Submitted:
02 September 2023
Posted:
04 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
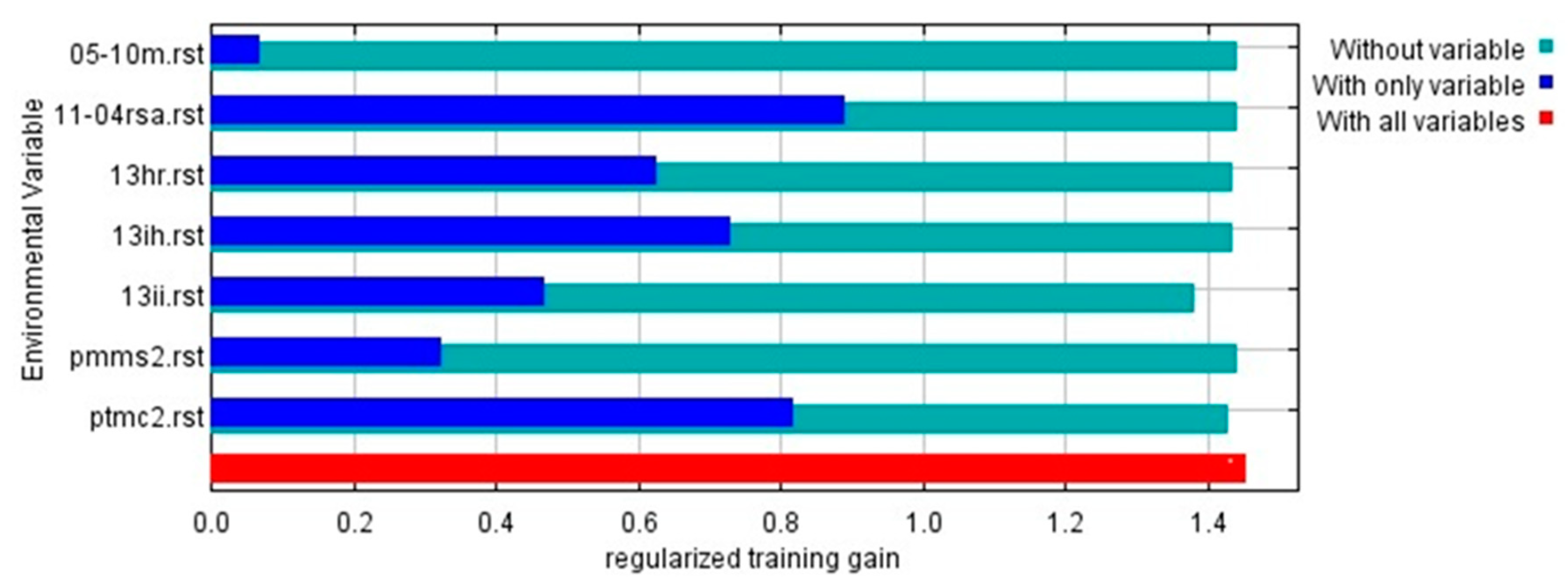
2.1. Selection of environmental variables
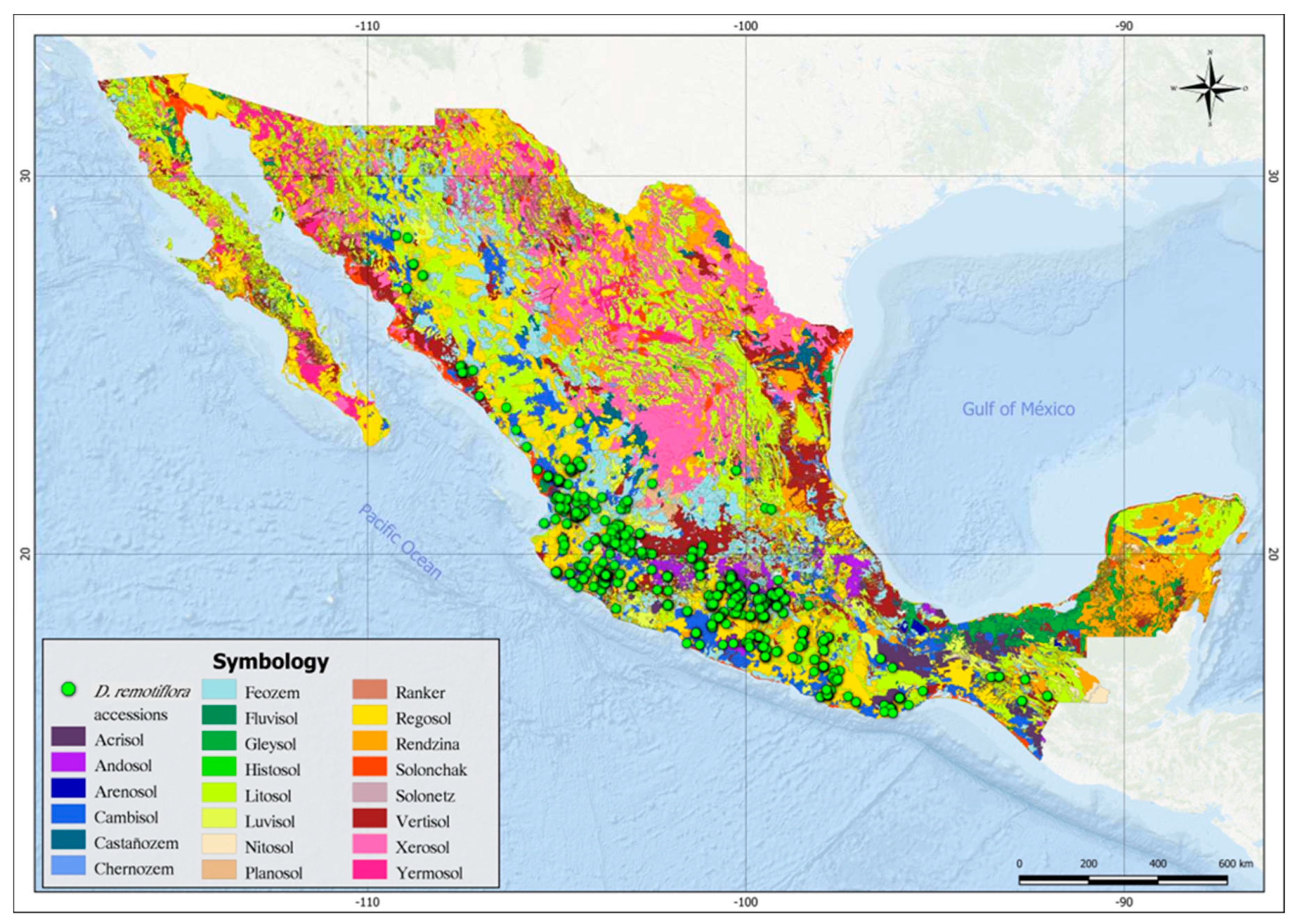
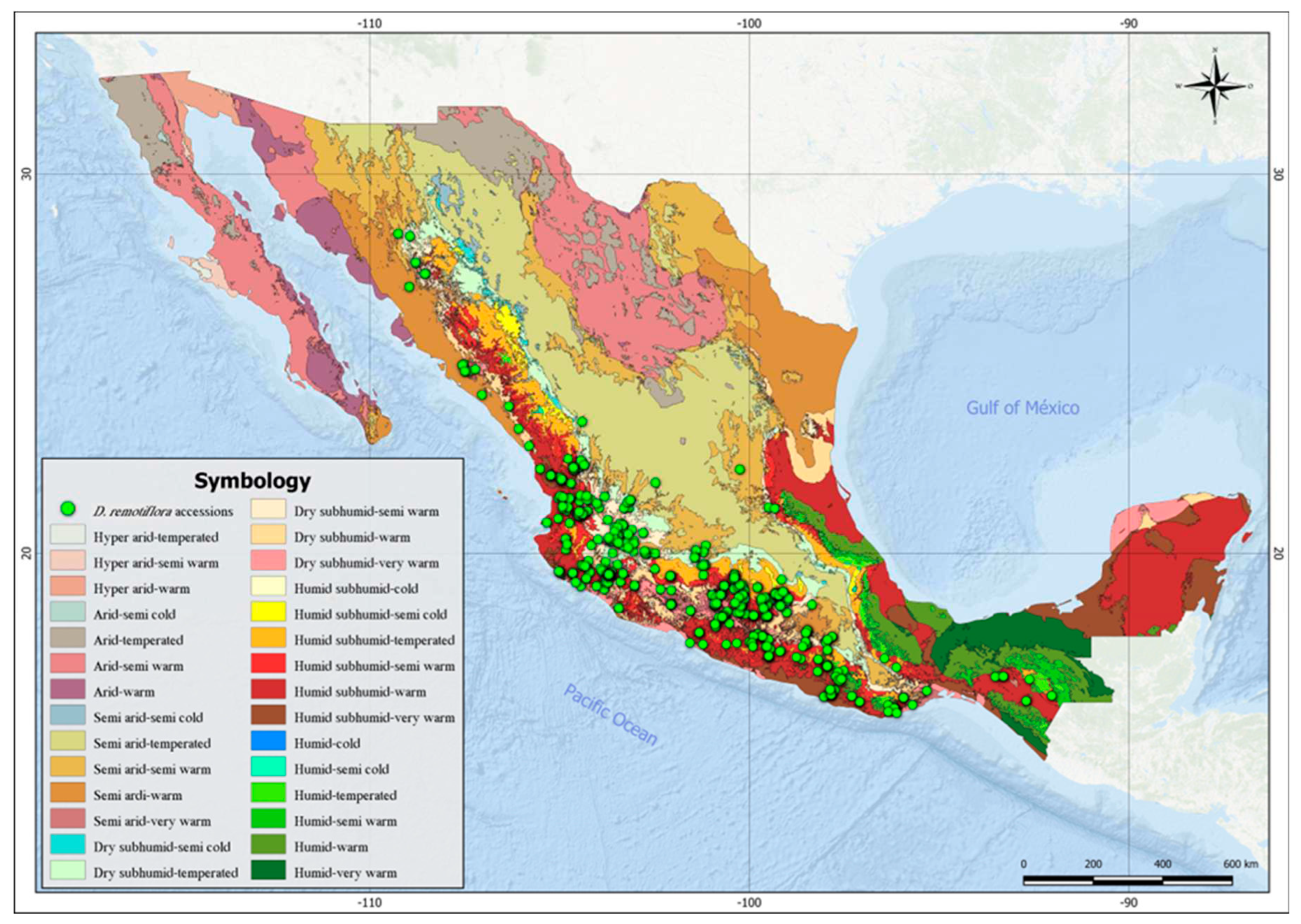
2.2. Current distribution, climatic adaptation and ecological descriptors
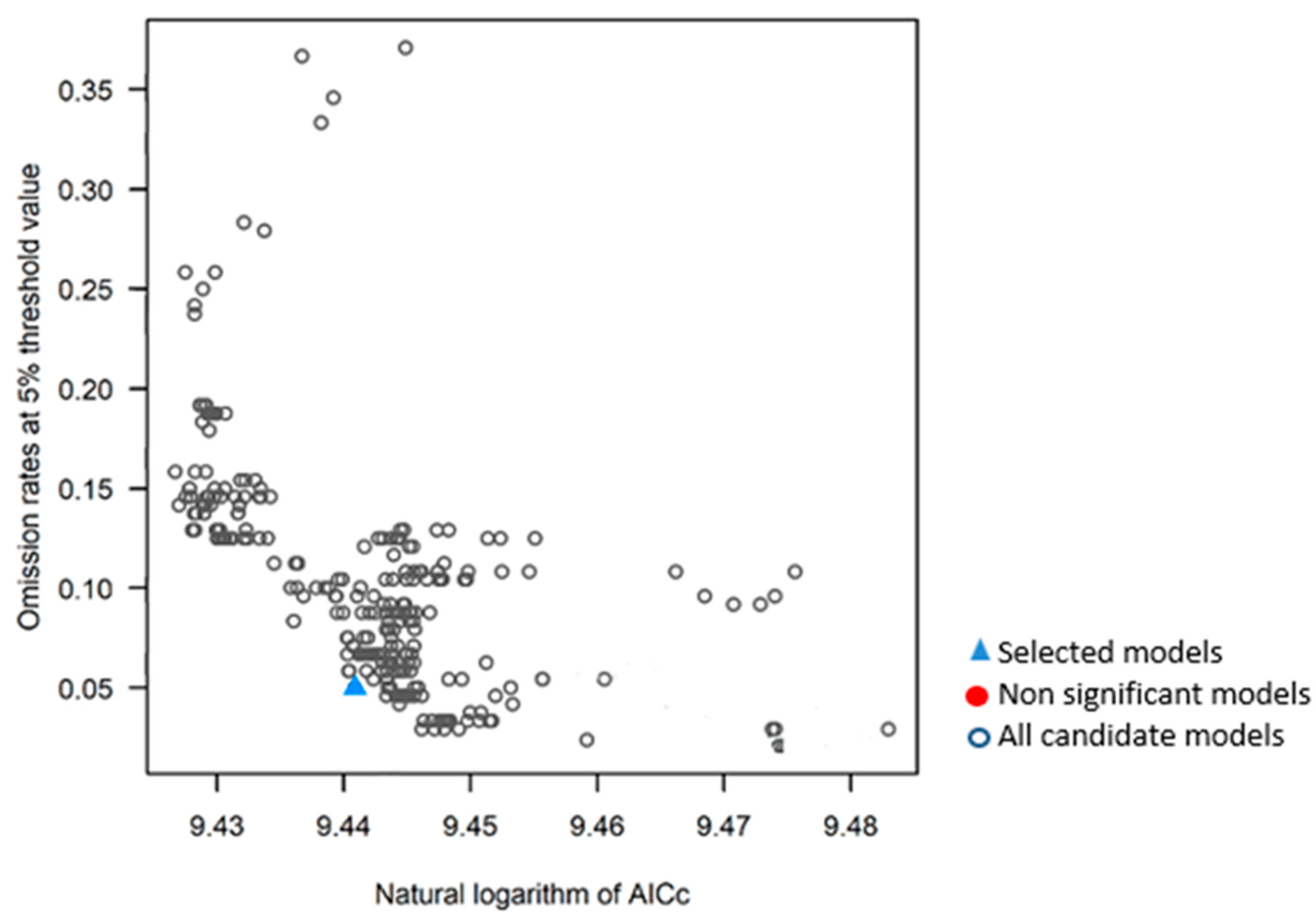
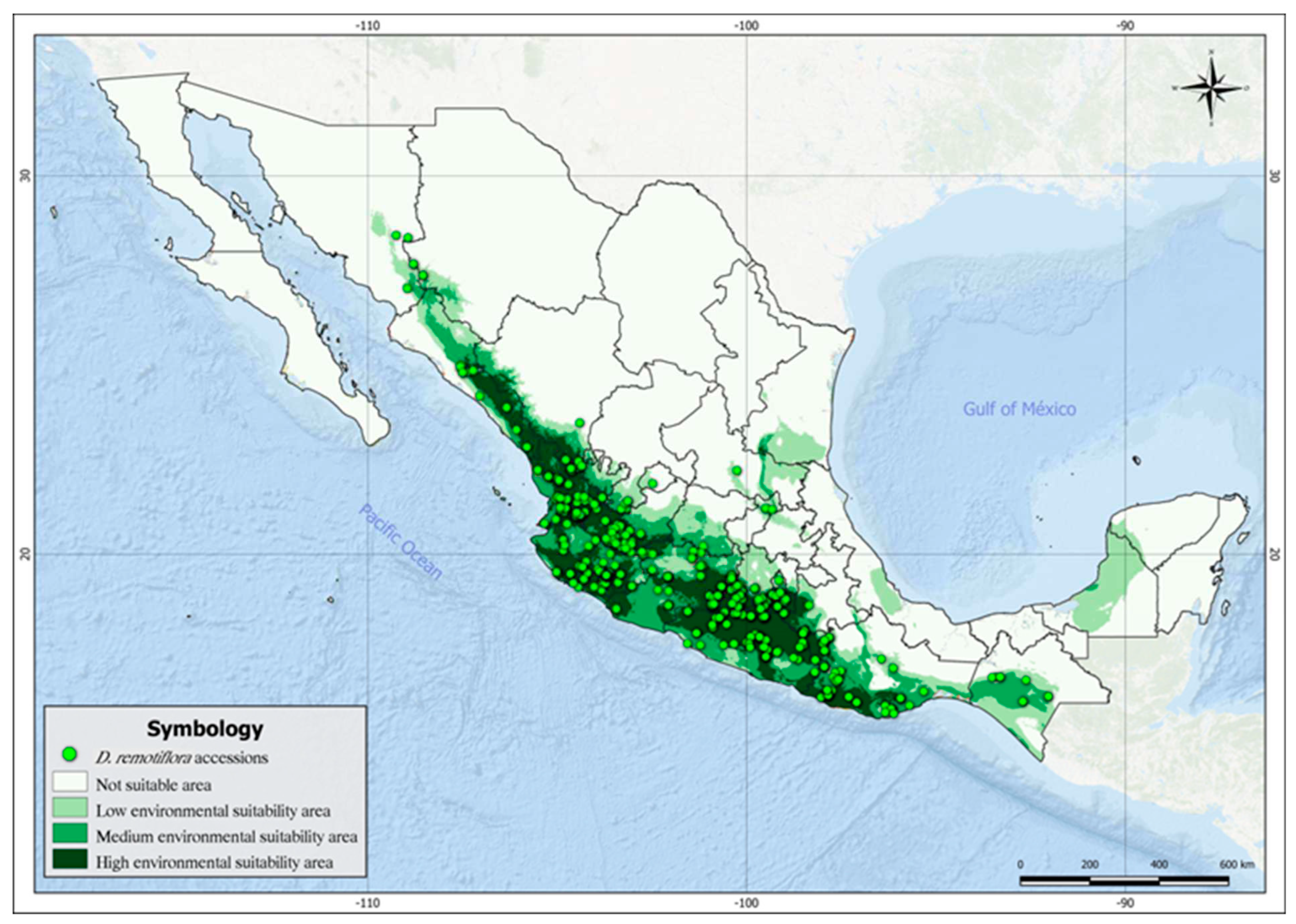
2.3. Modeling distribution niches of D. remotiflora
3. Discussion
3.1. Current distribution, climate adaptation and ecological descriptors
3.2. Modeling of distribution niches of D. remotiflora
3.3. D. remotiflora cultivation prospects
4. Materials and Methods
4.1. Occurrence data
4.2. Climatic data
4.3. Environmental characterization of the occurrence sites
4.4. Selection of environmental variables
4.5. Characterization of the adaptive capacity of D. remotiflora.
4.6. Ecological niche modeling
5. Conclusions
References
- Hussein, I.; Mengs, B.; Matiwos, T. Effect of Plant Growth Regulators on in Vitro Propagation of Yam Landraces (Dioscorea Species) Using Nodal Segments. Journal of Biology Agriculture and Healthcare 2018, 8, 13–23, https://core.ac.uk/ download/pdf/234662712.pdf. [Google Scholar]
