1. Introduction
Mineral water is defined as natural, uncontaminated groundwater sourced from deep underground or artificially exposed groundwater. It naturally contains a specific concentration of mineral salts, trace elements, and sometimes carbon dioxide gas [
1]. In addition, mineral water, as a special resource that combines the characteristics of mineral and water resources, has recoverability and fluidity, a special material composition, and relatively stable dynamic characteristics. Furthermore, as a high-quality water resource, it is a natural beverage that is beneficial to human health [
2].
Previous studies have demonstrated the ubiquitous presence of nanoparticles in various forms in aquatic environments, including groundwater, lakes, and rivers [
3]. Although these water resources comprise less than 1% of the planet’s total water supply, they are the most crucial due to their indispensability for human survival, including drinking water and agricultural use. However, they are facing mounting pressure due to the rapidly growing population [
4,
5,
6]. However, to the best of our knowledge, there have been no studies on environmental nanoparticles in natural mineral water resources.
The reason nanomaterials have generated significant interest is due to the unique behavior exhibited by materials when at least one spatial dimension is constrained within the nanoscale size range [
7]. This effect has been the driving force behind the rapid growth of nanotechnology, as evidenced by the numerous industrial applications of synthetic Fe-oxide nanoparticles [
8,
9,
10]. However, over billions of years, nature has also produced its own iron oxide nanoparticles. These nanoparticles function as carriers of elements and compounds in rivers and groundwater, facilitating their transport over long distances. Additionally, they play a vital role in the critical zone, being one of its primary components. The critical zone is responsible for various dynamic environmental processes, including soil formation, element cycling, and water quality maintenance [
3].
In this study, we aimed to identify if natural mineral water contained environmental nanoparticles. These nanoparticles not only had macroelements in their composition, such as O, Mg, Ca, Si, Fe, Ti, and P, but they also contained many trace elements, such as F, V, S, and Mn. In addition, the morphology and structure characteristics of these nanoparticles in mineral water were studied. The findings of this study can be used as a reference for future research to understand element cycling and find solutions for water quality.
2. Materials and Methods
2.1. Sampling Location and Geological Background
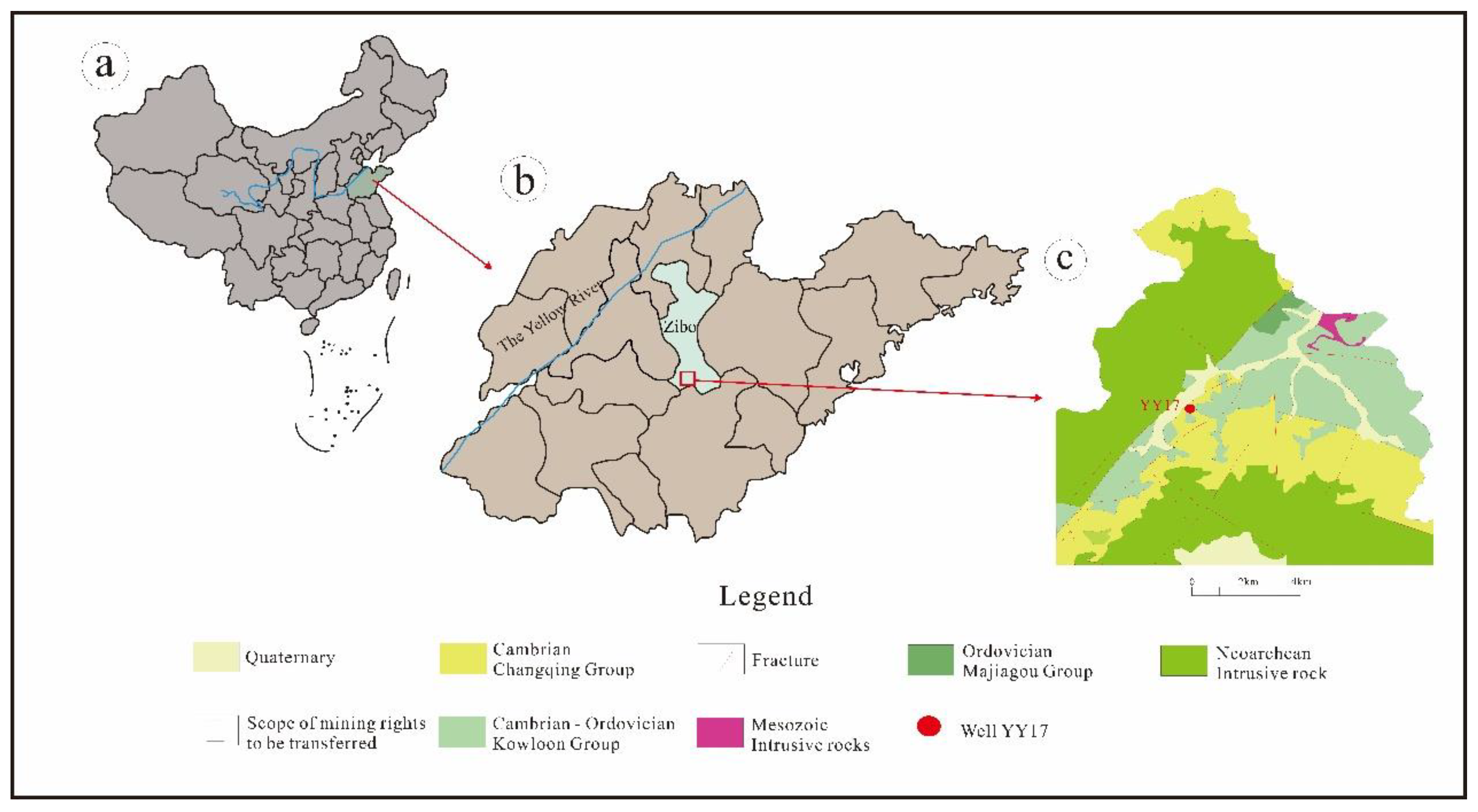
The mineral water samples were obtained from well YY17 in Yiyuan County, Shandong Province, eastern China. Yiyuan County is in the central part of Shandong Province (
Figure 1), and there are three types of landforms (low mountains, hills, and mountain plains) in this area. The altitude of this area ranges from 150 to 300 m. The study area is located in a warm temperate zone with a semi-humid continental monsoon climate. It experiences four distinct seasons throughout the year. In spring, the climate is characterized by dry and windy conditions. The summer is characterized by high humidity, hot temperatures, and frequent rainfall. Autumn is typically sunny, with pleasant weather. Winter is cold and dry in this region. The average annual temperature is 13.6 ℃, the average precipitation (1971–2017) is 724.90 mm, and the average annual evaporation is 1578.9 mm.
The strata in the study area are mainly composed of Cenozoic Quaternary Strata (clay and gravel sand), Ordovician Strata (limestone, dolomite, and siltstone), and Cambrian Strata (limestone, marl, dolomite, siltstone, and shale). The basement in this area is composed of the Neoarchean Taishan group (amphibolite). The fault structures in the region are extremely developed, with the northeast-trending faults being the most developed. Several springs in the region are also distributed in this direction. The intrusive rocks in the region are also relatively developed. Furthermore, the formation age is mainly Neoarchean, and the lithology is mainly amphibolite, hornblende quartz diorite, syenogranite, and monzogranite.
