Submitted:
31 August 2023
Posted:
04 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Synthesis of MOF
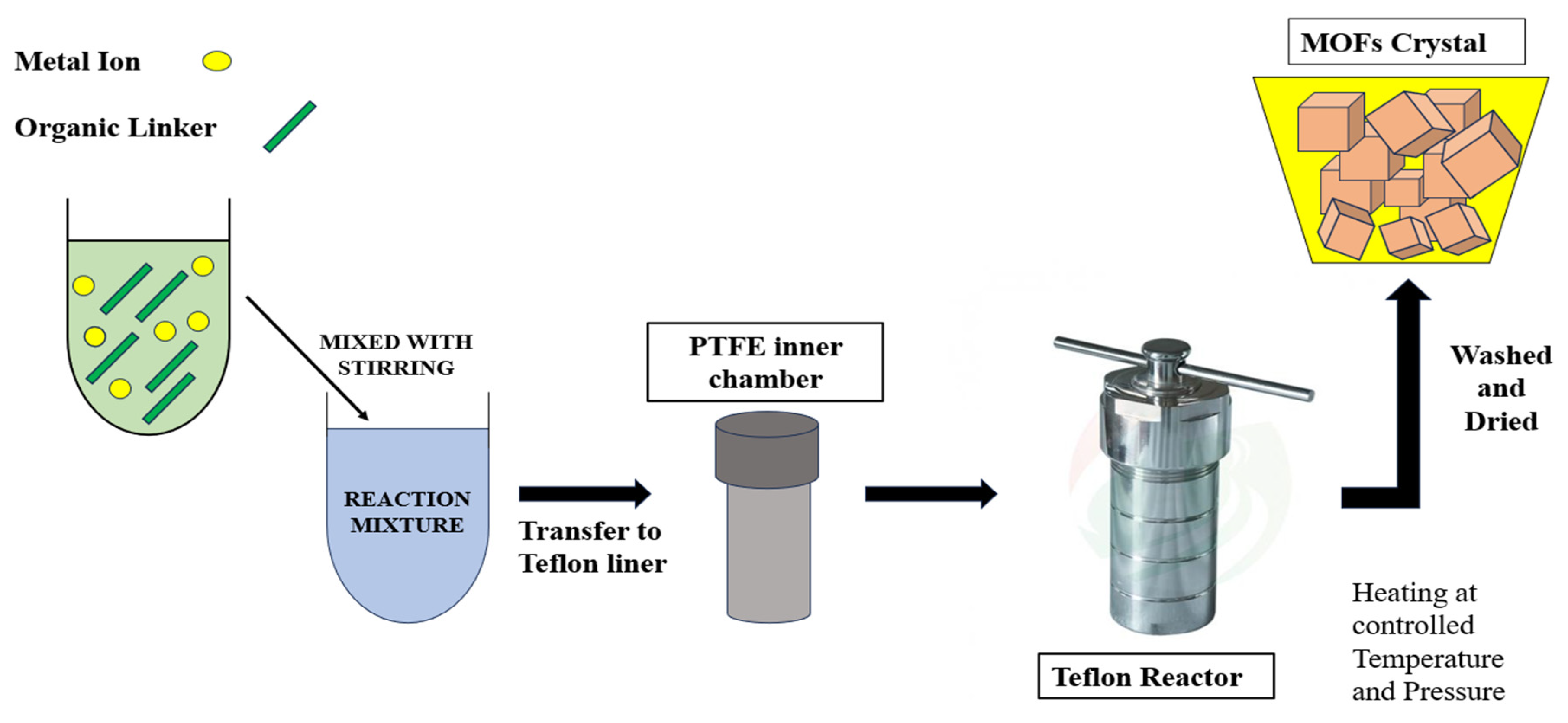
2.1. Hydrothermal/Solvothermal Synthesis
2.2. Ultrasonic methods
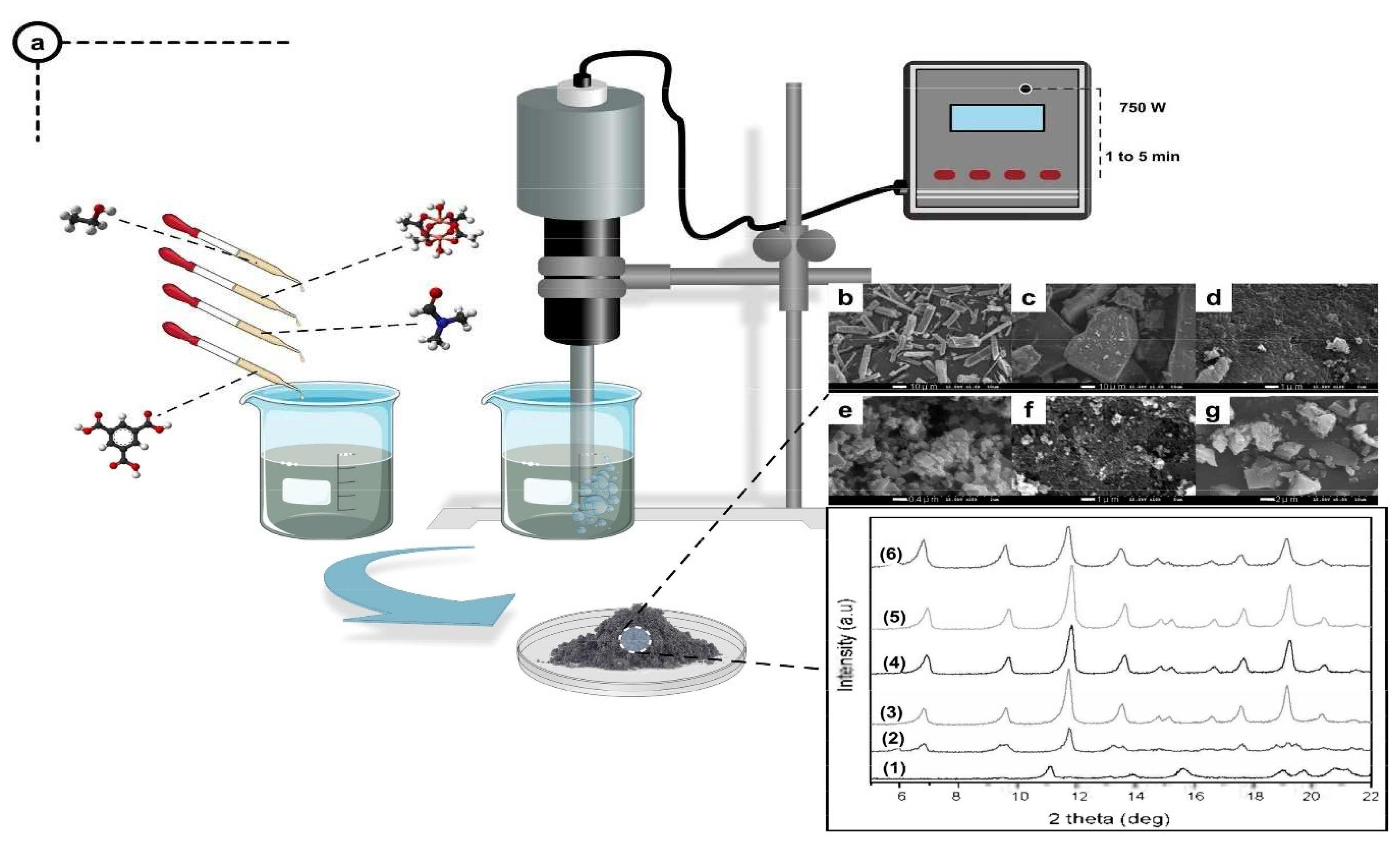
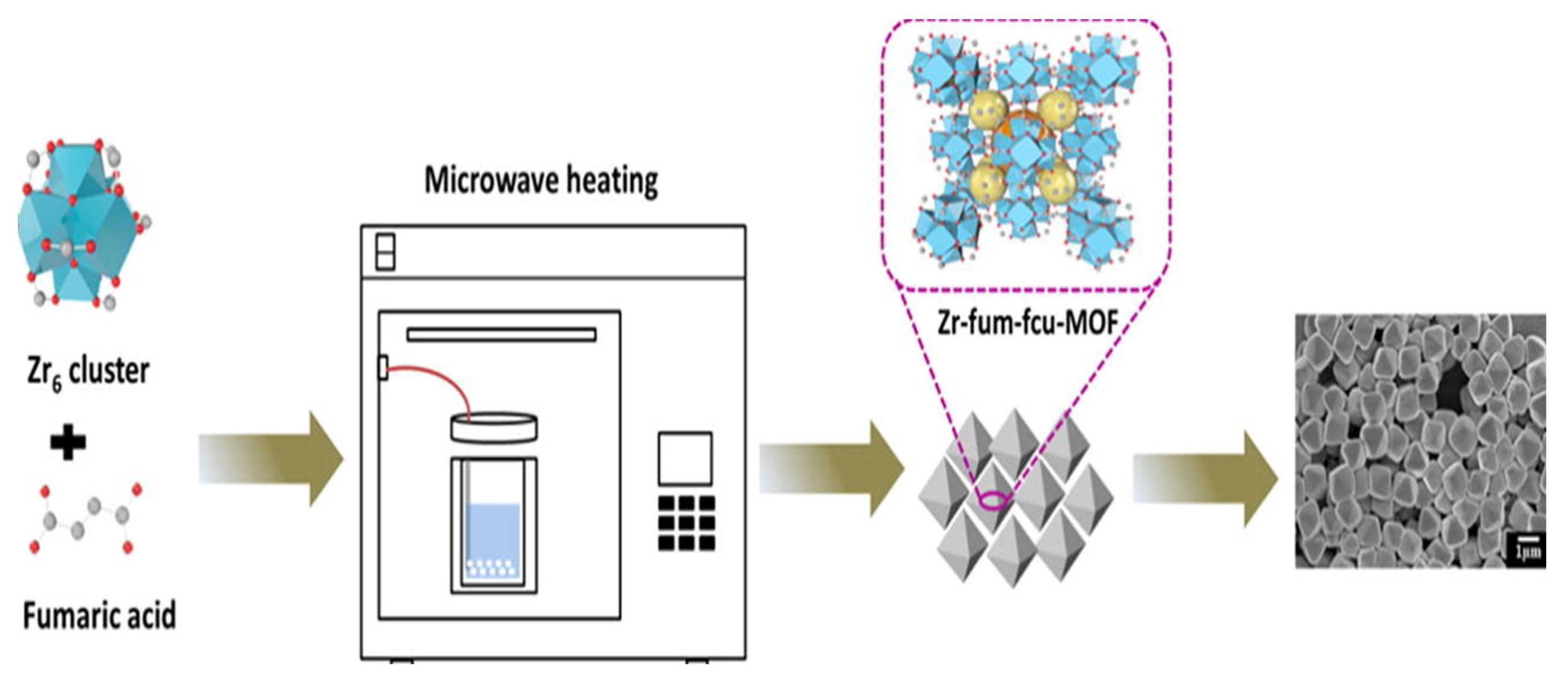
2.3. Microwave-aided synthesis
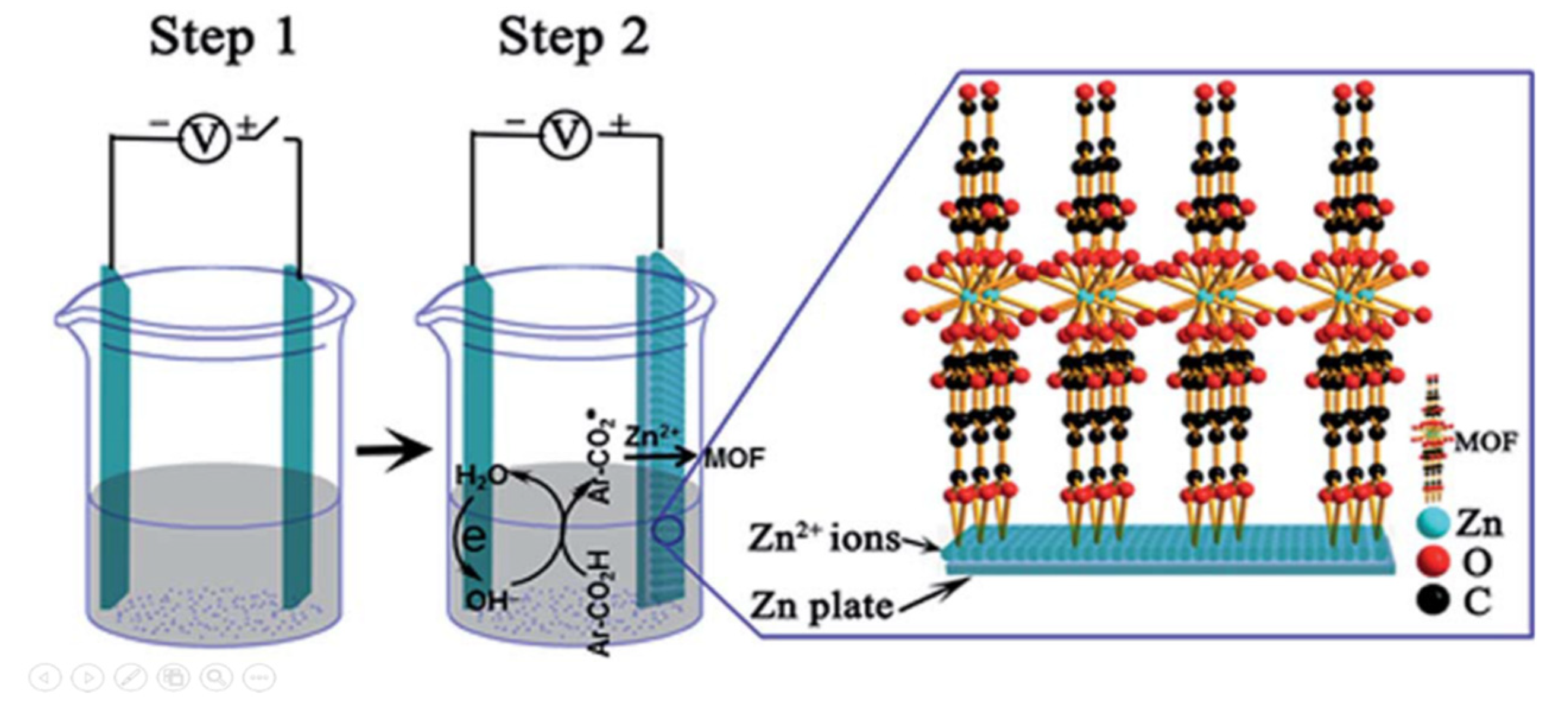
2.4. Electrochemical synthesis
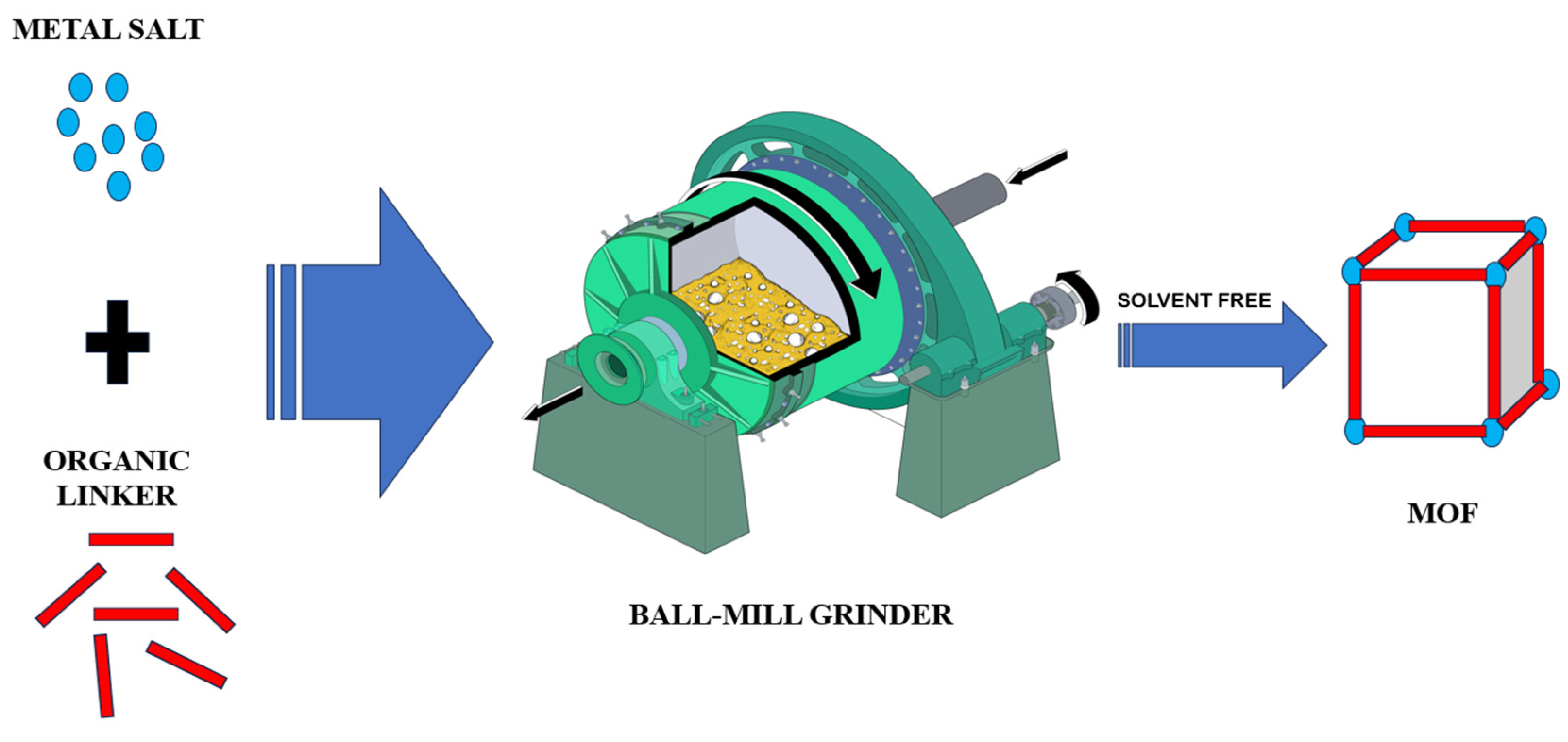
2.5. Mechanochemical synthesis
3. Design and fabrication of MOF-based sensors
3.1. Carbon based electrode modification by MOF
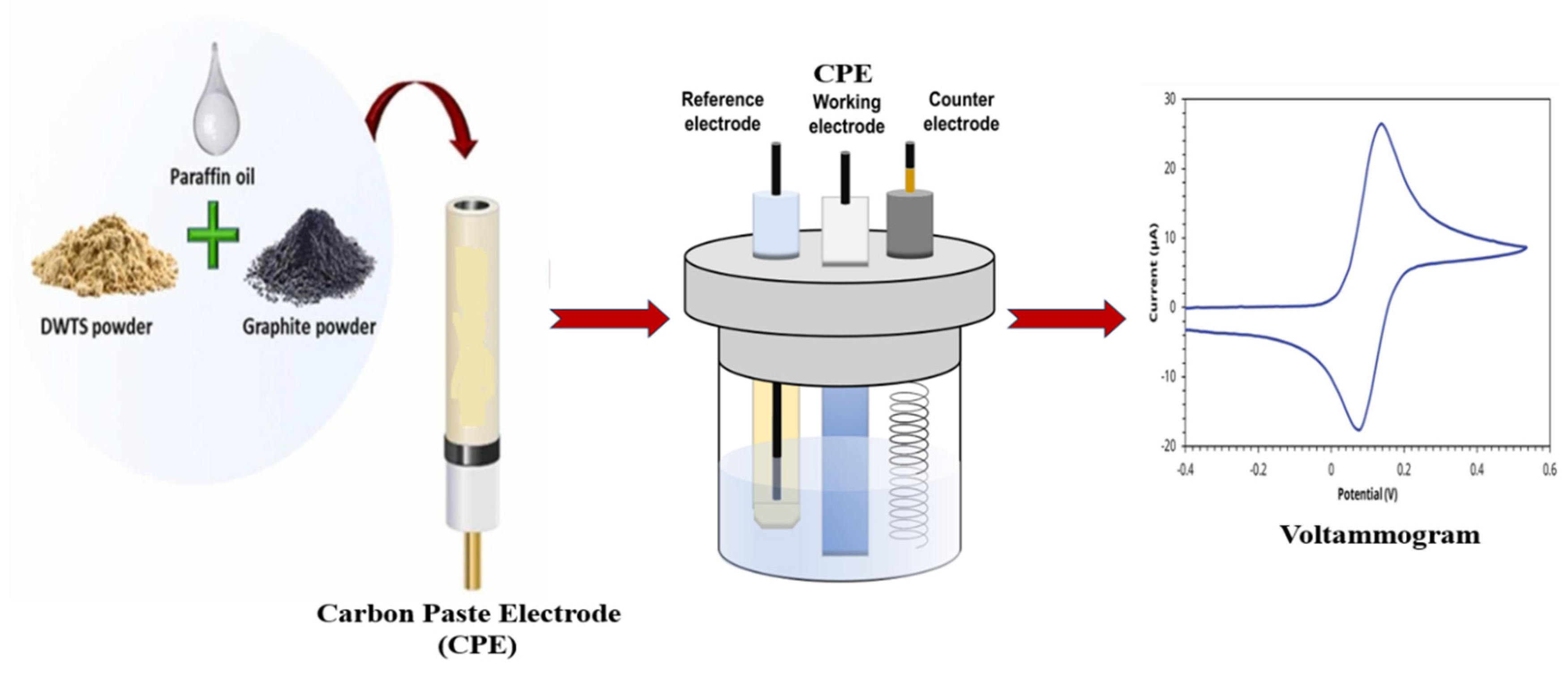
3.1.1. MOF modified carbon paste electrode (CPE)
3.1.2. MOF modified glassy carbon electrode (GCE)
4. Application of MOF in electrochemical sensing
4.1. Biomolecules sensing
4.2. Detection of hydrogen peroxide
4.3. Organic pollutant sensing
4.4. Heavy metals sensing
5. Conclusion and Future perspective
- Challenges encompass the synthesis of advanced materials to control the shape and size of MOFs; this may result in uniform growth of nanostructures with a significant increase in surface area.
- Homogeneous dispersion of active metals on the surface of MOF-derived carbons remains challenging.
- Although many MOFs are used to fabricate electrodes for heavy metal detection, the insights into electrochemical sensing mechanisms could enable greater implications for device development.
- MOF stability in an aqueous media remains challenging; studies on coupling hydrophobic ligands with high valence metal ions could provide newer research areas for suitable applications.
- Since MOFs' pore width and geometry play an essential role in the highly selective determination of food contaminants, synthesizing functionalized MOFs could enable required selectivity towards the target analyte species in complex matrices.
References
- Wiśniewska, P.; Haponiuk, J.; Saeb, M.R.; Rabiee, N.; Bencherif, S.A. Mitigating Metal-Organic Framework (MOF) Toxicity for Biomedical Applications. Chem. Eng. J. 2023, 471, 144400. [Google Scholar] [CrossRef]
- Ettlinger, R.; Lächelt, U.; Gref, R.; Horcajada, P.; Lammers, T.; Serre, C.; Couvreur, P.; Morris, R.E.; Wuttke, S. Toxicity of Metal–Organic Framework Nanoparticles: From Essential Analyses to Potential Applications. Chem. Soc. Rev. 2022, 51, 464–484. [Google Scholar] [CrossRef]
- Fan, W.; Yuan, S.; Wang, W.; Feng, L.; Liu, X.; Zhang, X.; Wang, X.; Kang, Z.; Dai, F.; Yuan, D.; et al. Optimizing Multivariate Metal–Organic Frameworks for Efficient C2H2/CO2 Separation. J. Am. Chem. Soc. 2020, 142, 8728–8737. [Google Scholar] [CrossRef]