- Rodríguez, R.R.; Téllez, V.O. Las Dioscoreas (Dioscoreaceae) del Estado de Morelos, México. Anales del Instituto de Biología. Serie Botánica 1992, 63, 67–99, https://www.redalyc.org/pdf/400/40063104.pdf. [Google Scholar]
- Castañeda, NJJ; Santacruz, RF; de Jesús SGJ; Parra, JR; De la Cruz, LL; Barba, R Shading and Container Effects on the Weight of the Dioscorea sparsiflora Tuber. Agronomy Journal 2017, 109, 33–38. [CrossRef]
- Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). Available online: https://enciclovida.mx/especies/192190-dioscorea-remotiflora: (accessed on 9 September 2022).
- Obidiegwu, JE; Akpabio, EM The geography of yam cultivation in southern Nigeria: Exploring its social meanings and cultural functions. Journal of Ethnic Foods 2017, 4, 28–35. [CrossRef]
- Akissoé, N; Hounhouigan, J; Mestres, C; Nago, M How blanching and drying affect the color and functional characteristics of yam (Dioscorea cayenensis-rotundata) flour. Food Chemistry 2003, 82, 257–264. [CrossRef]
- Montañez, SJL; Venegas, GJ; Bernardno, NA; González, CL; Yañez, FJ Chemical Characterization and Nutritional Evaluation of Mountain’s yam (Dioscorea remotiflora Kunth). Tubers 2014, 5, 153–160.
- Bernabe, AA; Santacruz, RF; Cruz, SF Effect of plant growth regulators on plant regeneration of Dioscorea remotiflora (Kunth) through nodal explants. Plant Growth Regulation 2012, 68, 293–301. [CrossRef]
- Obidiegwu, JE; Lyons, JB; Chilaka, CA The Dioscorea Genus (Yam) An Appraisal of Nutritional and Therapeutic Potentials. Foods 2020, 9, 1304. [CrossRef]
- Cobos, ME; Peterson, AT; Osorio, OL; Jiménez GD; An exhaustive analysis of heuristic methods for variable selection in ecological niche modeling and species distribution modeling. Ecological Informatics 2019, 53, 100983. [CrossRef]
- Chandra, A; Naithani, HB; Verma, PK; Saxena, J; & Prajapati, S. Plant diversity assessment of selected forest sites of Gaya district of Bihar, India. Journal of Applied and Natural Science 2021, 13, 424-432. [CrossRef]
- Ghorbani, A; Bahrami, B The influence environmental factors on the distribution of plant species in the southeast rangelands of Sabalan. Watershed Management Research Journal 2017, 30, 15–29. [CrossRef]
- Sánchez, GJDJ; Ruiz, CJA; García, GM; Ojeda, GR; Larios, LDLC; Holland, JB; García, RGE Ecogeography of teosinte. PLoS One 2018, 13, e0192676. /: https. [CrossRef]
- Aburto-Cansino, GN; Ruiz-Corral, JA; Sánchez González, JDJ; & González Eguiarte, DR Temperaturas cardinales de desarrollo del teocintle (Zea spp.). Revista mexicana de ciencias agrícolas 2018 9, 1269-1281. [CrossRef]
- Grativol, C; Silva HA; and Gomes, FP. Genetic and epigenetic regulation of stress responses in natural plant populations. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 2012, 1819, 176-185. [CrossRef]
- Ashapkin, VV; Kutueva, LI; Aleksandrushkina, NI; & Vanyushin, BF Epigenetic mechanisms of plant adaptation to biotic and abiotic stresses. International journal of molecular sciences 2020, 21, 7457. [CrossRef]
- Sosa, V; Nova, J Linajes de angiospermas endémicas en México: zonas de alto endemismo para la conservación. Acta Botánica Mexicana 2012, 100, 293–315, https://abm.ojs.inecol.mx/index.php/abm/article/view/38.