2.2. The occurrence and hydrochemistry characteristics of the mineral water
The well YY17 of natural mineral water was mainly located in the limestone and siltstone in the Cambrian Strata, and the roof and floor were impermeable shale in the Cambrian Strata and Neoarchean amphibolite, respectively. The strontium content of the well YY17 mineral water was 2.64–3.01 mg/L, with a chromaticity of less than five degrees and a turbidity of less than 2 NTU. The volatile phenol content of this mineral water was < 0.002 mg/L and the cyanide content was < 0.002 mg/L. The anionic synthetic detergent content of the mineral water was < 0.050 mg/L and the mineral oil content was < 0.005 mg/L. The nitrite (NO
2-) content ranged from 0.0172 to 0.054 mg/L, and the total content of the radioactivity β was 0.06–0.1032 Bq/L. There was no odor or objects in the water. In addition, the test result for coliform bacteria, fecal streptococci,
Pseudomonas aeruginosa, and
Clostridium perfringens was 0 [
11]. These results indicated that the well YY17 natural mineral water was high-quality strontium-type natural mineral water.
2.3. Analytical Methods
2.3.1. Nanoparticle tracking analysis
All the samples were diluted in phosphate-buffered saline (PBS) to a final volume of 1 mL. The optimal measurement concentrations were determined by pre-testing the ideal particle per frame value (140–200 particles/frame). The default software settings provided by the manufacturer for the nanoparticles were selected accordingly. Each measurement involved three cycles, scanning 11 particle positions and capturing 60 frames per position (video setting: high), under the following settings: focus, autofocus; camera sensitivity, 92.0; shutter, 70; scattering intensity, 4.0; and cell temperature, 25 °C. Subsequently, the videos were analyzed using the built-in ZetaView Software 8.02.31, employing the following analysis parameters: maximum particle size, 1000; minimum particle size, 5; and minimum particle brightness, 20. An embedded laser (40 mW at 488 nm) and a CMOS camera were employed in the experiment. The number of completed tracks in the nanoparticle tracking analysis (NTA) measurements was always greater than the proposed minimum of 1000 to minimize skewed data based on single large particles [
12].
2.3.2. Characteristics of the nanoparticle analysis
The hot spring samples were first pre-treated before being analyzed using high-resolution transmission electron microscopy (HRTEM). The pre-processing of these samples followed the procedures described by Li et al. (2016) [
13]. To ensure even dispersion of nanoparticles in the aqueous solution, the collected water samples were lightly shaken before being transferred onto the HRTEM grid using a pipette. This shaking procedure was repeated four to five times. All grid samples were tested at the Sinoma Institute of Materials Research (Guangzhou) Co. Ltd. using an HRTEM facility (Tecnai G2F20) equipped with energy-dispersive spectrometry (EDS) at 200 kV. The HRTEM had a dot resolution of 0.20 nm and a linear resolution of 0.102 nm. The maximum magnification for HRTEM and scanning transmission electron microscopy was 1 million and 2.3 million times, respectively.
3. Results
3.1. The number and size distribution of the environmental nanoparticles in the well YY17 mineral water
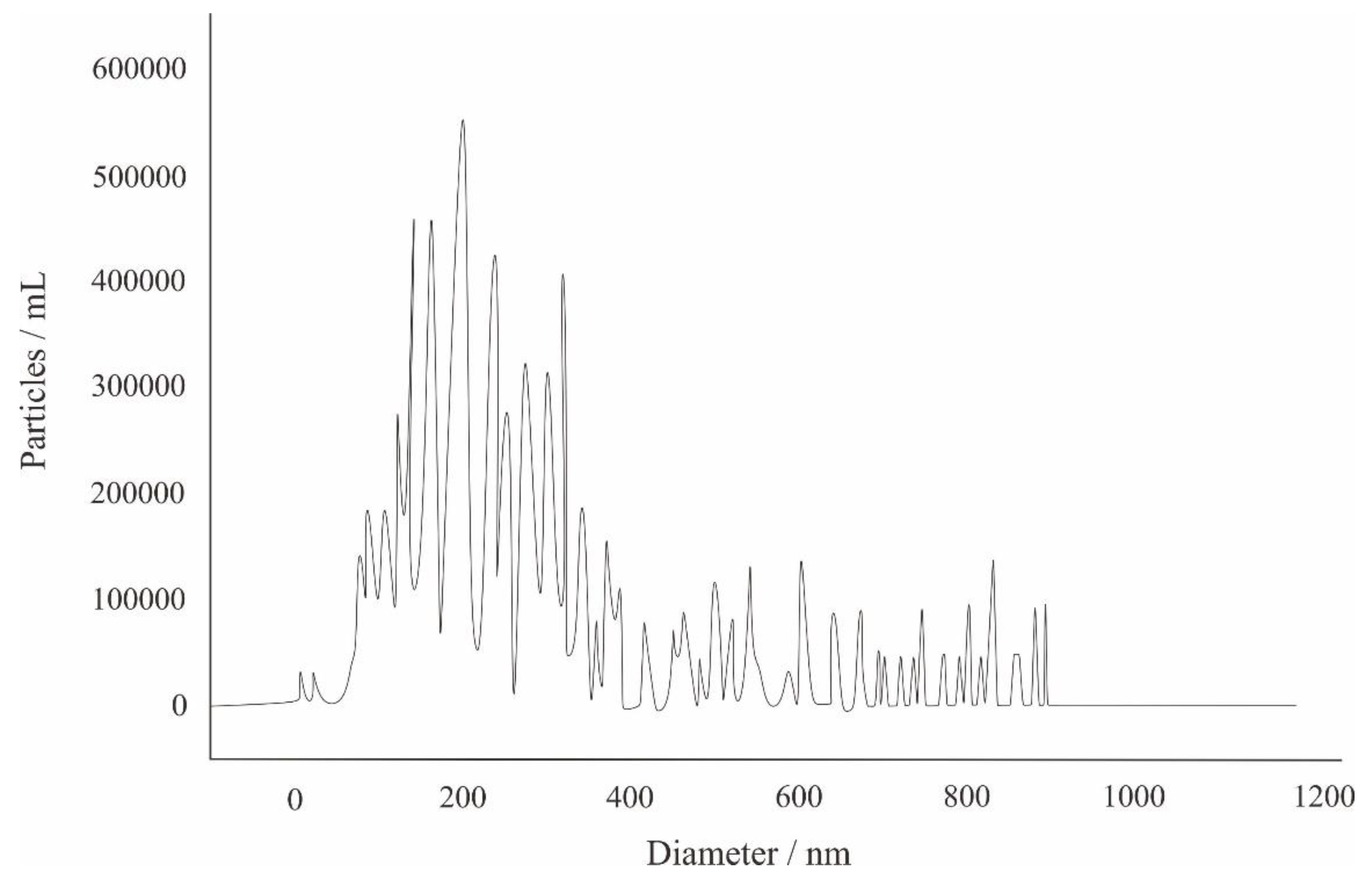
The NTA of the well YY17 mineral water showed the size distribution with a peak diameter of 180 nm (
Figure 2), and the concentration of the particles was 5.5 × 10
6 particles/mL (
Figure 2).
