1. Introduction
COVID-19, a contagious disease caused by the novel coronavirus SARS-CoV-2, emerged in 2019 and quickly became a pandemic, infecting more than 700 million people worldwide. Despite the emergence of new SARS-CoV-2 variants, the number of new cases, morbidity and mortality from COVID-19 are declining and based on current trends, World Health Organization estimates that by the end of 2023, the COVID-19 emergency could be ended worldwide [
1] . A pivotal factor that has led to this positive perspective is the rapid response of the scientific community and pharmaceutical industry to the pandemic with the development of effective vaccines. Despite great disparities in vaccination among Europeans, currently most EU countries have a significant immunization level. The vaccines from various platforms (mRNA, viral vectors, protein base, and inactivated) contributed to decreased incidence, severity, and mortality. SARS-CoV-2 vaccines usually induce minor local or systemic adverse effects, such as injection site pain, swelling and redness, or fatigue, fever, headaches, joint and muscle pain [
2]. More severe adverse events including anaphylaxis, myocarditis and vaccine-induced immune thrombotic thrombocytopenia are rare [
3,
4,
5].
Thyroid disorders post COVID-19 vaccinations have been rarely reported [
6]. Most of these cases occurred post immunization with mRNA-based vaccinations (68.7%), followed by viral vector vaccines (15.7%) and inactivated vaccines (14.5%). Jafarzadeh et al [
6], reviewed 83 cases of thyroid abnormalities post COVID 19 vaccinations from the literature and found that subacute thyroiditis, also known as de Quervain’s thyroiditis, was the most prevalent accounting for 60.2% of all cases, followed by Grave’s disease (GD) (25.3% of cases). Thyroid eye disease (TED) is an extrathyroidal manifestation of GD and is a very rare complication following COVID-19 vaccination; only few cases have been reported in the literature [
7,
8,
9]. All TED cases followed mRNA-based vaccinations [
7] but two new onset mild TED cases post viral vector vaccine (ChAdox1nCoV-19) have also been reported [
