1. Introduction
In recent years, there has been a great deal of interest in microfluidics and lab-on-a-chip-based research as a result of the global focus on biological phenomena. One of the most comprehensive categories of these investigations involves microfluidic studies on PDMS as a silicon-based polymer [
1,
2]. PDMS has a number of special properties due to the fact that it is chemically neutral, biocompatible, transparent, and flexible. PDMS is an inexpensive polymer that can be molded using photolithography-based methods and micrometer resolution [
3]. The above-mentioned properties and ease of access make this polymer widely used in biotechnology, microfluidic chips, and electronics [
4,
5]. The hydrophobicity of this polymer, however, limits its application in some applications, despite its advantages [
6]. In treating PDMS using the method, the most significant problem is the rapid recovery of the polymer's hydrophobicity after treatment.
Some applications require properties such as hydrophobicity and hydrophilicity. A PDMS chip's hydrophobic and hydrophilic properties affect surface adhesion, bioactive reactions, and fluid flow within the chip [
7,
8,
9]. This polymer has therefore been modified using multi objective optimization methods [
10,
11,
12] in order to increase its surface hydrophilicity. Plasma treatment can be an effective method of modifying the surface's hydrophilicity and hydrophobicity [
13]. To increase the surface hydrophilicity, APJ and DBD plasmas have been used [
14]. Plasma jets are easy to use due to the fact that they do not depend on the type, shape, or size of the treatment; this makes them more practical. Due to its greater applicability and relatively low cost, air as the process atmosphere has significant advantages over other options when it comes to plasma processing [
15,
16].
Many studies have been conducted on the performance of the PDMS surface hydrophilizing process due to its importance [
17,
18,
19,
20,
21,
22].
Nascimento et al. determined the water contact angle as a function of plasma process time for PDMS surfaces. A study by Nascimento et al. [
23] used pulsed dielectric barrier discharge plasmas to treat polydimethylsiloxane (PDMS) surfaces and found that surface roughness was reduced more rapidly than the water contact angle was reduced. In the bonding process, the water contact angle did not change since PDMS is processed with almost no discharge. By statistical design of experiments, Jofre-Reche et al. [
24] optimized operating conditions, and they found that an increase in oxygen content on the treated surface of PDMS increased its hydrophilicity.
Li et al. [
25] conducted an investigation to assess the biomedical effects of cold atmospheric plasma treatment on PDMS thickness. There is a thickness-dependent relationship between the permeability of CAPs with PDMS thickness less than 2.5 mm. In order to study the surface modification process of bound polydimethylsiloxane microchannels, Bashir et al. [
26] used a dielectric barrier corona discharge at atmospheric pressure. In accordance with the results, the surface of the channel exposed to plasma became hydrophilic. The optimization of microwave plasma treatment conditions on PDMS was also investigated by Aymes-Chodur et al. [
27]. Analytical methods were used to demonstrate that the radicals formed to serve as initiators for thiol grafting.
As part of their investigation of the treatment of organic pollutants in water, Patinglag et al. [
28] examined the potential of a microfluidic plasma reactor with a dielectric barrier discharge. Using methylene blue in solution, plasma-induced degradation of dissolved organic compounds has been studied in microfluidic devices. A flow rate of 35 liters per minute, a barrier thickness of 2 mm, and a channel depth of 50 m resulted in 97% degradation when air was used as the carrier gas.
The surface modification of PDMS has been studied experimentally and numerically [
29,
30,
31,
32], but little is known about the optimal conditions for imparting hydrophilicity to the PDMS surface. Accordingly, the present study investigates how parameters such as plasma treatment duration, voltage variation (of the plasma source), sample distance from the nozzle tip, and hydrophilicity properties of PDMS samples are affected by cold atmospheric plasma treatment. It was our goal in this study to identify and determine the optimum conditions for making PDMS surfaces hydrophilic as well as the variation in the duration of hydrophobic recovery. Furthermore, APJ and DBD plasma were compared for their ability to increase the surface hydrophilicity of the samples, as well as their ability to preserve the hydrophilicity of the treated surfaces.
2. Experimental Section
2.1. Material and Methods
Silgard 184 Polymer was provided by Sigma Corporation along with its curing agent. As part of the preparation of the samples, the polymer was mixed with a curing agent at a weight ratio of 10:1 and then vacuum-dried to remove bubbles. To complete the preparation process, the oven was incubated at 90 °C for 45 minutes. Samples of PDMS were prepared with dimensions of 25×25mm. From the nozzle tips of APJ, samples were placed at intervals of 5 to 25 mm. DBD was used to treat samples by placing them between two electrodes with an air gap of 4 mm. Each plasma source was tested using uniform and close electrical power selection to compare its efficacy.
A total of four groups of tests were conducted to investigate the influence of parameters such as treatment duration, sample distance from the plasma torch tip, source voltage, and aging duration on the surface hydrophilicity of the samples. In addition, DBD plasma was used to treat the samples, and the results were compared with other samples.
2.2. Atmospheric Pressure Plasma Treatment
As a source of low-temperature and non-equilibrium atmospheric pressure plasmas, atmospheric pressure plasma jets have been established. As a result of its remote operation (jet is not constrained by electrodes), this plasma tool has a scalable dimension that can be adjusted from several centimeters down to sub-millimeters, allowing for local treatment of 3D surfaces, such as inner walls, trenches, or cavities.
There are a number of advantages to using air plasma jets for the treatment of low-temperature and non-equilibrium atmospheric pressure plasmas [
1]. It is the ability to process complex shapes and 3D surfaces that makes plasma jets so valuable [
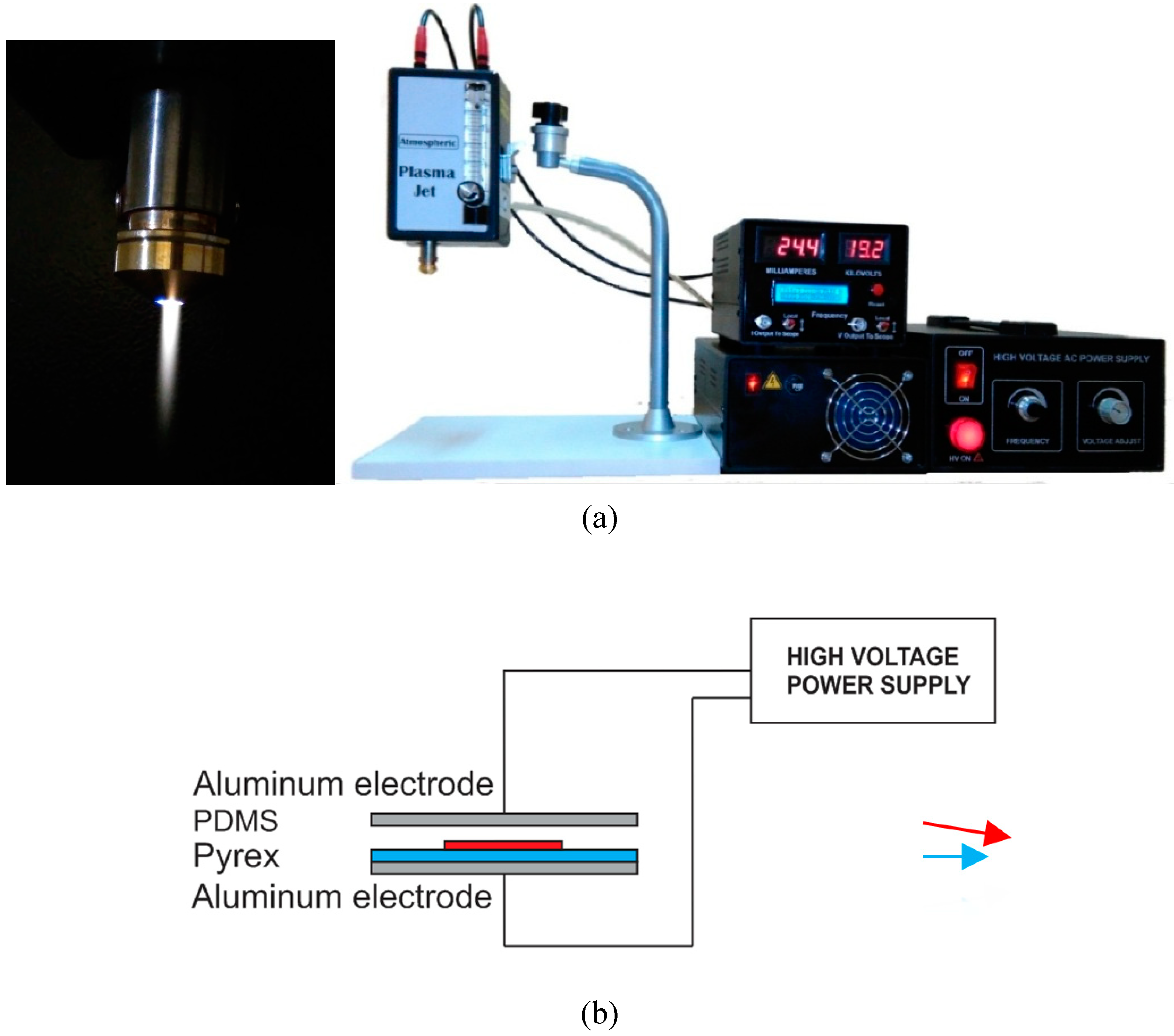
33]. Consequently, the plasma created by it is not confined to a limited area. A plasma jet device, manufactured by Megavolt Co., consisted of a jet nozzle with a zero-dielectric inner electrode and a ground-up setup which was equipped with an 8 kHz high voltage power supply and a 0 to 22 kV sinusoidally pure voltage supply. Plasma jets were supplied by a compressor with a flow rate of approximately 10 liters per minute. The APJ systems are illustrated in
Figure 1.
