Submitted:
01 September 2023
Posted:
04 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
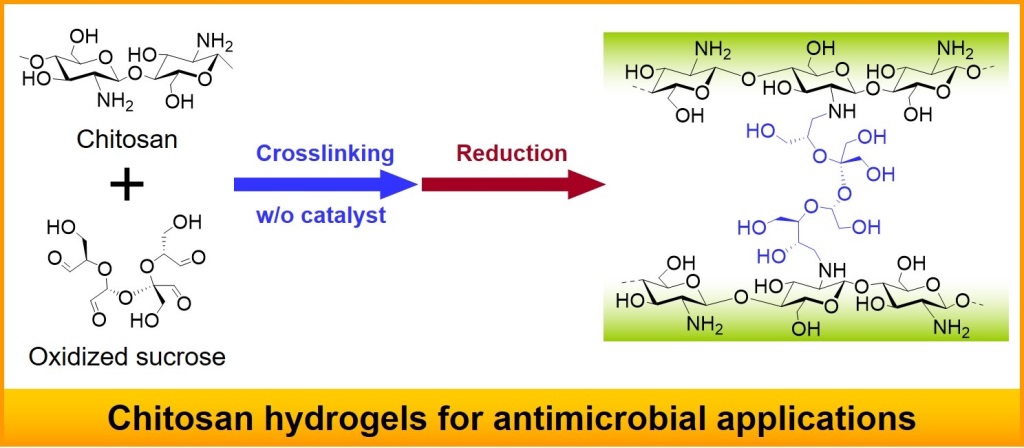
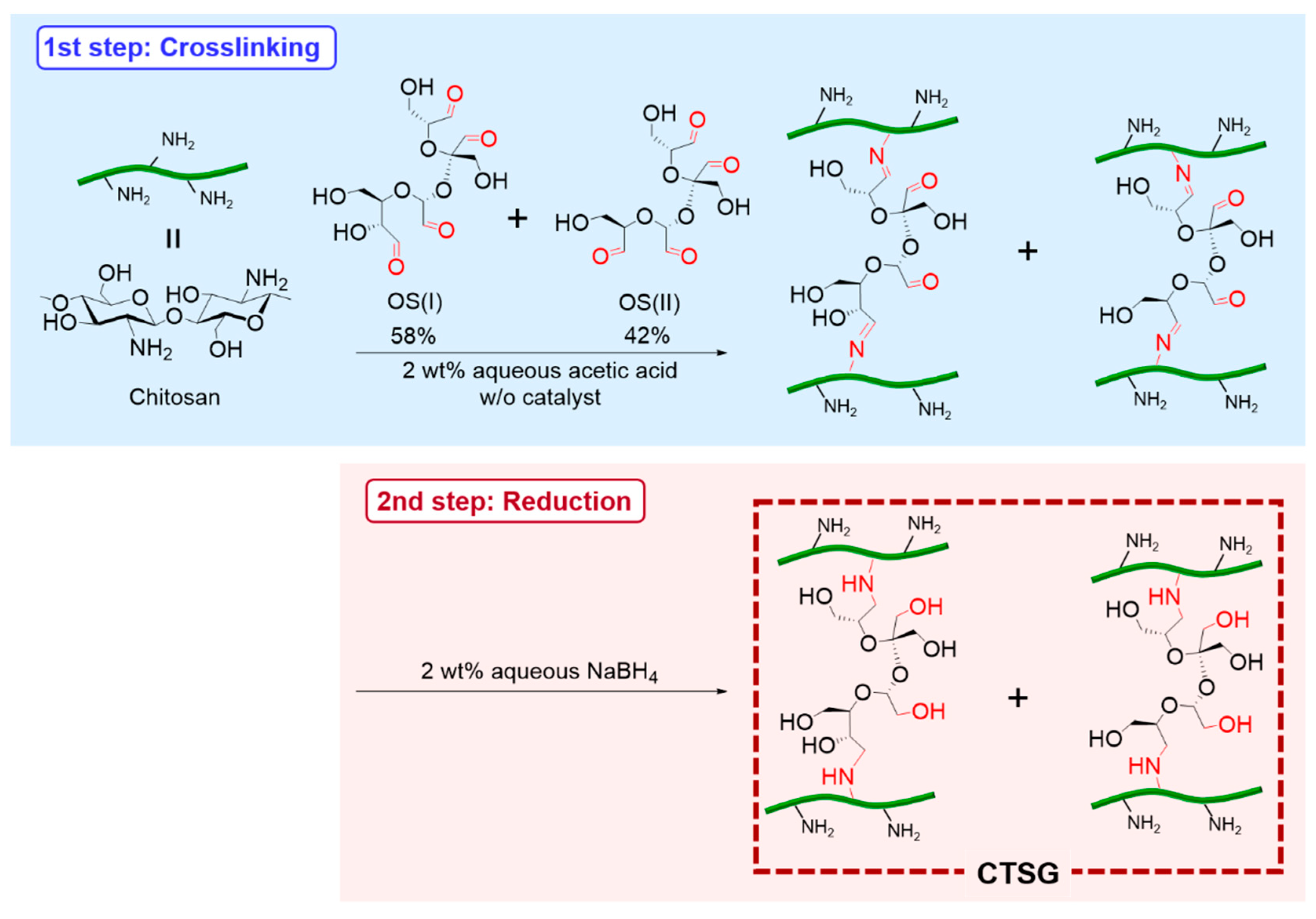
2.1. Preparation of CTSGs
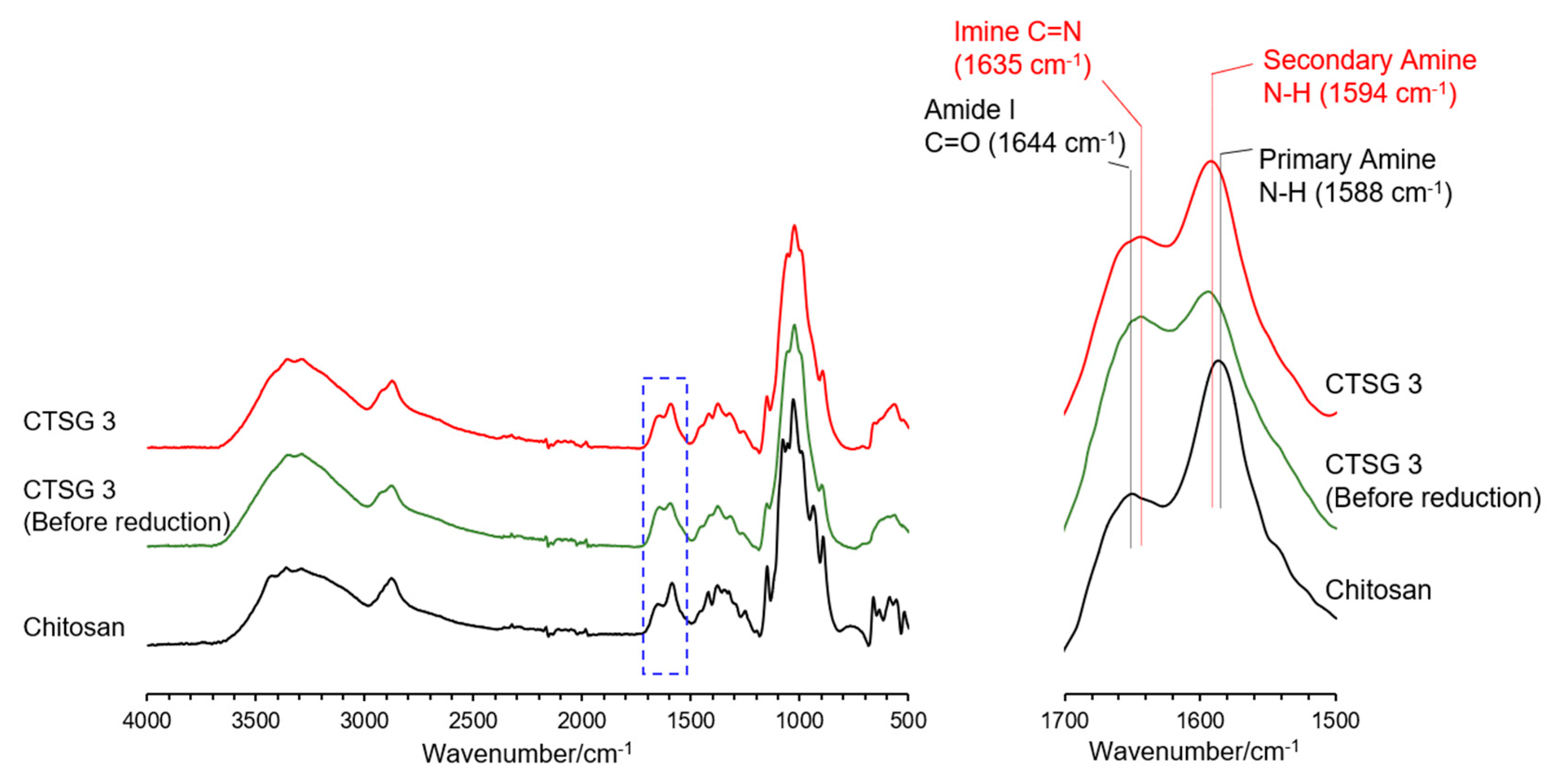
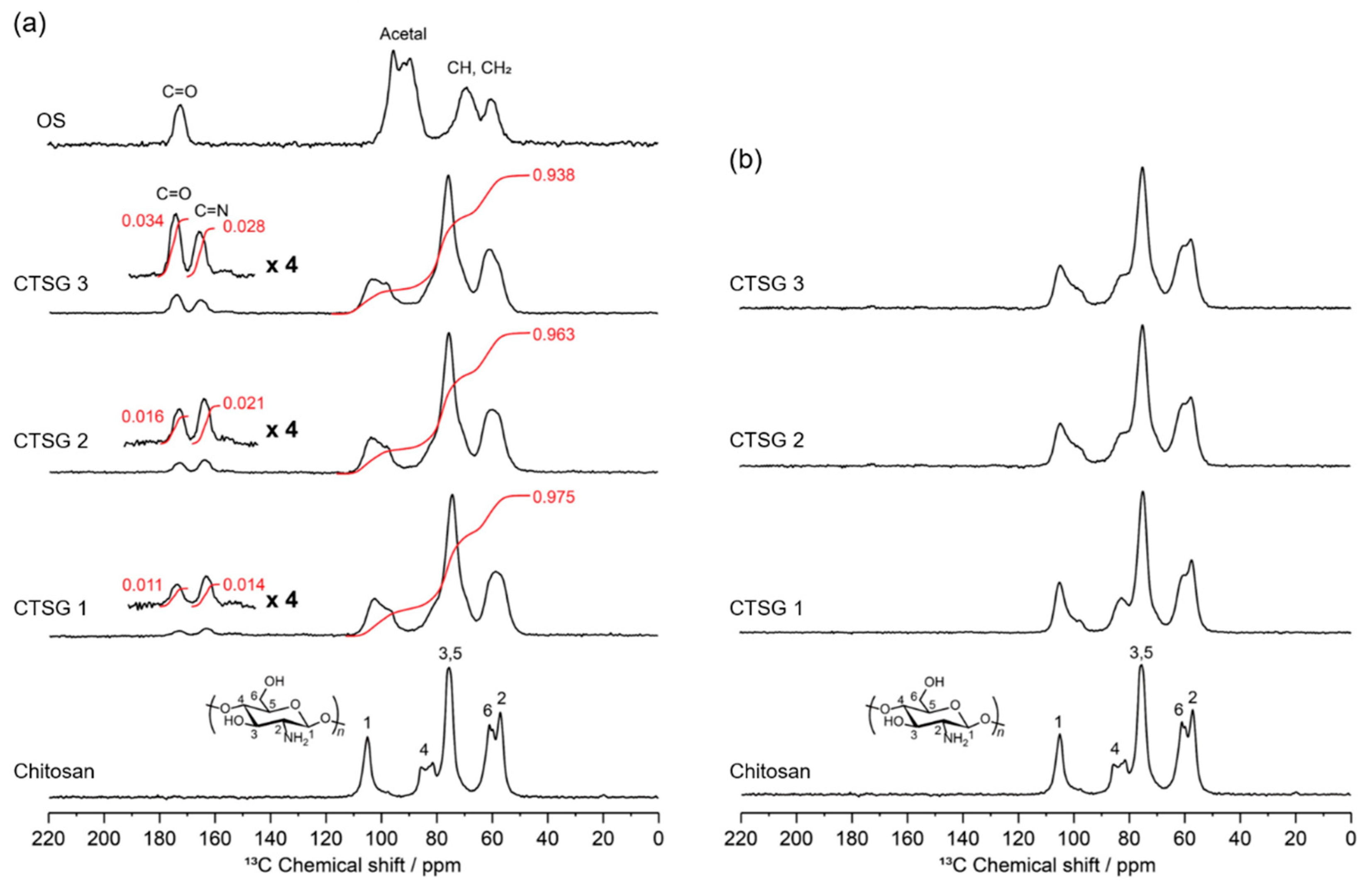
2.2. Structural Characterizations of CTSGs
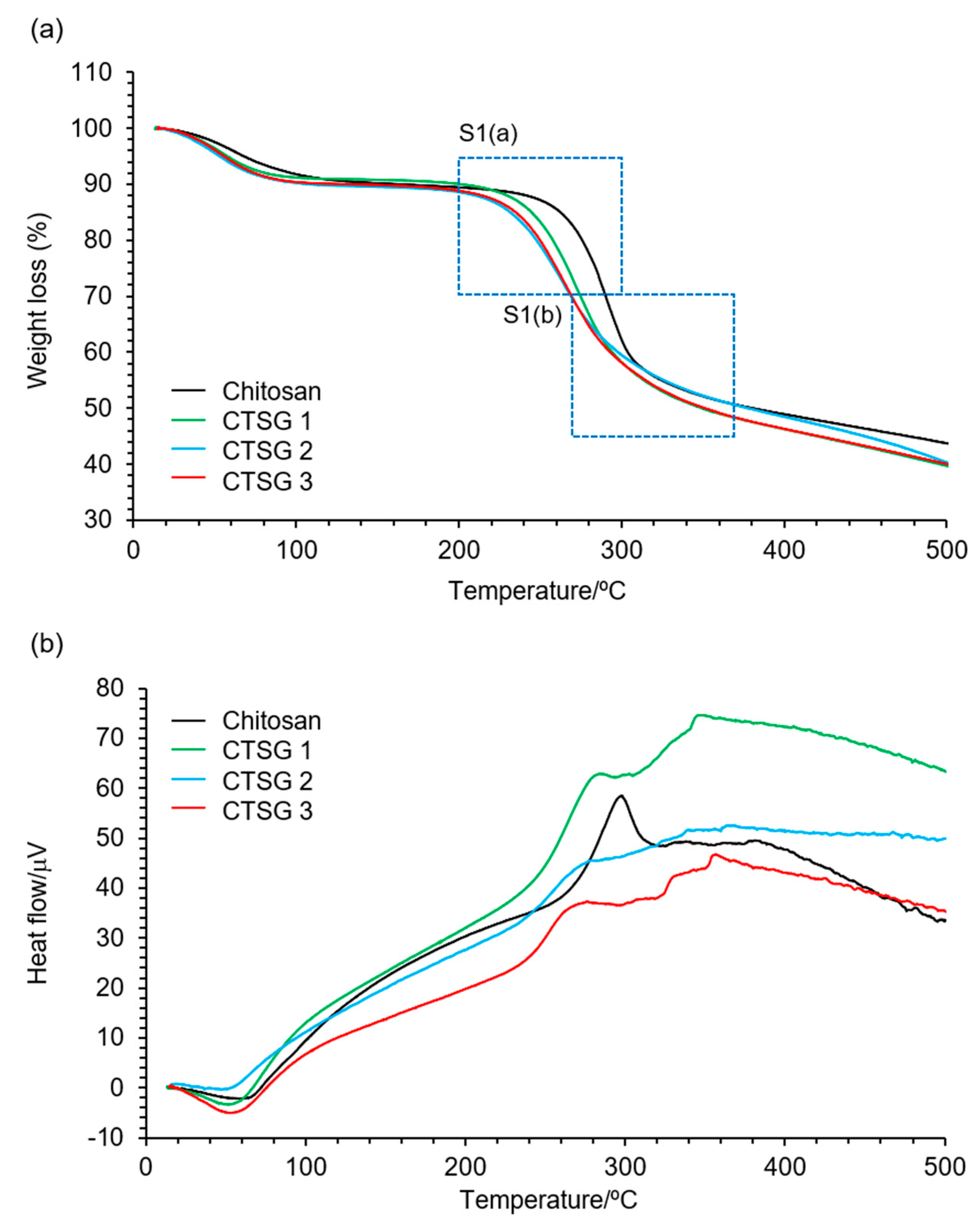
2.3. Thermal Properties of CTSG
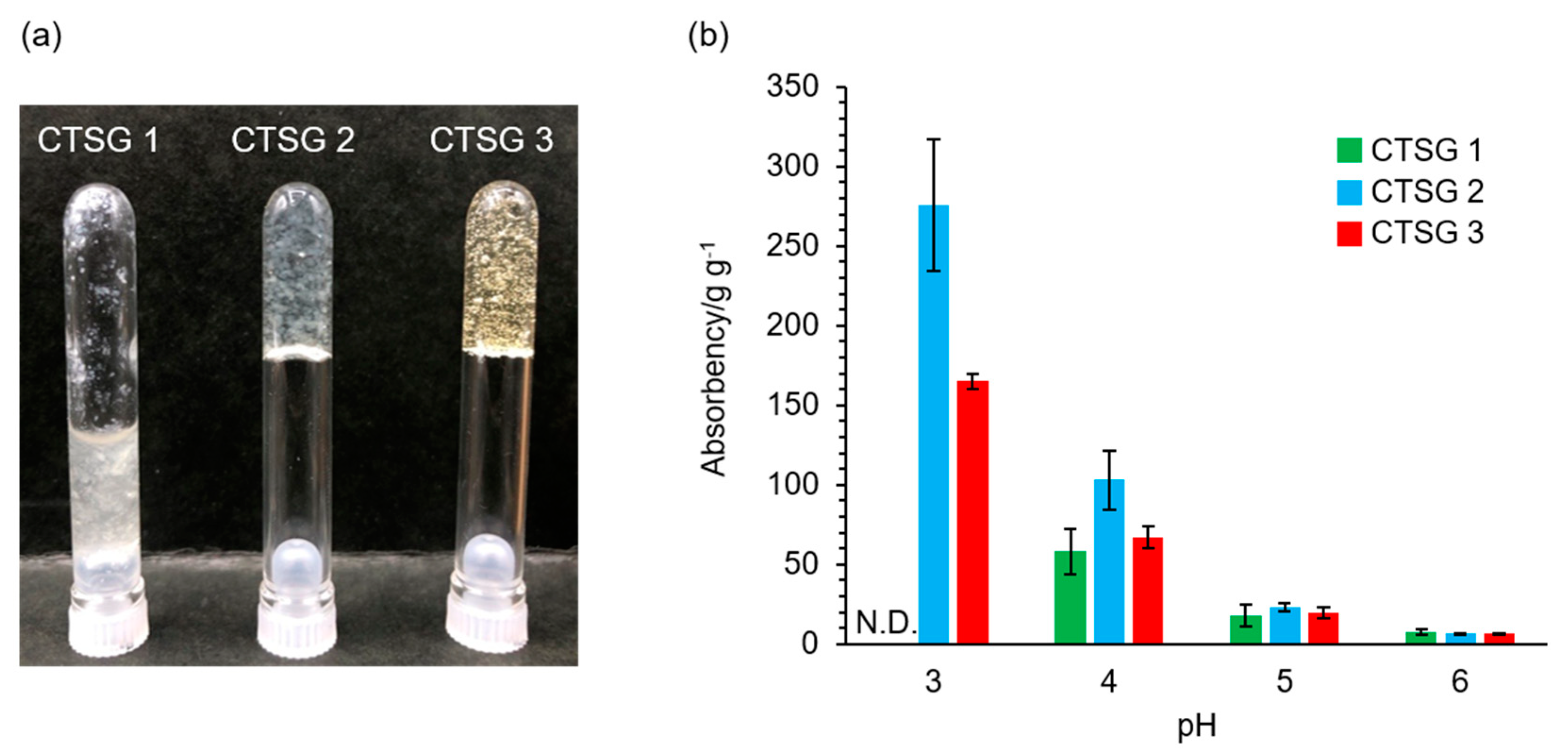
2.4. Water Absorbencies of CTSG 1–3 with Respect to pH
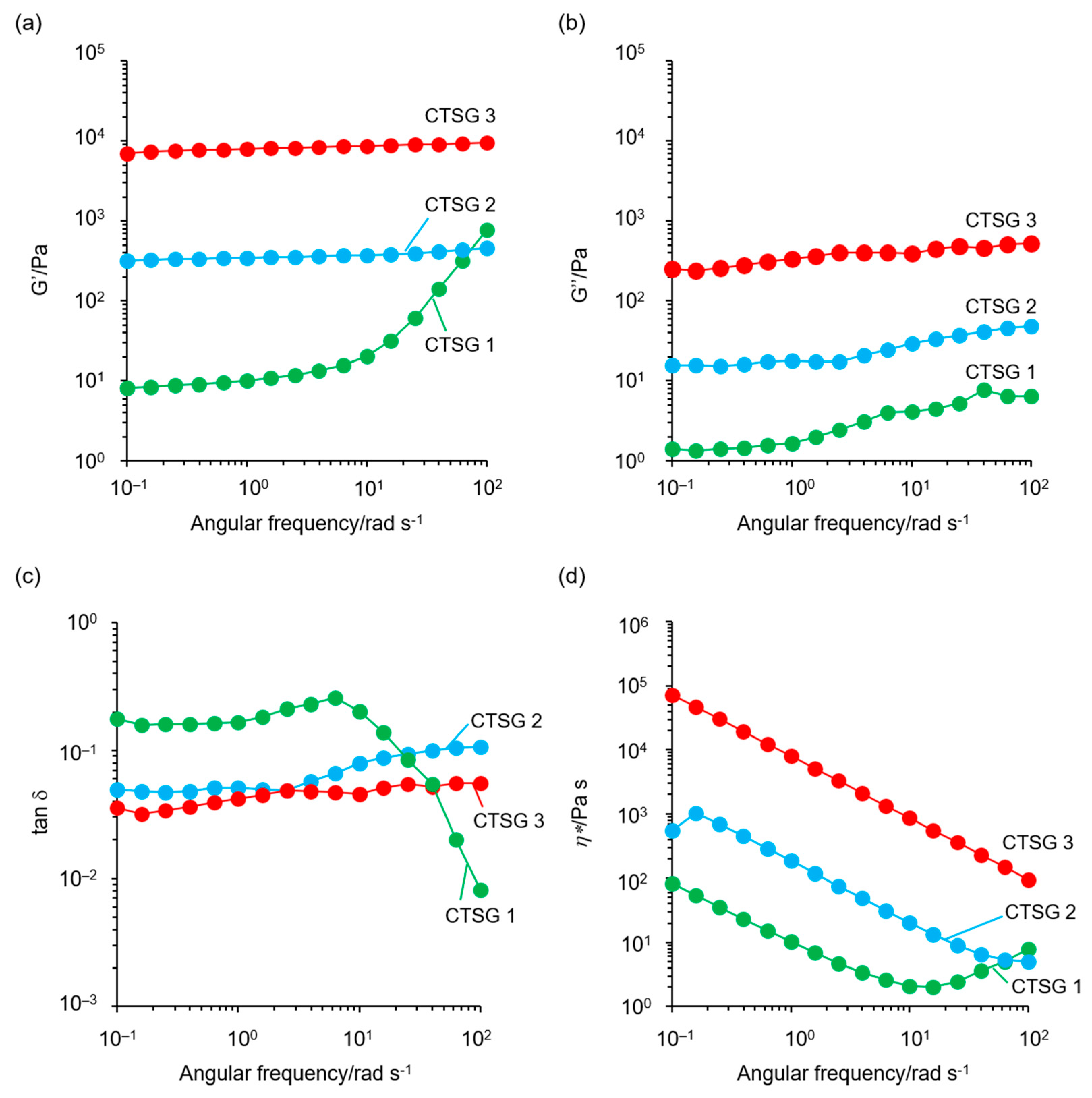
2.5. Rheological Properties of CTSGs
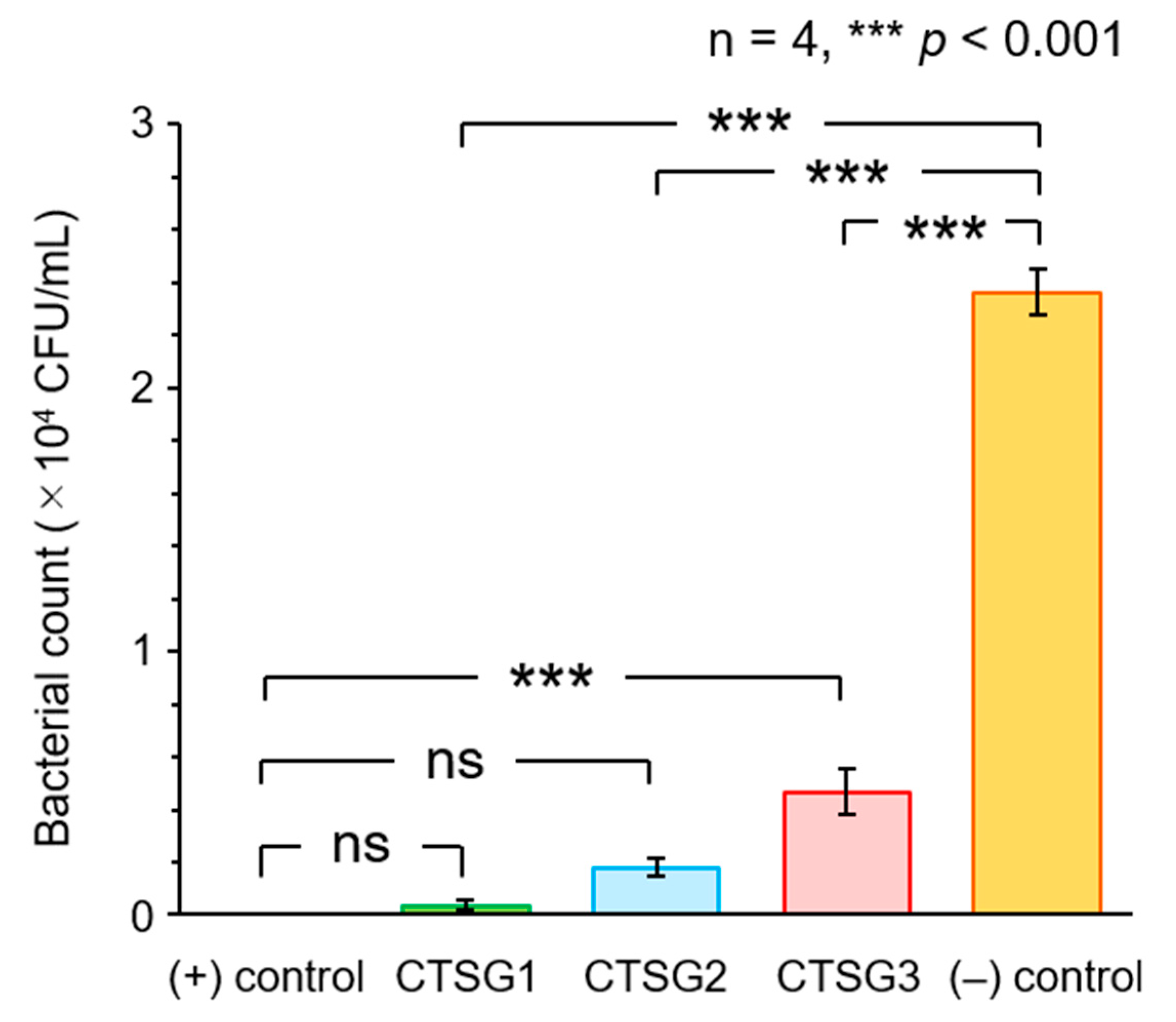
2.6. Antimicrobial Activities of CTSGs
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of OS
4.3. Synthesis of CTSGs
4.4. Structural Characterization
4.5. TG/DTA
4.6. Water absorbency
4.7. Rheological Measurements
4.8. Determination of Antimicrobial Activities
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, S.; Zhang, S.; Zuo, J.; Liang, S.; Yang, J.; Wang, J.; Wei, D. Progress in preparation and properties of chitosan-based hydrogels. Int. J. Biol. Macromol. 2023, 242, 124915. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; Bonis, A.D.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 2, 8084. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as a preservative for fruits and vegetables: A review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z. Chitosan-based agronanochemicals as a sustainable alternative in crop protection. Molecules 2020, 25, 1611. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; d’Ayala, G.G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.I.L.; Heras Caballero, A. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, M.; Xu, M.; Miao, F.; Merzougui, C.; Zhang, X.; Wei, Y.; Chen, W.; Huang, D. The fabrication of antibacterial hydrogels for wound healing. Eur. Polym. J. 2021, 146, 110268. [Google Scholar] [CrossRef]
- Lin, P.; Zhang, W.; Chen, D; Yang, Y.; Sun, T.; Chen, H.; Zhang, J. Electrospun nanofibers containing chitosan-stabilized bovine serum albumin nanoparticles for bone regeneration. Colloids Surf. B 2022, 217, 112680. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-based scaffold for mineralized tissues regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Kahya, N.; Adımcılar, V.; Kaygusuz, H.; Torlak, E.; Akın-Evingür, G.; Erim, F.B. Antibacterial nano cerium oxide/chitosan/cellulose acetate composite films as potential wound dressing. Eur. Polym. J. 2020, 133, 109777. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.-Y.; Lu, S.-T.; Li, P.-W.; Li, S.-D. Chitosan-based composite materials for prospective hemostatic applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Yang, X.; Pan, W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017, 155, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, S.; Duan, H.; He, J.; Luo, Y. Removal of anionic and cationic dyes using porous chitosan/carboxymethyl cellulose-PEG hydrogels: optimization, adsorption kinetics, isotherm and thermodynamics studies. Int. j. Biol. Macromol. 2023, 231, 123213. [Google Scholar] [CrossRef] [PubMed]
- Bratskaya, S.; Privar, Y.; Nesterov, D; Modin, E.; Kodess, M.; Slobodyuk, A.; Marinin, D.; Pestov, A. Chitosan gels and cryogels cross-linked with diglycidyl ethers of ethylene glycol and polyethylene glycol in acidic media. Biomacromolecules 2019, 20, 1635–1643. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-crosslinked chitosan gels and scaffolds for tissue engineering and regeneration of cartilage and bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef]