10].
GD is the most common cause of hyperthyroidism in iodine sufficient areas, with an annual incidence of 20 to 50 cases per 100,000 persons [
11]. It is a humoral autoimmune disorder that causes goiter and hyperthyroidism and could be accompanied by extrathyroidal manifestations such as TED and pretibial myxedema. Forty percent of patients with GD may develop TED but only 6% and 0.5% will have moderate-to-severe and sight-threatening disease, respectively [
12,
13]. The time course of TED has been described by Rundle in 1957 [
14]. It has an initial inflammatory phase that lasts for 6 to 24 months, followed by a short stable phase, and then it transitions into the last fibrotic, quiescent phase [
15]. Early medical intervention in the inflammatory phase (with glucocorticoids or other immunomodulatory therapies) prevents the progression of the disease whereas such therapy is not reasonable in the quiescent phase. The approach of each patient with TED includes the assessment of the activity and the severity of the disease to plan its management. The disease activity is evaluated with several clinical tools. The most widely used is the Clinical Activity Score (CAS) adopted by the European Group on Graves’ Orbitopathy (EUGOGO) [
16]. CAS is a 7-point score combining subjective (patient symptoms) and objective (patient evaluation) evidence. The most affected eye is used to score a patient and a CAS score ≥3 indicates active disease, corresponding to TED initial phase.
Herein, we present for the first time a case of a new onset, moderate to severe TED, resistant to medical therapy, which developed within ten days post a viral vector COVID-19 vaccine.
2. Case presentation
A 63-years old woman was referred to our tertiary outpatient endocrinology clinic on April 2022 from a private practicing endocrinologist, due to moderate to severe and active TED unresponsive to intravenous glucocorticoids. The woman received the first dose of SARS-CoV-2 vaccination with a viral vector vaccine (Vaxzevria COVID-19 Vaccine ChAdOx1-S[recombinant] - previously known as COVID-19 Vaccine AstraZeneca), on March 9