- Herbst, A.; Janiak, C. MOF Catalysts in Biomass Upgrading towards Value-Added Fine Chemicals. Cryst. Eng. Comm 2017, 19, 4092–4117. [Google Scholar] [CrossRef]
- Pornea, A.M.; Kim, H. Synthesis of Hybrid Dual-MOF Encapsulated Phase-Changing Material for Improved Broadband Light Absorption and Photothermal Conversion Enabling Efficient Solar Energy Storage. Sol. Ene. Mat and Sol. Cells. 2022, 244, 111817. [Google Scholar] [CrossRef]
- Dong, Z.; Sun, Y.; Chu, J.; Zhang, X.; Deng, H. Multivariate Metal–Organic Frameworks for Dialing-in the Binding and Programming the Release of Drug Molecules. J. Am. Chem. Soc. 2017, 139, 14209–14216. [Google Scholar] [CrossRef]
- Tchalala, M.R.; Bhatt, P.M.; Chappanda, K.N.; Tavares, S.R.; Adil, K.; Belmabkhout, Y.; Shkurenko, A.; Cadiau, A.; Heymans, N.; De Weireld, G.; et al. Fluorinated MOF Platform for Selective Removal and Sensing of SO 2 from Flue Gas and Air. Nat. Com. 2019, 10. [Google Scholar]
- Kannangara, Y.Y.; Rathnayake, U.A.; Song, J.-K. Redox Active Multi-Layered Zn-PPDA MOFs as High-Performance Supercapacitor Electrode Material. Electrochem. Acta. 2019, 297, 145–154. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly Porous Metal-Organic Framework. Nature. 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Sohrabi, H.; Ghasemzadeh, S.; Ghoreishi, Z.; Majidi, M.R.; Yoon, Y.; Dizge, N.; Khataee, A. Metal-Organic Frameworks (MOF)-Based Sensors for Detection of Toxic Gases: A Review of Current Status and Future Prospects. Mat. Chem. and Phy. 2023, 299, 127512. [Google Scholar] [CrossRef]
- Chui, S.S.-Y.; Lo, S.M.-F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Devic, T.; Serre, C. High Valence 3p and Transition Metal Based MOFs. Chem. Soc. Rev. 2014, 43, 6097–6115. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, T.; Volkringer, C.; Haouas, M.; Taulelle, F.; Férey, G. Crystal Chemistry of Aluminium Carboxylates: From Molecular Species towards Porous Infinite Three-Dimensional Networks. Comptes Rendus Chimie 2015, 18, 1350–1369. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Shen, C.; Wang, C.; Hu, X.; Wang, G. An Electrochemical Sensor on the Hierarchically Porous Cu-BTC MOF Platform for Glyphosate Determination. Sens. and Act. B: Chemical 2019, 283, 487–494. [Google Scholar] [CrossRef]
- Rana, A.; Baig, N.; Saleh, T.A. Electrochemically Pretreated Carbon Electrodes and Their Electroanalytical Applications – A Review. Jou.l of Electroanal. Chem. 2019, 833, 313–332. [Google Scholar] [CrossRef]
- Rupam, T.; Palash, M.L.; Jahan, I.; Harish, S.; Saha, B. GREEN SYNTHESIS AND ADSORPTION CHARACTERISATION OF AN ALUMINIUM BASED METAL ORGANIC FRAMEWORK; 2019.
