Preprint
Article
Idiopathic Nephrotic Syndrome in Children in Chad: Epidemiology and Clinical Outcome
Altmetrics
Downloads
135
Views
59
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
30 August 2023
Posted:
04 September 2023
You are already at the latest version
Alerts
Abstract
Introduction: Nephrotic syndrome (NS) remains the most frequent mode of presentation of glo-merular diseases in children; in addition, the NS is primitive, i.e., idiopathic in 90% of cases with an average age of onset between 2 and 10 years. The objective of our study was to describe the char-acteristics and outcome of NS in children from 3 major hospitals from one of the poorest countries worldwide (Chad).
Patients and Methods: Observational, cross-sectional, descriptive and multicenter study which took place over a period of 36 months (January 1,2019-December 31,2021) carried out in 3 hospitals in N’Djamena (Chad). All children aged 1-15 years presenting with NS were included.
Results: Out of 16,776 children hospitalized or followed-up in outpatient clinics, 24 cases of NS were identified, giving a prevalence of 0.14%. The median age at presentation was 6.16(1-10) years. Nineteen children were male (sex ratio 3.8). There were 8 impure NS (33.3%). Edema was present in all patients, oliguria in 29.16%(n=7), and arterial hypertension in 20.83%(n=5) of cases. Mean proteinuria, albuminemia and total proteins were 2.86g/L, 19.13g/L and 30.41g/L respectively. Median serum creatinine was 87.3 µmol/L(75-1375µmol/L); three patients had acute renal failure on admission. Four patients had secondary NS. All patients (n=20) with idiopathic NS had received corticosteroid therapy; steroid-sensitivity was found in 90% of patients. Those who were not ster-oid-sensitive or relapsed underwent kidney biopsy (n=7); the most represented histological lesion was focal segmental glomerulosclerosis (FSGS; n=4), followed by minimal change disease (n=2), and membranoproliferative glomerulonephritis (n=1). The median length of hospitalization stay was 10.67 (5-27) days. None of the patients with idiopathic NS died. At last follow-up sixteen patients (80%) achieved long-term complete remission with normal renal function; however, 4 had subse-quent relapses. 16 patients. One patient with secondary NS died.
Conclusion: In Chad childhood idiopathic nephrotic syndrome involves mostly young male; steroid sensitivity is as high as 95% and in the long-term 80% of the patients are in remission with normal renal function.
Keywords:
Subject: Medicine and Pharmacology - Urology and Nephrology
1. Introduction
Postinfectious glomerulonephritis, Henoch-Schönlein purpura nephritis, and minimal change disease (MCD) remain the most common causes of glomerular disease in children and can be diagnosed clinically without need for biopsy [1]. IgA nephropathy is the most common pediatric glomerular disease diagnosed by kidney biopsy and is considered the most common chronic glomerulopathy worldwide [1]. Childhood nephrotic syndromes are most commonly caused by one of two idiopathic diseases: minimal-change nephrotic syndrome and focal segmental glomerulosclerosis (FSGS) [2]. Membranous nephropathy, is rare in children; other causes of isolated nephrotic syndrome can be subdivided into two major categories: rare genetic disorders, and secondary diseases associated with drugs, infections, or neoplasia. Nephrotic syndrome (NS) is defined by nephrotic-range proteinuria (≥40 mg/m2/hour or urine protein/creatinine ratio ≥200 mg/mL or 3+ protein on urine dipstick), hypoalbuminemia (<25 g/L) and edema. The incidence of idiopathic nephrotic syndrome is 1·15-16·9 per 100 000 children, varying by ethnicity and region. The cause remains unknown but the pathogenesis of idiopathic NS is thought to involve immune dysregulation, systemic circulating factors, or inherited structural abnormalities of the podocyte. Genetic risk is more commonly described among children with steroid-resistant disease [3]. The mainstay of therapy is prednisone for the vast majority of patients who are steroid responsive, i.e., steroid-sensitive [4,5]; however, the disease can run a frequently relapsing course, necessitating the need for alternative immunosuppressive agents, e.g., cyclosporine, tacrolimus, cyclophosphamide, rituximab, … [6,7]. Infection and venous thromboembolism are the main complications of NS with also increased risk of acute kidney injury. Prognosis in terms of long-term kidney outcome overall is excellent for steroid-responsive disease, and steroid resistance is an important determinant of future risk of chronic (CKD) or end-stage kidney disease (ESKD).