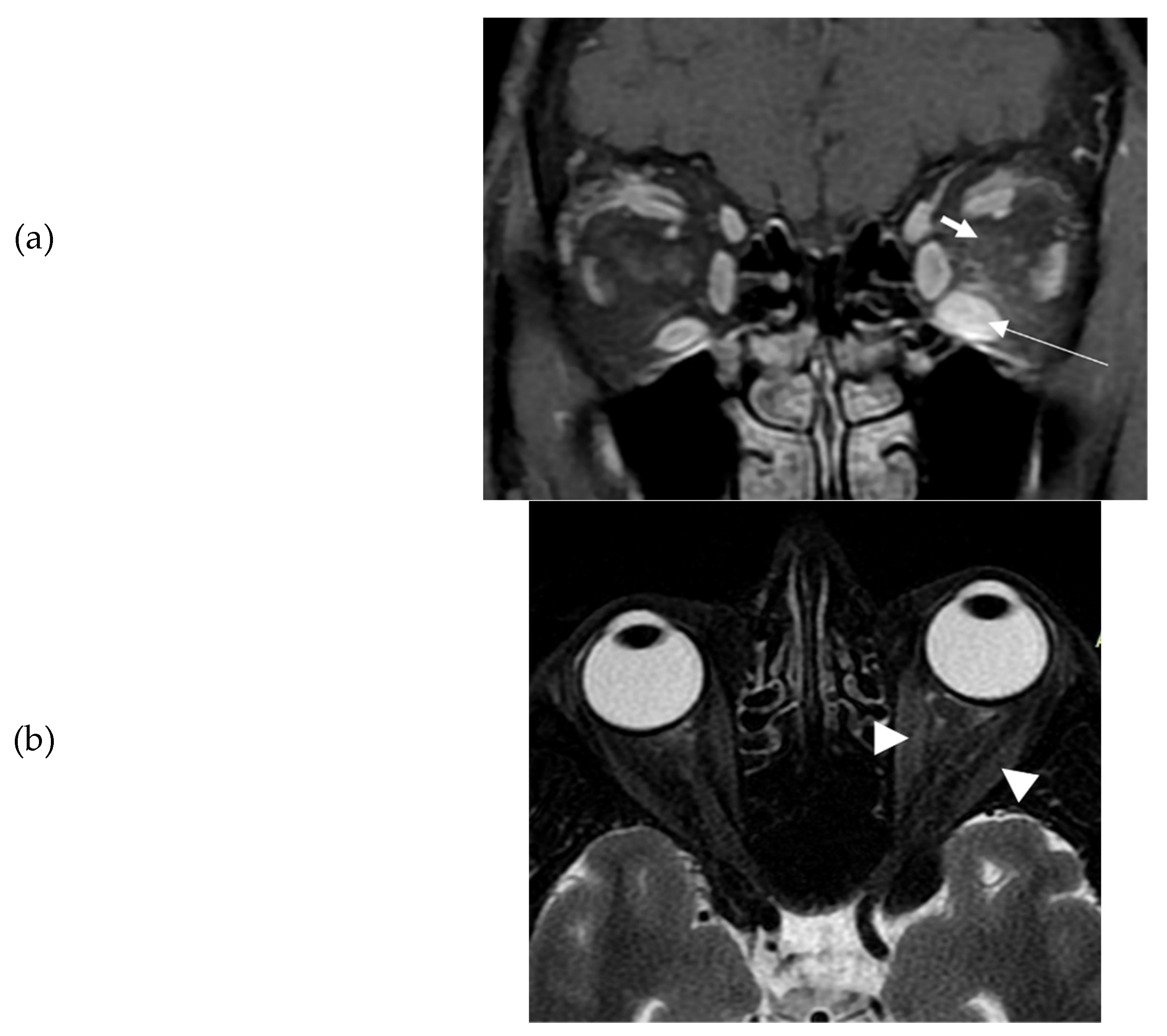
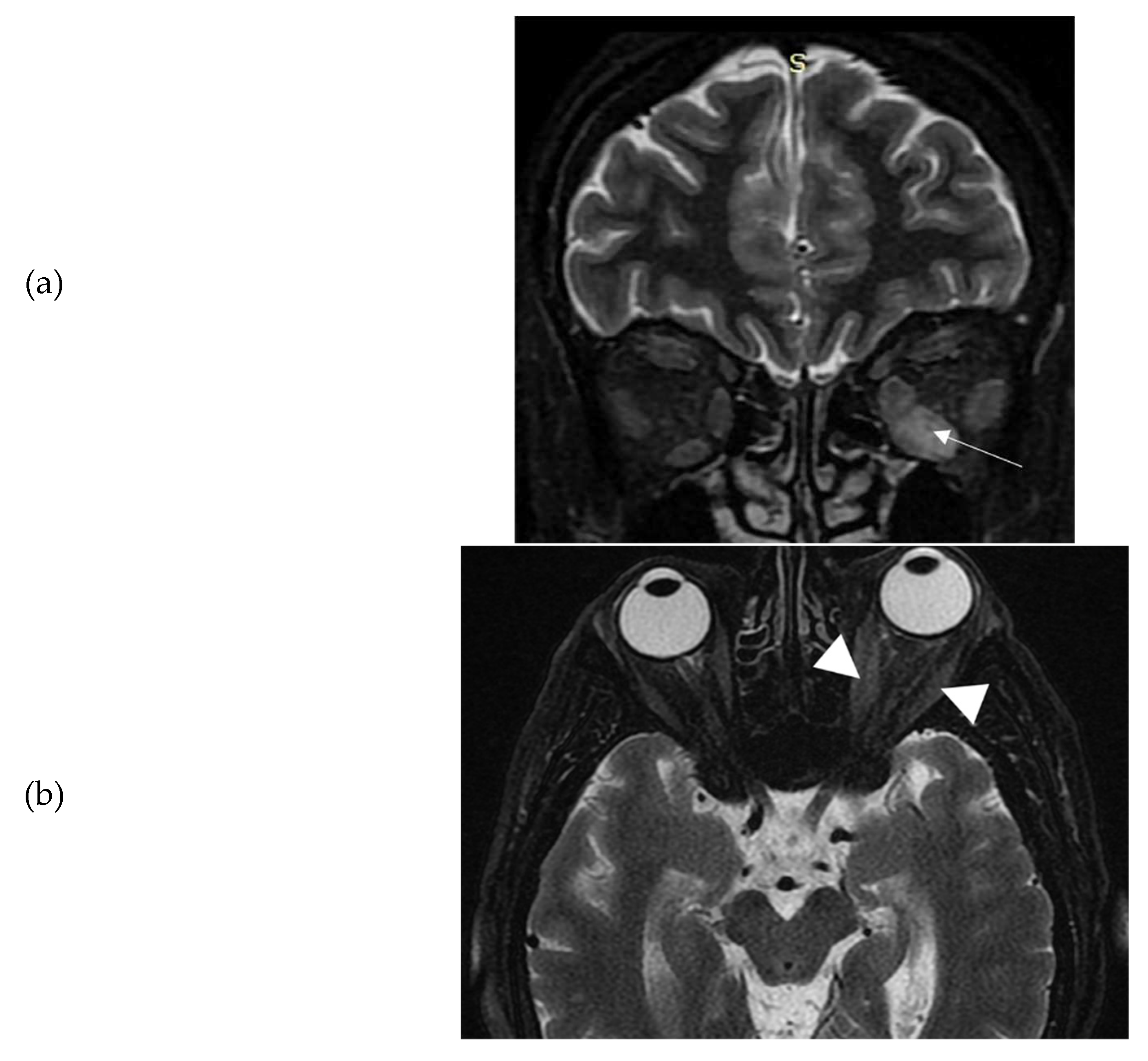
th, 2021. Ten days later, she presented spontaneous orbital pain, redness and swelling of the eyelids, conjunctival erythema, and restriction of ocular movements of her left eye leading to constant diplopia. So, the TED CAS score was 4/7 and the severity of the disease moderate to severe. She also had tremor, tachycardia and mild goiter. She did not have a known history of Graves' disease, other chronic diseases, or recent infection. She was a heavy smoker (90 pack-years). There was no history of autoimmune diseases or thyroid disorders in the family. At that time, thyroid hormone levels were: TSH <0.005 mIU/L (normal range 0.27-4.20 mIU/L); free thyroxine (FT4): 3.5 ng/dl (normal range: 0.8-2 ng/dl), triiodothyronine (Τ3): 3 ng/ml (normal range: 0.8-2 ng/ml), Thyrotropin Receptor Antibodies (TRAbs): 4.43 U/L (normal range <1.0 U/L). Furthermore, serum acetylcholine receptor and muscle-specific tyrosine kinase antibodies were measured and found negative. Sonography of the thyroid gland revealed a non-nodular goiter, with mild hypoechogenicity and increased vascularity. Due to markedly asymmetric ophthalmopathy, she underwent an orbital MRI (1 month following the initial insult). A remarkable enlargement of the inferior rectus and to a lower extent of the medial as well as the lateral rectus of the left eye was found. The rest of the extraocular muscles were found normal (
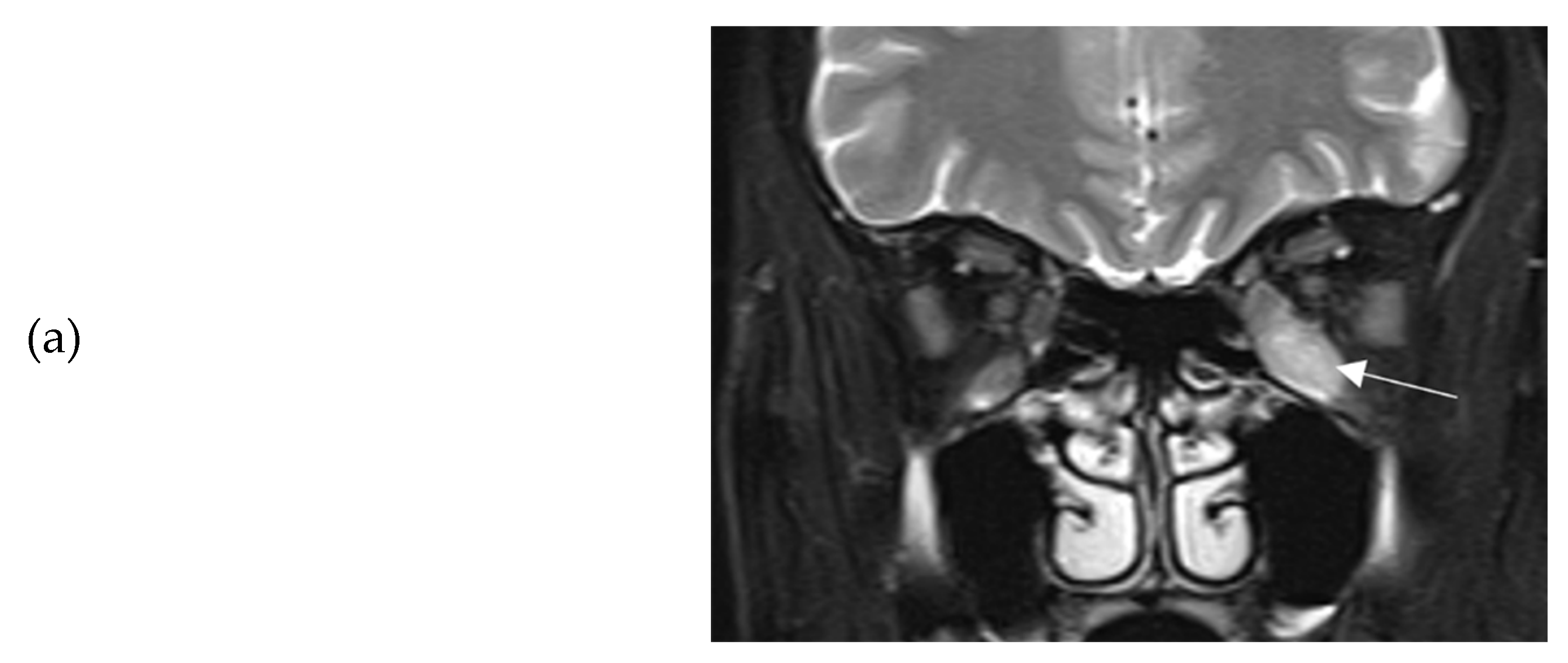
Figure 1a-b). Barrett’s index (BI) was found to be 50%, (strong indicator of absence of dysthyroid optic neuropathy). The patient was initially treated with methimazole (40 mg/day), propranolol and selenium (100 μg twice daily for 6 months). Intravenous glucocorticoids (IVGC) were started on May 2021 (500 mg methylprednisolone once weekly for 6 weeks and then 250 mg once weekly for another 6 weeks). Moreover, she quit smoking. Due to non-remission of diplopia and TED activity after IVGC treatment, repeated orbital MRI was performed, and the imaging findings were almost identical to those of the initial one (
Figure 2a-b).