- Santacruz, RF; Casas, SJF; Pérez, PR; Rodríguez, GE; Torres, MMI; Castillo, HC; Iturbe SI; Conservación, manejo y aprovechamiento del camote de cerro (Dioscorea spp.) en el estado de Jalisco, México. Avances de la Investigación Científica en el CUCBA. XVI: 2005, 179-183. http://www.floradejalisco.cucba.udg.mx /sites/default/files/publicaciones1/avances/avances_2005/Agronomia/SantacruzRuvalcabaFernando/SantacruzRuvalcabaFernando.pdf.
- Miranda, AG; Soto, JLM; Ruiz, IG. Parcial caracterización de nuevos almidones obtenidos del tubérculo de camote del cerro (Dioscorea spp). Revista Iberoamericana de Tecnología Postcosecha 2008, 9, 81–88, https://www.redalyc.org /pdf/813/81311226011.pdf.
- González, VME El ñame (Dioscorea spp.) Características, usos y valor medicinal. Aspectos de importancia en el desarrollo de su cultivo. Cultivos Tropicales 2012, 33, 5–15, http://scielo.sld.cu/scielo.php?pid=S0258-59362012000400001& script=sci_ arttext&tlng=en.
- Ruiz, CJA; Medina, GG; García, RGE Sistema de información agroclimática para México-Centroamérica. Revista Mexicana de Ciencias Agrícolas 2018, 9, 1–10. [CrossRef]
- Barrera-Sánchez, CF; Ruiz-Corral, JA; Zarazúa-Villaseñor, P; Lépiz-Ildefonso, R; & González-Eguiarte, DR Cambio climático y distribución potencial de frijol lima en Mesoamérica y Aridoamérica. Revista Mexicana Ciencias Agrícolas 2020 11, 1361-1375. [CrossRef]
- Dormann, CF; McPherson, JM; Araujo, MB; Bivand, R; Bolliger, J; Carl, G Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 2007, 30, 609–628. [CrossRef]
- Peterson, AT; Nakazawa, Y Environmental data sets matter in ecological niche modelling: an example with Solenopsis invicta and Solenopsis richteri. Global Ecology and Biogeography 2008, 17, 135–144. [CrossRef]
- Feng, X. , Park, DS; Liang, Y; Pandey, R; Papeş, M Collinearity in ecological niche modeling: Confusions and challenges. Ecology and evolution 2019, 9, 10365–10376. [Google Scholar] [CrossRef]
- Xu, Y; Zhu, R; Gao, L; Huang, D; Fan, Y; Liu, C; Chen, J. Predicting the current and future distributions of Pennisetum alopecuroides (L.) in China under climate change based on the MaxEnt model. Plos one 2023, e0281254. [CrossRef]
- UNEP (United Nations Environmental Program). World Atlas of Desertification 2 ed.; Middleton N. and Thomas D. S. G: London, UK,1997. pp 1-182.
- Rodríguez, W Botánica, domesticación y fisiología del cultivo de ñame (Dioscorea alata). Agronomía Mesoamericana 2000, 11, 133–152, https://www.redalyc.org/pdf/437/ 43711221.pdf.
- Avalo, DMR; García, MB Producción y diversificación sostenible del cultivo de ñame (Dioscorea spp.) en condiciones de sequía agrícola en el municipio de Jiguaní. Agrisost 2021, 27, 1–7, https://revistas.reduc.edu.cu/index.php/agrisost/article/view/ e10333-1.
- Velázquez, HJM; Durán, PN; Ruiz, CJA; González, EDR; Santacruz, RF; Gallegos, RA. Distribución geográfica y usos de especies del género Dioscorea. E-cucba 2022, 19, 141–150. [CrossRef]
- Mueller, L; Schindler, U; Mirschel, W; Shepherd, TG; Ball, BC; Helming, K; Rogasik, J; Eulenstein, F; Wiggering, H Assessing the productivity function of soils. A review. Sustainable Agriculture, 2010; 30, 601–614. [CrossRef]
- INEGI Suelos. Instituto Nacional de Estadística y Geografía. D. F México 2007. pp 36 https://apps1. semarnat.gob.mx:8443/dgeia/informe_12/pdf/Cap3_suelos.pdf.