A large single center retrospective study has evaluated the outcome of 372 children diagnosed with idiopathic NS (FSGS: 57%; MCD 20.6%; diffuse mesangial proliferation: 21.9%) who had a follow-up of at least 5-years after diagnosis [8]. Two hundred ninety-nine of the patients (80.4%) were steroid responsive and 73 (19.6%) were not. Steroid sensitivity was higher in patients with MCD and under the age of five years. Resistance to steroids was higher in children with FSGS. Complete remission was achieved in 96% of patients who were sensitive to steroids and in 46.6% who were resistant. Fifteen percent of patients who were steroid resistant developed chronic kidney disease. From the PodoNet Registry, 1354 children with steroid-resistant NS were followed-up; of these 612 had documented responsiveness to intensified immunosuppression (IIS); it was found that responsiveness to initial IIS and detection of a hereditary podocytopathy were prognostic indicators of favorable and poor long-term outcome, respectively [9].
In tropical Africa, it has recently been reported that children with NS now survive better than before, reflecting improved access to healthcare and transition to a clinical pattern favoring idiopathic NS and increased sensitivity to corticosteroids [10]. However, in sub-Saharan Africa, glomerular nephrotic syndrome, is the leading cause of chronic kidney disease and end-stage kidney disease in children. Despite limited reports from Nigeria and Sudan, the overall incidence of NS in Africa is unknown [11]. In Chad, a study carried out in 2012 on nephrotic syndrome in children in the pediatric department of the Mother and Child Hospital of N’Djamena reported a prevalence of 1.36% [12]. Ten years after that study, we decided to conduct a multicenter study across the 3 main hospitals in N’Djamena (Chad) with the objective to describe the characteristics and outcome of pediatric idiopathic nephrotic syndrome in one of the poorest countries in the world (https://hdr.undp.org/data-center/human-development-index#/indicies/HDI).
2. Patients and Methods
The study was carried out in 3 hospitals in N’Djamena (Chad), i.e., the pediatric department of the “Mother and Child” Hospital and University Center, the “Chad-China Friendship” Hospital and the Nephrology department of the “Renaissance” Hospital and University Center. This was an observational, cross-sectional, descriptive and multicenter study which took place over a period of 36 months from January 1, 2019 to December 31, 2021. From the medical charts of 16,776 children either hospitalized or followed-up in outpatient clinics we identified 24 children with NS aged between 1 and 10 years. The NS was defined by proteinuria greater than 50 mg/kg/d, hypoalbuminemia less than 30 g/L and hypoprotidemia less than 60 g/L [13]. The NS is impure when it is associated with at least one of these elements which are hematuria, arterial hypertension, non-selective proteinuria (albuminuria < 85%) with or without (acute) renal failure. Those with idiopathic NS were place on steroid therapy, i.e., prednisone 2mg/kg/d for 4 weeks, then a taper of 2 mg every 2 days for 2 months til 60 mg/d, then 45 mg/d for 2 weeks, then 30 mg/d for 2 weeks and then 15 mg/d for the last 2 weeks.
Corticosensitivity is defined by complete remission within 4 weeks of administration of prednisone/prednisolone at the standard dose (60 mg/m2/day or 2 mg/kg/day, maximum 60 mg/day). Children who do not show complete remission of proteinuria following 4–8 weeks treatment with corticosteroids are considered to have steroid-resistant nephrotic syndrome. Steroid dependence is defined by a relapse during the decrease of steroid therapy or within less than three months after stopping it.
Data were gathered and collected from patient records using a pre-established questionnaire. Data were entered using Excel 2016 software and analyzed using Sphinx V5 software. The results were given in terms of frequency and percentage for the qualitative variables and by calculating the means with their standard deviation for the quantitative variables or median (ranges) where appropriate. The analytical study was made with cross tables. The means with their standard deviation and the percentages were compared using the Student test and the Chi 2 test, according to their conditions of applicability, with a significance threshold if the “p” < 0.05.