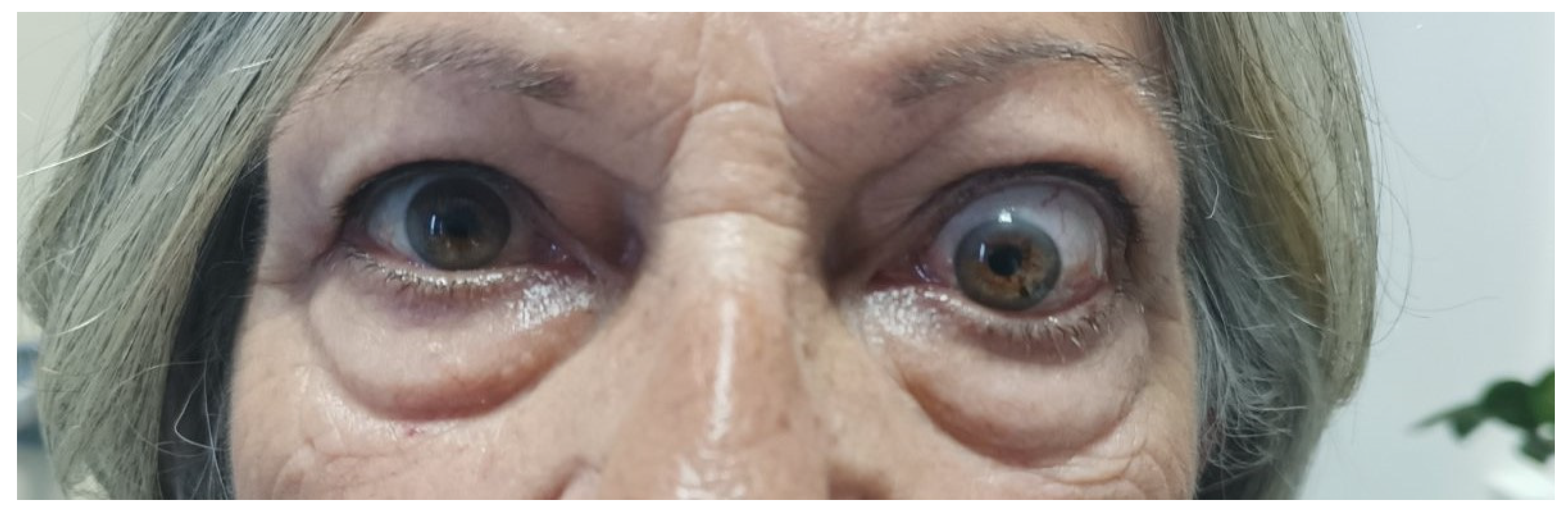
A few months later, she was referred to our clinic. At presentation, she had active disease, since the CAS score was 4/7 (spontaneous orbital pain, orbital pain upon eye movement, eyelid swelling, conjunctival redness); she also had unilateral exophthalmos of the left eye (Hertel exophthalmometer: right eye 14mm and left eye 20mm), ocular motility restriction and constant diplopia (
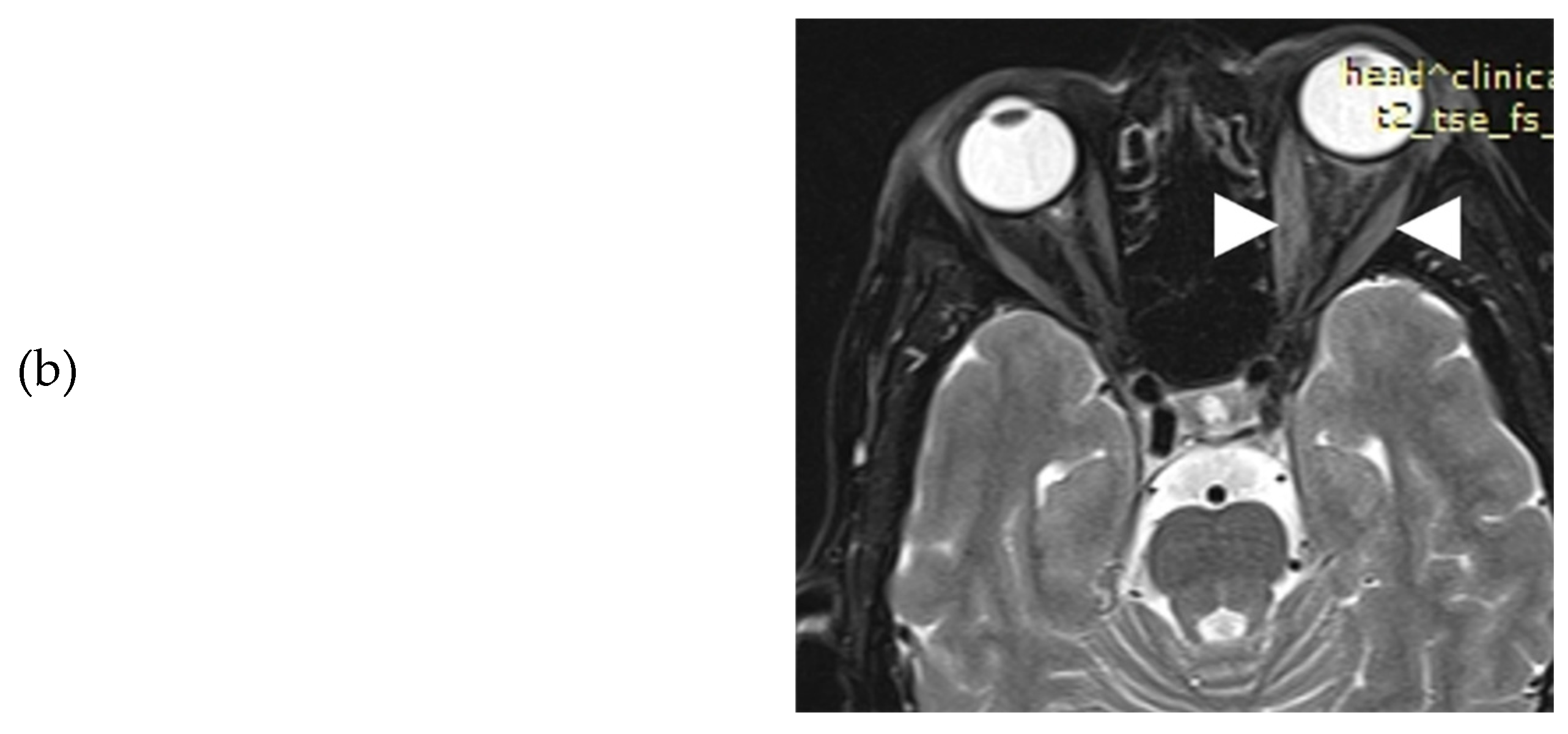
Figure 3). Her thyroid function tests, under methimazole 20 mg/day and levothyroxine 125 μg/day, were: TSH: 1.77 mIU/L, T3: 0.887 ng/ml, FT4: 1.61 ng/dl and positive TRAbs: 3.87 U/L. Serum T3, FT4, TSH and TRAbs concentrations were determined by the electrochemiluminescence immunoassay method, using Roche Diagnostic's kits and Roche/Hitachi Cobas e-411 analyzers (GmbH, Mannheim, Germany). Liver function tests (LFT), complete blood count, and renal function tests (RFT) were normal whereas low density lipoprotein (LDL) was 190 mg/dL. Hepatitis serology and purified protein derivative (PPD) test for tuberculosis were negative. Another orbital MRI (3rd in total) was performed to assess the severity of extraocular muscle involvement at presentation to our clinic. The imaging findings were practically unchanged when compared to the previous studies (