- López, FAJ; Hernández, CD Cambio climático y agricultura: una revisión de la literatura con énfasis en América Latina. El trimestre económico. 2016, 83, 459–496. [CrossRef]
- Ruiz, CJA.; Medina, GG. González, AIJ. Flores, LHE. Ramírez, OG. Ortiz, TC. Byerly, MKF. Requerimientos agroecológicos de cultivos, 3a ed.; INIFAP-Prometeo Editores: Jalisco, México, 2013; pp. 564. https://www.researchgate.net/publication/343047223_REQUERIMIENTOS_AGROECOLOGICOS_DE_CULTIVOS_2da_Edicion.
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen (Para adaptarlo a las condiciones de la República Mexicana), 5ª Edición.; Instituto de Geografía-UNAM: Ciudad de México, México, 2004; pp. 90. http://www.publicaciones.igg.unam.mx/index.php/ig/catalog/book/83.
- Thompson, AK; Oduro, I. Yams: Botany, Production and Uses, 1aed.; CABI: Boston, USA, 2021; pp. 296. [CrossRef]
- Patricio, VEG; Rodrigo, ARJ; Isaías, MAR; Cristina, LVI; del Pilar, PMN; Santiago, EVJ; Inhibición de la brotación del tubérculo de papa: una revisión de los métodos empleados Inhibition of potato tuber sprouting: a review of the methods employed. Journal of the Selva Andina Biosphere 2018, 6, 55–64, http://scielo.org.bo/pdf/jsab/v6n2/v6n2_a04.pdf.
- Coursey, D. Yams: Dioscorea spp, 2nd Edition.; In: Wiley: London, UK, 1967; pp. 70–74. https://agris.fao.org/ agrissearch/search.do?recordID=US201303063121.
- Yang, J; Huang, Y; Jiang, X; Chen, H; Liu, M; Wang, R Potential geographical distribution of the endangered plant Isoetes under human activities using MaxEnt and GARP. Global Ecology and Conservation 2022, e02186. [CrossRef]
- Abdelaal, M; Fois, M; Fenu, G., Bacchetta, G Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecological informatics 2019, 50, 68–75. [CrossRef]
- Muscarella, R; Galante, PJ; Soley, GM; Boria, RA; Kass, JM; Uriarte, M; Anderson, RP ENMeval: an R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods in Ecology and Evolution 2014, 5, 1198–1205. [CrossRef]
- Fois, MA; Cuena, LG; Fenu, G Bacchetta Using species distribution models at local scale to guide the search of poorly known species: review, methodological issues and future directions. Ecol. Model. 2018, 124–132. [CrossRef]
- Shen, L; Xu, J; Luo, L; Hu, H; Meng, X; Li, X.; Chen, S Predicting the potential global distribution of diosgenin-contained Dioscorea species. Chinese medicine 2018, 13, 58. [CrossRef] [PubMed]
- Yin, DS; Chen, F; Chen, Z; Guan WF Morpho- anatomical and physiological responses of two Dendranthema species to waterlogging. Environmental and Experimental Botany. 2010, 68, 122–130, https://doi.org/10.1016/j.envexpbot.2009.11.008. [CrossRef]
- Jiménez, JDLC; Moreno, LP; Magnitskiy, S Respuesta de las plantas a estrés por inundación. Una revisión. Revista Colombiana de Ciencias Hortícolas 2012, 6, 96–109, https://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S2011-21732012000 100010.
- Jiménez, JDLC; Moreno, LP; Magnitskiy, S Respuesta de las plantas a estrés por inundación. Una revisión. Revista Colombiana de Ciencias Hortícolas 2012, 6, 96–109, https://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S2011-21732012000 100010.
- Medina, GC; Giménez, deJ; Velázquez, MA Las comunidades vegetales del bosque de coníferas altimontano en el macizo del Tancítaro, Michoacán, México. Acta botánica mexicana 2020, p.127. [CrossRef]
- Schaal, BA; Hayworth, DA; Olsen, KM; Rauscher, JT; Smith, WA Phylogeographic studies in plants: problems and perspectives. Molecular Ecology 1998, 465–474. https://www.researchgate.net/profile/Kenneth-Olsen-4/publication/227339 790_Phylogeographic_studies_in_plants_Problems_and_prospects/links/5dfd016da6fdcc2837318eee/Phylogeographic-studies-in-plants-Problems-and-prospects.pdf. /: https.
- Dillon, MO; Tu, T; Xie, L; Quipuscoa, SV; Wen, J Biogeographic diversification in Nolana (Solanaceae), a ubiquitous member of the Atacama and Peruvian Deserts along the western coast of South America. Journal of Systematics and Evolution. 2009, 47, 457–476. [CrossRef]
- Schmidt, JR; Sytsma, KJ Phylogenetics of Puya (Bromeliaceae): placement, major lineages, and evolution of Chilean species. American Journal of Botany 2010, 97, 337–356. [CrossRef]
- Gao, X; Liu, J; Huang, Z The impact of climate change on the distribution of rare and endangered tree Firmiana kwangsiensis using the Maxent modeling. Ecology and Evolution 2022, 12, e9165. [CrossRef]
- Zhao, Y; Deng, X; Xiang, W; Chen, L; Ouyang, S Predicting potential suitable habitats of Chinese fir under current and future climatic scenarios based on Maxent model. Ecological Informatics. 2021, 64, 101393. [CrossRef]
- Ramírez, OG; Peralta, IE; Rodríguez, GE; Sahagún, CJ; Chávez, SJL; Medina, HTC; Rodríguez, PJE Edaphoclimatic Descriptors of Wild Tomato Species (Solanum Sect. Lycopersicon) and Closely Related Species (Solanum Sect. Juglandifolia and Sect. Lycopersicoides) in South America. Frontiers in genetics 2021, 12. [CrossRef]
- vironmental Systems Research Institute (ESRI) ArcGIS Desktop: Release 10. Environmental Systems Research Institute. Redlands, CA, USA. 2010, 1 p5. https://earthobservations.org/about_geo.shtml.