3.2. Characteristics of the environmental nanoparticles in the well YY17 mineral water
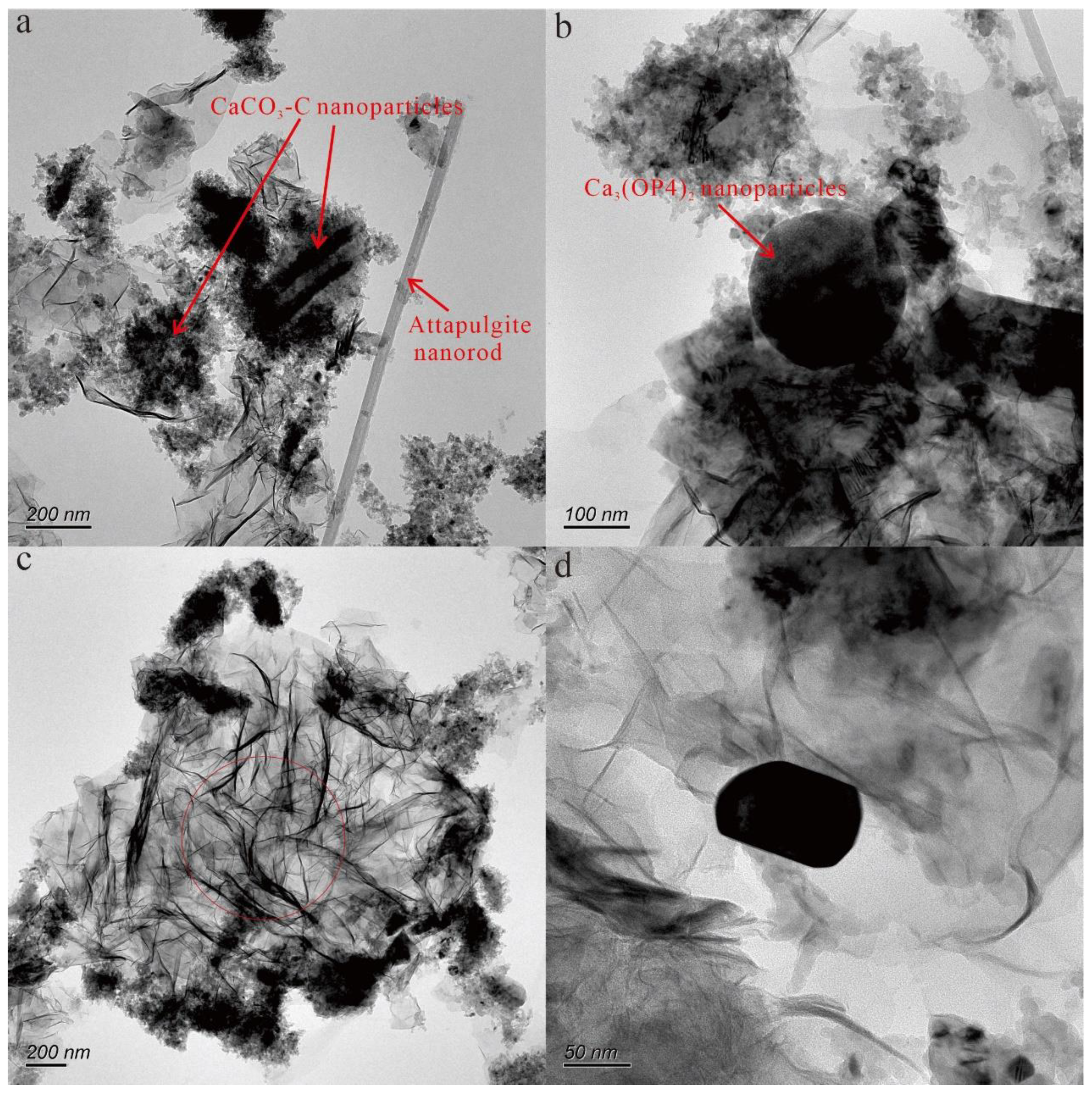
According to the morphological map of the nanoparticles (
Figure 3), it can be observed that these nanoparticles exhibit various shapes and sizes. Specifically, the samples consist of granular, pinniform, rodlike, and flakey nanoparticles. Furthermore, some of the nanoparticles are observed in the form of aggregates, while a few are present as monomers. Among these nanoparticles, the smaller ones were only 50 × 60 nm in size, while the larger ones reached 300 × 500 nm in size. Moreover, the elemental compositions of these nanoparticles varied, and the elements included O, Mg, Ca, Si, Fe, Ti, P, F, V, S, and Mn. Among them, the representative nanoparticles are discussed below.
3.2.1. Ca-bearing nanoparticles
As shown by the topography (
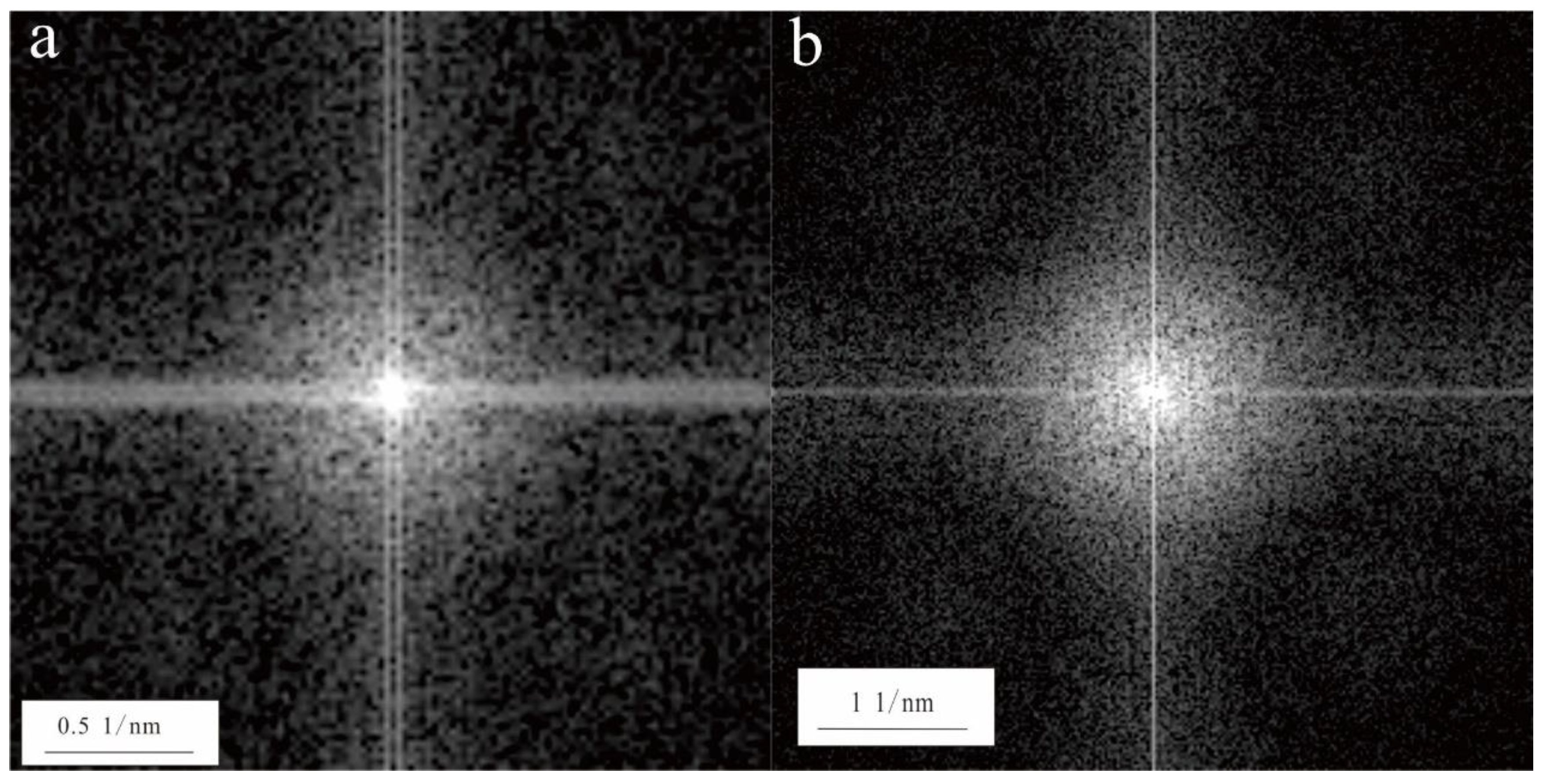
Figure 3a,b), the Ca-bearing nanoparticles mainly occurred as aggregates. According to the results of the TEM-EDS, the Ca-bearing nanoparticles mainly included two types: Ca-O-C nanoparticles and Ca-O-P nanoparticles. The Ca-O nanoparticles mainly occurred as irregular shapes that were 100 to 400 nm in size, and the Ca-O-P nanoparticles were spheres that were 200 nm in diameter. The selected area electron diffraction patterns of the Ca-bearing nanoparticles contained no obvious diffraction spots (
Figure 4), indicating that they were amorphous. Based on the composition of these nanoparticles, the Ca-O-C and Ca-O-P nanoparticles were CaCO
3 and Ca
3(PO
4)
2 nanoparticles, respectively.