- Hu, C.; Xiao, J.-D.; Mao, X.-D.; Song, L.-L.; Yang, X.-Y.; Liu, S.-J. Toughening Mechanisms of Epoxy Resin Using Aminated Metal-Organic Framework as Additive. Mat. Let. 2019, 240, 113–116. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Zhao, D. Modulated Hydrothermal Chemistry of Metal–Organic Frameworks. Acc. Mater. Res. 2022, 3, 1106–1114. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Abbas, Qaisar. ; Mouselly, M.; Alawadhi, H.; Olabi, A.G. High-Performance Effective Metal–Organic Frameworks for Electrochemical Applications. Jou. of Sc: Adv. Mat. and Dev. 2022, 7, 100465. [Google Scholar]
- Liu, B.; Vellingiri, K.; Jo, S.-H.; Kumar, P.; Ok, Y.S.; Kim, K.-H. Recent Advances in Controlled Modification of the Size and Morphology of Metal-Organic Frameworks. Nano Res. 2018, 11, 4441–4467. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. Jou. of the Amer. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Qi, Y.; Luo, F.; Che, Y.; Zheng, J. Hydrothermal Synthesis of Metal-Organic Frameworks Based on Aromatic Polycarboxylate and Flexible Bis(Imidazole) Ligands. Crys. Grow. and Des. 2008, 8, 606–611. [Google Scholar] [CrossRef]
- Horcajada, P.; Surblé, S.; Serre, C.; Hong, D.-Y.; Seo, Y.-K.; Chang, J.-S.; Grenèche, J.-M.; Margiolaki, I.; Férey, G. Synthesis and Catalytic Properties of MIL-100(Fe), an Iron(III) Carboxylate with Large Pores. Chem. Com. 2007, 2820–2822. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, H.; Fujita, T.; Hirata, A.; Zhang, W.; Inoue, A.; Chen, M. Nanoporous PdNi Bimetallic Catalyst with Enhanced Electrocatalytic Performances for Electro-Oxidation and Oxygen Reduction Reactions. Adv. Funct. Mat. 2011, 21, 4364–4370. [Google Scholar] [CrossRef]
- Wu, H.; Li, M.; Wang, Z.; Yu, H.; Han, J.; Xie, G.; Chen, S. Highly Stable Ni-MOF Comprising Triphenylamine Moieties as a High-Performance Redox Indicator for Sensitive Aptasensor Construction. Analytica Chimica Acta 2019, 1049, 74–81. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Diao, Y.; He, Q.; Lu, C.; Singh, A.; Kumar, A.; Liu, J.; Lan, Q. Recent Advances in the Electrochemical Applications of Ni-Based Metal Organic Frameworks (Ni-MOFs) and Their Derivatives. Chemosphere 2022, 307, 135729. [Google Scholar] [CrossRef]
- Ahmadi, A.; Nezamzadeh-Ejhieh, A. A Comprehensive Study on Electrocatalytic Current of Urea Oxidation by Modified Carbon Paste Electrode with Ni(II)-Clinoptilolite Nanoparticles: Experimental Design by Response Surface Methodology. J. Electroanal. chem. 2017, 801, 328–337. [Google Scholar] [CrossRef]
- Pourtaheri, A.; Nezamzadeh-Ejhieh, A. Enhancement in Photocatalytic Activity of NiO by Supporting onto an Iranian Clinoptilolite Nano-Particles of Aqueous Solution of Cefuroxime Pharmaceutical Capsule. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy 2015, 137, 338–344. [Google Scholar] [CrossRef]
- Sheikh-Mohseni, M.H.; Nezamzadeh-Ejhieh, A. Modification of Carbon Paste Electrode with Ni-Clinoptilolite Nanoparticles for Electrocatalytic Oxidation of Methanol. Electrochimica Acta 2014, 147, 572–581. [Google Scholar] [CrossRef]
- Liu, Y.; Myers, E.J.; Rydahl, S.A.; Wang, X. Ultrasonic-Assisted Synthesis, Characterization, and Application of a Metal–Organic Framework: A Green General Chemistry Laboratory Project. J. Chem. Educ. 2019, 96, 2286–2291. [Google Scholar] [CrossRef]
- Vinoth, V.; Wu, J.J.; Asiri, A.M.; Anandan, S. Sonochemical Synthesis of Silver Nanoparticles Anchored Reduced Graphene Oxide Nanosheets for Selective and Sensitive Detection of Glutathione. Ultrason. Sonochem. 2017, 39, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Areerob, Y.; Cho, J.Y.; Jang, W.K.; Oh, W.-C. Enhanced Sonocatalytic Degradation of Organic Dyes from Aqueous Solutions by Novel Synthesis of Mesoporous Fe3O4-Graphene/ZnO@SiO2 Nanocomposites. Ultrason. Sonochem. 2018, 41, 267–278. [Google Scholar] [CrossRef]
- Qiu, L.-G.; Li, Z.-Q.; Wu, Y.; Wang, W.; Xu, T.; Jiang, X. Facile Synthesis of Nanocrystals of a Microporous Metal–Organic Framework by an Ultrasonic Method and Selective Sensing of Organoamines. Chem. Commun. 2008, 3642–3644. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Jhung, S.H. Facile Syntheses of Metal-Organic Framework Cu3(BTC)2(H2O)3 under Ultrasound. Bulletin of the Korean Chem. Soc. 2009, 30, 2921–2926. [Google Scholar] [CrossRef]
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-Organic Frameworks for Advanced Drug Delivery. Acta Pharmaceutica Sinica B 2021, 11, 2362–2395. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, A.; Rahimpour, A.; Rastgar, M.; Sadrzadeh, M. Ultrasonically Synthesized MOFs for Modification of Polymeric Membranes: A Critical Review. Ultrason. Sonochem. 2022, 90, 106202. [Google Scholar] [CrossRef] [PubMed]
- Iron-Based Metal-Organic Framework: Synthesis, Structure and Current Technologies for Water Reclamation with Deep Insight into Framework Integrity. Chemosphere 2021, 284, 131171. [CrossRef] [PubMed]
- Ge, J.; Wu, Z.; Huang, X.; Ding, M. An Effective Microwave-Assisted Synthesis of MOF235 with Excellent Adsorption of Acid Chrome Blue K. J.l of Nanomat. 2019, 2019, e4035075. [Google Scholar] [CrossRef]
- Ahn, W.S.; Kang, K.K.; Kim, K.Y. Synthesis of TS-1 by Microwave Heating of Template-Impregnated SiO2–TiO2 Xerogels. Catalysis Letters. 2001, 72, 229–232. [Google Scholar] [CrossRef]
- Jin, T.; Hwang, Y.K.; Hong, D.-Y.; Jhung, S.H.; Hwang, J.-S.; Park, S.-E.; Kim, Y.H.; Chang, J.-S. Microwave Synthesis, Characterization and Catalytic Properties of Titanium-Incorporated ZSM-5 Zeolite. Res Chem Intermed 2007, 33, 501–512. [Google Scholar] [CrossRef]
- Komarneni, S.; Rajha, R.K.; Katsuki, H. Microwave-Hydrothermal Processing of Titanium Dioxide1Dedicated to Professor Shigeyuki Somiya.1. Mater. Chem. and Phys. 1999, 61, 50–54. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Conner, W.C.; Yngvesson, K.S. Microwave Synthesis of Nanoporous Materials. Chemphyschem. 2006, 7, 296–319. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Khan, N.A.; Kim, C.M.; Jhung, S.H. Syntheses of Metal–Organic Frameworks and Aluminophosphates under Microwave Heating: Quantitative Analysis of Accelerations. Crystal Growth & Design 2011, 11, 4413–4421. [Google Scholar]
- Ni, Z.; Masel, R.I. Rapid Production of Metal−Organic Frameworks via Microwave-Assisted Solvothermal Synthesis. J. Am. Chem. Soc. 2006, 128, 12394–1239. [Google Scholar] [CrossRef]
- Parnham, E.R.; Morris, R.E. Ionothermal Synthesis of Zeolites, Metal–Organic Frameworks, and Inorganic–Organic Hybrids. Acc. Chem. Res. 2007, 40, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Son, W.-J.; Kim, J.; Ahn, W.-S. Metal–Organic Framework MOF-5 Prepared by Microwave Heating: Factors to Be Considered. Microporous and Mesoporous Mater. 2008, 116, 727–731. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Zhou, C.; Mu, B.; Chen, L. Microwave-Assisted Synthesis of Zr-Based Metal–Organic Framework (Zr-Fum-Fcu-MOF) for Gas Adsorption Separation. Chem. Phys. Let. 2021, 780, 138906. [Google Scholar] [CrossRef]
- Ameloot, R.; Stappers, L.; Fransaer, J.; Alaerts, L.; Sels, B.F.; De Vos, D.E. Patterned Growth of Metal-Organic Framework Coatings by Electrochemical Synthesis. Chem. Mater. 2009, 21, 2580–2582. [Google Scholar] [CrossRef]
- Bétard, A.; Fischer, R.A. Metal–Organic Framework Thin Films: From Fundamentals to Applications. Chem. Rev. 2012, 112, 1055–1083. [Google Scholar] [CrossRef]
- Al-Kutubi, H.; Dikhtiarenko, A.; Zafarani, H.R.; Sudhölter, E.J.R.; Gascon, J.; Rassaei, L. Facile Formation of ZIF-8 Thin Films on ZnO Nanorods. CrystEngComm. 2015, 17, 5360–5364. [Google Scholar] [CrossRef]
- Asghar, A.; Iqbal, N.; Noor, T.; Kariuki, B.M.; Kidwell, L.; Easun, T.L. Efficient Electrochemical Synthesis of a Manganese-Based Metal–Organic Framework for H2 and CO2 Uptake. Green Chem. 2021, 23, 1220–1227. [Google Scholar] [CrossRef]
- Guerrero, V.V.; Yoo, Y.; McCarthy, M.C.; Jeong, H.-K. HKUST-1 Membranes on Porous Supports Using Secondary Growth. J. of Mat. Chem. 2010, 20, 3938–3943. [Google Scholar] [CrossRef]
- Neto, O.J. de L.; Frós, A.C. de O.; Barros, B.S.; Monteiro, A.F. de F.; Kulesza, J. Rapid and Efficient Electrochemical Synthesis of a Zinc-Based Nano-MOF for Ibuprofen Adsorption. New J. Chem. 2019, 43, 5518–5524. [Google Scholar] [CrossRef]
- Li, W.-J.; Lü, J.; Gao, S.-Y.; Li, Q.-H.; Cao, R. Electrochemical Preparation of Metal–Organic Framework Films for Fast Detection of Nitro Explosives. J. Mater. Chem. A 2014, 2, 19473–19478. [Google Scholar] [CrossRef]
- Nadizadeh, Z.; Naimi-Jamal, M.R.; Panahi, L. Mechanochemical Solvent-Free in Situ Synthesis of Drug-Loaded {Cu2(1,4-Bdc)2(Dabco)}n MOFs for Controlled Drug Delivery. J. of Solid State Chem. 2018, 259, 35–42. [Google Scholar] [CrossRef]
- Tanaka, S. Chapter 10 - Mechanochemical Synthesis of MOFs. In Metal-Organic Frameworks for Biomedical Applications; Mozafari, M., Ed.; Woodhead Publishing. 2020; pp. 197–222.