- O’brien, RM Caution Regarding Rules of Thumb for Variance Inflation Factors. Qual Quant 2007, 41, 673–690. [CrossRef]
- Core, T.R. R.A., Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna.Austria. (Versión 4.2.1). 2021. https://www.R-project.org/.
- INEGI., 2017. Conjunto de datos vectoriales de edafología serie 4, escala 1:250,000. Aguascalientes: Instituto Nacional de Estadística y Geografía. https://www. inegi.org.mx/temas/edafologia/.
- Thouverai, E; Marcantonio, M; Lenoir, J; Galfré, M; Marchetto, E; Bacaro, G; Rocchini, D Integrals of life: Tracking ecosystem spatial heterogeneity from space through the area under the curve of the parametric Rao’s Q index. Ecological Complexity 2023, 52, 101029. [CrossRef]
- Phillips, SJ; Anderson, RP; Schapire, RE Maximum entropy modeling of species geographic distributions. Ecological Modelling 2006, 190, 231–259. [CrossRef]
- Elith, J. , Phillips, SJ; Hastie, T; Dudík, M; Chee, YE; Yates, CJA Statistical explanation of MaxEnt for ecologists. Diversity and Distributions 2011, 171, 43–57. [Google Scholar] [CrossRef]
- Warren, DL; Seifert, SN Ecological niche modeling in Maxent: the importance of model complexity and the performance of model selection criteria. Ecological applications 2011, 21, 335–342. [CrossRef]
- Mazzolari, AC; Millán, EN; Bringa, EM; Vázquez, DP Modeling habitat suitability and spread dynamics of two invasive rose species in protected areas of Mendoza, Argentina. Ecological Complexity 2020, 44, 100868. [CrossRef]
- Gull E; Fareen, A; Mahmood, T; Bodlah, I; Rashid, A; Khalid, A; Mahmood, S. Modeling potential distribution of newly recorded ant, Brachyponera nigrita using Maxent under climate change in Pothwar region, Pakistan. PLoS One 2022, 17, e0262451. [CrossRef]
- Cao, Y; DeWalt, RE; Robinson, JL; Tweddale, T; Hinz, L; Pessino, M Using Maxent to model the historic distributions of stonefly species in Illinois streams: The effects of regularization and threshold selections. Ecological Modelling 2013, 259, 30–39. [CrossRef]
| Agroclimatic Region | Annual moisture availability index | Annual mean temperature (°C) | Total Accessions |
|---|---|---|---|
| Semiarid very warm | 0.2 - 0.5 | >26 | 17 |
| Semiarid warm | 0.2 - 0.5 | 22 - 26 | 22 |
| Semiarid semi-warm | 0.2 - 0.5 | 18 a 22 | 14 |
| Semiarid temperate | 0.2 - 0.5 | 12 a 18 | 3 |
| Dry-subhumid very warm | 0.5 - 0.65 | > 26 | 20 |
| Dry-subhumid warm | 0.5 - 0.65 | 22 – 26 | 66 |
| Dry-subhumid semi-warm | 0.5 - 0.65 | 18 – 22 | 88 |
| Dry-subhumid temperate | 0.5 - 0.65 | 12 – 18 | 7 |
| Humid-subhumid very warm | 0.5 - 0.65 | >26 | 19 |
| Humid-subhumid warm | 0.65 - 1.0 | 22 - 26 | 67 |
| Humid-subhumid semi-warm | 0.65 - 1.0 | 18 – 22 | 98 |
| Humid-subhumid temperate | 0.65 - 1.0 | 12 - 18 | 11 |
| Humid very warm | >1.0 | >26 | 2 |
| Humid warm | >1.0 | 22 - 26 | 26 |
| Humid semi-warm | >1.0 | 18 - 22 | 12 |
| Humid temperate | >1.0 | 12 - 18 | 6 |
| Humid semi-cold | >1.0 | 5 - 12 | 3 |
| FAO Soil Unit | Soil Texture | Total Accessions |
|---|---|---|
| Lithosol | Coarse | 108 |
| Regosol calcaric | Coarse | 57 |
| Regosol eutric | Coarse | 209 |
| Faozem haplic | Coarse | 34 |
| Vertisol cromic | Fine | 39 |
| Solonchak ortic | Fine | 22 |
| Fluvisol eutric | Medium | 10 |
| Fluvisol calcaric | Coarse | 1 |
| Environmental variables | Min | Max | Optimum |
|---|---|---|---|
| 1.Precipitation of the warmest quarter (mm) | 240 | 1,204 | 400-884 |
| 2.Precipitation of the driest month (mm) | 1 | 73 | 1-7 |
| 3.Annual mean precipitation (mm) | 444 | 2,886 | 700-1299 |
| 4.May-October mean precipitation (mm) | 344 | 1,943 | 700-1199 |
| 5.November-April mean precipitation | 23 | 863 | 30-100 |
| 6.Annual moisture availability index | 0.27 | 2.32 | 0.40-0.99 |
| 7.November-April availability index | 0.026 | 1.83 | 0.030-1,300 |
| 8.May-October availability index | 0.005 | 1.47 | 0.009-1.4 |
| 9.Maximum maximorum temperature (°C) | 24.61 | 41.17 | 29-37 |
| 10.Minimum minimorum temperature (°C) | 1.7 | 18.2 | 5-15 |
| 11.Annual mean temperature (°C) | 14.66 | 28.51 | 19-27 |
| 12.May-October mean temperature | 9.13 | 29.88 | 19-26 |
| 13.November-April mean temperature | 7.95 | 27.83 | 19-26 |
| 14.Annual thermal oscillation (°C) | 10.42 | 19.54 | 13.16 |
| 15.Annual temperature range (°C) | 1.54 | 14.52 | 3-7 |
| 16.Soil texture | Arenoso | Fina | Media |
| 17.May-October mean photoperiod (h) | 12.5 | 12.9 | 12.6-12.9 |