3. Results
Out of a total of 16,776 children hospitalized in pediatrics or followed-up in the outpatient clinic during the study period, 24 cases of nephrotic syndrome were recorded, i.e., a prevalence of 0.14%. The median age at diagnosis was 6.16 (1-10) years. Nineteen children (79%) were male, i.e., a sex ratio of 3.8. Sickle cell disease background (heterozygozy) was found in 12.52% (n=3) of cases. The average patient weight was 22.6 kg (11-42) and the average height was 113.21 cm (131-145). Arterial hypertension was noted in 20.9% (n=5). It was found that 91.7% of the patients presented with an alteration of the general status. The average diuresis was 1983.3 ml/24h (2001-3500) with oliguria found in 33.3% (n=7). All the patients had lower limb edema and proteinuria on the urine dipstick of more than 2 ++ (Table 1). Edemas were associated with pericarditis in 54.1% and anasarca in 28.1% of cases. Regarding biological parameters, median serum creatinine was 87.3 µmol/L (75-1375.1 µmol/L); it was abnormal in 3 children. The mean proteinuria was 2.86 g/d (2.17-3.90). Protidemia was less than 40 g/L in 20 patients (83.3%).
Fifty percent of the patients presented with an albuminemia between 10 and 20 g/L; in one patient, serum albumin was < 10 g/L; it was between 20 and 30 g/L in 11 patients (55%). Seven patients (29.1%) had a corrected calcemia < 88 mg/L (i.e., hypocalcemia). Prerenal acute renal failure was noted in 2 patients (8.3%). In the Addis count, 12.5% of patients (n=3) had leukocyturia and 4.1% (n=1) hematuria. On admission, 4 children (16.6%) had developed an Escherichia coli urinary tract infection identified on cytobacteriological examination of urine. Serum complement testing was only perfomed in a single patient, i.e., a 7-year girl presenting with NS, hematuria and hypertension: it was found low levels of C4 and CH50 fractions ; furthermore there were the presence of anti-factor H and factor I autoantibodies. Eight children (33.3%) presented kidneys of increased size on renal ultrasound. The NS was impure in 6 patients (25%). A kidney biopsy was performed in 7 patients (29.1%) whose indication was the persistence of impure nephrotic syndrome (hematuria and hypertension) in the setting of steroid-dependency in 2 patients (8.3%), steroid-resistance in 2 children (8.3%) and frequent relapses in 3 cases (12.5%). The most represented histological lesion (Figure 1) was focal segmental glomerulosclerosis (FSGS) in 4 children (16.6%), 2 cases (8.2%) of minimal change disease (MCD) and one case (4.1%) of membranoproliferative glomerulonephritis (MPGN). In 4 children (16.6%) the nephrotic syndrome was not idiopathic, i.e., 2 cases of sickle cell nephropathy, one case of atypical hemolytic uremic syndrome and one case of Burkitt’s lymphoma. Regarding therapy, the 20 patients with idiopathic NS had received corticosteroid therapy based on prednisone (see above). The average length of hospitalization was 10.67 days with extremes ranging from 5 to 27 days. The median duration of steroid therapy was 114 days (62-155).
Table 2 and Figure 2 summarized patients’outcome. Eighteen patients underwent complete remission (90%); however, two patients had 1-2 relapses per year with normal renal function at last follow-up, and 2 patients had more than 3 relapses per year and developed CKD stage 3a. Finally, long-term remission was achieved with normal renal function in 16 patients (80% of the cohort). Only one patient died; it was the one with secondary NS due to atypical hemolytic and uremic syndrome. Death occurred in a setting of rapidly progressive acute renal failure and sepsis.
4. Discussion
There are very few studies addressing idiopathic nephrotic syndrome in children in sub-Saharan countries. Ours is one of the largest and demonstrate steroid sensitivity in idiopathic NS in 90% of cases with long-term remission in 80% of them.