- Xu, H.; Canisag, H.; Mu, B.; Yang, Y. Robust and flexible films from 100% starch cross-linked by biobased disaccharide derivative. ACS Sustain. Chem. Eng. 2015, 3, 2631–2639. [Google Scholar] [CrossRef]
- Xu, H.; Liu, P.; Mi, X.; Xu, L.; Yang, Y. Potent and regularizable crosslinking of ultrafine fibrous protein scaffolds for tissue engineering using a cytocompatible disaccharide derivative. J. Mater. Chem. B 2015, 3, 3609–3616. [Google Scholar] [CrossRef]
- Wang, P.; Sheng, F.; Tang, S.W.; ud-Din, Z.; Chen, L.; Nawaz, A.; Hu, C.; Xiong, H. Synthesis and characterization of corn starch crosslinked with oxidized sucrose. Starch 2019, 71, 1800152. [Google Scholar] [CrossRef]
- El-Feky, G.S.; Zayed, G.M.; Elshaier, Y.A.M.M.; Alsharif, F.M. Chitosan-gelatin hydrogel crosslinked with oxidized sucrose for the ocular delivery of timolol maleate. J. Pharma. Sci. 2018, 107, 3098–3104. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Chang, Y.; Xu, H.; Yang, Y. Valorization of keratin from food wastes via crosslinking using non-toxic oligosaccharide derivatives. Food Chem. 2019, 300, 125181. [Google Scholar] [CrossRef]
- Kono, H.; Noda, J.; Wakamori, H. Detailed structural characterization of oxidized sucrose and its application in the fully carbohydrate-based preparation of a hydrogel from carboxymethyl chitosan. Molecules 2022, 27, 6137. [Google Scholar] [CrossRef] [PubMed]
