA8 | > 500 | 100 | > 500 | 500 | 500 | 125 |
| HA10 | 250-500 | 100 | 250-500 | 250 | > 500 | 125 |
| HA12 | > 500 | 100-500 | > 500 | > 500 | 125 | 250 |
| HA17 | > 500 | > 500 | > 500 | ≥ 500 | ≥ 500 | > 500 |
| FeCl2 | > 500 | > 500 | > 500 | 500 | > 500 | > 500 |
| FeCl3 | > 500 | > 500 | > 500 | 500 | > 500 | > 500 |
| NiCl2 | > 200 | > 500 | 500 | ≥ 500 | > 500 | 250 |
| CuCl2 | > 200 | 200 | > 200 | 250 | > 500 | 500 |
| ZnCl2 | > 500 | > 500 | > 500 | 250 | > 500 | 125-250 |
| Compounds | MIC (μg/mL)/FICI |
|---|---|
| Vancomycin | 750/- |
| HA10FeCl | 48.18-96.37/- |
| HA10FeCl (46.35 μg/mL) + Vancomycin | 125/0.176 |
| Vancomycin (50 μg/mL) + HA10FeCl | 11.59/0.125-0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