In 2014 a study reviewed the documents of all children presenting with renal diseases between January 2010 and December 2012 in two major pediatric hospitals in the Niger Delta region of Nigeria, i.e., university hospital in Oghara and GN Children’s Clinic in Warri. They found that renal diseases (n=110) accounted for 1.6% of all admissions during the same period. Out of 110 patients there were 73 males (66.3%), and about half the patients were aged 5-10 years. The commonest presentations were nephrotic syndrome (30%), acute glomerulonephritis (18.2%), urinary tract infection (16.3%), acute kidney injury (10.9%), chronic kidney diseases (7.3%) and obstructive uropathy (7.3%). Regarding the outcome of renal diseases, 80% of the patients achieved full recovery, and 14.6% died [14]. Conversely, in the same country Lapedo et al. reported that kidney diseases accounted for 8.9% of pediatric admissions with a prevalence of 22.3 admissions per 1000 child-admissions per year. Nephrotic syndrome, acute kidney injury and nephroblastoma accounted for almost 70% of admissions. The overall mortality was 14.4% with acute kidney injury accounting for 36% of this [15].
In 2016 Muoneke et al. reported on a single center study from Nigeria. Over 3-year period 1780 children were admitted, of which only 4.4% (79/1780) had renal disorders. They found that nephrotic syndrome was the most common, i.e., present in 32.9% (26/79). The association between treatment mode and outcome of the treatment was statistically significant (P = 0.03) [16].
Also in 2016, Kebele Mola et al. reported on a single center study from Ethiopia. Out of 14,521 pediatric ward admissions in the study period, kidney diseases accounted for 473 admissions in 381 children, i.e., 3.3% of all admissions. The three most common renal diseases observed were congenital anomalies of the kidney and urinary tract (CAKUT) seen in 127 children (26.8%), followed by nephrotic syndrome in 80 children (16.9%) and acute glomerulonephritis in 58 children (12.2%). Out of 381 children 207 (54.3%) recovered normal renal function, 20 (5.2%) remained with proteinuria, 13(3.4%) progressed to chronic kidney disease and 11(2.9%) died. In addition, sixty-one nephrotic children (76.3%) achieved remission, but 17 children (21.3%) remained with proteinuria; one steroid-resistant NS child died of end stage renal disease [17].
From these studies it is concluded that in sub-saharan countries the prevalence of renal disorders in children admitted in pediatric departments ranges from 1.6 to 8.9%. However, we do not have yet any data regarding the prevalence/incidence of idiopathic nephrotic syndrome in sub-saharan countries. It is recognized that in most of North Africa, as well as among White and Indian populations in South Africa, the epidemiology and treatment of childhood nephrotic syndrome closely resembles that of European and North American populations. However, historically, secondary causes of nephrotic syndrome (eg, quartan malaria nephropathy and hepatitis B-associated nephropathy) were predominant among Blacks in Africa. Meanwhile, over time, the proportion of secondary cases has decreased, along with rates of steroid resistance. However, focal segmental glomerulosclerosis increasingly has been reported among patients with steroid resistance [18]. Recently, a bicentric study from Lagos (Nigeria) has shown that among 209 Black children, the proportion with steroid sensitive NS is comparable to proportions described in children of Asian and European descent. Furthermore, they observed that children with steroid sensitive NS (85.9% of the study cohort) had lower serum creatinine and higher glomerular filtration rate than those with steroid resistant NS [19]. In addition, among those aged 0-5 years, 92.6% were steroid sensitive NS compared with 69.2% in those aged 11-17 years at the time of diagnosis. In addition, the proportion of children with steroid sensitive NS increased from 73.8% between year 2010 and 2012 to 88.4% afterwards [19]. In a previous study the same group reported on a retrospective study (from January 2008 to April 2013) in which 108 children (median age: 5.9 years, peak: 1-2 years) with NS were included; 90.2% of whom had idiopathic nephrotic syndrome [20]. Nephrotic syndrome was diagnosed based on the following: 24-hour urine protein > 40 mg/m2/hr or spot urine protein: creatinine ratio > 200 mg/mmol, hypoalbuminemia (serum albumin < 25 g/L), generalized oedema, and hypercholesterolemia (serum cholesterol > 5.2 mmol/L). Steroid sensitivity was 82.8% among children with idiopathic nephrotic syndrome but 75.9% overall. Median time to remission was 7 days. Median age was significantly lower in steroid sensitive compared with resistant patients. Finally, the predominant histologic finding in resistant cases was focal segmental glomerulosclerosis (53.3%). No cases of quartan malaria nephropathy or hepatitis B virus nephropathy were diagnosed. Overall mortality was 6.5% [20].