2.3. DBD Plasma Treatment
A Dielectric Barrier Discharge plasma (DBD) is a non-thermal plasma that establishes a uniform and stable plasma at atmospheric pressure, which can be generated in open air or in a closed chamber. There is an electrical discharge between two electrodes, one covered with a dielectric plate. There are various applications for DBD plasma, such as improving wettability and improving the adhesion of surfaces [
34,
35,
36]. DBD plasma operates at atmospheric pressure and has a low temperature suitable for interacting with sensitive surfaces of materials. For the treatment, a DBD plasma generator with a dielectric layer of Pyrex was used.
Figure 2 illustrates a plasma generator with an operating voltage of 20 kV, a current output of 15 mA, and an operating frequency of 8 kHz that produces uniform plasma.
2.4. Plasma characterization
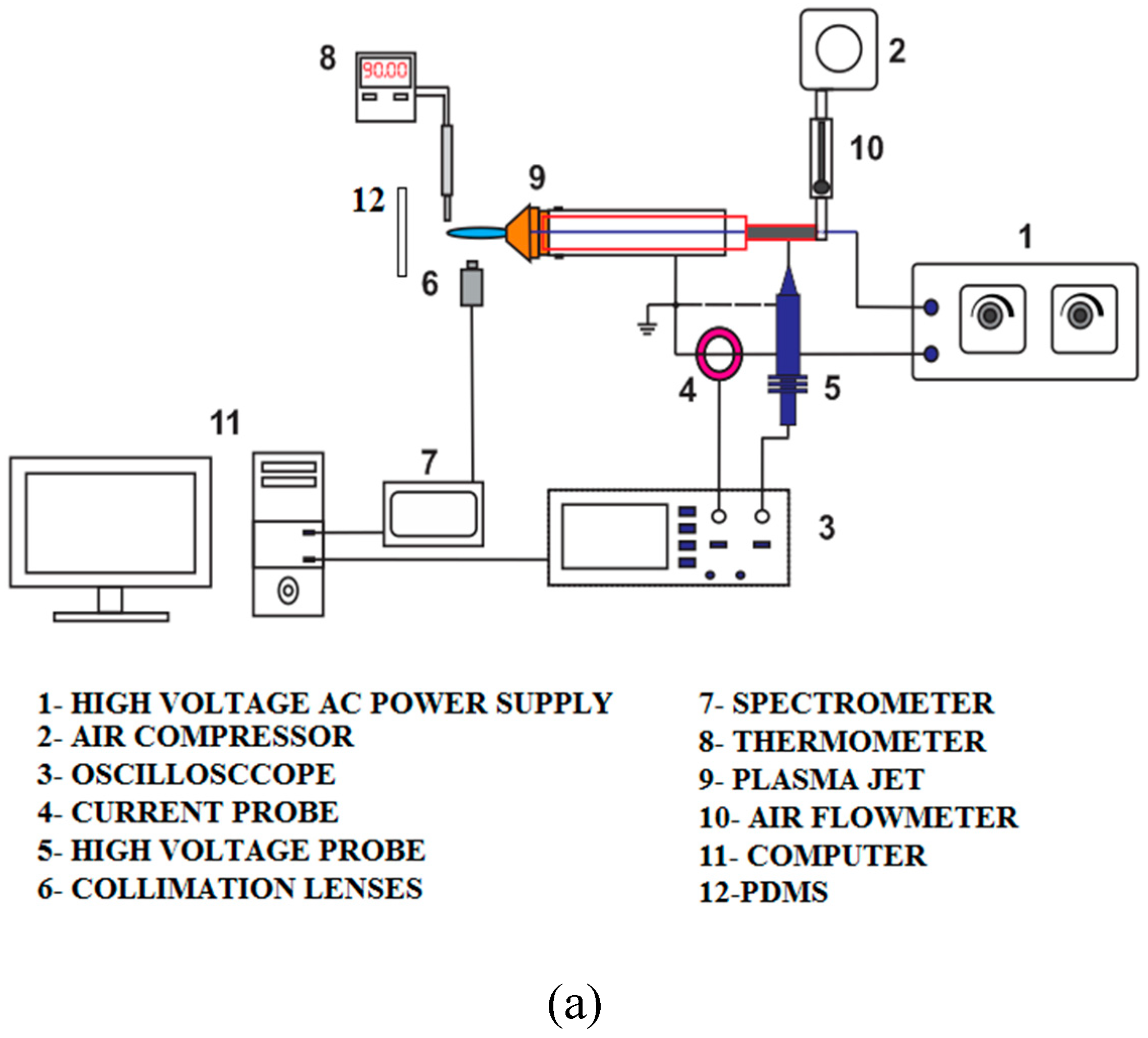
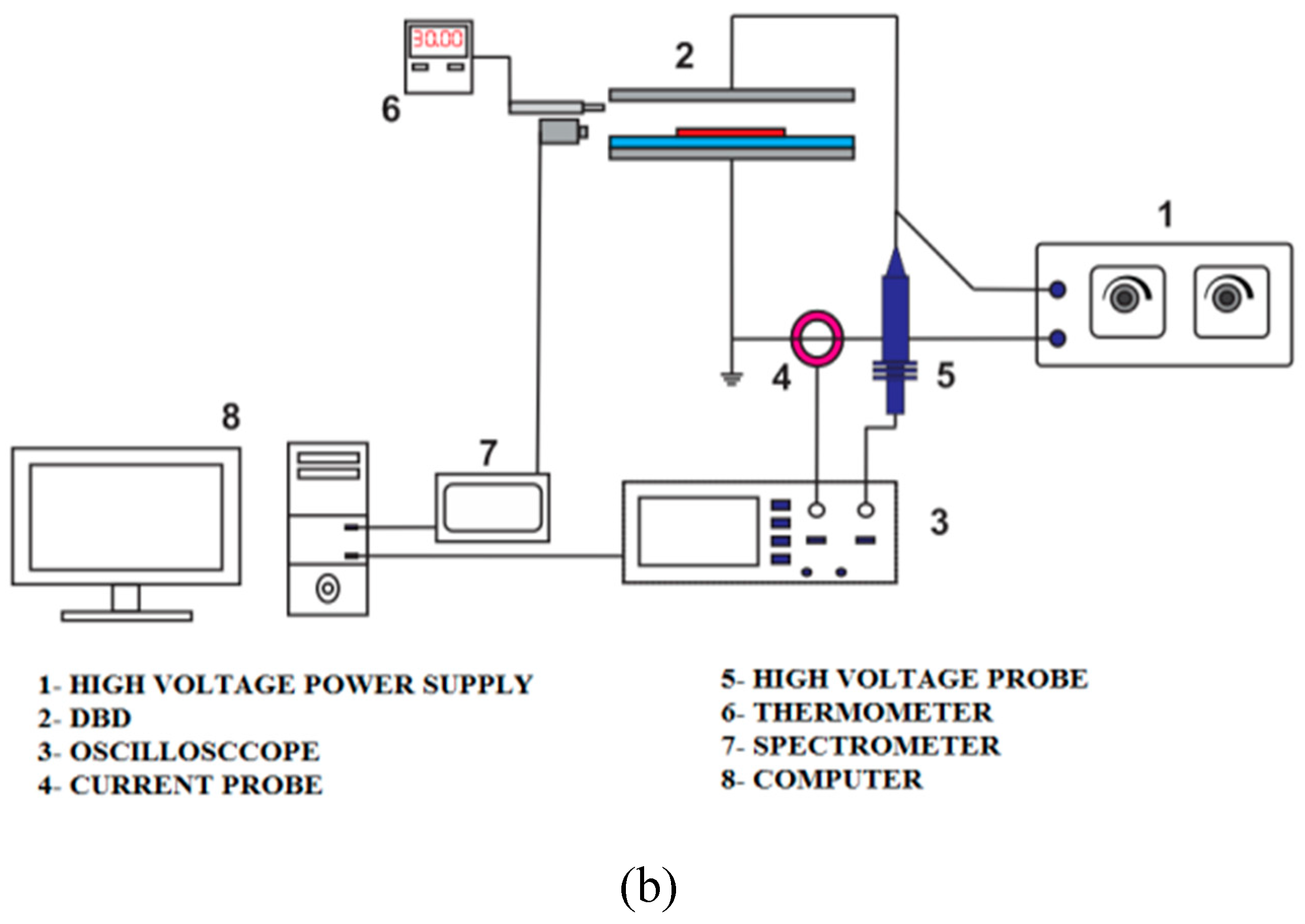
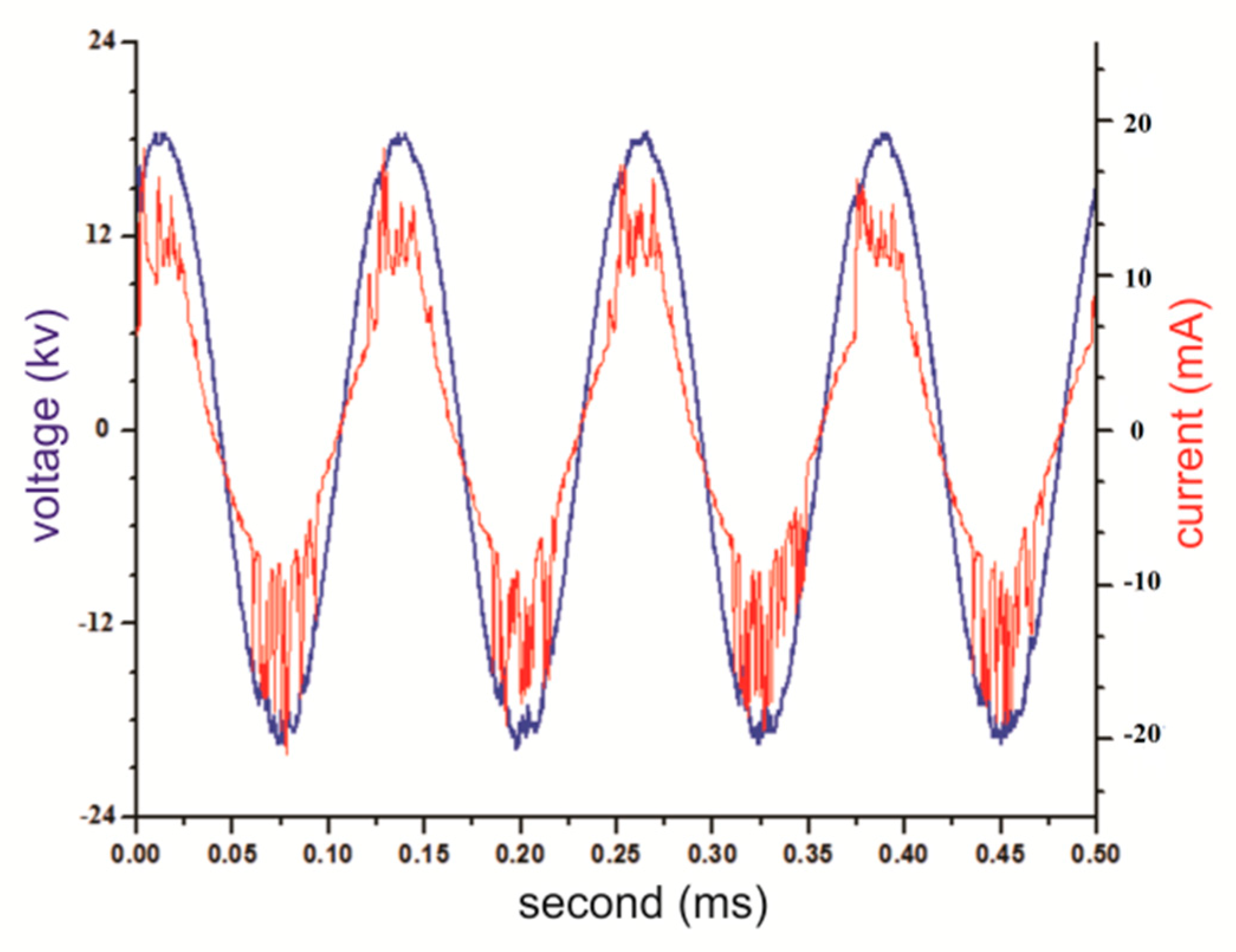
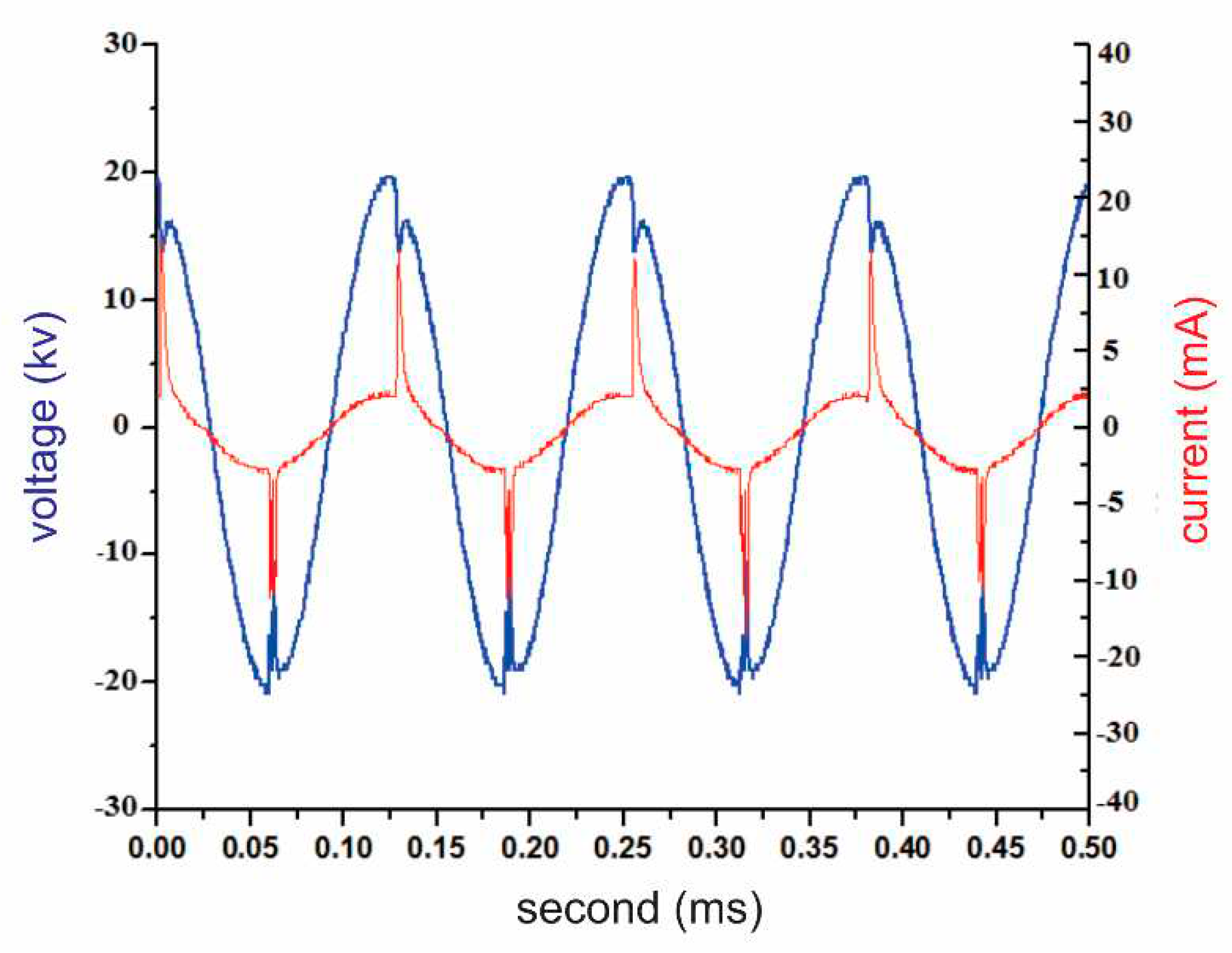
Tektronix P6015A high voltage probe and CC-65 Hantek current clamp used to measure and display voltage and current of the high voltage power supply on the Tektronix 2024c oscilloscope.