- Aguanell, A.; del Pozo, M.L.; Pérez-Martín, C.; Pontes, G.; Bastida, A.; Fernández-Mayoralas, A.; García-Junceda, E.; Revuelta, J. Chitosan sulfate-lysozyme hybrid hydrogels as platforms with fine-tuned degradability and sustained inherent antibiotic and antioxidant activities. Carbohydr. Polym. 2022, 291, 119611. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Bai, Z.; Zhu, Y. Fast removal of Co(II) from aqueous solution using porous carboxymethyl chitosan beads and its adsorption mechanism. RSC Adv. 2018, 8, 13370–13387. [Google Scholar] [CrossRef]
- Fujita, S.; Sakairi, N. Water soluble EDTA-linked chitosan as a zwitterionic flocculant for pH sensitive removal of Cu(ii) ion. RSC Adv 2016, 6, 10385–10392. [Google Scholar] [CrossRef]
- Islam, N.; Wang, H.; Maqbool, F.; Ferro, V. In vitro enzymatic digestibility of glutaraldehyde-crosslinked chitosan nanoparticles in lysozyme solution and their applicability in pulmonary drug delivery. Molecules 2019, 24, 1271. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Feng, M.; Hu, X.; Yin, Y.; Liang, Y.; Niu, J.; Yao, J. Structural analysis of oxidized sucrose and its application as a crease-resistant crosslinking agent. Polymers 2022, 14, 2842. [Google Scholar] [CrossRef]