In our study we observed a male prevalence (79% of the cohort, sex ratio 3.8). Other studies have also reported a male prevalence, e.g., male-female ratio of 1.4 In Nigeria [19] or in Ivory Coast [21] and Algeria [22].
Renal type edema was present in all our patients (100%). Ndongo et al. [21] and Mabiala-Babela et al. [22] found the same results in their studies. The presence of edema is a major and almost constant clinical sign of childhood nephrotic syndrome and is the main reason for consultation.
With regards to idiopathic NS we observed that nearly all the patients (90%) were steroid sensitive. This is higher than rates reported in Sudan (66%) (25), Ethiopia (73.3%) (17), Senegal (77%) (26), or in Nigeria ranging from 82.8 to 85.9% (19, 20). However, at last follow-up we found that only 80% of the patients were in remission with normal renal function. In one study from Ethiopia in the long-term a higher number of patients (21.3%) remained with high rate of proteinuria (17).
At last follow-up we observed that 80% of patients with idiopathic NS were still in remission. Indeed, the others were either steroid dependent or resistant. This is the reason why 7 out of our 20 patients have had a kidney biopsy. Indeed, in 6 cases we found either FSGS lesions (n=4) or MCD lesions (n=2). Indeed, Ladapo et al. have found in Nigerian children with steroid resistant NS that in most of the cases the histological lesions were FSGS (20). Two studies from Algeria and Sudan found FSGS lesions in 57.4% (27) and 44% (25) of cases respectively. It is recognized that those presenting with FSGS lesions might evolve to CKD; for example, children with FSGS who do not achieve partial or complete remission have a 50% risk of progression to end-stage renal disease within 5 years whereas those who enter complete remission have a 5-year kidney survival rate of 90% (28).
The strength of our study is that across three main hospitals from Chad we were able to retrieve all the cases of idiopathic NS over a 3-year recent period. In addition, they were all managed the same way. Moreover, for those who were steroid dependent or resistant we always performed a kidney biopsy. The limitation of the study is that we were limited to perform as many lab tests as we would have wished due to financial constraints.
5. Conclusions
The prevalence of childhood idiopathic nephrotic syndrome is poorly addressed in sub-saharan countries. In this cohort of 20 children with idiopathic NS from Chad steroid sensitivity was as high as 90% and in the long-term 80% of the patients were in remission with normal renal function.
Author Contributions
MAHAMAT ABDERRAMAN Guillaume: recruiting, managing patients, data collection, data management, statistical analysis, paper writing, DJIDITA HAGRE Youssouf: recruiting and managing patients, MAHAMAT Hissein Ali: recruiting and managing patients, CHARFADINE Senoussi: recruiting and managing patients, AMNE Ali Sakine: recruiting and managing patients, KHADIDJA Adoum Attimer: recruiting and managing patients, ROSTAING Lionel: paper writing and paper editing.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study due to the fact that all the children were managed as per international guidelines, i.e., standard of care.
Informed Consent Statement
Informed consent was obtained from all participants under age 16, from a parent and/or legal guardian. There were NO experimental protocols, i.e., all the children were looked after according to international guidelines regarding the management of idiopathic nephrotic syndrome.
Data Availability Statement
Data are available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
NS: nephrotic syndrome; FSGS: focal segmental glomerulosclerosis; MCD, minimal change disease; CKD, chronic kidney disease; ESKD, end-stage kidney disease.