Figure 4a-b).
The patient was administered 2 intravenous doses of rituximab (1 gr/dose, given 2 weeks apart), since only this type of second line treatment is available in our country. She was advised to start rosuvastatin 10 mg once daily, while she remained euthyroid under treatment with 5 mg methimazole per day; levothyroxine was discontinued. Improvement in CAS value from 4 to 2 was noted, however constant diplopia remained. Thus, she was referred for surgical correction of diplopia, after her TED had remained inactive for 6 months.
3. Review of the literature
So far, TED following SARS-CoV-2 vaccination has been previously reported in 23 patients [
7,
8,
9,
10,
17,
18,
19,
20,
21,
22,
23], 16 of which were women (69.6%) and 7 men (30.4%), with a median age of 50 years [IQR 43-59] (
Table 1). Of note, 8 out of 23 patients (34.7%) had a personal history of autoimmunity. mRNA vaccine was administered in 21 out of 23 patients (91.3%), while viral vector vaccine was administered in 2 (8.7%). Concerning mRNA vaccine, TED manifested after the first vaccine dose in 8 out of 21 patients (38%), after the second dose in 12 out of 21 patients (57%), while in 1 case the timing of TED onset was not clarified. Regarding the viral vector vaccine, both TED cases manifested within two weeks after the first dose.