| 18.November-April mean photoperiod (h) | 10.97 | 11.47 | 11.10-11.39 |
| 19.Growing season | 120-190 | ||
| 20.Altitude (mm) | 6 | 4,295 | 200-1800 |
| Environmental variables | Contribution (%) | Permutation importance (%) |
|---|---|---|
| Precipitation of the warmest quarter (mm) | 42.4 | 49 |
| Pprecipitation of the driest month (mm) | 17.5 | 2.8 |
| Minimum temperature of the coldest month (°C) | 15 | 31 |
| November-April mean solar radiation (w/m2) | 10 | 0.8 |
| Annual mean relative humidity (%) | 8.5 | 3.6 |
| Annual moisture availability index | 5.7 | 7.4 |
| May-October mean temperature (°C) | 0.9 | 5.3 |
| Institution/source | Institution/Department | Accessions |
|---|---|---|
| Universidad Nacional Autónoma de México (UNAM). | Instituto de Biología | 169 |
| Instituto de Ecología (INECOL). | Xalapa Veracruz | 30 |
| Universidad Autónoma de Querétaro (UAQ). | Facultad de Ciencias Naturales | 3 |
| Instituto Nacional de Estadística y Geografía (INEGI). | Departamento de Botánica | 2 |
| Universidad Autónoma de Aguascalientes (UAA). | Centro de Ciencias Básicas | 2 |
| Universidad Autónoma de Veracruz (UPAV) (CIB). | Instituto de Investigaciones Biológicas | 1 |
| Universidad Autónoma de San Luis Potosí (UASLP). | Instituto de Investigación de Zonas Desérticas | 3 |
| Colegio de la Frontera Sur (ECO SUR). | Herbario San Cristóbal | 3 |
| Universidad de Guadalajara (CUCBA, CUC SUR). | Herbario IBUG, Herbario ZEA | 6 |
| Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Michoacán. | Herbario Facultad de Biología Universidad Michoacana de San Nicolás de Hidalgo | 6 |
| Artículos científicos/Inventarios florísticos de los estados de Oaxaca, Chiapas, Veracruz, Tabasco, Guerrero, Puebla, Jstor Plant Science. | 20 | |
| Universidad Autónoma de Nuevo León (UNL). | Facultad de Ciencias Biológicas | 1 |
| La Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). | Herbario digital de CONABIO | 3 |
| Trópicos.org. | 3 | |
| Red de Herbarios del Noroeste de México. | 13 | |
| GBIF | 215 | |
| Total | 480 |
| Variable | Description | Temporal scale |
|---|---|---|
| BIO01 | (Annual mean temperature) | Annual |
| BIO02 | Mean diurnal range | Variation |
| BIO03 | Isothermality | Variation |
| BIO04 | Temperature seasonality | Variation |
| BIO05 | (Maximum temperature of the warmest month) | Month |
| BIO06 | (Minimum temperature of the coldest month) | Month |
| BIO07 | Temperature annual range | Annual |
| BIO08 | (Mean temperature of the wettest quarter) | Quarter |
| BIO09 | (Mean temperature of the driest quarter) | Quarter |
| BIO10 | (Mean temperature of the warmest quarter) | Quarter |
| BIO11 | (Mean temperature of the coldest quarter) | Quarter |
| BIO12 | Annual precipitation | Annual |
| BIO13 | Precipitation of the wettest month | Month |
| BIO14 | Precipitation of the driest month | Month |
| BIO15 | Precipitation seasonality | Variation |
| BIO16 | Precipitation of the wettest quarter | Quarter |
| BIO17 | Precipitation of the driest quarter | Quarter |
| BIO18 | Precipitation of the warmest quarter | Quarter |
| BIO19 | Precipitation of the coldest quarter | Quarter |
| N-AMT | November-April mean temperature | Seasonal |
| M-OMT | May-October mean temperature | Seasonal |
| M-OXT | Maximum temperature May-October | Seasonal |
| N-AXT | November-April maximum temperature | Seasonal |
| AXT | Annual maximum temperature | Annual |
| M-OIT | May-October minimum temperature | Seasonal |
| N-AIT | November-April minimum temperature | Seasonal |
| AIT | Annual minimum temperature | Annual |
| ATO | Annual thermal oscillation | Annual |
| M-OP | May-October precipitation | Seasonal |
| N-AP | November-April Precipitation | Seasonal |
| M-OPH | May-October photoperiod | Seasonal |
| N-APH | November-April photoperiod | Seasonal |
| AMI | Annual moisture index | Annual |
| M-OMI | May-October mean moisture index | Seasonal |
| N-AMI | November-April mean moisture index | Seasonal |
| ASR | Annual mean solar radiation | Annual |
| M-OSR | May-October mean solar radiation | Seasonal |
| N-ASR | November-April solar radiation | Seasonal |
| ARH | Annual relative humidity | Annual |
| M-ORH | May-October relative humidity | Seasonal |
| N-ARH | November-April relative humidity | Seasonal |
| GSL | Growing season length | Seasonal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