3.2.2. Attapulgite nanorod
The particle with a rodlike shape in
Figure 3a mainly contained Si, Mg, and O, indicating that the nanorod may be attapulgite. The nanorod had two dimensions that were about 30 nm in diameter and 1–3 μm in length. The selected area electron diffraction pattern of the attapulgite nanorod contained no obvious diffraction spots (
Figure 5), indicating that it was amorphous.
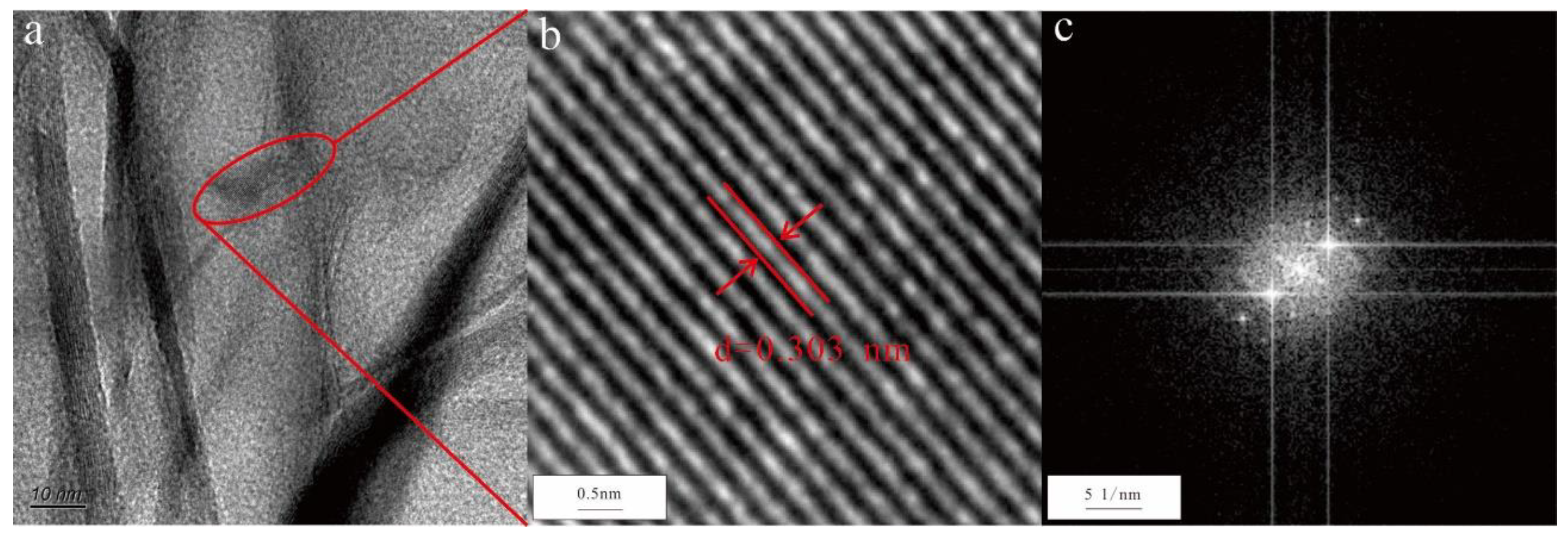
3.2.3. MnO2 nanosheets
In
Figure 3c, there are many nanosheet aggregates in the mineral water. Based on the EDS, the nanosheets mainly contained Mn and O. The HRTEM image showed that the lattice of this nanosheet was 0.303 nm (
Figure 6a,b). In addition, the selected area electron diffraction pattern had obvious diffraction spots, which were characterized by 110 (d = 0.303 nm) main reflections (
Figure 6c). These results indicated that the nanosheet was pyrolusite (MnO
2).