- Friščić, T. Supramolecular Concepts and New Techniques in Mechanochemistry: Cocrystals, Cages, Rotaxanes, Open Metal–Organic Frameworks. Chem. Soc. Rev. 2012, 41, 3493–3510. [Google Scholar] [CrossRef]
- Lv, D.; Chen, Y.; Li, Y.; Shi, R.; Wu, H.; Sun, X.; Xiao, J.; Xi, H.; Xia, Q.; Li, Z. Efficient Mechanochemical Synthesis of MOF-5 for Linear Alkanes Adsorption. J. of Chem. and Eng. Data. 2017, 62, 2030–2036. [Google Scholar] [CrossRef]
- Kaur, G.; Anthwal, A.; Kandwal, P.; Sud, D. Mechanochemical Synthesis and Theoretical Investigations of Fe (II) Based MOF Containing 4,4′-Bipyridine with Ordained Intercalated p-Aminobenzoic Acid: Application as Fluoroprobe for Detection of Carbonyl Group. Inorganica Chimica Acta. 2023, 545, 121248. [Google Scholar] [CrossRef]
- Prochowicz, D.; Sokołowski, K.; Justyniak, I.; Kornowicz, A.; Fairen-Jimenez, D.; Friščić, T.; Lewiński, J. A Mechanochemical Strategy for IRMOF Assembly Based on Pre-Designed Oxo-Zinc Precursors. Chem. Commun. 2015, 51, 4032–4035. [Google Scholar] [CrossRef]
- Kujawa, J.; Al-Gharabli, S.; Muzioł, T.M.; Knozowska, K.; Li, G.; Dumée, L.F.; Kujawski, W. Crystalline Porous Frameworks as Nano-Enhancers for Membrane Liquid Separation – Recent Developments. Coordin. Chem. Rev. 2021, 440, 213969. [Google Scholar] [CrossRef]
- Mu, X.; Jiang, J.; Chao, F.; Lou, Y.; Chen, J. Ligand Modification of UiO-66 with an Unusual Visible Light Photocatalytic Behavior for RhB Degradation. Dalton Trans. 2018, 47, 1895–1902. [Google Scholar] [CrossRef]
- Srinivasan, R.; Elaiyappillai, E.; Nixon, E.J.; Sharmila Lydia, I.; Johnson, P.M. Enhanced Electrochemical Behaviour of Co-MOF/PANI Composite Electrode for Supercapacitors. Inorganica Chimica Acta. 2020, 502, 119393. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Li, Q.; Zhao, Y.; Liu, C.-S.; Pang, H. NiO Nanoparticles Decorated Hexagonal Nickel-Based Metal-Organic Framework: Self-Template Synthesis and Its Application in Electrochemical Energy Storage. J. Colloid. Interface Sci. 2021, 581, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Chen, Q.; Yuan, H.; Hu, R.; Liang, C.; Liu, F.; Pan, L.; Zhang, Y.; Wu, X.; Yang, S.-T. Reversible Environmental Impacts of Iron-Based Metal-Organic Framework MIL-53(Fe) on Nitrogen-Fixing Bacterium Azotobacter Vinelandii. J.Env. Chem. Eng. 2022, 10, 107794. [Google Scholar] [CrossRef]
- Pu, C.; Zhao, H.; Hong, Y.; Zhan, Q.; Lan, M. Elution-Free Ultra-Sensitive Enrichment for Glycopeptides Analyses: Using a Degradable, Post-Modified Ce-Metal–Organic Framework. Analytica Chimica Acta 2019, 1045, 123–131. [Google Scholar] [CrossRef]
- Rezki, M.; Septiani, N.L.W.; Iqbal, M.; Harimurti, S.; Sambegoro, P.; Adhika, D.R.; Yuliarto, B. Amine-Functionalized Cu-MOF Nanospheres towards Label-Free Hepatitis B Surface Antigen Electrochemical Immunosensors. J. Mater. Chem. B 2021, 9, 5711–5721. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Shao, J.; Zhang, L.; Cui, Q.; Wang, H. Study on Heat Conduction and Adsorption/Desorption Characteristic of MIL-101/Few Layer Graphene Composite. J Porous Mater 2021, 28, 1197–1213. [Google Scholar] [CrossRef]
- Burgaz, E.; Erciyes, A.; Andac, M.; Andac, O. Synthesis and Characterization of Nano-Sized Metal Organic Framework-5 (MOF-5) by Using Consecutive Combination of Ultrasound and Microwave Irradiation Methods. Inorganica Chimica Acta 2019, 485, 118–124. [Google Scholar] [CrossRef]
- Cho, H.-Y.; Kim, J.; Kim, S.-N.; Ahn, W.-S. High Yield 1-L Scale Synthesis of ZIF-8 via a Sonochemical Route. Microporous and Mesoporous Materials 2013, 169, 180–184. [Google Scholar] [CrossRef]
- Yang, D.-A.; Cho, H.-Y.; Kim, J.; Yang, S.-T.; Ahn, W.-S. CO2 Capture and Conversion Using Mg-MOF-74 Prepared by a Sonochemical Method. Energy Environ. Sci. 2012, 5, 6465–6473. [Google Scholar] [CrossRef]
- Brainer, N.S.; dos Santos, T.V.; D. E.S. Barbosa, C.; Meneghetti, S.M.P. Simple and Fast Ultrasound-Assisted Synthesis of Sn-MOFs and Obtention of SnO2. Materials Letters. 2020, 280, 128512. [Google Scholar] [CrossRef]
- Azad, F.N.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Pezeshkpour, V. Ultrasonically Assisted Hydrothermal Synthesis of Activated Carbon–HKUST-1-MOF Hybrid for Efficient Simultaneous Ultrasound-Assisted Removal of Ternary Organic Dyes and Antibacterial Investigation: Taguchi Optimization. Ultrasonics Sonochem. 2016, 31, 383–393. [Google Scholar] [CrossRef]
- Wu, X.; Bao, Z.; Yuan, B.; Wang, J.; Sun, Y.; Luo, H.; Deng, S. Microwave Synthesis and Characterization of MOF-74 (M=Ni, Mg) for Gas Separation. Microporous and Mesoporous Materials. 2013, 180, 114–122. [Google Scholar] [CrossRef]
- Yoo, Y.; Lai, Z.; Jeong, H.-K. Fabrication of MOF-5 Membranes Using Microwave-Induced Rapid Seeding and Solvothermal Secondary Growth. Microporous and Mesoporous Materials. 2009, 123, 100–106. [Google Scholar] [CrossRef]
- Jung, D.-W.; Yang, D.-A.; Kim, J.; Kim, J.; Ahn, W.-S. Facile Synthesis of MOF-177 by a Sonochemical Method Using 1-Methyl-2-Pyrrolidinone as a Solvent. Dalton Trans. 2010, 39, 2883–2887. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.T.; Phong, N.H.; Van Duc, H.; Khieu, D.Q. Microwave Synthesis and Voltammetric Simultaneous Determination of Paracetamol and Caffeine Using an MOF-199-Based Electrode. J Mater Sci 2018, 53, 2453–2471. [Google Scholar] [CrossRef]
- Han, Y.; Cui, J.; Yu, Y.; Chao, Y.; Li, D.; Wang, C.; Wallace, G.G. Efficient Metal-Oriented Electrodeposition of a Co-Based Metal-Organic Framework with Superior Capacitive Performance. Chem. Sus. Chem. 2022, 15, e202200644. [Google Scholar] [CrossRef]
- Worrall, S.D.; Bissett, M.A.; Hill, P.I.; Rooney, A.P.; Haigh, S.J.; Attfield, M.P.; Dryfe, R.A.W. Metal-Organic Framework Templated Electrodeposition of Functional Gold Nanostructures. Electrochimica Acta. 2016, 222, 361–369. [Google Scholar] [CrossRef]
- Tian, N.; Gao, Y.; Wu, J.; Luo, S.; Dai, W. Water-Resistant HKUST-1 Functionalized with Polydimethylsiloxane for Efficient Rubidium Ion Capture. New J. Chem. 2019, 43, 15539–15547. [Google Scholar] [CrossRef]
- Naseri, M.; Fotouhi, L.; Ehsani, A.; Dehghanpour, S. Facile Electrosynthesis of Nano Flower like Metal-Organic Framework and Its Nanocomposite with Conjugated Polymer as a Novel and Hybrid Electrode Material for Highly Capacitive Pseudocapacitors. J. Colloid. Interface Sci. 2016, 484, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.R.; Karimi, M.; Daasbjerg, K. Efficient Removal of Crystal Violet and Methylene Blue from Wastewater by Ultrasound Nanoparticles Cu-MOF in Comparison with Mechanosynthesis Method. Ultrason. Sonochem. 2017, 37, 182–191. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J. 3D-Superstructured Networks Comprising Fe-MIL-88A Metal-Organic Frameworks Under Mechanochemical Conditions. Euro. J. Inorganic Chem. 2019, 2019, 4597–4600. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Liu, Z.; Sun, X.; Xia, Q.; Li, Z. Liquid-Assisted Mechanochemical Synthesis of Copper Based MOF-505 for the Separation of CO2 over CH4 or N2. Ind. Eng. Chem. Res. 2018, 57, 703–709. [Google Scholar] [CrossRef]
- Prochowicz, D.; Nawrocki, J.; Terlecki, M.; Marynowski, W.; Lewiński, J. Facile Mechanosynthesis of the Archetypal Zn-Based Metal–Organic Frameworks. Inorg. Chem. 2018, 57, 13437–13442. [Google Scholar] [CrossRef]
- Liu, C.-S.; Li, J.; Pang, H. Metal-Organic Framework-Based Materials as an Emerging Platform for Advanced Electrochemical Sensing. Coordin. Chem. Rev. 2020, 410, 213222. [Google Scholar] [CrossRef]
- Liu, W.; Yin, X.-B. Metal–Organic Frameworks for Electrochemical Applications. TrAC Trends in Analytical Chemistry 2016, 75, 86–96. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, A.; Zhong, M.; Zhang, Z.; Zhang, X.; Zhou, Z.; Bu, X.-H. Metal–Organic Frameworks (MOFs) and MOF-Derived Materials for Energy Storage and Conversion. Electrochemical Energy Reviews 2019, 2, 29–104. [Google Scholar] [CrossRef]
- Jaouen, F.; Morozan, A. Metal-Organic Frameworks: Electrochemical Properties. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd., 2014; pp. 1–24 ISBN 978-1-119-95143-8.
- Morozan, A.; Jaouen, F. Metal Organic Frameworks for Electrochemical Applications. Energy Environ. Sci. 2012, 5, 9269–9290. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, F.; Zeng, B. One-Step Synthesis of a Copper-Based Metal–Organic Framework–Graphene Nanocomposite with Enhanced Electrocatalytic Activity. RSC Advances 2015, 5, 22060–22065. [Google Scholar] [CrossRef]
- Shams-Eldin, R.; Ali, A.A.; Hani, A.; Haikal, R.R.; Fahmy, H.M.; El Nashar, R.M.; Alkordi, M.H. Metal–Organic Framework Mediated Ni-Deposition on MWCNTs for Direct Methanol Fuel Cell Catalysis. SN Appl. Sci. 2023, 5, 166. [Google Scholar] [CrossRef]
- Stassen, I.; Styles, M.; Van Assche, T.; Campagnol, N.; Fransaer, J.; Denayer, J.; Tan, J.-C.; Falcaro, P.; De Vos, D.; Ameloot, R. Electrochemical Film Deposition of the Zirconium Metal–Organic Framework UiO-66 and Application in a Miniaturized Sorbent Trap. Chem. Mater. 2015, 27, 1801–1807. [Google Scholar] [CrossRef]
- Ye, W.; Li, Y.; Wang, J.; Li, B.; Cui, Y.; Yang, Y.; Qian, G. Electrochemical Detection of Trace Heavy Metal Ions Using a Ln-MOF Modified Glass Carbon Electrode. J. Solid State Chem. 2020, 281, 121032. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electochemistry: Wang/Electrochemistry 2E E-Bk; John Wiley & Sons, Inc.: New York, USA, 2000; ISBN 978-0-471-22823-3. [Google Scholar]
- Cruz-Navarro, J.A.; Hernandez-Garcia, F.; Alvarez Romero, G.A. Novel Applications of Metal-Organic Frameworks (MOFs) as Redox-Active Materials for Elaboration of Carbon-Based Electrodes with Electroanalytical Uses. Coordin. Chem. Rev. 2020, 412, 213263. [Google Scholar] [CrossRef]
- Švancara, I.; Kalcher, K. Carbon Paste Electrodes. In Electrochemistry of Carbon Electrodes; John Wiley & Sons, Ltd., 2015; pp. 379–424 ISBN 978-3-527-69748-9.
- Švancara, I.; Vytřas, K.; Kalcher, K.; Walcarius, A.; Wang, J. Carbon Paste Electrodes in Facts, Numbers, and Notes: A Review on the Occasion of the 50-Years Jubilee of Carbon Paste in Electrochemistry and Electroanalysis. Electroanalysis 2009, 21, 7–28. [Google Scholar] [CrossRef]
- Alipour, Z.; Hassaninejad-Darzi, S.K.; Asadollahi-Baboli, M. Construction of Pd-Ag/g-C3N4/CPE Electrochemical Nanosensor for Simultaneous Determination of Clozapine and Quetiapine Antipsychotic Drugs. J.Alloys and Comp. 2023, 941, 168958. [Google Scholar] [CrossRef]
- Svancara, I.; Kalcher, K.; Walcarius, A.; Vytras, K. Electroanalysis with Carbon Paste Electrodes; CRC Press, 2012; ISBN 978-1-4398-3019-2.
- Fazl, F.; Gholivand, M.B. High Performance Electrochemical Method for Simultaneous Determination Dopamine, Serotonin, and Tryptophan by ZrO2–CuO Co-Doped CeO2 Modified Carbon Paste Electrode. Talanta. 2022, 239, 122982. [Google Scholar] [CrossRef]
- Bi, W.; Wang, G.; Hu, X. Fabrication of Zn-MOF Derived Graphitic Carbon Materials with Mesoporous Structure for Adsorptive Removal of Ceftazidime from Aqueous Solutions. Colloid. Surf. A: Physicochem. Eng. A. 2022, 652, 129758. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Q.; Meng, Y.; Jin, Z.; Fang, Z.; Fu, Q.; Gao, W.; Xu, L.; Song, Y.; Lu, F. Efficient Detection of Hazardous Catechol and Hydroquinone with MOF-RGO Modified Carbon Paste Electrode. J. Hazard. Mat. 2018, 353, 151–157. [Google Scholar] [CrossRef]
- Kumari, N.; Sareen, S.; Verma, M.; Sharma, S.; Sharma, A.; Sohal, H.S.; Mehta, S.K.; Park, J.; Mutreja, V. Zirconia-Based Nanomaterials: Recent Developments in Synthesis and Applications. Nanoscale Adv 4, 4210–4236.