Figure 3 illustrates the layout schematic for the APJ and DBD characterizations.
Based on equation (1), DBD's electrical power was approximately 300 watts at 8 kHz, 20 kV applied voltage, 15 mA current, and 20 kV applied voltage. Furthermore, the plasma jet was operated at an applied voltage of 18 kV and an output current of 17 mA. This resulted in an output power of 306 watts. Due to the fact that the plasma jet relies on a direct electric discharge without a dielectric layer, the operating voltage of the DBD plasma is greater than that of the plasma jet. According to the equation (1), plasma jet power can be calculated as follows:
The temperature of the plasma torch of APJ was measured at intervals of 5, 10, 15, 20, and 25 mm using a grounded metal shield thermocouple sensor on the DM6801A thermometer (
Table 1). A thermocouple sensor with a 32°C temperature range was used to measure the plasma temperature for DBD between the two electrodes.
2.5. Plasma spectrum Characterization
A dual spectroscopy device from Avantes-Avesepec was used to measure optical emission spectroscopy (OES). More light was obtained by using a collimator lens (COL-UV / VIS-25).
2.5.1. Water Contact Angle Measurements
In order to measure the hydrophilicity of the treated surfaces, the water contact angle test was conducted. During the measurement, Dataphysics Company's OCA-20 water contact angle measurement setup was used. In less than 30 seconds following the treatment process, samples were evaluated based on the droplet contact angle. The test was conducted by dropping 5 microliters of distilled water at 25 degrees Celsius onto the sample and measuring the contact angle between the droplet and the surface. For this test, a distance of 5mm should be maintained between the sample and the nozzle tip. In both plasma sources, the electric power was identical with 20 kV and 15 mA in DBD and 18 kV and 17 mA in APJ.
According to the surface contact angle measurement test, the surface wettability decreases with increasing distance from the nozzle tip in APJ. Generally, the best results were observed at a distance of 5 mm from the tip of the nozzle, which results in a lower contact angle.