The proportion of new TED cases was 56.5% (13 out of 23 patients), and the rest 43.5% (10 out of 23 patients) concerned patients with recurrence/worsening of TED. Of the new TED cases in 84.6% (11 out of 13 patients) TED presented after mRNA vaccine and in 15.4% after viral vector vaccine, while thyrotoxicosis was present in 69.2% of these cases. Concerning patients with TED recurrence/worsening, all were vaccinated with mRNA vaccine, while thyrotoxicosis was present in 10% of them (1 out of 10). Finally, the median time interval between vaccination and TED appearance was 14 days (
Table 1).
4. Discussion
To the very best of our knowledge, this is the first description of a new onset, moderate to severe TED along with GD, following the first dose of the viral vector ChAdOx1 COVID-19 vaccine. Our patient was a heavy smoker, middle-aged woman without known history of GD or any other thyroid or autoimmune disease. She did not receive any medication at presentation, but severe untreated hypercholesterolemia was found. She did not have a documented previous COVID-19 infection. In the literature, only few cases with TED post COVID-19 vaccination have been reported; all were after mRNA-based vaccines, but two mild cases post viral-vector have also been reported.
Orbital magnetic resonance imaging (MRI) is a very helpful tool for the initial diagnosis and follow-up of TED. It definitely helps in the assessment of disease activity and the prediction of the response to treatment [
16]. Moreover, due to quantitative evaluation, it can reliably predict dysthyroid optic neuropathy (DON), by measuring Barrett’s index (BI). This is an assessment of muscle expansion as a percentage of horizontal or vertical extraocular muscles occupied by the height or width axis [
24], and it accurately predicts DON, a sight-threatening complication of TED. A BI ≥60% is highly sensitive and specific for DON, whereas a BI <50% excludes optic nerve compression [
24]. In our case BI was 50%, compatible with the absence of optic nerve damage in the course of our patient’s TED.
GD is caused by the production of antibodies against thyroid-stimulating hormone (TSH) receptor (TSHR), expressed on cell membrane of thyroid follicular cells. The antibodies stimulate the TSHR, mimicking TSH action and causing goiter and hyperthyroidism. TSHR is also expressed in the orbital adipocytes and fibroblasts [
12]. TSHR antibodies activate retroocular fibroblasts, which proliferate and secrete hydrophilic glycosaminoglycans (GAG), mostly hyaluronic acid, and differentiate into mature adipocytes. TSHR is co-localized with insulin-like growth factor 1 receptor on the membranes of orbital fibroblasts, and synergistically regulate signaling cascades in TED. The accumulation of GAG in orbital adipose tissue and extra ocular muscles leads to oedema and in conjunction with adipogenesis results in proptosis, diplopia and impaired venous drainage [
12,
25].
The initiation of the autoimmune response in GD and TED is not fully explained. Genetic and environmental triggers are implicated [
26]. Thus, specific polymorphisms of several genes, such as human leukocyte antigen genes (HLA-DRβ-Arg74), the protein tyrosine phosphatase, non-receptor type 22 (PTPN22), thyroglobulin and thyrotropin confer a susceptibility for developing GD [
27]. Unfortunately, no molecular analysis was performed in our patient. Smoking, severe stress and radioactive iodine ablation of the thyroid gland are considered risk factors for TED in predisposed patients with GD [
28,
29,
30,
31]; our patient was a heavy smoker. Furthermore, hypercholesterolemia has been suggested as a risk factor for TED and, therefore, management of blood lipid levels should be included in the management of TED [
32]. The culpability of the vaccination to the development of TED could not be unequivocally proven in our patient. However, the temporal correlation may be indicative for such a relation, similarly to all other cases in the literature, where the causality was suspected due to the appearance of TED within a few days from vaccination. It should be noted that we cannot exclude the possibility of pre-existent undiagnosed GD, since the disease is 5-10 times more prevalent in women [
33]. Viral or bacterial infections could precede autoimmune diseases, and hepatitis C virus, enterovirus and reovirus have been postulated to trigger GD [
33]. No such history or laboratory evidence was found in our case.
On the other hand, vaccines contain inactivated viruses or share viral antigens, so they may cause autoimmune reactions. Several SARS-CoV-2 proteins, such as the spike protein, the nucleoprotein, and the membrane protein have an homology with the enzyme thyroidal peroxidase (TPO), thus they could trigger GD and/or TED via molecular mimicry in predisposed subjects [
34]. Another potential pathogenic mechanism could be the direct injury of follicular cells by SARS-CoV-2, leading to the release of antigens and initiation of autoimmune response. The thyroid epithelial cells express the receptor used by SARS-CoV-2 to enter cells, namely the angiotensin-converting enzyme 2 (ACE2), as well as a transmembrane protease, the serine 2 (TMPRSS2), thus facilitating virus infectivity.
Lastly, the vaccine‘s adjuvants can lead to an overstimulation of the immune system, resulting in the well-known autoimmune/inflammatory syndrome (ASIA), described in 2011 [
35]. Most types of vaccines also contain adjuvants which may enhance the immune response to the infectious antigen and, consequently, could provoke autoimmunity. mRNA-based vaccines lack adjuvants [
36], thus the basis for this hypothesized immune system overstimulation is not known. Some authors suggest that mRNA has intrinsic immunostimulatory properties, while another speculation is that lipid nanoparticles may act as adjuvants, triggering autoimmunity processes [
37].