- Chikere, Chrys. O.; Faisal, N.H.; Kong-Thoo-Lin, P.; Fernandez, C. Interaction between Amorphous Zirconia Nanoparticles and Graphite: Electrochemical Applications for Gallic Acid Sensing Using Carbon Paste Electrodes in Wine. Nanomaterials (Basel) 2020, 10, 537. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Synthesis, Characterization, and Ammonia Adsorption Properties of Mesoporous Metal–Organic Framework (MIL(Fe))–Graphite Oxide Composites: Exploring the Limits of Materials Fabrication. Adv. Funct. Mat. 2011, 21, 2108–2117. [Google Scholar] [CrossRef]
- Wu, L.; Lu, Z.; Ye, J. Enzyme-Free Glucose Sensor Based on Layer-by-Layer Electrodeposition of Multilayer Films of Multi-Walled Carbon Nanotubes and Cu-Based Metal Framework Modified Glassy Carbon Electrode. Biosensors and Bioelectronics. 2019, 135, 45–49. [Google Scholar] [CrossRef]
- Sharma, S. Glassy Carbon: A Promising Material for Micro- and Nanomanufacturing. Materials. 2018, 11, 1857. [Google Scholar] [CrossRef]
- Arul, P.; John, S.A. Silver Nanoparticles Built-in Zinc Metal Organic Framework Modified Electrode for the Selective Non-Enzymatic Determination of H2O2. Electrochimica Acta. 2017, 235, 680–689. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Dhanjai; Sinha, A. ; Lu, X.; Wu, L.; Tan, D.; Li, Y.; Chen, J.; Jain, R. Voltammetric Sensing of Biomolecules at Carbon Based Electrode Interfaces: A Review. TrAC Trends Anal. Chem. 2018, 98, 174–189. [Google Scholar]
- Park, S.; Boo, H.; Chung, T.D. Electrochemical Non-Enzymatic Glucose Sensors. Analytica Chimica Acta 2006, 556, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Sarma, A.K.; Vatsyayan, P.; Goswami, P.; Minteer, S.D. Recent Advances in Material Science for Developing Enzyme Electrodes. Biosensors and Bioelectronics. 2009, 24, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
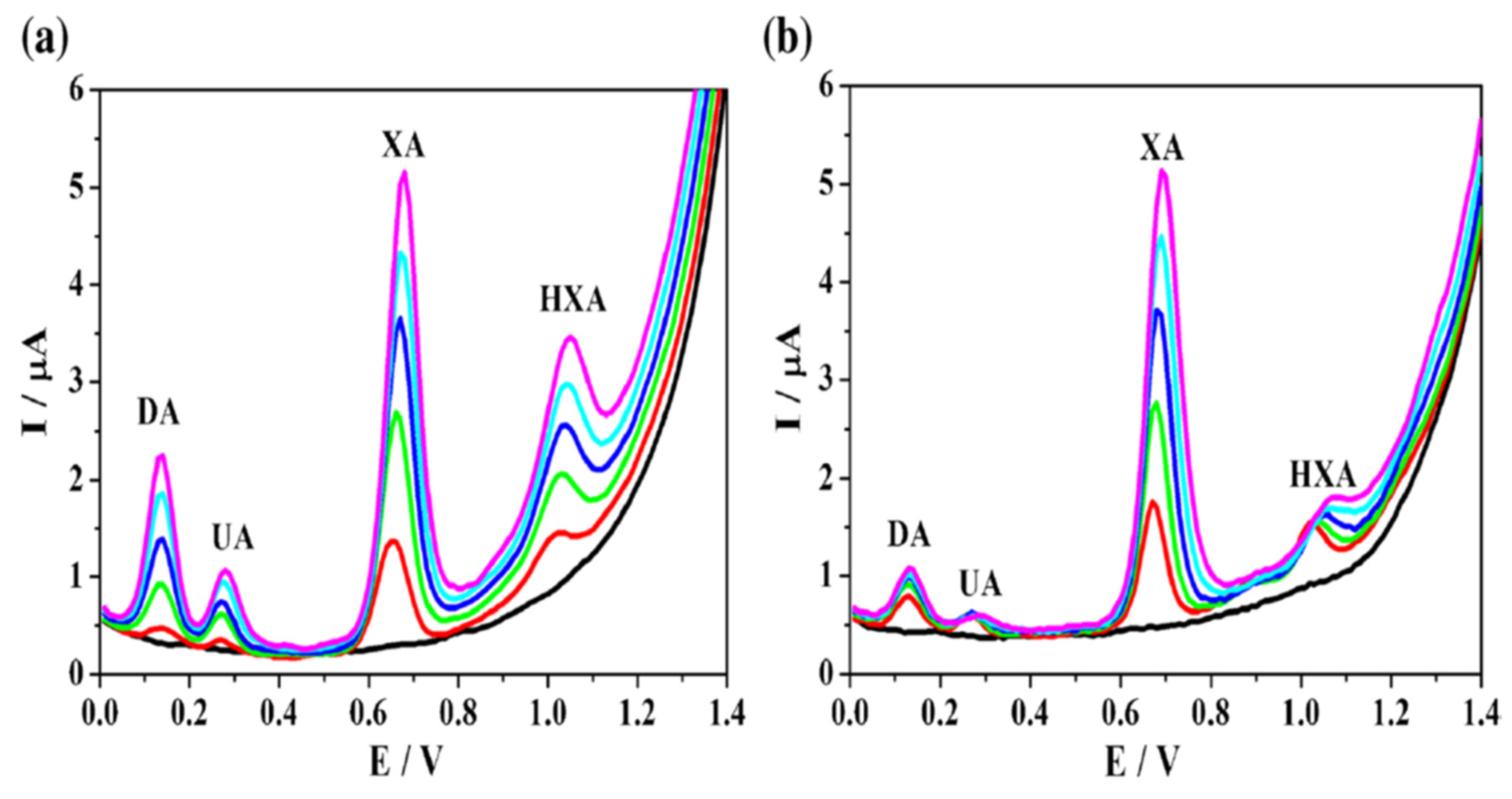
- Zhang, L.; Li, S.; Xin, J.; Ma, H.; Pang, H.; Tan, L.; Wang, X. A Non-Enzymatic Voltammetric Xanthine Sensor Based on the Use of Platinum Nanoparticles Loaded with a Metal-Organic Framework of Type MIL-101(Cr). Application to Simultaneous Detection of Dopamine, Uric Acid, Xanthine and Hypoxanthine. Microchim Acta. 2018, 186, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhuang, X.; Wu, Q.; Jin, M.; Zheng, C.; Jiang, Y.; Lou, Y.; Zheng, L. Cu-MOF@Pt 3D Nanocomposites Prepared by One-Step Wrapping Method with Peroxidase-like Activity for Colorimetric Detection of Glucose. Colloids and Surfaces B: Biointerfaces. 2022, 216, 112601. [Google Scholar] [CrossRef]
- Yin, H.; Zhu, J.; Chen, J.; Gong, J.; Nie, Q. MOF-Derived in Situ Growth of Carbon Nanotubes Entangled Ni/NiO Porous Polyhedrons for High Performance Glucose Sensor. Materials Letters. 2018, 221, 267–270. [Google Scholar] [CrossRef]
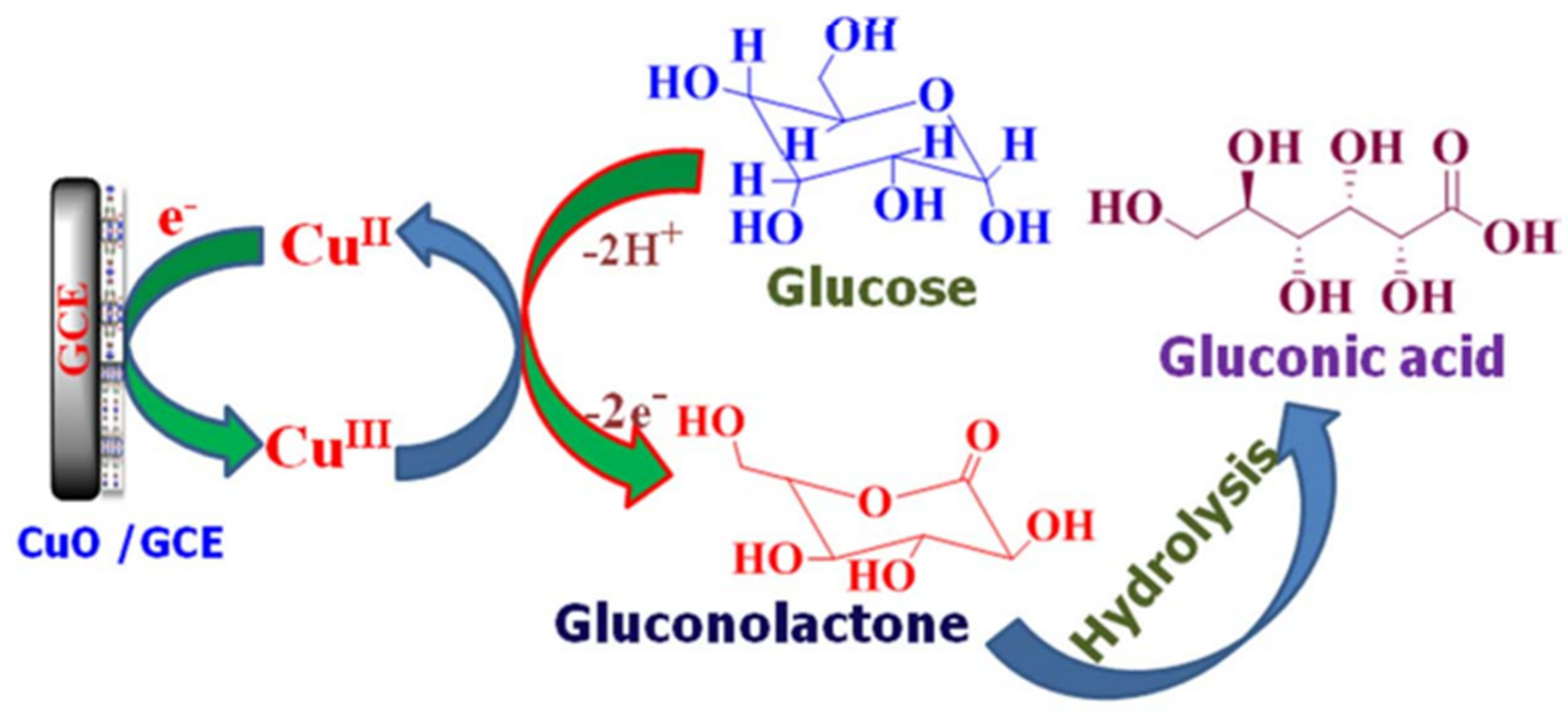
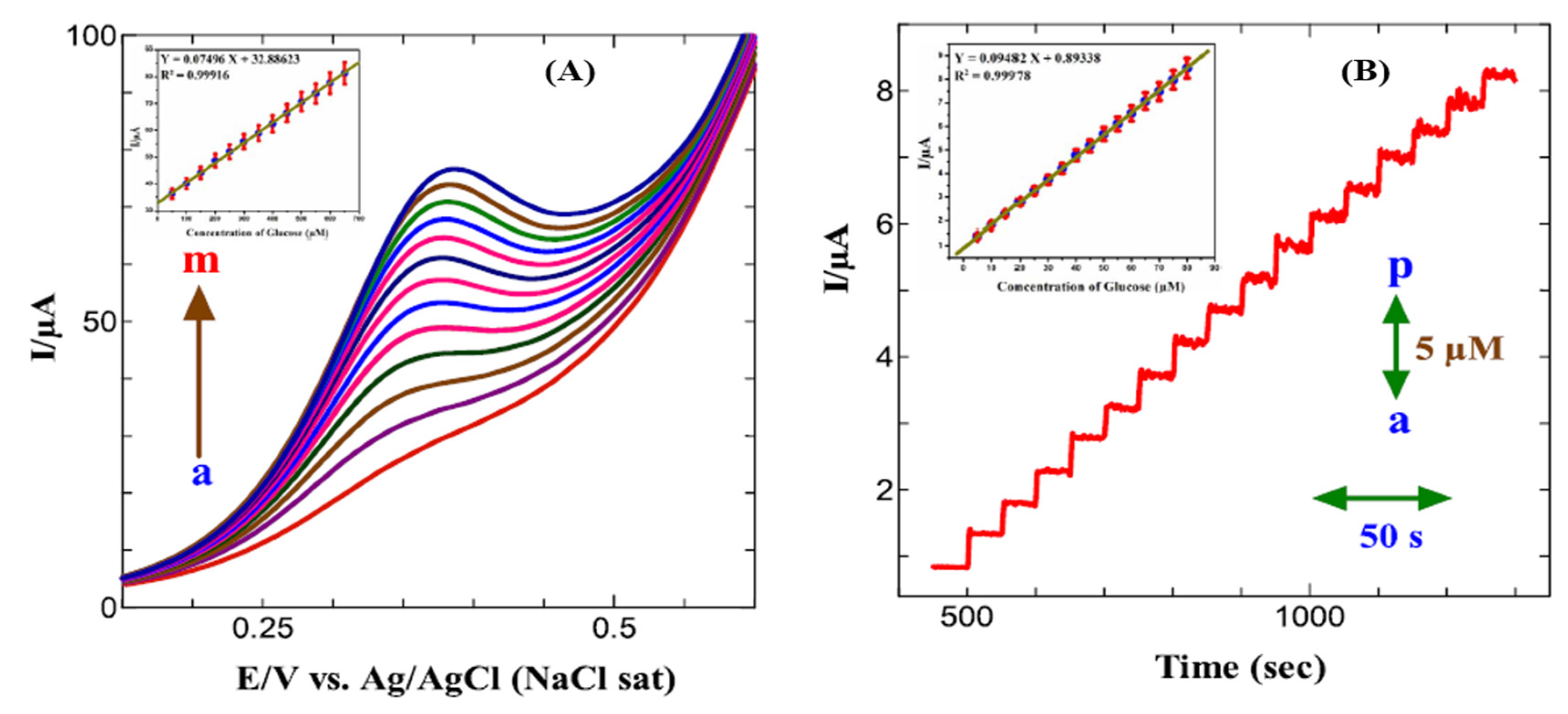
- Arul, P.; JOHN, S.A. Electrodeposition of CuO from Cu-MOF on Glassy Carbon Electrode: A Non-Enzymatic Sensor for Glucose. J. Electroanal. Chem. 2017, 799. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Khaki Sanati, E.; Hosseini, H. Direct Growth of Metal-Organic Frameworks Thin Film Arrays on Glassy Carbon Electrode Based on Rapid Conversion Step Mediated by Copper Clusters and Hydroxide Nanotubes for Fabrication of a High Performance Non-Enzymatic Glucose Sensing Platform. Biosensors and Bioelectronics 2018, 112, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lu, Z.-W.; Ma, Y.; Zhang, J.-J.; Mo, G.-Q.; Du, H.-J.; Ye, J.-S. Cu(II) Metal-Organic Framework Encapsulated in Carbon Paste Electrode for High-Performance Non-Enzymatic Glucose Sensing. Chinese J. Anal. Chem. 2020, 48, e20038–e20046. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Xin, J.; Ma, H.; Pang, H.; Tan, L.; Wang, X. A Non-Enzymatic Voltammetric Xanthine Sensor Based on the Use of Platinum Nanoparticles Loaded with a Metal-Organic Framework of Type MIL-101(Cr). Application to Simultaneous Detection of Dopamine, Uric Acid, Xanthine and Hypoxanthine. Microchim Acta. 2018, 186, 9. [Google Scholar] [CrossRef]
- Feng, X.; Yin, X.; Bo, X.; Guo, L. An Ultrasensitive Luteolin Sensor Based on MOFs Derived CuCo Coated Nitrogen-Doped Porous Carbon Polyhedron. Sensors and Actuators B: Chemical 2019, 281, 730–738. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Li, C.-X.; Ding, X.-T.; Yang, Q.; Qi, Y.-M.; Zhang, H.-M.; Qu, L.-T. Detection of Dopamine at Graphene-ZIF-8 Nanocomposite Modified Electrode. Chinese Chemical Letters 2017, 28, 1473–1478. [Google Scholar] [CrossRef]
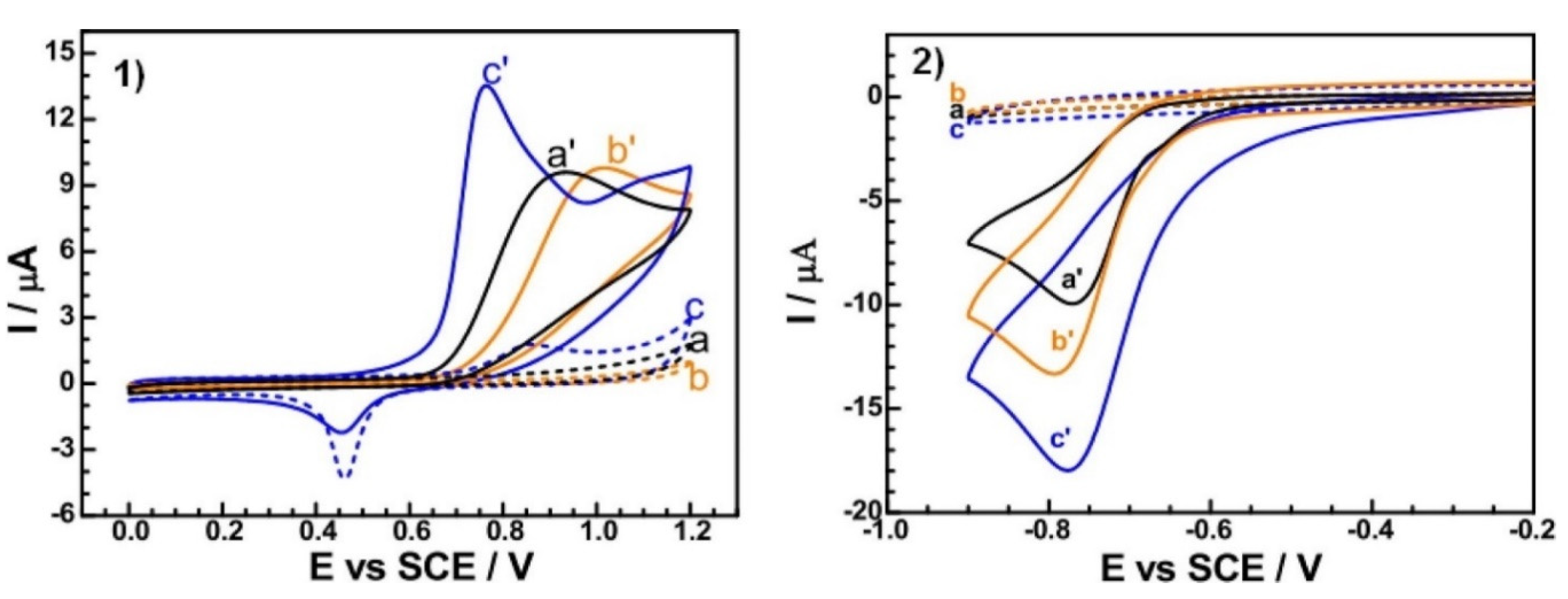
- Hosseini, H.; Ahmar, H.; Dehghani, A.; Bagheri, A.; Tadjarodi, A.; Fakhari, A.R. A Novel Electrochemical Sensor Based on Metal-Organic Framework for Electro-Catalytic Oxidation of L-Cysteine. Biosensors and Bioelectronics 2013, 42, 426–429. [Google Scholar] [CrossRef]
- Duan, D.; Si, X.; Ding, Y.; Li, L.; Ma, G.; Zhang, L.; Jian, B. A Novel Molecularly Imprinted Electrochemical Sensor Based on Double Sensitization by MOF/CNTs and Prussian Blue for Detection of 17β-Estradiol. Bioelectrochemistry 2019, 129, 211–217. [Google Scholar] [CrossRef]
- He, J.; Yang, H.; Zhang, Y.; Yu, J.; Miao, L.; Song, Y.; Wang, L. Smart Nanocomposites of Cu-Hemin Metal-Organic Frameworks for Electrochemical Glucose Biosensing. Sci Rep 2016, 6, 36637. [Google Scholar] [CrossRef]
- Singh, R.; Musameh, M.; Gao, Y.; Ozcelik, B.; Mulet, X.; M. Doherty, C. Stable MOF@enzyme Composites for Electrochemical Biosensing Devices. J. Mat. Chem. 2021, 9, 7677–7688. [Google Scholar]
- Hou, C.; Zhao, D.; Wang, Y.; Zhang, S.; Li, S. Preparation of Magnetic Fe3O4/PPy@ZIF-8 Nanocomposite for Glucose Oxidase Immobilization and Used as Glucose Electrochemical Biosensor. J. Electroanal. Chem. 2018, 822, 50–56. [Google Scholar] [CrossRef]
- Arul, P.; Gowthaman, N.S.K.; John, S.A.; Tominaga, M. Tunable Electrochemical Synthesis of 3D Nucleated Microparticles like Cu-BTC MOF-Carbon Nanotubes Composite: Enzyme Free Ultrasensitive Determination of Glucose in a Complex Biological Fluid. Electrochimica Acta. 2020, 354, 136673. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Y.; Zhang, Y.; Zeng, T.; Wan, Q.; Lai, G.; Yang, N. A UiO-66-NH2/Carbon Nanotube Nanocomposite for Simultaneous Sensing of Dopamine and Acetaminophen. Analytica Chimica Acta. 2021, 1158, 338419. [Google Scholar] [CrossRef]
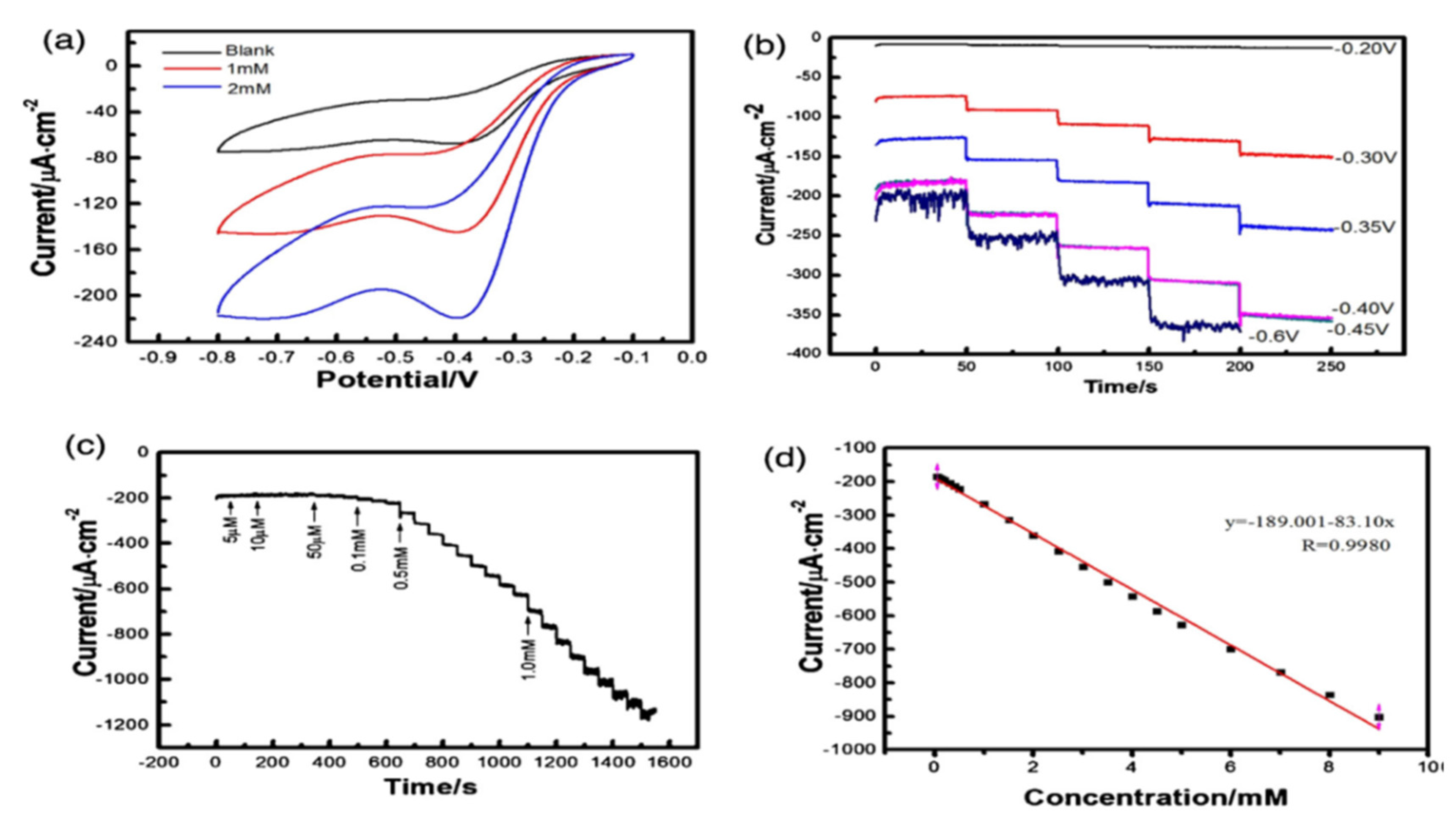
- Tran, T.Q.N.; Das, G.; Yoon, H.H. Nickel-Metal Organic Framework/MWCNT Composite Electrode for Non-Enzymatic Urea Detection. Sensors and Actuators B: Chemical. 2017, 243, 78–83. [Google Scholar] [CrossRef]
- Dang, W.; Sun, Y.; Jiao, H.; Xu, L.; Lin, M. AuNPs-NH2/Cu-MOF Modified Glassy Carbon Electrode as Enzyme-Free Electrochemical Sensor Detecting H2O2. J. Electroanal. Chem. 2020, 856, 113592. [Google Scholar] [CrossRef]
- Yang, L.; Xu, C.; Ye, W.; Liu, W. An Electrochemical Sensor for H2O2 Based on a New Co-Metal-Organic Framework Modified Electrode. Sensors and Actuators B: Chemical 2015, 215, 489–496. [Google Scholar] [CrossRef]