Increased voltage and higher bombardment rates increased plasma density and ionization. Comparison of APJ and DBD for a treatment time of 10 seconds and a distance of 5 mm from the nozzle tip in APJ. Electric power was the same in both plasma sources, at 20 kV and 15 mA in DBD and 18 kV and 17 mA in APJ. The surface hydrophilicity of the APJ surface increased with increasing voltage of the APJ power supply. The minimum surface contact angle for DBD was 4.1 degrees and for the plasma jet 4.9 degrees. This was when the voltage was increased to 22 kV.
2.5.2. Hydrophobic Recovery
After treatment with APJ and DBD plasma, the surface aging time is maintained for up to five hours. In order to determine the surface hydrophilicity of the surface, surface contact angle measurements were performed up to 24 hours after treatment. PDMS surface aging by DBD treatment is slower than aging by APJ treatment, as shown in
Figure 4.
3. Results and Discussion
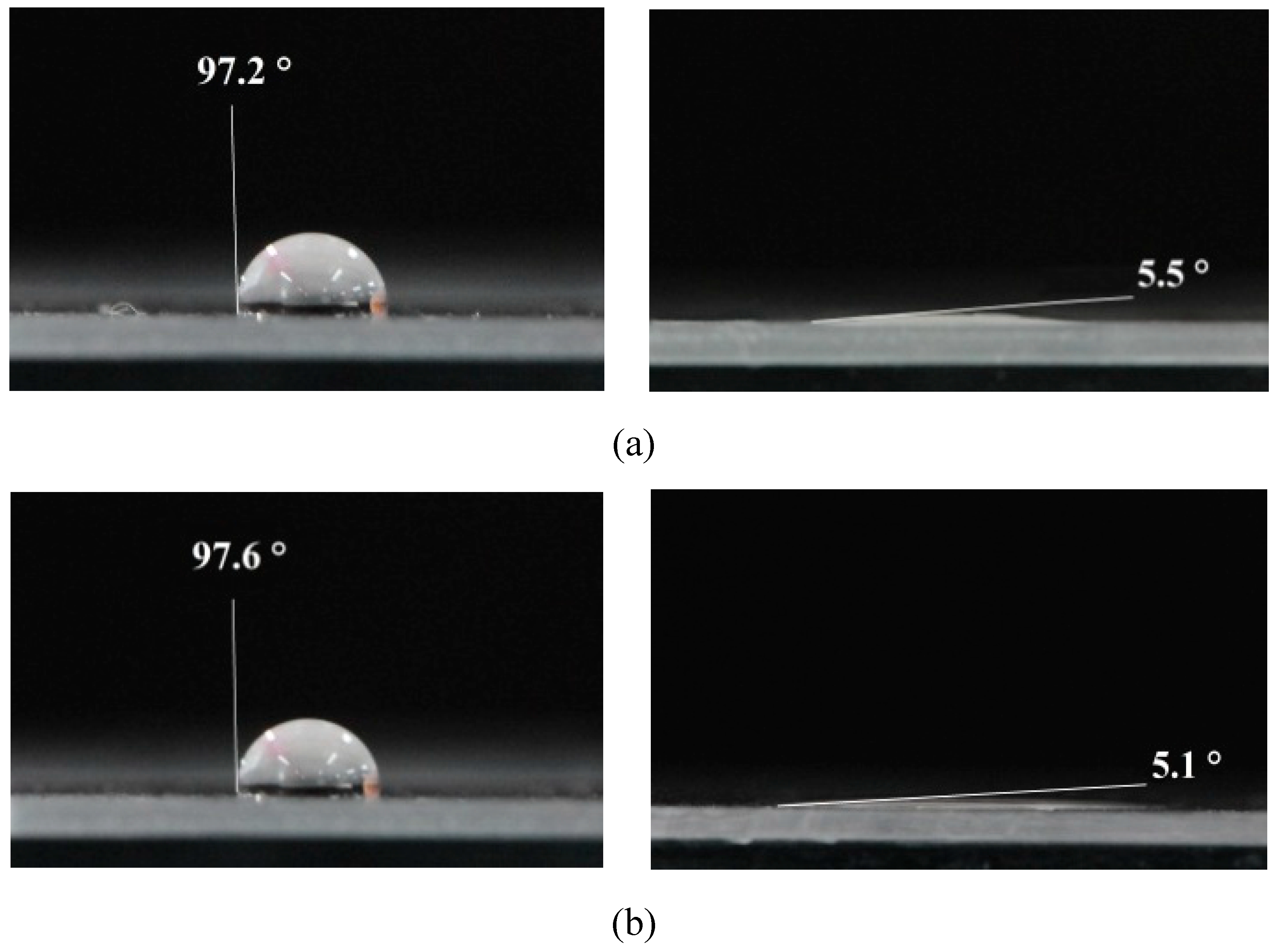
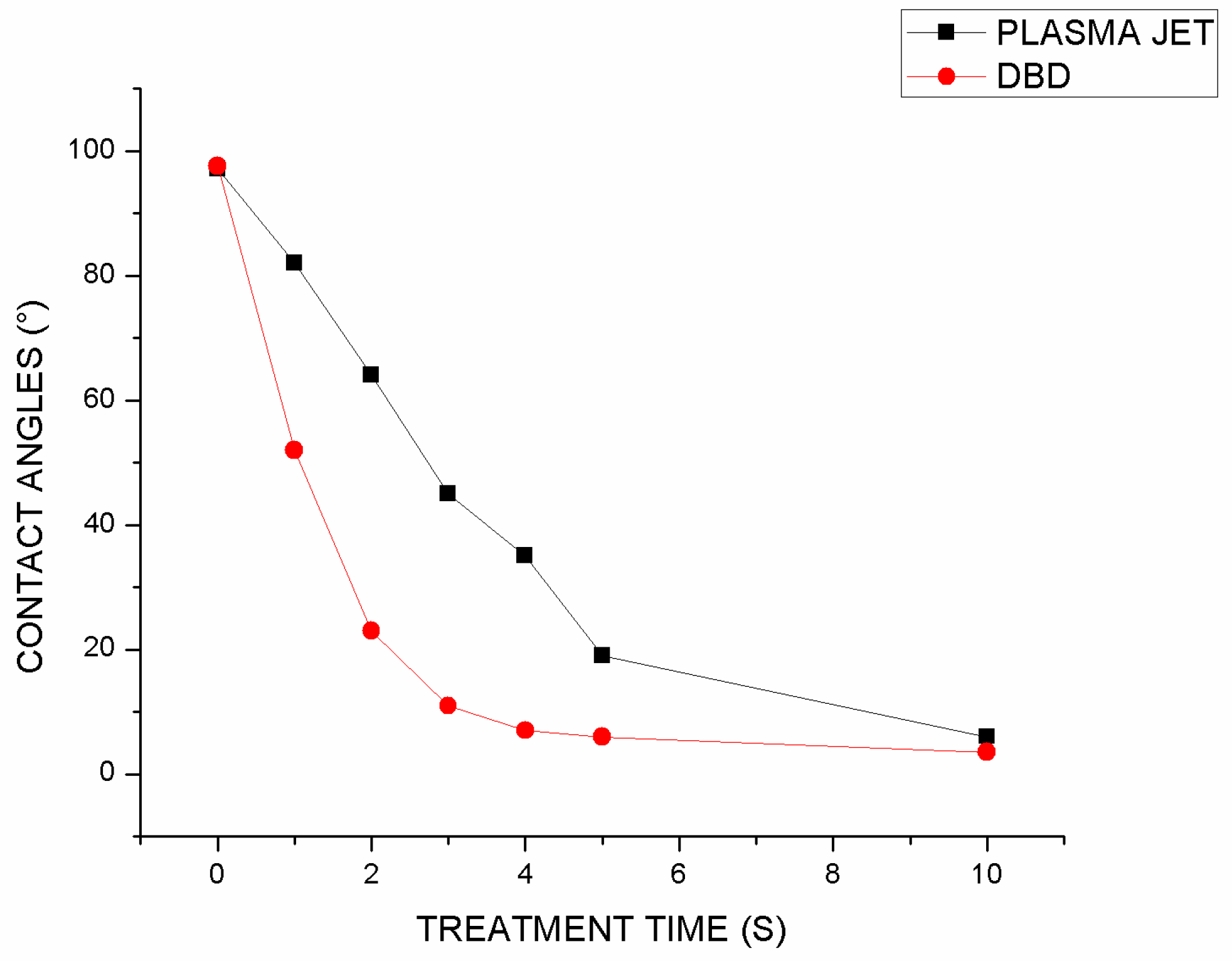
On a variety of specimens, the drop angle test was conducted approximately 20 seconds after the treatment. The untreated sample was also used as a criterion for comparison. This sample had a contact angle of 97.4 degrees based on the results of the contact angle test (
Figure 5). We examined the effects of parameters such as treatment duration, voltage, and sample distance from the plasma torch on three sample groups. In the results, it is evident that the contact angle between the droplet and the surface decreases with an increase in plasma treatment duration, such that by 10 seconds of plasma jet treatment, the contact angle reaches 5.5 degrees and by DBD treatment it reaches 5.1 degrees. As shown in
Figure 5, DBD plasma has a greater effect on increasing hydrophilicity than plasma jet, reaching a contact angle of 7 degrees after 4 seconds of treatment as compared to 35 degrees after treatment with plasma jet for an equal duration.
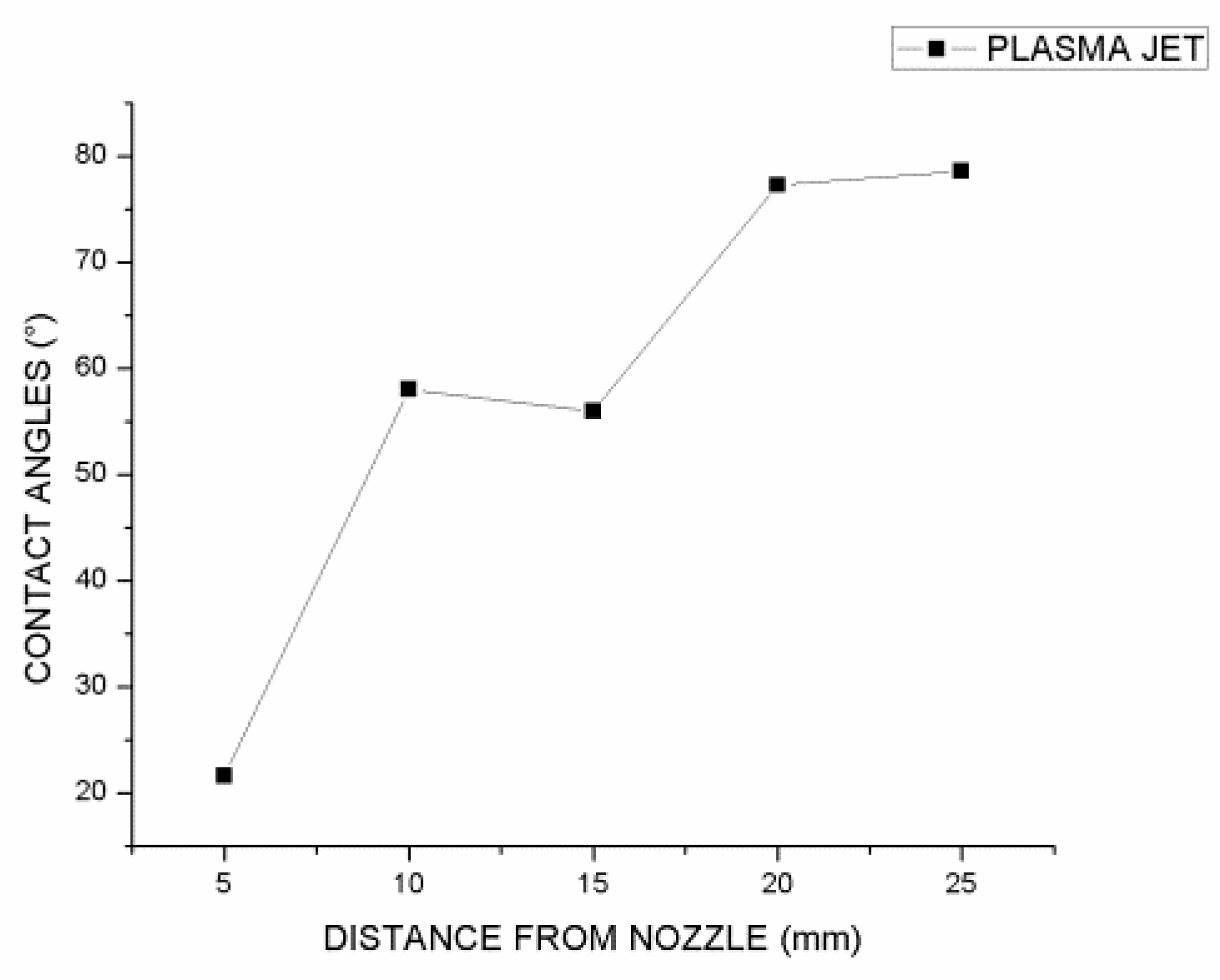
Five samples were subjected to APJ treatment at a frequency of 8 kHz and a voltage of 18 kV to investigate the effect of specimen distance from the plasma jet tip. In
Figure 6, it can be seen that reducing the distance between the specimen and the tip of the nozzle resulted in an increase in hydrophilicity, resulting in an increase in contact angle of 22 degrees for the sample with the shortest distance from the tip of the nozzle.