- Kono, H.; Oeda, I.; Nakamura, T. The preparation, swelling characteristics, and albumin adsorption and release behaviors of a novel chitosan-based polyampholyte hydrogel. React. Funct. Polym. 2013, 73, 97–107. [Google Scholar] [CrossRef]
- Kraskouski, A.; Hileuskaya, K.; Nikalaichuk, V.; Ladutska, A.; Kabanava, V.; Yao, W.; You, L. Chitosan-based Maillard self-reaction products: Formation, characterization, antioxidant and antimicrobial potential. Carbohydr. Polym. Technol. Appl. 2022, 4, 100257. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Bourakhouadar, M. Kinetics and mechanism of the thermal degradation of biopolymers chitin and chitosan using thermogravimetric analysis. Polym. Degrad. Stab. 2016, 130, 1–9. [Google Scholar] [CrossRef]
- Bayazit, N.; Cakran, H.S.; Çabir, A.; Akışcan, Y.; Demetgül, C. Synthesis, characterization and antioxidant activity of chitosan Schiff base derivatives bearing (−)-gossypol. Carbohydr. Polym. 2020, 240, 116333. [Google Scholar] [CrossRef] [PubMed]
- Zong, Z.; Kimura, Y.; Takahashi, M.; Yamane, H. Characterization of chemical and solid state structures of acylated chitosans. Polymer 2000, 41, 899–906. [Google Scholar] [CrossRef]
- Ma, G.; Yang, D.; Zhou, Y.; Xiao, M.; Kenn, J.F.; Nie, J. Preparation and characterization of water-soluble N-alkylated chitosan. Carbohydr. Polym. 2008, 74, 121–126. [Google Scholar] [CrossRef]
- Abd El-Hady, M.M.; Saeed, S.E.-S. Antibacterial properties and pH sensitive swelling of insitu formed silver-curcumin nanocomposite based chitosan hydrogel. Polymers 2020, 12, 2451. [Google Scholar] [CrossRef]
- Kono, H. Carboxymethyl cellulose-based hydrogels. In Cellulose and Cellulose Derivatives: Synthesis, Modification and Applications, 1st ed.; Mondal, M.I.H., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2015; pp. 243–258. [Google Scholar]
- Li, J.; Zhao, L.; Wu, Y.; Rajoka, M.S.R. Insights on the ultra high antibacterial activity of positionally substituted 2′-O-hydroxypropyl trimethyl ammonium chloride chitosan: A joint interaction of -NH2 and -N+(CH3)3 with bacterial cell wall. Colloids Surf. B 2019, 173, 429–436. [Google Scholar] [CrossRef]