References
- Wenderfer, S.E.; Gaut, J.P. Glomerular Diseases in Children. Adv Chronic Kidney Dis 2017, 24, 364–371. [Google Scholar] [CrossRef]
- Eddy, A.A. Symons JM: Nephrotic syndrome in childhood. Lancet 2003, 362, 629–639. [Google Scholar] [CrossRef]
- Noone, D.G.; Iijima, K.; Parekh, R. Idiopathic nephrotic syndrome in children. Lancet 2018, 392, 61–74. [Google Scholar] [CrossRef]
- Vivarelli, M.; Emma, F. How I Treat Steroid-Sensitive Nephrotic Syndrome in Children. Clin J Am Soc Nephrol 2022, 17, 1685–1687. [Google Scholar] [CrossRef]
- Horinouchi, T.; Nozu, K.; Iijima, K. An updated view of the pathogenesis of steroid-sensitive nephrotic syndrome. Pediatr Nephrol 2022, 37, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.D.; Willis, N.S.; Craig, J.C.; Hodson, E.M. Interventions for idiopathic steroid-resistant nephrotic syndrome in children. Cochrane Database Syst Rev 2019, 2019, CD003594. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Khan, S.; Davalos, C.; Avanthika, C.; Jhaveri, S.; Babu, A.; et al. Management of Steroid-Resistant Nephrotic Syndrome in Children. Cureus 2021, 13, e19363. [Google Scholar] [CrossRef] [PubMed]
- Özlü, S.G.; Demircin, G.; Tökmeci, N.; Yılmaz, A.C.; Aydoğ, O.; Bülbül, M.; et al. Long-term prognosis of idiopathic nephrotic syndrome in children. Ren Fail 2015, 37, 672–677. [Google Scholar] [CrossRef]
- Trautmann, A.; Schnaidt, S.; Lipska-Ziętkiewicz, B.S.; et al. PodoNet Consortium. Long-Term Outcome of Steroid-Resistant Nephrotic Syndrome in Children. J Am Soc Nephrol 2017, 28, 3055–3065. [Google Scholar] [CrossRef]
- Olowu, W.A.; Ademola, A.; Ajite, A.B.; Saad, Y. M. Childhood nephrotic syndrome in tropical Africa: then and now. Paediatrics and International Child Health, 2017, 37, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Esezobor, C.; Ademola, A.D.; Adetunji, A.E.; Anigilaje, EA.; Batte, A.; Jiya-Bello, F.N.; et al. for Human Hereditary and Health in Africa Kidney Disease Research Network. Management of idiopathic childhood nephrotic syndrome in sub-Saharan Africa: Ibadan consensus statement. Kidney Int 2021, 99, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Mahoi, K. « Le Syndrome néphrotique de l’enfant: Aspect clinique et évolutif dans le service de pédiatrie de N’Djamena », Thèse de doctorat en médecine, Faculté des Sciences de la Santé Humaine de N’Djamena, (2012).
- Waugh, A.; Grant, A. editors. Anatomie & physiologie normales et pathologiques. Ross Wilson. Traduction 12e édition originale. Paris: Elsevier Masson ;2014 Apr 28. p 522.