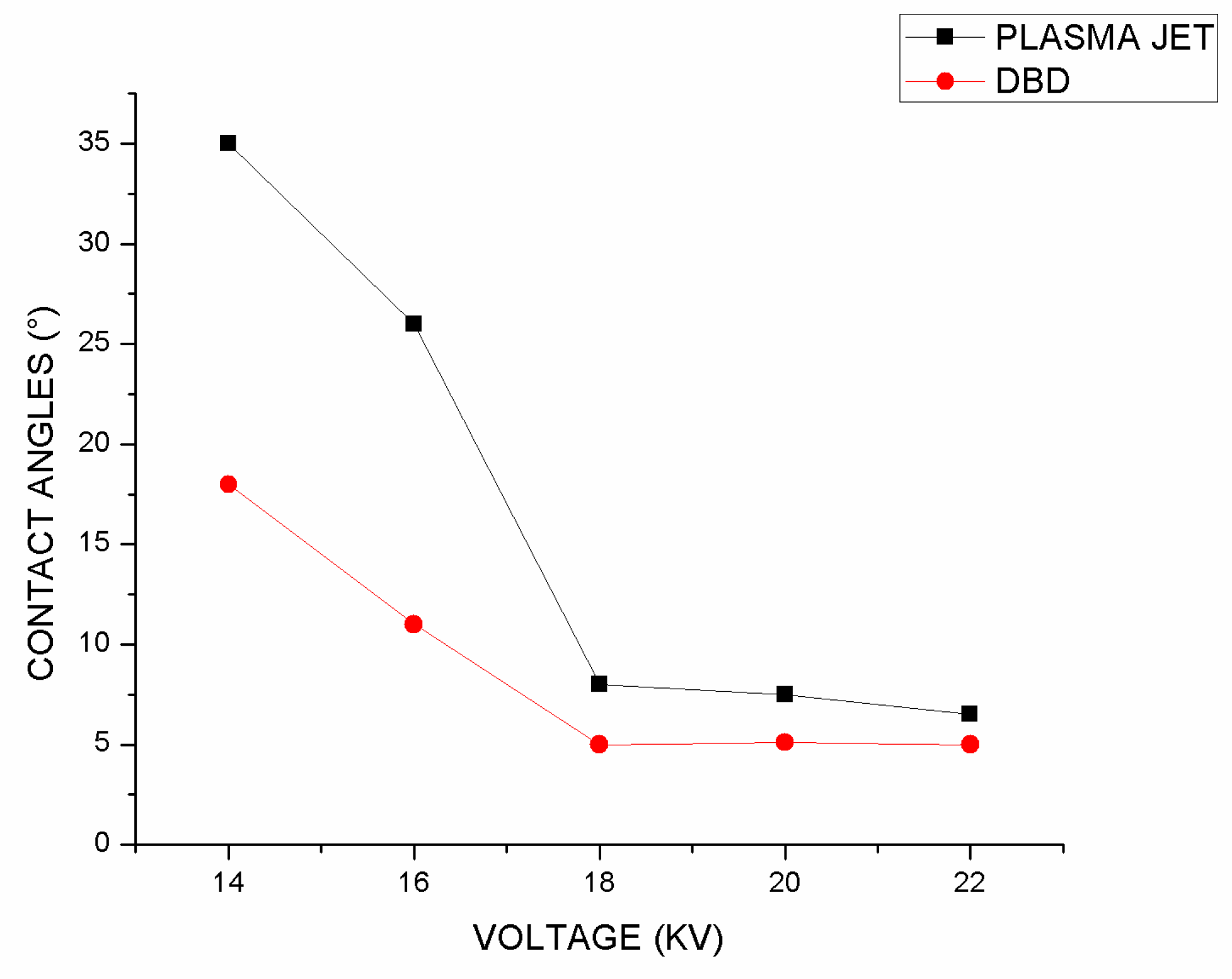
Following that, the effect of voltage variations on the contact angle was investigated, and it can be seen in
Figure 7 that the contact angle decreases with increasing voltage. DBD plasma performed better than plasma jet at increasing hydrophilicity, especially at lower voltages, based on the results of this study.
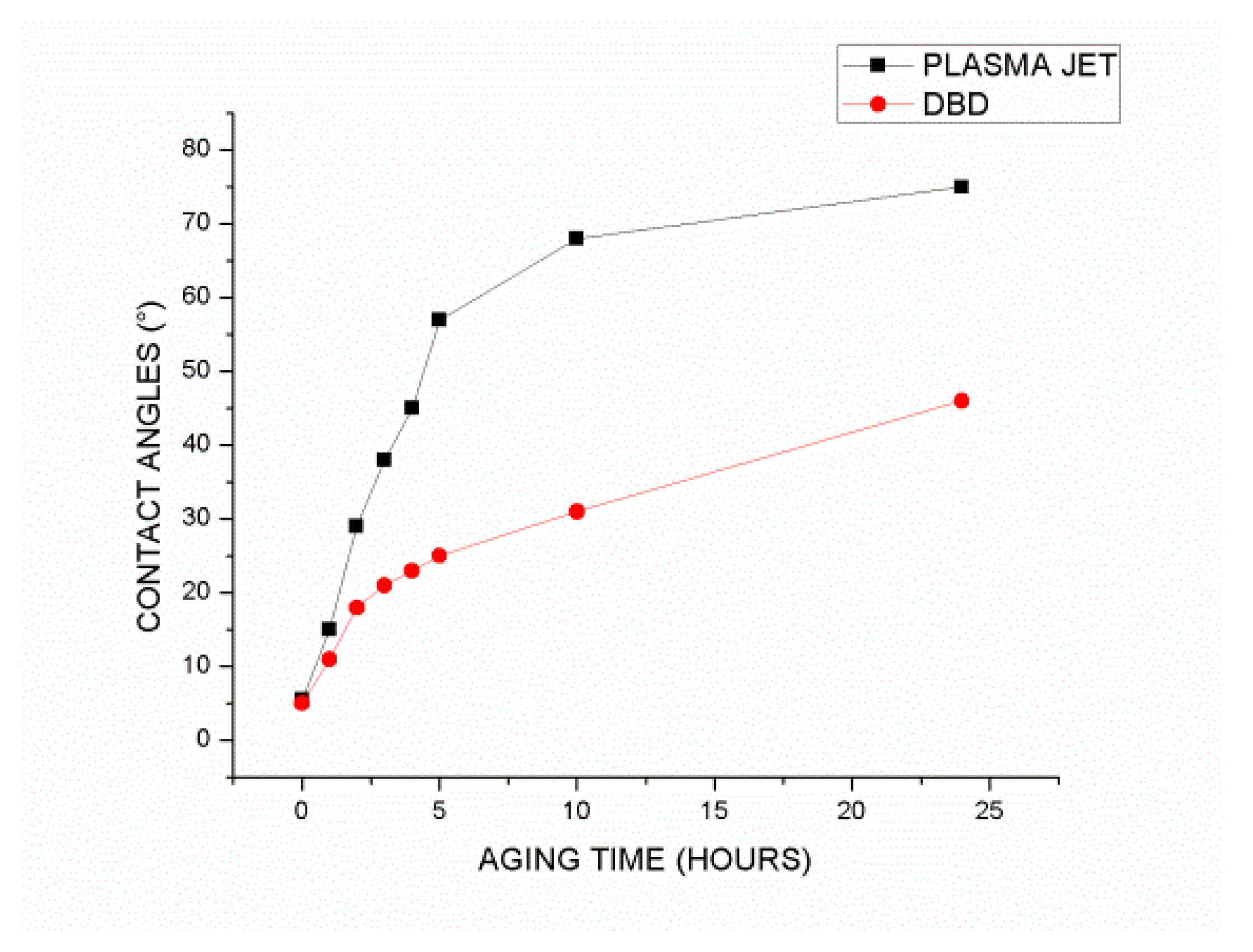
A contact angle test was used to determine the rate of hydrophobic recovery by two groups of specimens treated with two different sources.
Figure 8 illustrates the mean contact angle for three samples treated under identical conditions. Based on prior investigations, the cross-linked chains are displaced from bulk to the polymer surface over time to restore the hydrophobicity of the surface.