- Lisanti, M.P.; Martinez-Outschoorn, U.E.; Lin, Z.; Pavlides, S.; Whitaker-Menezes, D.; Pestell, R.G.; Howell, A.; Sotgia, F. Hydrogen Peroxide Fuels Aging, Inflammation, Cancer Metabolism and Metastasis. Cell Cycle. 2011, 10, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Asadian, E.; Shahrokhian, S.; Iraji Zad, A. Highly Sensitive Nonenzymetic Glucose Sensing Platform Based on MOF-Derived NiCo LDH Nanosheets/Graphene Nanoribbons Composite. J. Electroanal. Chem. 2018, 808, 114–123. [Google Scholar] [CrossRef]
- Sherino, B.; Mohamad, S.; Abdul Halim, S.N.; Abdul Manan, N.S. Electrochemical Detection of Hydrogen Peroxide on a New Microporous Ni–Metal Organic Framework Material-Carbon Paste Electrode. Sensors and Actuators B: Chemical. 2018, 254, 1148–1156. [Google Scholar] [CrossRef]
- Li, C.; Wu, R.; Zou, J.; Zhang, T.; Zhang, S.; Zhang, Z.; Hu, X.; Yan, Y.; Ling, X. MNPs@anionic MOFs/ERGO with the Size Selectivity for the Electrochemical Determination of H2O2 Released from Living Cells. Biosensors and Bioelectronics. 2018, 116, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, K.; Youliwasi, N.; Guo, H.; Yang, G.; Jiao, F.; Dong, B.; Chai, Y.; Mintova, S.; Liu, C. Highly Sensitive H2O2 Sensor Based on Porous Bimetallic Oxide Ce1−xTbxOy Derived from Homeotypic Ln-MOFs. Applied Surface Science. 2019, 470, 91–98. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.; Liu, L.; Yang, X.; Xu, X. Electrochemical Investigation of a New Cu-MOF and Its Electrocatalytic Activity towards H2O2 Oxidation in Alkaline Solution. Electrochemistry Communications. 2013, 33, 131–134. [Google Scholar] [CrossRef]
- Naseri, M.; Fotouhi, L.; Ehsani, A. Nanostructured Metal Organic Framework Modified Glassy Carbon Electrode as a High Efficient Non-Enzymatic Amperometric Sensor for Electrochemical Detection of H2O2. J. Electrochem. Sci. Technol. 2018, 9, 28–36. [Google Scholar] [CrossRef]
- Meng, W.; Xu, S.; Dai, L.; Li, Y.; Zhu, J.; Wang, L. An Enhanced Sensitivity towards H2O2 Reduction Based on a Novel Cu Metal–Organic Framework and Acetylene Black Modified Electrode. Electrochimica Acta. 2017, 230, 324–332. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Zhang, R.; Shi, H.; Guo, Y.; Guo, X.; Li, S.; Yuan, B. 3D Porous Metal-Organic Framework as an Efficient Electrocatalyst for Nonenzymatic Sensing Application. Talanta. 2015, 144, 1176–1181. [Google Scholar] [CrossRef]
- Cobos, M.; De-La-Pinta, I.; Quindós, G.; Fernández, M.J.; Fernández, M.D. Graphene Oxide–Silver Nanoparticle Nanohybrids: Synthesis, Characterization, and Antimicrobial Properties. Nanomaterials. 2020, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, J.; Shi, H.; Guo, X.; Guo, Y.; Zhang, R.; Yuan, B. Redox-Active Microsized Metal-Organic Framework for Efficient Nonenzymatic H2O2 Sensing. Sensors and Actuators B: Chemical. 2015, 221, 224–229. [Google Scholar] [CrossRef]
- Lopa, N.S.; Rahman, Md.M.; Ahmed, F.; Chandra Sutradhar, S.; Ryu, T.; Kim, W. A Base-Stable Metal-Organic Framework for Sensitive and Non-Enzymatic Electrochemical Detection of Hydrogen Peroxide. Electrochimica Acta. 2018, 274, 49–56. [Google Scholar] [CrossRef]
- Wang, M.-Q.; Zhang, Y.; Bao, S.-J.; Yu, Y.-N.; Ye, C. Ni(II)-Based Metal-Organic Framework Anchored on Carbon Nanotubes for Highly Sensitive Non-Enzymatic Hydrogen Peroxide Sensing. Electrochimica Acta. 2016, 190, 365–370. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Lian, M.; Chen, X.; Lu, Y.; Yang, W. Enzyme Immobilization on ZIF-67/MWCNT Composite Engenders High Sensitivity Electrochemical Sensing. J. Electroanal. Chem. 2019, 833, 505–511. [Google Scholar] [CrossRef]
- Real-Time Electrochemical Quantification of H2O2 in Living Cancer Cells Using Bismuth Based MOF. J. Electroanal. Chem. 2022, 914, 116255. [CrossRef]
- Liu, B.; Wang, X.; Zhai, Y.; Zhang, Z.; Liu, H.; Li, L.; Wen, H. Facile Preparation of Well Conductive 2D MOF for Nonenzymatic Detection of Hydrogen Peroxide: Relationship between Electrocatalysis and Metal Center. J. Electroanal. Chem. 2020, 858, 113804. [Google Scholar] [CrossRef]
- Yadav, D.K.; Ganesan, V.; Sonkar, P.K.; Gupta, R.; Rastogi, P.K. Electrochemical Investigation of Gold Nanoparticles Incorporated Zinc Based Metal-Organic Framework for Selective Recognition of Nitrite and Nitrobenzene. Electrochimica Acta. 2016, 200, 276–282. [Google Scholar] [CrossRef]
- Ioi, J.D.; Zhou, T.; Tsao, R.; F. Marcone, M. Mitigation of Patulin in Fresh and Processed Foods and Beverages. Toxins. 2017, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Afzali, Z.; Mohadesi, A.; Ali Karimi, M.; Fathirad, F. A Highly Selective and Sensitive Electrochemical Sensor Based on Graphene Oxide and Molecularly Imprinted Polymer Magnetic Nanocomposite for Patulin Determination. Microchemical Journal. 2022, 177, 107215. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Wang, Y.; Huang, H.; Ma, J. Amperometric Sensing of Paracetamol Using a Glassy Carbon Electrode Modified with a Composite of Water–Stable Metal–Organic Framework and Gold Nanoparticles. Int. J. Electrochem. Sci. 2018, 13, 7643–7654. [Google Scholar] [CrossRef]
- Hadi, M.; Poorgholi, H.; Mostaanzadeh, H. Determination of Metformin at Metal-Organic Framework (Cu-BTC) Nanocrystals/Multi-Walled Carbon Nanotubes Modified Glassy Carbon Electrode : Research Article. South African Journal of Chemistry. 2016, 69, 132–139. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, R.; Yuan, Q.; Wang, F. Highly Sensitive Electrochemical Sensor for Chloramphenicol Based on MOF Derived Exfoliated Porous Carbon. Talanta. 2017, 167, 39–43. [Google Scholar] [CrossRef]
- Kung, C.-W.; Chang, T.-H.; Chou, L.-Y.; Hupp, J.T.; Farha, O.K.; Ho, K.-C. Porphyrin-Based Metal–Organic Framework Thin Films for Electrochemical Nitrite Detection. Electrochemistry Communications. 2015, 58, 51–56. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, J.; Zhang, R.; Shi, H.; Wang, N.; Li, J.; Ma, F.; Zhang, D. Cu-Based Metal–Organic Framework as a Novel Sensing Platform for the Enhanced Electro-Oxidation of Nitrite. Sensors and Actuators B: Chemical. 2016, 222, 632–637. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, X.; Nsabimana, A.; Han, C.; Li, M.; Guo, L. Electrocatalytically Active Cobalt-Based Metal–Organic Framework with Incorporated Macroporous Carbon Composite for Electrochemical Applications. J. Mater. Chem. A. 2014, 3, 732–738. [Google Scholar] [CrossRef]