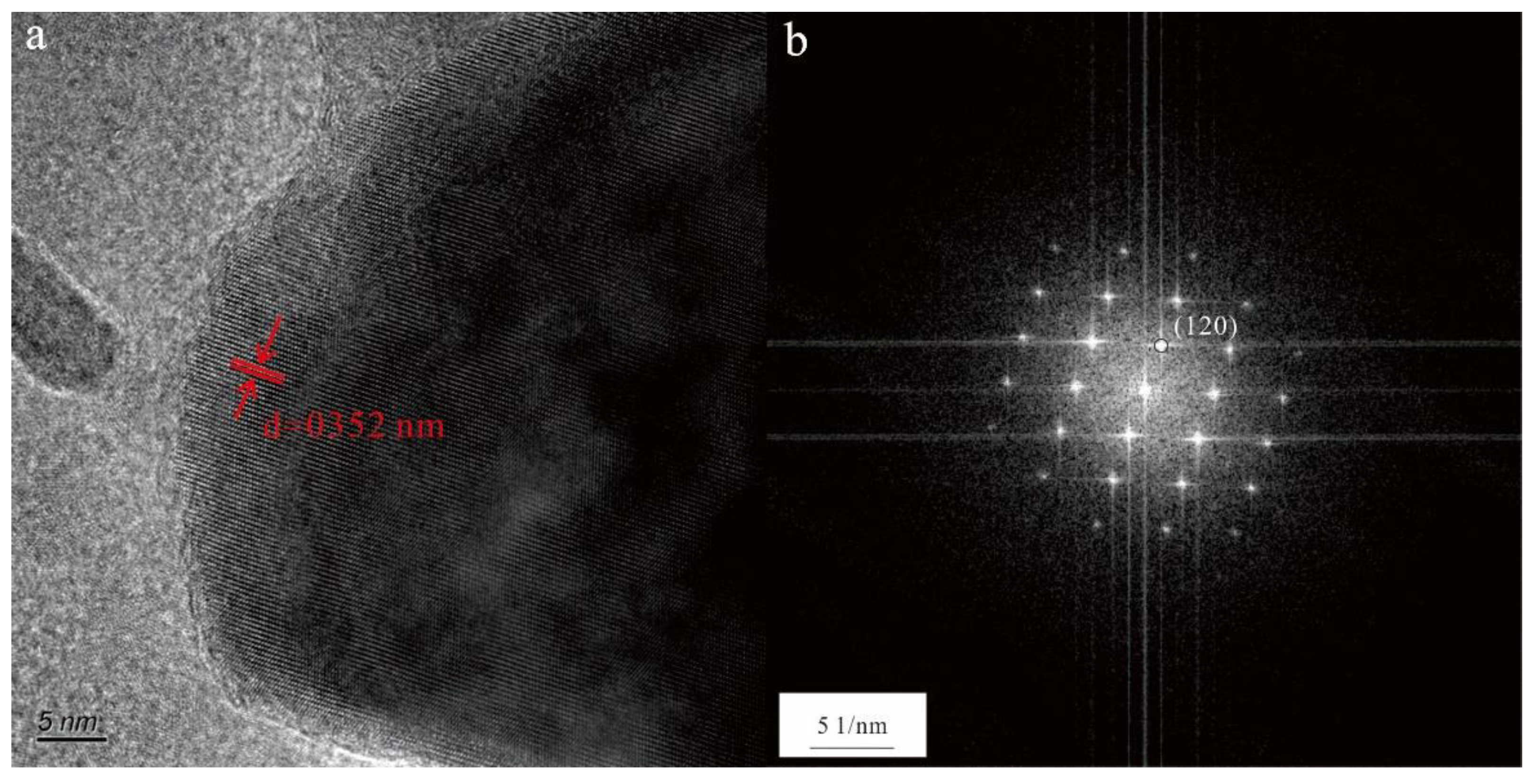
3.2.4. TiO2 nanoparticle
Figure 3d shows a nanoparticle with a composition of mainly Ti and O. The high-resolution transmission electron microscopy (HRTEM) image shows the ordered arrangement of the atoms with a lattice spacing of 0.352 nm (
Figure 7a). In addition, the selected area electron diffraction pattern showed some diffraction spots that were characterized by 120 main reflections (d = 0.352 nm;
Figure 7b), indicating that this nanoparticle was crystalline. When combined with the results of the EDS, it suggested that this nanoparticle was a TiO
2 nanoparticle.
4. Discussion
4.1. A new form of elements in mineral water
For elements in groundwater, most researchers have only focused on the ionic forms, such as Ca
2+, Mg
2+, K
+, Na
+, Cl
-, SO
42-, HCO
3-, and NO
3-. However, researchers are now discovering that naturally occurring environmental nanoparticles can exist stably in natural waters [
3]. In addition, nanoparticles can play a crucial role in determining important chemical properties and overall water quality in natural environments. In this study, numerous nanoparticles were found in mineral water, which had a concentration of about 5.5 × 10
6 particles/mL. These results indicate a new form of elements in mineral water, especially Ca, Si, and Mg. The findings also confirm that natural nanoparticles can exist stably in mineral water and the elements can be transported in a nanoparticle form. The nanoparticles may also serve as one of the primary constituents of mineral water and element cycling [
3].
4.1.1. Natural CaCO3 nanoparticles in mineral water
Calcium carbonate (CaCO
3) is one of the most abundant biominerals in the Earth’s crust, ranking fifth in terms of abundance [
14]. It is a crucial substance that can be found in geological sources such as limestone, chalk, and marble [
15], as well as in biological organisms including corals, pearls, mollusk shells, and eggshells [
16]. Biogenic calcium carbonate can take on six different forms: three anhydrous crystalline polymorphs (calcite, aragonite, and vaterite) [
17], and three hydrated forms (amorphous calcium carbonate, calcium carbonate hexahydrate [CaCO
3·6H
2O], and calcium carbonate monohydrate [CaCO
3·H
2O]) [
18]. In this study, we also found the CaCO
3 nanoparticle occurred in the mineral water. They were amorphous and usually occurred as an aggregation, indicating that the aggregation-based growth of the CaCO
3 nanoparticle may exist in this water system. In addition, the occurrence of the CaCO
3 nanoparticle in mineral water also confirmed that it is widely distributed in the environment.
4.1.2. Natural Ca3(PO4)2 nanoparticles in mineral water
The most abundantly produced phosphate mineral is carbonated hydroxyapatite, which is also called dahllite, and it is closely associated with bones and teeth [
19]. Amorphous calcium phosphate (Ca
3(PO
4)
2) is often found during the formation of calcium phosphates in aqueous systems. It is believed to be a precursor to hydroxyapatite [
20,
21]. In addition, as calcium phosphate exhibits excellent biocompatibility and has a nano-scale particle size, the research on Ca
3(PO
4)
2 nanoparticles mainly focuses on its application in medicine for biomaterials (e.g., bone regeneration and tooth substitutes) and drug delivery vehicles [
22].
In natural water systems, the occurrence of phosphorus mainly exits as dissolved phosphorus (PO
43+) and particulate phosphorus (> 450 nm; [
23]). Moreover, Ca
3(PO
4)
2 is the precipitation of corrosion products and is the one of most costly design and operating problems in water recycling systems [
24,
25]. Therefore, either in natural water systems or in industrial water systems, there has been a lack of studies on Ca
3(PO
4)
2 nanoparticles. Our findings could indicate an important occurrence of phosphorus in water systems.
4.1.3. Natural attapulgite nanorods in mineral water
Attapulgite, also known as palygorskite, has gained significant attention in recent years due to its unique properties such as large specific surface area, nanochannel structure, exchangeable cations, ultra-high adsorption capacity, and colloidal properties [
26]. Its versatile applications include being used as nanofillers [
27,
28] in clay nanocomposites, supporting high-performance catalysts [
29,
30], sensor devices [
31], and for clay-biological interfaces in tissue engineering [
32]. Attapulgite is a naturally occurring crystalloid hydrated magnesium-aluminum silicate clay, which is classified as part of the sepiolite family in mineralogy due to its similar microscopic structure and morphology [
33]. It has been observed that natural attapulgite mainly consists of laths (the smallest structure), rods (the oriented arrangement of laths), and bundles (the aggregation of rods; [
34,
35,
36]).
The nano attapulgite in the mineral water occurred as rods (two-dimensional), indicating that the attapulgite nanorod may be relatively widespread in the environment. In addition, the attapulgite nanorod also demonstrated a new occurrence of a clay mineral in mineral water. As clay is widely available on Earth, a rock-water reaction commonly occurs between the groundwater and the clay layer. Therefore, there are likely a lot of clay minerals entering the groundwater, and our findings provide strong evidence that numerous nano-scale clay minerals may occur in groundwater.