According to the results, hydrophobic recovery has been significantly faster for treated samples using plasma jet than for treated samples using DBD, as the contact angle increased by over 50 degrees after 5 hours of plasma jet treatment, whereas this change was approximately 20 degrees for treated surfaces using DBD. Moreover, after 25 hours of treatment, plasma jet-treated samples recovered their hydrophobicity to a great extent, whereas DBD-treated samples with contact angles of 45 degrees retained a considerable degree of hydrophilicity.
As the gas gets ionized, due to the voltage and current waveforms shown in
Figure 9, the ionization current is added to the normal current and the current waveform becomes noisy.
A voltage drop occurs at the point where the voltage reaches a peak, as shown in
Figure 10. Furthermore, the electrical current reaches its maximum only when the voltage peaks, when an electrical discharge occurs between the electrodes and the barrier between them, causing the plasma to operate in the bright discharge region.
APJ spectrum generally consists of two types of transitions related to oxygen and nitrogen, with nitrogen being the dominant transition. Nitric oxide is a free radical formed by electrical discharges into the atmosphere that has a peak at 321.1 nm and 440.1 nm. As a result of the collision of high-energy electrons with oxygen molecules during plasma generation, atomic oxygen species can be observed in the spectra of 400.80nm and 415.6nm.
The PDMS polymer is treated with plasma to remove hydrocarbon groups and form silanol groups in their place. Due to the breaking of hydrocarbon bonds attached to the Si atoms by the excited oxygen and hydrogen atoms in the plasma's atmosphere, silanol groups are formed on the Si atoms in the PDMS polymer chains. As opposed to non-polar hydrocarbon groups, silanol groups are polar and interact with polar water molecules, resulting in a high degree of hydrophilicity on the surface of polymers containing silanol functional groups. As a result, the hydrophilic property of the PDMS surface is significantly enhanced during the plasma treatment process. After the treatment process, the hydrophilicity of the surface decreases. Due to the displacement of oligomers from the bulk to the surface and the increase in hydrocarbon groups on the surface, this change has occurred. Over time, the hydrophobic properties of PDMS polymer can be recovered.
4. Conclusion
An investigation of the effects of multiple parameters on the surface hydrophilicity of PDMS after plasma treatment is presented in this paper. As part of the investigation, plasma treatment duration, voltage variation, sample distance from the nozzle tip, and hydrophilicity properties of the PDMS samples were examined. Additionally, the paper compares the ability of APJ and DBD plasma to increase the surface hydrophilicity of PDMS samples. The purpose of this paper is to provide a comprehensive understanding of the factors that affect the surface hydrophilicity of PDMS after plasma treatment. In order to impart hydrophilicity to PDMS surfaces, this information can be used for optimizing the plasma treatment process. To minimize the effects of hydrophobic recovery, it is also important to understand the mechanisms involved. Using this information, strategies can be developed to preserve the hydrophilicity of PDMS surfaces following plasma treatment. As a result of cold air atmospheric plasma, the time required to hydrophilize the PDMS surface is reduced. Therefore, plasma DBD produces a greater effect than APJ, resulting in a shorter development time for hydrophilicity on PDM surfaces, and a shorter scan time in DBD, which results in uniform treatment across all PDM surfaces. The distance between the plasma torch and the surface is also an important parameter in APJ. A lower level of surface hydrophilicity will result in a higher level of surface hydrophilicity. An increase in surface hydrophilicity was observed with an increase in voltage. A decrease in surface hydrophilicity was observed when the sample distance was increased from the plasma jet nozzle tip. As a result of increasing the voltage on DBD to 22 kV, the surface contact angle was reduced to 4.1 degrees.
References
- S. Gupta, A. Vilouras, and R. Dahiya, “Polydimethylsiloxane as polymeric protective coating for fabrication of ultra-thin chips,” Microelectron. Eng., vol. 221, p. 111157, Jan. 2020. https://doi.org/10.1016/J.MEE.2019.111157. [CrossRef]
- M. T. Frassica, S. K. Jones, P. Diaz-Rodriguez, M. S. Hahn, and M. A. Grunlan, “Incorporation of a silicon-based polymer to PEG-DA templated hydrogel scaffolds for bioactivity and osteoinductivity,” Acta Biomater., vol. 99, pp. 100–109, Nov. 2019. https://doi.org/10.1016/J.ACTBIO.2019.09.018. [CrossRef]
- B. H. Jo, L. M. Van Lerberghe, K. M. Motsegood, and D. J. Beebe, “Three-dimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer,” J. Microelectromechanical Syst., vol. 9, no. 1, pp. 76–81, Mar. 2000. https://doi.org/10.1109/84.825780. [CrossRef]
- D. Lee and S. Yang, “Surface modification of PDMS by atmospheric-pressure plasma-enhanced chemical vapor deposition and analysis of long-lasting surface hydrophilicity,” Sensors Actuators B Chem., vol. 162, no. 1, pp. 425–434, Feb. 2012. https://doi.org/10.1016/J.SNB.2011.12.017. [CrossRef]
- J. A. Juárez-Moreno, A. Ávila-Ortega, A. I. Oliva, F. Avilés, and J. V. Cauich-Rodríguez, “Effect of wettability and surface roughness on the adhesion properties of collagen on PDMS films treated by capacitively coupled oxygen plasma,” Appl. Surf. Sci., vol. 349, pp. 763–773, Sep. 2015. https://doi.org/10.1016/J.APSUSC.2015.05.063. [CrossRef]
- A. Vesel, R. Zaplotnik, G. Primc, and M. Mozetič, “Evolution of the Surface Wettability of PET Polymer upon Treatment with an Atmospheric-Pressure Plasma Jet,” Polym. 2020, Vol. 12, Page 87, vol. 12, no. 1, p. 87, Jan. 2020. https://doi.org/10.3390/POLYM12010087. [CrossRef]
- S. E. Hosseini and A. Keshmiri, “Experimental and numerical investigation of different geometrical parameters in a centrifugal blood pump,” Res. Biomed. Eng., vol. 38, no. 5, pp. 423–437, 2022. https://doi.org/10.1007/s42600-021-00195-8. [CrossRef]
- V. Sharma, M. Dhayal, Govind, S. M. Shivaprasad, and S. C. Jain, “Surface characterization of plasma-treated and PEG-grafted PDMS for micro fluidic applications,” Vacuum, vol. 81, no. 9, pp. 1094–1100, May 2007. https://doi.org/10.1016/J.VACUUM.2007.02.004. [CrossRef]
- Z. He, Y. Zhuo, F. Wang, J. He, and Z. Zhang, “Design and preparation of icephobic PDMS-based coatings by introducing an aqueous lubricating layer and macro-crack initiators at the ice-substrate interface,” Prog. Org. Coatings, vol. 147, p. 105737, Oct. 2020. https://doi.org/10.1016/J.PORGCOAT.2020.105737. [CrossRef]
- M. H. Shojaeefard, S. E. Hosseini, and J. Zare, “Numerical simulation and multi-objective optimization of the centrifugal pump inducer,” Modares Mech. Eng., vol. 17, no. 7, pp. 205–216, 2018.