In conclusion, we described a very rare case of moderate to severe TED as the initial manifestation of GD following the first shot of the ChAdOx1 COVID-19 vaccine, unfortunately with unfavorable outcome. Although the causal relationship between the vaccination and TED cannot be unequivocally proven, clinicians should be aware of such potential autoimmune side effects of COVID vaccination.
Author Contributions
Conceptualization, M.A.M.; methodology, A.K.A.; formal analysis, A.K.A.; investigation, A.K.A., A.S. and M.A.M.; data curation, A.K.A.; writing—original draft preparation, A.K.A., K.S. and M.A.M.; writing—review and editing, G.K.M., P.Z., S.F.A. and M.A.M.; images interpretation, P.Z.; supervision, M.A.M.; project administration, M.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the University Hospital of Patras, Greece.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Data are available upon request.
Acknowledgments
The publication fees of this manuscript have been financed by the funding programme “MEDICUS”, of the University of Patras and by the Research Council of the University of Patras.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, J. Novel statistics predict the COVID-19 pandemic could terminate in 2022. J. Med Virol. 2022, 94, 2845–2848. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, H.A.; Khedr, A.; Koritala, T.; Bartlett, B.N.; Jain, N.K.; Khan, S.A. A review of adverse effects of COVID-19 vaccines. Infez. Med. 2022, 30, 1–10. [Google Scholar] [CrossRef]
- Kim MA, Lee YW, Kim SR, Kim JH, Min TK, Park HS, et al. COVID-19 Vaccine-associated Anaphylaxis and Allergic Reactions: Consensus Statements of the KAAACI Urticaria/Angioedema/Anaphylaxis Working Group. Allergy Asthma Immunol Res. 2021 Jul;13(4):526–44. [CrossRef]
- Ribeiro, M.I.; Pimenta, I.; Conde, I.; Gonzalez, F.A. Vaccine-induced immune thrombocytopaenia and thrombosis (VITT) after COVID-19 vaccination. BMJ Case Rep. 2022, 15, e247346. [Google Scholar] [CrossRef] [PubMed]
- Voleti, N.; Reddy, S.P.; Ssentongo, P. Myocarditis in SARS-CoV-2 infection vs. COVID-19 vaccination: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 951314. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Nemati, M.; Jafarzadeh, S.; Nozari, P.; Mortazavi, S.M.J. Thyroid dysfunction following vaccination with COVID-19 vaccines: a basic review of the preliminary evidence. J. Endocrinol. Investig. 2022, 45, 1835–1863. [Google Scholar] [CrossRef] [PubMed]
- Abeillon-du Payrat J, Grunenwald S, Gall E, Ladsous M, Raingeard I, Caron P. Graves’ orbitopathy post-SARS-CoV-2 vaccines: report on six patients. J Endocrinol Invest. 2023 Mar;46(3):617–27. [CrossRef]
- Rubinstein, T.J. Thyroid Eye Disease Following COVID-19 Vaccine in a Patient With a History Graves’ Disease: A Case Report. Ophthalmic Plast. Reconstr. Surg. 2021, 37, e221–e223. [Google Scholar] [CrossRef]
- Patrizio A, Ferrari SM, Antonelli A, Fallahi P. Worsening of Graves’ ophthalmopathy after SARS-CoV-2 mRNA vaccination. Autoimmun Rev. 2022 Jul;21(7):103096. [CrossRef]
- Chaudhary S, Dogra V, Walia R. Four cases of Graves’ disease following viral vector severe acute respiratory syndrome corona virus-2 (SARS-CoV-2) vaccine. Endocr J. 2022 Dec 28;69(12):1431–5. [CrossRef]
- Pn, T.; D, A.; A, S.; G, G.-B.; Jh, L.; Cm, D.; Oe, O. Global epidemiology of hyperthyroidism and hypothyroidism. Yearb. Paediatr. Endocrinol. 2018. [Google Scholar] [CrossRef]
- Smith TJ, Hegedüs L. Graves’ Disease. N Engl J Med. 2016 Oct 20;375(16):1552–65.