4.1.4. MnO2 nanosheets in the mineral water
Manganese is abundant in the crust (ranked No. 10), and it is the most common transition element that is second only to iron [
37]. The migration of Mn (II) that is generated by rock weathering is prevalent, and it is oxidized into a variety of manganese minerals after entering the surface and water environment. As a natural oxidant and catalyst, manganese oxide minerals are widely involved in the oxidation-reduction and catalytic reactions of various organic and inorganic compounds in nature [
38,
39,
40,
41,
42]. In addition, due to the formation of manganese oxide minerals at the interface between the lithosphere, hydrosphere, atmosphere, or biosphere, they are the products of interactions between these layered systems. Their formation and transformation carry rich environmental information and are potential indicators of system evolution [
37]. Therefore, the formation of manganese oxide minerals has important significance in the environmental geochemical cycle of organic and inorganic compound migration and transformation. Additionally, due to its large specific capacitance and environment-friendly nature [
43,
44,
45], MnO
2 has attracted interest for low-cost and green energy storage devices. Therefore, artificial MnO
2 materials (e.g., MnO
2 nanosheets) are very common in the industrial field.
In this study, the MnO2 materials also occurred in the mineral water. These MnO2 materials mainly exited as nanosheets in shape, which is the same as the MnO2 materials that are synthesized in industry. In composition, there were other elements (e.g., F, Mg, Si, S, and Fe) occurring in these MnO2 nanosheets, indicating that the MnO2 nanosheets can act as carriers of elements in the mineral water. In addition, MnO2 nanosheets are widely distributed in mineral water and thus can be considered one of the key components in groundwater. Their presence in groundwater plays a significant role in dynamic environmental processes such as soil formation, element cycling, and water quality regulation.
4.1.5. TiO2 nanoparticles in the mineral water
TiO
2 nanoparticles are widely used in various industries such as paints, ceramics, rubber, plastics, cosmetics, and water purification [
46,
47]. Although there have been numerous studies on the safety and characteristics of engineered nanoparticles due to their increased usage and discharge, research on naturally occurring nanoparticles is scarce [
48]. Given that Ti is one of the most abundant elements in the earth’s crust, it can be speculated that TiO
2 nanoparticles, predominantly composed of TiO
2, are naturally distributed in the environment through natural processes. Furthermore, several studies have estimated the environmental concentration of nanomaterials, with TiO
2 nanoparticles consistently showing the highest levels in all regions [
49,
50,
51,
52,
53]. Despite this, research on naturally formed TiO2 nanoparticles has been limited.
Naturally formed TiO2 nanoparticles were found to be widely distributed in the mineral water in this study, which also provides strong evidence that TiO2 nanoparticles can be produced in nature. Additionally, the TiO2 nanoparticles were found to be present alongside other common elements found on the Earth’s surface, including Mg, Al, and Si. This coexistence suggests that during the formation and movement of the TiO2 nanoparticles in the natural environment, they interacted with various elements present in the mineral water. These interactions led to the aggregation of these elements with the TiO2 particles, resulting in a more complex chemical composition for the overall particle.
4.2. The origins of environmental nanoparticles in mineral water
As was discussed above, many nanoparticles can be synthesized in industry, such as CaCO3 nanoparticles, MnO2 nanosheets, and TiO2 nanoparticles. Therefore, these nanoparticles are considered to be engineering nanoparticles. However, regardless of the preparation methods used, the engineering nanoparticles exhibited consistent granular size and pure chemical composition, with a narrow range of size distribution. In contrast, the nanoparticles investigated in this study displayed varying diameters and compositions, indicating that they were not artificially produced.
Environmental nanoparticles are often generated through three main processes: weathering of minerals, biogenic products of microbial activity, or as growth nuclei in super-saturated fluids [
3]. Some of the nanoparticles (e.g., Ca-bearing nanoparticles and MnO
2 nanosheets) in the mineral water exited as irregular shapes. As the nanoparticles that are formed as biogenic products of microbial activity are usually regular shaped, such as a sphere with smoothed edges (e.g., [
54]), nanoparticles with irregular shapes cannot be products of biogenesis. In addition, the concentration of microorganisms (e.g., coliform bacteria,
Enterococcus faecalis,
Pseudomonas aeruginosa, and
Clostridium perfringens) in the mineral water was low [
11]. Therefore, the nanoparticles in the mineral water were unlikely to have been formed by organisms. Moreover, all the mineral (e.g., anhydrite, aragonite, calcite, chalcedony, chrysotile, dolomite, and gypsum) concentrations did not reach saturation, indicating that these minerals were unsaturated in this mineral water. Therefore, the nanoparticles were also unlikely to have formed as growth nuclei in super-saturated fluids.
We suspect that these environmental nanoparticles in the mineral water are the weathering byproducts of minerals. The well YY17 mineral water mainly existed in limestone and siltstone, and the roof and floor were shale and amphibolite, respectively. Therefore, strata and igneous rocks can be a source of Ca, P, Ti, Mn, Mg, and Si elements in mineral water during water-rock reactions. These findings also support that elements can be transported as nanoparticles during water-rock reactions, which may be an important form for element cycling between water and rocks.
4.3. Perspectives on the advancement of environmental nanoparticles in mineral water
4.3.1. The opportunity to understand the long-term stability and transport mechanisms of nanoparticles
As was discussed above, the nanoparticles that occurred in the natural mineral water were also found in other systems, such as Ca
3(PO
4)
2 and CaCO
3 nanoparticles in vivo and TiO
2 nanoparticles and MnO
2 nanosheets in engineered systems. However, the research on their long-term stability and transport mechanisms is relatively limited. Although the system in this study is different from in vivo and engineered systems, the behavior of nanoparticles may be the same in these systems due to the surface effect of nanoparticles [
7]. Thus, the findings of this study can assist with understanding the long-term stability and transport mechanisms of nanoparticles in nature and in vivo and engineered systems.
4.3.2. Environmental nanoparticles in drinking water systems
Particulate species in drinking water are defined as particles larger than 0.45 μm. Colloids, on the other hand, range in size from 0.1 to 0.45 μm. Soluble fractions, which are also known as dissolved substances, have a size smaller than 0.1 μm, equivalent to 100 nm. This study has presented compelling evidence that nanoparticles can be found in mineral water, which is renowned for its high quality. Furthermore, other investigations have demonstrated the presence of environmental nanoparticles in various natural aquatic systems, ranging from subsurface aquifers to surface lakes and rivers [
3]. Therefore, this supports that nanoparticles widely occur in drinking water systems. Based on this study, the nanoparticles in drinking water systems should be varied, and some of these nanoparticles have potential environmental risks, such as being associated with toxic elements. Therefore, the presence of environmental nanoparticles in water distribution systems may have an impact on water quality and the efficacy of drinking water treatment processes. Thus, the safety of drinking water for human consumption should be considered when environmental nanoparticles are present in treated waters, and treatment methods to remove the environmental nanoparticles should also be considered. Consequently, this study provides a foundation for understanding the behavior of nanoparticles in drinking water systems.