- M. H. Shojaeefard, S. E. Hosseini, and J. Zare, “CFD simulation and Pareto-based multi-objective shape optimization of the centrifugal pump inducer applying GMDH neural network, modified NSGA-II, and TOPSIS,” Struct. Multidiscip. Optim., vol. 60, no. 4, pp. 1509–1525, May 2019. https://doi.org/10.1007/s00158-019-02280-0. [CrossRef]
- S. E. Hosseini, O. Karimi, and M. A. AsemanBakhsh, “Experimental Investigation and Multi-objective Optimization of Savonius Wind Turbine Based on modified Non-dominated Sorting Genetic Algorithm-II,” Preprints, Aug. 2023. https://doi.org/10.20944/PREPRINTS202308.1937.V1. [CrossRef]
- S. Bhattacharya, A. Datta, J. M. Berg, and S. Gangopadhyay, “Studies on surface wettability of poly(dimethyl) siloxane (PDMS) and glass under oxygen-plasma treatment and correlation with bond strength,” J. Microelectromechanical Syst., vol. 14, no. 3, pp. 590–597, Jun. 2005. https://doi.org/10.1109/JMEMS.2005.844746. [CrossRef]
- Z. Fang, J. Yang, Y. Liu, T. Shao, and C. Zhang, “Surface treatment of polyethylene terephthalate to improving hydrophilicity using atmospheric pressure plasma jet,” IEEE Trans. Plasma Sci., vol. 41, no. 6, pp. 1627–1634, 2013. https://doi.org/10.1109/TPS.2013.2259508. [CrossRef]
- J. Zare, S. E. Hosseini, and M. R. Rastan, “Airborne dust-induced performance degradation in NREL phase VI wind turbine: a numerical study,” Int. J. Green Energy, pp. 1–20, Aug. 2023. https://doi.org/10.1080/15435075.2023.2246544. [CrossRef]
- J. Zare, S. E. Hosseini, and M. R. Rastan, “NREL Phase VI wind turbine in the dusty environment,” arXiv Prepr. arXiv2304.06285, Apr. 2023. https://doi.org/10.48550/arXiv.2304.06285. [CrossRef]
- F. Jahangiri, T. Hakala, and V. Jokinen, “Long-term hydrophilization of polydimethylsiloxane (PDMS) for capillary filling microfluidic chips,” Microfluid. Nanofluidics 2019 241, vol. 24, no. 1, pp. 1–11, Nov. 2019. https://doi.org/10.1007/S10404-019-2302-2. [CrossRef]
- D. J. O’Brien, A. J. H. Sedlack, P. Bhatia, C. J. Jensen, A. Quintana-Puebla, and M. Paranjape, “Systematic Characterization of Hydrophilized Polydimethylsiloxane,” J. Microelectromechanical Syst., vol. 29, no. 5, pp. 1216–1224, Oct. 2020. https://doi.org/10.1109/JMEMS.2020.3010087. [CrossRef]
- M. Amerian, M. Amerian, M. Sameti, and E. Seyedjafari, “Improvement of PDMS surface biocompatibility is limited by the duration of oxygen plasma treatment,” J. Biomed. Mater. Res. Part A, vol. 107, no. 12, pp. 2806–2813, Dec. 2019. https://doi.org/10.1002/JBM.A.36783. [CrossRef]
- T. Trantidou, Y. Elani, E. Parsons, and O. Ces, “Hydrophilic surface modification of PDMS for droplet microfluidics using a simple, quick, and robust method via PVA deposition,” Microsystems Nanoeng. 2017 31, vol. 3, no. 1, pp. 1–9, Apr. 2017. https://doi.org/10.1038/micronano.2016.91. [CrossRef]
- H. Hu et al., “Hydrophilic PDMS with a sandwich-like structure and no loss of mechanical properties and optical transparency,” Appl. Surf. Sci., vol. 503, p. 144126, Feb. 2020. https://doi.org/10.1016/J.APSUSC.2019.144126. [CrossRef]
- W. Zhang, M. Kalulu, X.-H. Wang, X.-K. Xia, X.-L. Han, and Y. Jiang, “Reverse hydrophobic PDMS surface to hydrophilic by 1-step hydrolysis reaction,” Polym. Adv. Technol., vol. 29, no. 7, pp. 2103–2109, Jul. 2018. https://doi.org/10.1002/PAT.4319. [CrossRef]
- F. do Nascimento, S. Moshkalev, M. Machida, and S. Parada, “Treatment of PDMS surfaces using pulsed DBD plasmas: comparing the use of different gases and its influence on adhesion,” Jpn. J. Appl. Phys., vol. 55, no. 2, Apr. 2015. https://doi.org/10.7567/jjap.55.021602. [CrossRef]
- J. A. Jofre-Reche, J. Pulpytel, F. Arefi-Khonsari, and J. M. Martín-Martínez, “Increased adhesion of polydimethylsiloxane (PDMS) to acrylic adhesive tape for medical use by surface treatment with an atmospheric pressure rotating plasma jet,” J. Phys. D. Appl. Phys., vol. 49, no. 33, p. 334001, Jul. 2016. https://doi.org/10.1088/0022-3727/49/33/334001. [CrossRef]
- Y. Li, X. Hu, H. Li, Y. Zhang, and H. Chen, “Investigation of cold atmospheric plasma treatment in polydimethylsiloxane microfluidic devices with a transmural method,” J. Phys. Condens. Matter, vol. 30, no. 38, p. 384001, Aug. 2018. https://doi.org/10.1088/1361-648X/AAD981. [CrossRef]
- S. Bashir, M. Bashir, X. C. i Solvas, J. M. Rees, and W. B. Zimmerman, “Hydrophilic Surface Modification of PDMS Microchannel for O/W and W/O/W Emulsions,” Micromachines 2015, Vol. 6, Pages 1445-1458, vol. 6, no. 10, pp. 1445–1458, Sep. 2015. https://doi.org/10.3390/MI6101429. [CrossRef]
- C. Aymes-Chodur, H. Salmi-Mani, D. Dragoe, N. Aubry-Barroca, M. Buchotte, and P. Roger, “Optimization of microwave plasma treatment conditions on polydimethylsiloxane films for further surface functionalization,” Eur. Polym. J., vol. 150, p. 110416, May 2021. https://doi.org/10.1016/J.EURPOLYMJ.2021.110416. [CrossRef]
- L. Patinglag, D. Sawtell, A. Iles, L. M. Melling, and K. J. Shaw, “A Microfluidic Atmospheric-Pressure Plasma Reactor for Water Treatment,” Plasma Chem. Plasma Process. 2019 393, vol. 39, no. 3, pp. 561–575, Mar. 2019. https://doi.org/10.1007/S11090-019-09970-Z. [CrossRef]
- B. Gong, J. C. Spagnola, and G. N. Parsons, “Hydrophilic mechanical buffer layers and stable hydrophilic finishes on polydimethylsiloxane using combined sequential vapor infiltration and atomic/molecular layer deposition,” J. Vac. Sci. Technol. A Vacuum, Surfaces, Film., vol. 30, no. 1, p. 01A156, Dec. 2011. https://doi.org/10.1116/1.3670963. [CrossRef]
- S. Hemmilä, J. V. Cauich-Rodríguez, J. Kreutzer, and P. Kallio, “Rapid, simple, and cost-effective treatments to achieve long-term hydrophilic PDMS surfaces,” Appl. Surf. Sci., vol. 258, no. 24, pp. 9864–9875, Oct. 2012. https://doi.org/10.1016/J.APSUSC.2012.06.044. [CrossRef]
- H. P. Long, C. C. Lai, and C. K. Chung, “Polyethylene glycol coating for hydrophilicity enhancement of polydimethylsiloxane self-driven microfluidic chip,” Surf. Coatings Technol., vol. 320, pp. 315–319, Jun. 2017. https://doi.org/10.1016/J.SURFCOAT.2016.12.059. [CrossRef]
- C. C. Lai and C. K. Chung, “Hydrophilicity and optic property of polyethylene glycol coating on polydimethylsiloxane for fast prototyping and its application to backlight microfluidic chip,” Surf. Coatings Technol., vol. 389, p. 125606, May 2020. https://doi.org/10.1016/J.SURFCOAT.2020.125606. [CrossRef]
- O. Karimi, M. K. Koopaee, A. reza Tavakolpour-Saleh, and S. E. Hosseini, “Investigating Overlap Ratio Effect on Performance of a Modified Savonius Wind Turbine: An Experimental Study,” Preprints, Aug. 2023. https://doi.org/10.20944/PREPRINTS202308.1853.V1. [CrossRef]
- M. Machida, “Ferrite Loaded DBD Plasma Device,” Brazilian J. Phys. 2014 451, vol. 45, no. 1, pp. 132–137, Dec. 2014. https://doi.org/10.1007/S13538-014-0293-8. [CrossRef]
- N. K. Bibinov, A. A. Fateev, and K. Wiesemann, “Variations of the gas temperature in He/N2 barrier discharges,” Plasma Sources Sci. Technol., vol. 10, no. 4, p. 579, Sep. 2001. https://doi.org/10.1088/0963-0252/10/4/306. [CrossRef]
- Z. Fang, J. Lin, H. Yang, Y. Qiu, and E. Kuffel, “Polyethylene terephthalate surface modification by filamentary and homogeneous dielectric barrier discharges in air,” IEEE Trans. Plasma Sci., vol. 37, no. 5, pp. 659–667, 2009. https://doi.org/10.1109/TPS.2009.2015322. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).