- Burch HB, Perros P, Bednarczuk T, Cooper DS, Dolman PJ, Leung AM, et al. Management of Thyroid Eye Disease: A Consensus Statement by the American Thyroid Association and the European Thyroid Association. Thyroid Off J Am Thyroid Assoc. 2022 Dec;32(12):1439–70. [CrossRef]
- Rundle, F.F. Management of exophthalmos and related ocular changes in Graves' disease.. Metabolism 1957, 6. [CrossRef]
- Anagnostis P, Boboridis K, Adamidou F, Kita M. Natural course of mild Graves’ orbitopathy: is it a chronic remitting or a transient disease? J Endocrinol Invest. 2017 Mar;40(3):257–61. [CrossRef]
- Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, Marinò M, Vaidya B, Wiersinga WM; EUGOGO. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur J Endocrinol. 2021 Aug 27;185(4):G43-G67. [CrossRef]
- Pierman G, Delgrange E, Jonas C. Recurrence of Graves’ Disease (a Th1-type Cytokine Disease) Following SARS-CoV-2 mRNA Vaccine Administration: A Simple Coincidence? Eur J Case Rep Intern Med. 2021;8(9):002807. [CrossRef]
- Bostan, H.; Ucan, B.; Kizilgul, M.; Calapkulu, M.; Hepsen, S.; Gul, U.; Unsal, I.O.; Cakal, E. Relapsed and newly diagnosed Graves’ disease due to immunization against COVID-19: A case series and review of the literature. J. Autoimmun. 2022, 128, 102809–102809. [Google Scholar] [CrossRef]
- Goblirsch TJ, Paulson AE, Tashko G, Mekonnen AJ. Graves’ disease following administration of second dose of SARS-CoV-2 vaccine. BMJ Case Rep. 2021 Dec 30;14(12):e246432. [CrossRef]
- Kikkawa, D.; Park, K.; Fung, S.; Ting, M.; Ozzello, D.; Yoon, J.; Liu, C.; Korn, B. Thyroid eye disease reactivation associated with COVID-19 vaccination. Taiwan J. Ophthalmol. 2022, 12, 93–96. [Google Scholar] [CrossRef]
- Chee YJ, Liew H, Hoi WH, Lee Y, Lim B, Chin HX, et al. SARS-CoV-2 mRNA Vaccination and Graves’ Disease: A Report of 12 Cases and Review of the Literature. J Clin Endocrinol Metab. 2022 Dec 17;107(6):e2324–30. [CrossRef]
- Mohamed A, Tzoulis P, Kossler AL, Dosiou C. New Onset or Deterioration of Thyroid Eye Disease After mRNA SARS-CoV-2 Vaccines: Report of 2 Cases and Literature Review. J Clin Endocrinol Metab. 2023 Mar 10;108(4):979–85. [CrossRef]
- Cheng, O.T.; Schlachter, D.M. Teprotumumab in advanced reactivated thyroid eye disease. Am. J. Ophthalmol. Case Rep. 2022, 26, 101484. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.; Glatt, H.J.; Burde, R.M.; Gado, M.H. Optic nerve dysfunction in thyroid eye disease: CT. Radiology 1988, 167, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Draman MS, Zhang L, Dayan C, Ludgate M. Orbital Signaling in Graves’ Orbitopathy. Front Endocrinol. 2021;12:739994. [CrossRef]
- Weetman, A.P. The Immunopathogenesis of Chronic Autoimmune Thyroiditis One Century after Hashimoto. Eur. Thyroid. J. 2012, 1, 243–250. [Google Scholar] [CrossRef]
- Bliddal, S.; Nielsen, C.H.; Feldt-Rasmussen, U. Recent advances in understanding autoimmune thyroid disease: the tallest tree in the forest of polyautoimmunity. F1000Research 2017, 6, 1776. [Google Scholar] [CrossRef]
- Kendler, D.L.; Lippa, J.; Rootman, J. The Initial Clinical Characteristics of Graves' Orbitopathy Vary With Age and Sex. Arch. Ophthalmol. 1993, 111, 197–201. [Google Scholar] [CrossRef]
- Stan, M.N.; Bahn, R.S.; Perros, P.; Tumminia, A.; Frittitta, L.; Buscema, M.; Palermo, F.; Sciacca, L.; Squatrito, S.; McAlinden, C.; et al. Risk Factors for Development or Deterioration of Graves' Ophthalmopathy. Thyroid® 2010, 20, 777–783. [Google Scholar] [CrossRef]
- Tallstedt, L.; Abraham-Nordling, M.; Andersson, T.; Berg, G.; Calissendorff, J.; Hallengren, B.; Hedner, P.; Lantz, M.; Ponjavic, V.; Taube, A.; et al. Thyroid-Associated Ophthalmopathy after Treatment for Graves’ Hyperthyroidism with Antithyroid Drugs or Iodine-131. J. Clin. Endocrinol. Metab. 2009, 94, 3700–3707. [Google Scholar] [CrossRef]
- Vestergaard, P. Smoking and thyroid disorders--a meta-analysis. Eur. J. Endocrinol. 2002, 146, 153–161. [Google Scholar] [CrossRef]
- Ye, X.Z.; Huang, S.S.; Liu, J.; Lu, B.; Shao, J.Q. [High serum cholesterol: a novel risk factor for thyroid associated ophthalmopathy?]. 2019, 58, 823–825. [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Ragusa, F.; Elia, G.; Paparo, S.R.; Ruffilli, I.; Patrizio, A.; Giusti, C.; Gonnella, D.; Cristaudo, A.; et al. Graves’ disease: Epidemiology, genetic and environmental risk factors and viruses. Best Pr. Res. Clin. Endocrinol. Metab. 2020, 34, 101387. [Google Scholar] [CrossRef] [PubMed]
- A V, D K. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol Orlando Fla [Internet]. 2020 Aug [cited 2023 Jul 27];217. Available from: https://pubmed.ncbi.nlm.nih.gov/32461193/.
- Shoenfeld Y, Agmon-Levin N. ‘ASIA’ - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011 Feb;36(1):4–8.
- Patrizio A, Ferrari SM, Elia G, Ragusa F, Paparo SR, Mazzi V, et al. Graves’ Disease Following SARS-CoV-2 Vaccination: A Systematic Review. Vaccines. 2022 Sep 1;10(9):1445.
- Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021 Apr;21(4):195–7.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).