4.3.3. Environmental nanoparticles science in drinking water systems
Recently, billions of dollars have been invested in nanoscience and technology research and development around the world [
3]. There is also considerable commercial funding in this field. However, the attention given to environmental nanoscience is only a small fraction of the total research conducted. As a result, the progress in environmental nanoscience has significantly fallen behind other sub-disciplines in the field of nanoscience. Nevertheless, it is anticipated that the environmental and health implications of nanoscience and technology will become increasingly significant as their potential costs become apparent.
Based on significant advancements in the field of nanoscience over the past few decades, it has been observed that nanoparticles exhibit properties and reactivity that vary depending on their size. These characteristics can have a considerable impact on the aqueous chemistry of both natural and engineered waters [
3]. Therefore, it is crucial to conduct fundamental research on the variability of physical properties and chemical reactivity of environmental nanoparticles as they relate to their size. Furthermore, comprehensive studies on the impact of environmental nanoparticles on aqueous chemical processes are also necessary. The behavior of materials changes as they transition from bulk material to a molecular state, and the rates of reaction measured vary significantly depending on the size of the nanoparticles. As a result, environmental nanoparticle science has expanded beyond conventional boundaries of aqueous colloid science, and these disciplines are becoming increasingly integrated. Additionally, environmental nanoparticle science should be prioritized as a new research direction for continued advancement in the areas of water safety, treatment, and remediation.
5. Conclusion
Using the NTA and HRTEM, the nature and composition of environmental nanoparticles in the mineral water from Zibo City, Shandong Province, eastern China was investigated. The concentration of the nanoparticles was 5.5 × 106 particles/mL, and the peak diameter of the size distribution was 180 nm. The shapes of these nanoparticles were granular, pinniform, rodlike, and flakey, and some of the nanoparticles were aggregated. The composition of these nanoparticles mainly included O, Mg, Ca, Si, Fe, Ti, and P. In addition, F, V, S, and Mn were also found in some of the nanoparticles. It was confirmed that there were Ca-bearing nanoparticles, attapulgite nanorods, MnO2 nanosheets, and TiO2 nanoparticles in the mineral water, indicating a new form of elements in the mineral water. The chemical and physical characteristics of the mineral water suggest that the environmental nanoparticles are the weathering byproducts of minerals. In the future, these findings can be used as a basis for understanding how environmental nanoparticles behave in natural aquatic settings and this knowledge can be applied to other systems. In addition, environmental nanoparticle science will be important in drinking water systems, and more research should be conducted in environmental nanoparticle science for water safety, treatment, and remediation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant numbers 42102076]; and the Natural Science Foundation of Shandong Province [grant number ZR2021QD037].
References
- Shi, N.N. : Zuo, W.S.; Liu, Z.F.; et al. Characteristics of the mineral water resources in Guangdong and suggestions for their exploitation and utilization. Tropical Geography. 2012, 32, 501–507. [Google Scholar]
- Xu, F. Development, utilization and management of mineral water in the Soviet Union. Journal of Environment and Health. 1988, 3, 62–64. [Google Scholar]
- Wigginton, N.S.; Haus, K.L.; Hochella Jr, M.F. Aquatic environmental nanoparticles. Journal of environmental monitoring. 2007, 9, 1306–1316. [Google Scholar] [CrossRef]
- Geldreich, E. E.; Nash, H.D.; Reasoner, D. J.; Taylor, R.H. The necessity of controlling bacterial populations in potable waters-bottled water and emergency water supplies. Journal-American Water Works Association. 1975, 67, 117–124. [Google Scholar]
- Warburton, D.W.; Peterkin, P.I.; Weiss, K.F.; et al. Microbiological quality of bottled water sold in Canada. Canadian journal of microbiology, 1986, 32, 891–893. [Google Scholar] [CrossRef]
- González, C.; Gutiérrez, C.; Grande, T. Bacterial flora in bottled uncarbonated mineral drinking water. Canadian Journal of Microbiology. 1987, 33, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Hochella Jr, M.F.; Mogk, D.W.; Ranville, J.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science. 2019, 363, 6434. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.; Cesar, I.; Grätzel, M. New benchmark for water photooxidation by nanostructured α-Fe2O3 films. Journal of the American Chemical Society, 2006, 128, 15714–15721. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Mikhaylova, M.; Zhang, Y.; et al. Protective coating of superparamagnetic iron oxide nanoparticles. Chemistry of Materials. 2003, 15, 1617–1627. [Google Scholar] [CrossRef]
- Miser, D.E.; Shin, E.J.; Hajaligol, M.R.; et al. HRTEM characterization of phase changes and the occurrence of maghemite during catalysis by an iron oxide. Applied Catalysis A: General. 2004, 258, 7–16. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Guan, Q. Research on the origin and water quality evaluation of natural mineral water in Yiyuan county, Shandong province. Groundwater. 2021, 43, 25–28. [Google Scholar] [CrossRef]
- Gardiner, C.; Ferreira, Y.J.; Dragovic, R.A.; et al. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. Journal of extracellular vesicles, 2013, 2: 19671.
- Li, Y.; Cao, J.; Hopke, P.K.; et al. The discovery of the metallic particles of groundwater from the Dongshengmiao polymetallic deposit, Inner Mongolia, and their prospecting significance. Journal of Geochemical Exploration. 2016, 161, 49–61. [Google Scholar] [CrossRef]
- Ramasamy, V.; Anand, P.; Suresh, G. Synthesis and characterization of polymer-mediated CaCO3 nanoparticles using limestone: a novel approach. Advanced Powder Technology. 2018, 29, 818–834. [Google Scholar] [CrossRef]
- Karimi, M.A.; Ranjbar, M. Hydrothermal synthesis and characterization ofhydrothermal synthesis and characterization of CaCO; nanostructure, Synth.React. Inorganic Met. Nano-Metal Chem. 2016, 46, 635–637. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mishra, S. Nano-calcium carbonate (CaCO3)/polystyrene (PS)core-shell nanoparticle: it’s effect on physical and mechanical properties ofhigh impact polystyrene (HIPS). Polym. Res. 2013, 20, 1–12. [Google Scholar] [CrossRef]
- Barhoum, A.; Van, L.; Lokeren, H.; Rahier, A.G. Van Dufresne, Assche,Roles ofin situ surface modification in controlling the growth and crystallization ofCaCO3 nanoparticles, and their dispersion in polymeric materials,J. Mater.Sci. 2015, 50, 7908–7918. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Palanisamy, K.; Subramani, K.; et al. Polymorphic and Morphological Transformations of CaCO3 under CO2 Atmosphere and under the Influence of EDTA at 60 °C. International Letters of Chemistry Physics and Astronomy. 2015, 53, 173–179. [Google Scholar] [CrossRef]
- Lowenstam, H.; Weiner, S. On Biomineralization. Oxford University Press, NewYork. 1989.