- Li, J.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. An Electrochemical Sensor Based on Copper-Based Metal-Organic Frameworks-Graphene Composites for Determination of Dihydroxybenzene Isomers in Water. Talanta. 2018, 181, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Chen, H.; Hu, X.; Ma, S. Fabrication of Highly Sensitive and Stable Hydroxylamine Electrochemical Sensor Based on Gold Nanoparticles and Metal–Metalloporphyrin Framework Modified Electrode. ACS Appl. Mater. Interfaces. 2016, 8, 18173–18181. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Chen, L. An Ultrasensitive Electrochemical Bisphenol A Sensor Based on Hierarchical Ce-Metal-Organic Framework Modified with Cetyltrimethylammonium Bromide. Sensors and Actuators B: Chemical. 2018, 261, 425–433. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, B.; Tan, C.-P.; Lai, O.-M.; Panpipat, W.; Cheong, L.-Z.; Shen, C. Hierarchical Macro-Microporous ZIF-8 Nanostructures as Efficient Nano-Lipase Carriers for Rapid and Direct Electrochemical Detection of Nitrogenous Diphenyl Ether Pesticides. Sensors and Actuators B: Chemical. 2020, 321, 128477. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Qiang, Y. Ultrasensitive Electrochemical Aptasensor for Ochratoxin A Detection Using AgPt Bimetallic Nanoparticles Decorated Iron-Porphyrinic Metal-Organic Framework for Signal Amplification. Sensors and Actuators B: Chemical. 2020, 312, 127964. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Fakhri, H.; Hajian, A.; Afkhami, A.; Bagheri, H. High-Performance Electrochemical Enzyme Sensor for Organophosphate Pesticide Detection Using Modified Metal-Organic Framework Sensing Platforms. Bioelectrochemistry. 2019, 130, 107348. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Frontiers in Pharmacology. 2021, 12. [Google Scholar] [CrossRef]
- Shamim, M.A.; Zia, H.; Zeeshan, M.; Khan, M.Y.; Shahid, M. Metal Organic Frameworks (MOFs) as a Cutting-Edge Tool for the Selective Detection and Rapid Removal of Heavy Metal Ions from Water: Recent Progress. J. Environ. Chem. Eng. 2022, 10, 106991. [Google Scholar] [CrossRef]
- Patel, P.D. (Bio)Sensors for Measurement of Analytes Implicated in Food Safety: A Review. TrAC Trends Anal. Chem. 2002, 21, 96–115. [Google Scholar] [CrossRef]
- Gurusamy, L.; Anandan, S.; Wu, J.J. Chapter 18 - Nanomaterials Derived from Metal-Organic Frameworks for Energy Storage Supercapacitor Application. In Metal-Organic Frameworks for Chemical Reactions; Khan, A., Verpoort, F., Asiri, A.M., Hoque, M.E., Bilgrami, A.L., Azam, M., Naidu, K.C.B., Eds.; Elsevier, 2021; pp. 441–470 ISBN 978-0-12-822099-3.
- Song, D.; Jiang, X.; Li, Y.; Lu, X.; Luan, S.; Wang, Y.; Li, Y.; Gao, F. Metal−organic Frameworks-Derived MnO2/Mn3O4 Microcuboids with Hierarchically Ordered Nanosheets and Ti3C2 MXene/Au NPs Composites for Electrochemical Pesticide Detection. J. Hazard. Mat. 2019, 373, 367–376. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Pan, Z.; Ye, B.; Xu, M. Determination of Malachite Green in Fish by a Modified MOF-Based Electrochemical Sensor. Food Anal. Methods. 2019, 12, 1246–1254. [Google Scholar] [CrossRef]
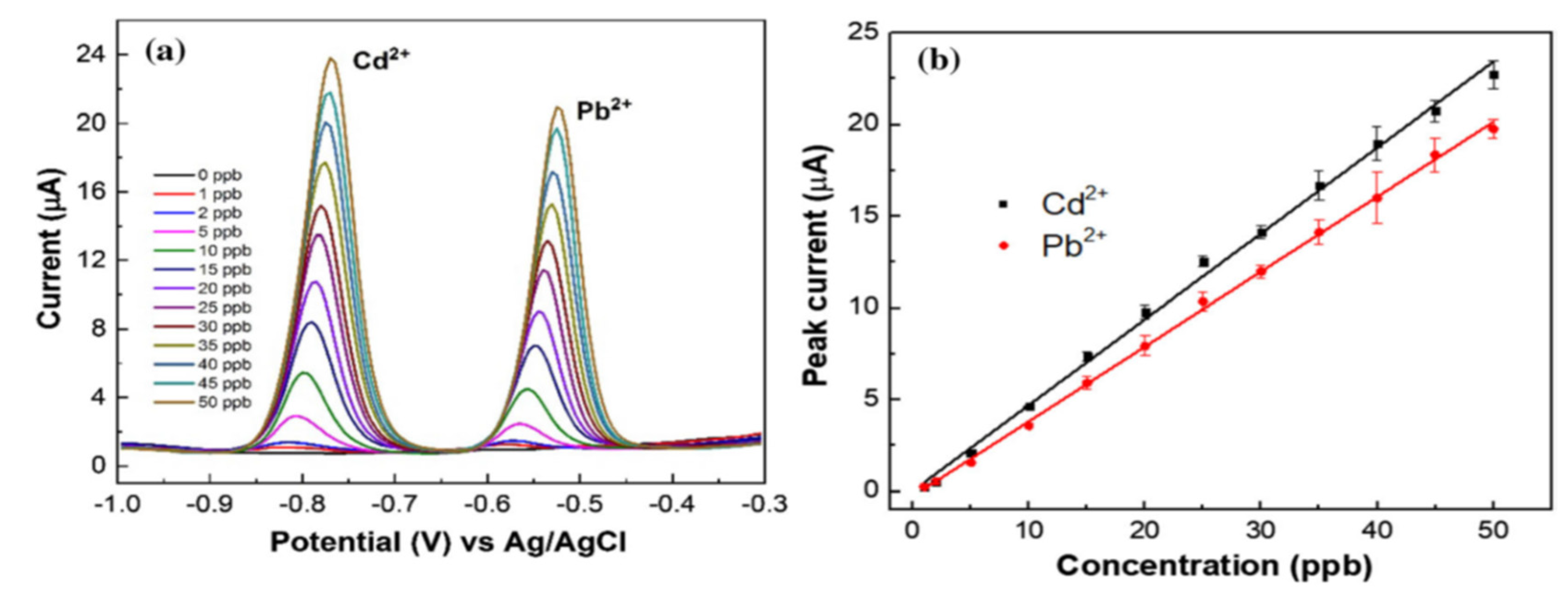
- Zhang, S.; Huang, W. Simultaneous Determination of Cd2+ and Pb2+ Using a Chemically Modified Electrode. Anal Sci. 2001, 17, 983–985. [Google Scholar] [CrossRef]
- Nguyen, M.B.; Nga, D.; Vu, T.T.; Piro, B.; PHAM TRUONG, T.N.; Yen, P.; Le, G.; Hung, L.; Vu, T.; Ha, V. Novel Nanoscale Yb-MOF Used as Highly Efficient Electrode for Simultaneous Detection of Heavy Metal Ions. J. Materials Sci. 2021, 56. [Google Scholar] [CrossRef]
- Roushani, M.; Valipour, A.; Saedi, Z. Electroanalytical Sensing of Cd2+ Based on Metal–Organic Framework Modified Carbon Paste Electrode. Sensors and Actuators B: Chemical. 2016, 233, 419–425. [Google Scholar] [CrossRef]
- Yin, H.; He, H.; Li, T.; Hu, M.; Huang, W.; Wang, Z.; Yang, X.; Yao, W.; Xiao, F.; Wu, Y.; et al. Ultra-Sensitive Detection of Multiplexed Heavy Metal Ions by MOF-Derived Carbon Film Encapsulating BiCu Alloy Nanoparticles in Potable Electrochemical Sensing System. Analytica Chimica Acta. 2023, 1239, 340730. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Xie, J.; Hu, X. Metal–Organic Framework Modified Carbon Paste Electrode for Lead Sensor. Sensors and Actuators B: Chemical. 2013, 177, 1161–1166. [Google Scholar] [CrossRef]
- Huo, D.; Zhang, Y.; Li, N.; Ma, W.; Liu, H.; Xu, G.; Li, Z.; Yang, M.; Hou, C. Three-Dimensional Graphene/Amino-Functionalized Metal–Organic Framework for Simultaneous Electrochemical Detection of Cd(II), Pb(II), Cu(II), and Hg(II). Anal. Bioanal. Chem. 2022, 414, 1575–1586. [Google Scholar] [CrossRef]
- Wang, F.-F.; Liu, C.; Yang, J.; Xu, H.-L.; Pei, W.-Y.; Ma, J.-F. A Sulfur-Containing Capsule-Based Metal-Organic Electrochemical Sensor for Super-Sensitive Capture and Detection of Multiple Heavy-Metal Ions. Chem. Eng. J. 2022, 438. [Google Scholar] [CrossRef]
- Ru, J.; Wang, X.; Cui, X.; Wang, F.; Ji, H.; Du, X.; Lu, X. GaOOH-Modified Metal-Organic Frameworks UiO-66-NH2: Selective and Sensitive Sensing Four Heavy-Metal Ions in Real Wastewater by Electrochemical Method. Talanta. 2021, 234, 122679. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ge, H.; Wu, Y.; Ye, G.; Chen, H.; Hu, X. Construction of an Electrochemical Sensor Based on Amino-Functionalized Metal-Organic Frameworks for Differential Pulse Anodic Stripping Voltammetric Determination of Lead. Talanta 2014, 129, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, J.-X.; Wang, J.-W.; Liu, Y.; Wang, L.-C.; Weerasooriya, R.; Wu, Y.-C. Doping ZIF-67 with Transition Metals Results in Bimetallic Centers for Electrochemical Detection of Hg(II). Electrochimica Acta. 2021, 387, 138539. [Google Scholar] [CrossRef]
- Niu, B.; Yao, B.; Zhu, M.; Guo, H.; Ying, S.; Chen, Z. Carbon Paste Electrode Modified with Fern Leave-like MIL-47(as) for Electrochemical Simultaneous Detection of Pb(II), Cu(II) and Hg(II). J. Electroanal. Chem. 2021, 886, 115121. [Google Scholar] [CrossRef]
- Quang Khieu, D.; Thi Thanh, M.; Vinh Thien, T.; Hai Phong, N.; Hoang Van, D.; Dinh Du, P.; Phi Hung, N. Synthesis and Voltammetric Determination of Pb(II) Using a ZIF-8-Based Electrode. J. Chem. 2018, 2018. [Google Scholar] [CrossRef]
- Lu, M.; Deng, Y.; Luo, Y.; Lv, J.; Li, T.; Xu, J.; Chen, S.-W.; Wang, J. Graphene Aerogel–Metal–Organic Framework-Based Electrochemical Method for Simultaneous Detection of Multiple Heavy-Metal Ions. Anal. Chem. 2019, 91, 888–895. [Google Scholar] [CrossRef] [PubMed]
- D. Pournara, A.; Margariti, A.; D. Tarlas, G.; Kourtelaris, A.; Petkov, V.; Kokkinos, C.; Economou, A.; S. Papaefstathiou, G.; J. Manos, M. A Ca 2+ MOF Combining Highly Efficient Sorption and Capability for Voltammetric Determination of Heavy Metal Ions in Aqueous Media. J. Materials Chem. A. 2019, 7, 15432–15443. [Google Scholar]
- Ma, L.; Zhang, X.; Ikram, M.; Ullah, M.; Wu, H.; Shi, K. Controllable Synthesis of an Intercalated ZIF-67/EG Structure for the Detection of Ultratrace Cd2+, Cu2+, Hg2+ and Pb2+ Ions. Chem. Eng. J. 2020, 395, 125216. [Google Scholar] [CrossRef]
- Yang, H.; Peng, C.; Han, J.; Song, Y.; Wang, L. Three-Dimensional Macroporous Carbon/Zr-2,5-Dimercaptoterephthalic Acid Metal-Organic Frameworks Nanocomposites for Removal and Detection of Hg(II). Sensors and Actuators B: Chemical. 2020, 320, 12844. [Google Scholar] [CrossRef]
| Sample | Metal | Ligand | Solvent | Condition | Ref. | |
|---|---|---|---|---|---|---|
| Hydrothermal synthesis | UiO-66 | ZrCl4 | H2BDC | DMF | 120℃, 24 h | [62] |
| Co-MOF | Co(NO3)2·6H2O | H3BTC | DMF | 100℃, 24 h | [63] | |
| Ni-MOF | Ni(NO3)2·6H2O | H3BTC | DMF | 80℃, 18 h | [64] | |
| MIL-53 | FeCl3·6H2O | H2BDC | DMF | 150℃, 15 h | [65] | |
| Ce-MOF | Ce(NO3)3·6H2O | H3BTC | DMF-Ethanol | 120℃, 2 h | [66] | |
| Cu-NH2BDC | Cu(NO3)2·3H2O | NH2BDC | DMF-Ethanol | 110℃, 20 h | [67] | |
| MIL-101 | Cr(NO3)3·9H2O | H2BDC | De-ionized water | 180℃, 5 h | [68] | |