- Yu, Q.; Yan, Y.; Huang, J.; Liang, Q.; Li, J.; Wang, B.; Ma, B.; Bianco, A.; Ge, S.; Shao, J. A multifunctional chitosan-based hydrogel with self-healing, antibacterial, and immunomodulatory effects as wound dressing. Int. J. Biol. Macromol. 2023, 231, 123149. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, P.; Kieft, T.L.; Ryan, S.J.; Baker, S.M.; Wiesmann, W.P.; Rogelj, S. Antibacterial action of a novel functionalized chitosan-arginine against Gram-negative bacteria. Acta Biomater. 2010, 6, 2562–2571. [Google Scholar] [CrossRef]
- Kono, H.; Teshirogi, T. Cyclodextrin-grafted chitosan hydrogels for controlled drug delivery. Int. J. Biol. Macromol. 2015, 72, 299–308. [Google Scholar] [CrossRef]
- Yang, X.; Lan, W.; Sun, X. Antibacterial and antioxidant properties of phenolic acid grafted chitosan and its application in food preservation: A review. Food Chem. 2023, 428, 136788. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
| Sample | Initial feed amount | Feed mass ratio of Amine/Aldehyde | CRa | nOSb | |
| chitosan (g) (Amine (mmol)) |
OS (g) (Aldehyde (mmol)) |
||||
| CTSG 1 | 0.30 (1.9) | 0.30 (0.4) | 0.2 | 0.09 | 0.04 |
| CTSG 2 | 0.30 (1.9) | 0.45 (0.6) | 0.3 | 0.14 | 0.06 |
| CTSG 3 | 0.30 (1.9) | 0.75 (0.9) | 0.5 | 0.20 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