- McGil Ugwu, G.I.; Nwajei, G.; Chinemelu, U. Pattern of Renal Diseases among Children in The Niger Delta Region, Nigeria. Arab J Nephrol Transplant 2014, 7, 49–50. [Google Scholar]
- Ladapo, T.A.; Esezobor, C.I.; Lesi, F.E. Pediatric kidney diseases in an African country: prevalence, spectrum and outcome. Saudi J Kidney Dis Transpl 2014, 25, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Muoneke, V.U.; Una, A.F.; Eke, C.B.; Anyanwu, O.U. The Burden and Outcome of Pediatric Renal Admissions at the Federal Teaching Hospital Abakaliki: A 3-year Review (2011-2013). Ann Med Health Sci Res 2016, 6, 243–250. [Google Scholar] [CrossRef]
- Kebede, M.; Damte, S. Pattern and outcome of renal diseases in hospitalized children in Tikur Anbessa teaching hospital, Addis-Ababa, Ethiopia. Ethiop Med J 2016, 54, 117–123. [Google Scholar]
- Ademola, A.D.; Asinobi, A.O.; Alao, M.A.; Olowu, W.A. Childhood Nephrotic Syndrome in Africa: Epidemiology, Treatment Trends, and Outcomes. Semin Nephrol 2022, 42, 151311. [Google Scholar] [CrossRef]
- Esezobor, C.I.; Solarin, A.U.; Gbadegesin, R. Changing epidemiology of nephrotic syndrome in Nigerian children: A cross-sectional study. PLoS One 2020, 15, e0239300. [Google Scholar] [CrossRef]
- Ladapo, T.A.; Esezobor, C.I.; Lesi, F.E. High Steroid Sensitivity among Children with Nephrotic Syndrome in Southwestern Nigeria. Int J Nephrol 2014, 2014, 350640. [Google Scholar] [CrossRef]
- Coulibaly, P.; Adonis Koffy, L.; Diarrassouba, G.; Timité Konan, M. La croissance staturale des enfants ayant un syndrome néphrotique cortico-sensible en Côte d’Ivoire. Néphrologie & Thérapeutique 2015, 11, 160–163. [Google Scholar]
- Oukrif, L.; Toudji, L.; Bekkat, D.F.; Zemiri, F.; Boukhil, K.S.; Messadi, W.; et al. Syndrome néphrotique idiopathique chez 408 enfants, Pédiatrie B, CHU Isaad Hassani de Beni-Messous, Alger, Algérie. Néphrologie & Thérapeutique 2017, 344–388. [Google Scholar]
- Ndongo, A.A.; Thiongane, A.; Keita, Y.; Boiro, D.; Basse, I.; Seck, N.; et al. Les particularités du syndrome néphrotique de l’enfant au Sénégal. ISSN 2017, 2424–7243. [Google Scholar]
- Mabiala-Babela, J.R.; Pecko, J.F.; Loumingou, R.; Diatoulou, F.B.; Senga, P. Le syndrome néphrotique chez l’adolescent congolais: aspects cliniques et histologiques. Arch Pediatr 2006, 13, 88–90. [Google Scholar] [CrossRef]
- Tigani, M.; Ali, M.A.; Hanna, F.K.M.; Mohamed, B.A.; Salwa, O.M.; Rachid, A.L. Childhood Idiopathic steroid—resistant nephritic syndrome at the single center in Kharthoum. Saudi J Kidney Dis Transpl 2017, 28, 851–859. [Google Scholar]
- Keita, Y.; Lemrabott, A.T.; Sylla, A.; Niang, B.; Ka, E.H.F.; Dial, C.M.; et al. Le syndrome néphrotique idiopathique (SNI) de l’enfant à Dakar: à propos de 40 cas. Pan Afr Med J 2017, 26, 161. [Google Scholar] [CrossRef] [PubMed]
- Azouaou, L.; Oukrif, L.; Bensnoussi, A.; Nemar, S.; Boukhedouma, L.; Chabani, L.; et al. Syndrome néphrotique idiopathique corticorésistant de l’enfant à Alger. Néphrologie & Thérapeutique 2015, 11, 355–356. [Google Scholar] [CrossRef]
- Sethna, C.B.; Gipson, D.S. Treatment of FSGS in Children. Adv Chronic Kidney Dis 2014, 21, 194–199. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Findings of renal pathology in 7 patients who have had a kidney biopsy. Abbreviations: FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; MPGN, membranoproliferative glomerulonephritis.
Figure 1.
Findings of renal pathology in 7 patients who have had a kidney biopsy. Abbreviations: FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; MPGN, membranoproliferative glomerulonephritis.

Figure 2.
Outcome of the patients following idiopathic NS treatment.