| Ultrasound | MOF-5 | Zn(NO3)2·6H2O | H2BDC | DMF | 90 W, 2 min | [69] |
| ZIF-8 | Zn(NO3)2·6H2O | MeIM | DMF | 300 W, 1 h | [70] | |
| MOF-74 | Mg(NO3)2·6H2O | H4dhtp | DMF | 500 W, 1 h | [71] | |
| Sn-BDC | SnSO4 | Na2BDC | De-ionized water | 155 W, 5 min | [72] | |
| HKUST-1 | Copper(II) nitrate hemipentahydrate | H3BTC | DMF | 130 W, 1 h | [73] | |
| Microwave method | Ni-MOF-74 | Ni(NO3)2·6H2O | DOT | DMF | 100℃, 90 min | [74] |
| Mg-MOF | Mg(NO3)2·6H2O | DOT | DMF | 125℃, 90 min | [74] | |
| MOF-5 | Zn(NO3)2·6H2O | H2BDC | DMF | 300 W, 2.5 min | [75] | |
| MOF-177 | Zn(NO3)2·6H2O | H3BTB | NMP | 800 W, 35 min | [76] | |
| MOF-199 | Cu(NO3)2·3H2O | H3BTC | DMF | 250 W, 30 min | [77] | |
| Electrochemical synthesis | Co-MOF | Co(NO3)2⋅6H2O | H3BTC | H2O, ethanol | Electrolyte (Et3NHCl)⋅6H2O | [78] |
| HKUST-1 | Cu foil electrode | H3BTC | DMSO, ethanol | Electrolyte (MTBAMS) | [79] | |
| HKUST-1 | Cu electrode | H3BTC | Methanol | Electrolyte (TBATFB) | [80] | |
| Cu-MOF | Cu(NO3)2·3H2O | H4BTEC | DMF, H2O | Electrolyte (TBATFB) | [81] | |
| Mechanochemical synthesis | Cu-MOF | Cu(OAc)2·H2O | H3BTC | No solvent | 15.0 min | [82] |
| MIL-88A | FeCl3·6H2O | Furmarate | No solvent | 10.0 min | [83] | |
| MOF-505 | Cu(OAc)2·H2O | H4bptc | DMF | 40.0 Hz, 80 min | [84] | |
| IRMOF-3 | Zn4(μ4O)(NHOCPh)6 | NH2BDC | No solvent | 30.0 Hz, 30 min | [85] |
| Electrode type | Analyte | MOF | MOF composite | Work potential | pH | LOD | Linear range (10−6 mol/L) | Real sample | References |
|---|---|---|---|---|---|---|---|---|---|
| NPCP | Leuteoline | ZIF-67 | CuCo@NPCP | 0.10V | 7.0 | 0.080 nM | 0.20 –2.50 | Human vaccine | [121] |
| G.C.E. | Dopamine | ZIF-8 | ZIF-8@G | 0.30V | 7.0 | 1.00 μM | 3.0 –1.00 | Cow vaccine | [122] |
| G.C.E. | L-Cysteine | HKUST-1 | Au-SH-SiO2@Cu-MOF | 0.40V | 5.0 | 0.0080 μM | 0.02.–300 | N/r | [123] |
| G.C.E. | Ascorbic acid | HKUST-1 | HKUST-1@GO | -0.02V | 7.0 | 20.0 nM | 0.50–6965 | N/r | [91] |
| G.C.E | Catechol | MIL-101 | MIL-101 (Cr)@rGO | N/r | 7.0 | 4.00 μM | 10.0–1400 | Lake | [103] |
| G.C.E | Xanthine | MIL-101 | Pt-NPs@MIL-101 | 0.280V | 7.0 | 0.420 μM | 0.50–162 | Human vaccine | [114] |
| G.C.E | 17β-estradiol | MIL-53 | MIP-Pb/MIL-53@CNT | 0.210V | 3.0 | 0.00615 pM | 0.010–1000 | Domestic | [124] |
| G.C.E | Glucose | GOD/Cu | Hemin | -0.25V | 7.0 | 2.73 μM | 9.10-36.0 | Human Serum | [125] |
| G.C.E | Glucose | ZIF-8@GOx | GO | 0.4V | 7.4 | 0.05 mM | 1-10 | Calf Serum | [126] |
| G.C.E | Glucose | ZIF-8 | Fe3O4/PPy/GOx | 0.6V | 7 | 0.333 μM | 1-2 | Human Serum | [127] |
| G.C.E | Glucose | Cu-MOF | MWCNTs | 0.55V | 7 | 0.4 μM | 0.5-11.84 | Human Serum | [128] |
| G.C.E | Dopamine | UiO-66-NH2 | CNTs | 0.0V | 7 | 15nM | 0.03-2 | Human Serum | [129] |
| G.C.E | Urea | Ni-MOF | MWCNT/ITO | 0.45V | 3.0 μM | 10-1120 | Urine | [130] |
| Electrode type | MOF | Reduction potential | pH | LOD | Linear range (10−6 mol/L) |
Real sample | Ref. |
|---|---|---|---|---|---|---|---|
| CPE | Ni-MOF | −0.250 V | 13 | 0.00090 mM | 0.0040–60 | Cleaning soln. | [135] |
| GCE | Y1-4-NDC-MOF | −0.50 V | 7 | 0.430 μM | 04.0–11000 | A549 cells | [136] |
| GCE | Ce1-xTbx-MOF | 0.750 V | 7 | 7.70 μM | 0.10 –4.2 | N/r | [137] |
| GCE | [Cu(adp)(BIB)(H2O)]n | N/r | 13 | 0.0680 μM | 0.100–2.750 | N/r | [138] |
| GCE | Cu(btec)0.5DMF | −0.20 V | 6.5 | 0.8650 μM | 5.0–8000 | N/r | [139] |
| GCE | {[Cu2(bep)(ada)2]H2O}n | −0.45 V | 13 | 0.014 μM | 0.05–3 | N/r | [140] |
| CPE | Cu-MOF | −0.2 V | 7.2 | 1.00 μM | 1.0 –0.99 | N/r | [141] |
| GCE | HKUST-1 | −0.4 V | 7 | 0.49 μM | 1.0–5.6 | Raw 264.7 cells | [142] |
| GCE | Zn-MOF | −0.80 V | 7.2 | 67 nM | 1 –5 | Milk | [109] |
| CPE | Co-MOF | −0.30 V | 7.2 | 0.50 μM | 1.0–823 | N/r | [143] |
| GCE | MIL-53-Cr(III) | −0.307 V | 13 | 3.520 μM | 25.0–500 | Human vaccine | [144] |
| GCE | Ni-MOF/CNTs | 0.5V | 13 | 2.1 μM | 10-5.600 | N/r | [145] |
| GCE | AuNPs-NH2/Cu-MOF | -0.15V | 7.4 | 1.2 μM | 5–850 | HeLa cells | [131] |
| GCE | ZIF-67 | -0.05V | 7 | 0.11 μM | 1.86-1050 | N/r | [146] |
| GCE | Ag-Bi–BDC (s) MOF | -0.4V | 7 | 0.02 μM | 10-5000 | THP-1 | [147] |
| GCE | 2D Co-MOF | 0.25V | 12 | 0.69 μM | 0.5-832 | N/r | [148] |
| CPE | AP-Ni-MOF | -0.25V | 7 | 0. 9 μM | 4–60000 | Lens cleaning solution | [135] |
| Electrode type | Analyte | MOF | Work potential | pH | LOD | Linear range (10−6 mol/L) | Real sample | References |
|---|---|---|---|---|---|---|---|---|
| GCE | Nitrobenzene | MOF-5 | −0.790 V | 7 | 15.3 μM | 20.0–500 | N/r | [149] |
| GCE | Nitrite | MOF-525 | 0.90 V | 8 | 2.10 μM | 20.0–800 | N/r | [155] |
| CPE | Nitrite | Cu-MOF | 0.9 V | 7.2 | 30 nM | 50 –712 | Lake water | [156] |
| GCE | Hydrazine | [Co2(4-ptz)2 (bpp)(N3)2]n | 0.20 V | N/r | N/r | 5.0–630 | N/r | [157] |
| GCE | Dihydroxybenzene | HKUST-1 | N/r | 7 | 0.590 μM | 1.0–1000 | Domestic | [158] |
| GCE | Hydroxylamine | MMPF-6 | 0.350 V | 7 | 0.004 μM | 1–20 | Domestic | [159] |
| GCE | BPA | Ce-MOF | 0.520 V | 7 | 02.0 nM | 0.005–5.00 | Milk | [160] |
| GCE | Paracetamol | HKUST-1 | −0.060 V | 6 | 0.01–100 μM | 0.01–100.0 | Commercial tabs | [152] |
| GCE | Metformin | HKUST-1 | 0.6 V | 13 | 5.0–25 μM | 5–25.0 | Commercial tabs | [153] |
| GCE | Chloramphenicol | IRMOF-8 | −0.10 V | 7.5 | 0.010–1.0 μM | 0.01–1.0 | Honey | [154] |
| GCE | Diphenylether | MAC-ZIF-8 | -0.4V | 7 | 0.46 Μm | 0-114 | Apricot | [161] |
| GE | Ochratoxin A | AgPt/PCN-223-Fe | -0.6V | 6 | 20-2000 | 14 | Red wine | [162] |
| GCE | Paraoxon | Ce/UiO-66@MWCNTs | 0.2V | 7.5 | 0.01-150 | 0.004 | Spinach | [163] |
| Electrode Type | Analyte | M.O.F | Penetration potential | pH | L.O.D | Linear Range (10−6 mol/L) | Real sample | Ref |
|---|---|---|---|---|---|---|---|---|
| CPE | Cd2+ | [Zn2(NH2-BDC)2(4-bpdh)]·3DMF | -1.0V | 3 | 0.2 μM | 0.7 – 120 | Tap water | [172] |
| GCE | Zn2+ | BiCux-ANPs@CF/SPCE | -1.2V | 4.5 | 35 μM | 150-600 | Urine | [173] |
| CPE | Pb2+ | MOF-5 | -0.9V | 5 | 4.9 μM | 10 – 1000 | Tap water | [174] |
| GCE | Hg2+ | 3DGO/UiO-66-NH2 | -1.1V | 7.4 | 3.1 μM | 0.01-3.5 | Rice and honey sample | [175] |
| GCE | Cu2+ | Co-TMC4R-BDC | -1.3V | 5 | 0.067 μM | 0.25-9 | Lake water | [176] |
| GCE | Cu2+ | Yb-MOF | -1.1V | 4.5 | 1.6 μM | 0-50 | River water | [171] |
| GCE | Hg2+ | UiO-66-NH2/GaOOH | -1.0V | 6 | 0.006 μM | 0.10-0.45 | Waste water | [177] |
| GCE | Pb2+ | NH2-CU3 (BTC)2 | -1.0V | 4.5 | 5.0 μM | 10 – 500 | Powder milk | [178] |
| GCE | Hg2+ | Fe1Co1 | -1.0V | 5 | 0.0078 μM | 0.1-1.1 | River water | [179] |
| CPE | Cu2+ | MIL -47 | -1.10V | 4.5 | 0.087 μM | 1-10 | Lake water | [180] |
| GCE | Hg2+ | ZJU -27 | -0.58V | 5 | 0.0013 μM | 0.5-2 | Lake water | [94] |
| GCE | Pb2+ | ZIF-8 | -1.2V | 4.7 | 4.16 μM | 12 – 100 | N/R | [181] |
| GCE | Cu2+ | GA -UiO -66 -NH2 | -1.3V | 5 | 0.008 μM | 0.01-1.6 | Vegetable | [182] |
| GPE | Cu2+ | Ca -MOF | -0.2V | 4.5 | 1.4 μM | 10-60 | Waste water | [183] |
| GCE | Hg2+ | ZIF -67/EG | -0.80V | 5 | 0.00129 μM | 0.5-3 | Waste water | [184] |
| CPE | Pb2+ | MOF-235 | N/r | N/r | 50 μM | N/r | Tap water | [96] |
| KSC | Hg2+ | Zr -DMBD MOF | -0.8V | 6 | 0.05 μM | 0.25-3.5 | River water | [185] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