- Brown, W.E.; Smithm, J.P.; Lehr, J.R.; et al. Octacalcium Phosphate and Hydroxyapatite: Crystallographic and Chemical Relations between Octacalcium Phosphate and Hydroxyapatite. Nature. 1962. [CrossRef]
- Suzuki, O.; Kamakura, S.; Katagiri, T. Surface chemistry and biological responses to synthetic octacalcium phosphate. Appl Biomater, 2010, 77B(1), 201-212. [CrossRef]
- Mok, Z.H.; Mylonas, P.; Austin, R.; et al. Calcium phosphate nanoparticles for potential application as enamel remineralising agent tested on hydroxyapatite discs. Nanoscale. 2021, 13, 20002–20012. [Google Scholar] [CrossRef]
- Fang, T.H.; Wang, C.W. Dissolved and particulate phosphorus species partitioning and distribution in the Danshuei River Estuary, Northern Taiwan. Marine Pollution Bulletin. 2020, 151, 110839. [Google Scholar] [CrossRef]
- Belton, D.; Stupp, S.I. Adsorption of ionizable polymers on ionic surfaces: Poly (acrylicacid). Macromolecules. 1983, 16, 1143–1150. [Google Scholar] [CrossRef]
- Shakkthivel, P.; Vasudevan, T. Acrylic acid-diphenylamine sul-phonic acid copolymer threshold inhibitor for sulfate and carbonatescales in cooling water systems. Desalination. 2006, 197, 179–189. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, J.P.; Chen, Z.W.; et al. Preparation, Characterization, Application and Structure Evolution of Attapulgite: From Nanorods to Nanosheets. Applied Surface Science. 2021, 565, 150398. [Google Scholar] [CrossRef]
- Chen, F.; Lou, D.; Yang, J.; Zhong, M. Mechanical and thermal properties of attapulgite clay reinforced polymethylmethacrylate nanocomposite. Polymers for Advanced Technologies, 2011, 22, 1912–1918. [Google Scholar] [CrossRef]
- Xu, H.; Yang, H.; Zhang, L.; et al. Preparation and properties of polycarbonate nanocomposites using attapulgite grafted poly (methyl methacrylate) as a potential nanofiller. Journal of Applied Polymer Science. 2015, 132, 1079–1083. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Chang, D.; Chen, D.; He, H.; Yuan, P.; Xie, J.; Frost, R.L. Characterization and catalytic performance of FegNis/palygorskite for catalyticcracking of benzene. Applied clay science. 2013, 74, 135–140. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Recent progress in dispersion of palygorskite crystal bundles for nanocomposites. Applied Clay Science. 2016, 119, 18–30. [Google Scholar] [CrossRef]
- Xu, J.; Han, W.; Yin, Q.; et al. Direct Electron Transfer of Glucose Oxidase and Glucose Biosensor Based on Nano-structural Attapulgite Clay Matrix. Chinese. Chem. 2009, 27, 2197–2202. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Fernandes, F.M.; et al. Fibrous clays based bionanocomposites. Progress in Polymer Science. 2013, 38, 1392–1414. [CrossRef]
- Wang, W.; Wang, A. Nanoscale clay minerals for functional ecomaterials: Fabrication, applications, and future trends. Handbook of Ecomaterials, Springer, Cham. 2019. [CrossRef]
- Chryssikos, G.D.; Gionis, V.; Kacandes, G.H.; Stathopoulou, E.T.; Suarez, M.; Garcia-Romero, E.; del Rio, M.S. Octahedral cation distribution in palygorskite. Am. Miner. 2009, 94, 200–203. [Google Scholar] [CrossRef]
- Garia-Romero, E.; Suarez, M. Sepiolite-palygorskite: Textural study and genetic considerations. Applied clay science. 2013, 86, 129–144. [Google Scholar] [CrossRef]
- Garcia-Romero, E.; Suarez, M. Sepiolite-palygorskite polysomatic series: Oriented aggregation as a crystal growth mechanism in natural environments. American Mineralogist. 2014, 99(8-9), 1653–1661. [CrossRef]
- Liu, F.; Feng, X.H.; Chen, X.H.; et al. , Advances in the study of biological genesis of manganese oxide minerals and their characteristics. Earth Science Frontiers. 2008, 15, 066–073. [Google Scholar]
- Post, J.E. Manganese oxide minerals: crystal structures and economic and environmental significance. Proceedings of the National Academy of Sciences. 1999, 96, 3447–3454. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; et al. Biogenic manganese oxides: properties and mechanisms of formation. Annual Review of Earth and Planetary Sciences. 2004, 32, 287328. [Google Scholar] [CrossRef]
- Lu, A.H.; Lu, X.Y.; Ren, Z.P.; et al. New advances in environmental mineralogy of natural oxides and hydroxides of iron and manganese. Earth Science Frontiers. 2000, 7, 473–483. [Google Scholar]
- Sunda, W.G.; Kieber, D.J. Oxidation of humic substances by manganese oxides yields low-molecular-weight organic substrates. Nature. 1994, 367, 62–64. [Google Scholar] [CrossRef]
- Chen, Z.; Kim, K.W.; Zhu, Y.G.; et al. Adsorption (AsIII, V) and oxidation (AsIII) of arsenic by pedogenic Fe–Mn nodules. Geoderma. 2006, 136, 566–572. [Google Scholar] [CrossRef]
- Arico, A.S.; Bruce, P.; Scrosati, B.; et al. Nanostructured materials for advanced energy conversion and storage devices. Nature materials. 2005, 4, 366–377. [Google Scholar] [CrossRef]
- Hall, P.J.; Mirzaeian, M.; Fletcher, S. I.; Sillars, F.B.; Rennie, A.J.R.; Shitta-Bey, G.O.; Wilson, G.; Cruden, A; Carter, R. Energy storage in electrochemical capacitors: designing functional materials to improve performance. Energy Environ. Sci. 2010, 3, 1238–1251. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. Journal of power sources. 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Warheit, D.B.; Webb, T.R.; Reed, K.L.; et al. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: differential responses related to surface properties. Toxicology. 2007, 230, 90–104. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, X.; Zhou, Z.; et al. Prenatal exposure to nanoparticulate titanium dioxide enhances depressive-like behaviors in adult rats. Chemosphere. 2014, 96, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Cao, J. Metal-containing nanoparticles derived from concealed metal deposits: an important source of toxic nanoparticles in aquatic environments. Chemosphere. 2019, 224, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.C.; Nowack, B. Exposure modeling of engineered nanoparticles in the environment. Environmental science & technology. 2008, 42, 4447–4453. [Google Scholar]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for differentregions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef]
- Gottschalk, F.; Scholz, R.W.; Nowack, B. Probabilistic material flow modeling for assessing the environmental exposure to compounds: Methodology and an application to engineered nano-TiO2 particles. Environmental Modelling & Software. 2010, 25, 320–332. [Google Scholar]
- Gottschalk, F.; Sun, T.Y. Nowack B. Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environmental pollution. 2013, 181, 287–300. [Google Scholar] [CrossRef]
- Arvidsson, R.; Molander, S.; Sandén, B.A. Particle flow analysis: exploring potential use phase emissions of titanium dioxide nanoparticles from sunscreen, paint, and cement. Journal of Industrial Ecology. 2012, 16, 343–351. [Google Scholar] [CrossRef]
- Shao, G.Y.; Li, C.S.; Liu, R.; et al. Silicified Microorganisms and Microorganism-Like Particlesin the Groundwater of an Abandoned Coal Mine. Mine Water and the Environment. 2023, 1–11. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).