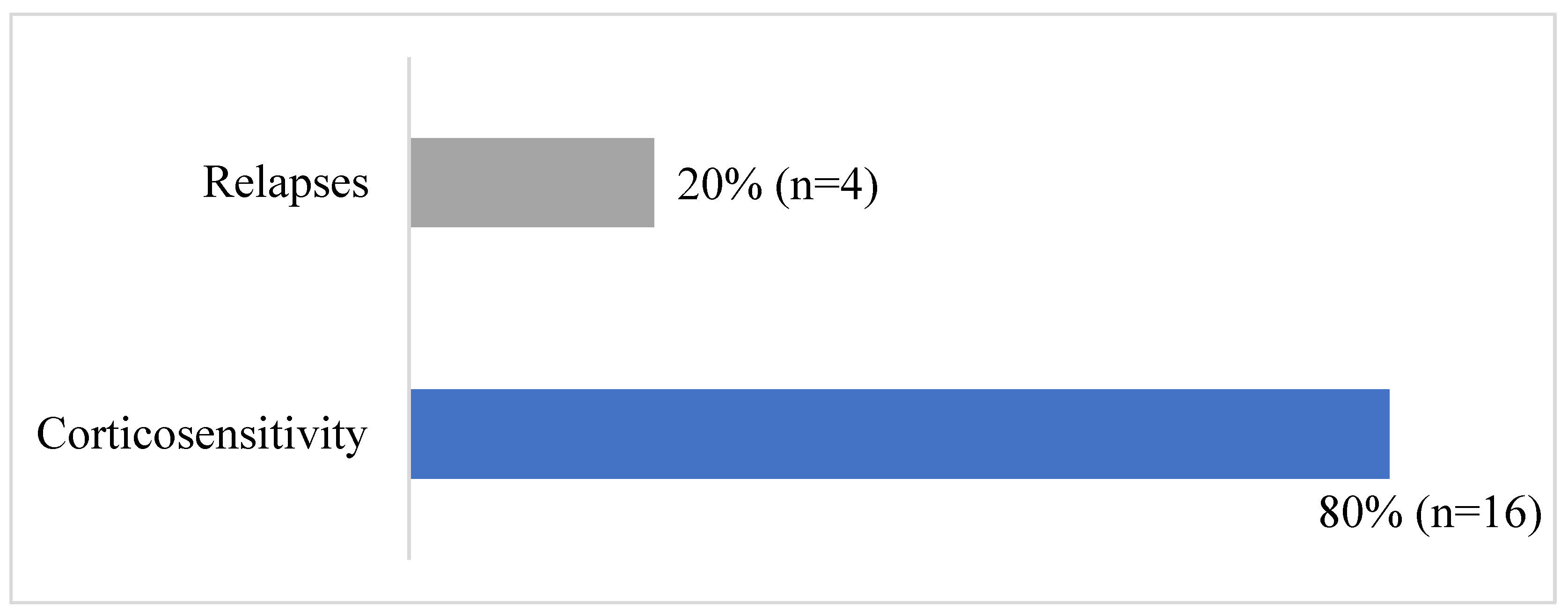
Table 1.
Socio-demographic and clinical characteristics at diagnosis.
| Variable | N=24 patients | Frequency (%) |
|---|---|---|
| Gender | ||
| Female | 5 | 21 |
| Male | 19 | 79 |
| Background | ||
| Sickle cell disease | 3 | 12.5 |
| Clinic | ||
| Proteinuria | 24 | 100 |
| Hematuria | 6 | 25.0 |
| Leukocyturia | 3 | 12.5 |
| Lower limb edema | 24 | 100 |
| Pericardial effusion | 13 | 54.17 |
| Anasarca | 7 | 29.17 |
| Ascitis | 9 | 37.5 |
| Pleural effusion | 3 | 12.5 |
| Infiltration of the external genitals | 4 | 16.7 |
| Oliguria | 8 | 33.3 |
| Hypertension | 5 | 20.8 |
Table 2.
Patients outcome following idiopathic NS treatment (20 patients).
| Number of patients (n) | Frequency (%) | |
|---|---|---|
| Long-term remission with normal kidney function | 16 | 80 |
| 1-2 relapses per year with regular monitoring in nephrology and normal renal function | 2 | 10 |
| > 3 relapses per year with evolution towards chronicity (eGFR< 60 mL/min) | 2 | 10 |
| Total | 20 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







