Submitted:
29 August 2023
Posted:
05 September 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and methods
Exclusion Criteria
Statistical analysis
Results
Discussion
Author contributions
Funding
Availability of data and materials
Acknowledgments
Availability of data and materials
Competing interests
Consent for publication
Ethical approval
References
- Rasmussen SA, Fernhoff PM, Scanlon KS. Vitamin B12 deficiency in children and adolescents. J Pediatr. 2001;138(1):10-17. [CrossRef]
- Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014;349: g5226.
- Koc A, Kocyigit A, Soran M, et al. High frequency of maternal vitamin B12 deficiency as an important cause of infantile vitamin B12 deficiency in Sanliurfa province of Turkey. Eur J Nutr. 2006;45(5):291-297. [CrossRef]
- Albayrak D, Albayrak C. Clinical follow-up of children with high vitamin B12 values: should we worry?. Turk J Pediatr. 2021;63(6):1064-1071. [CrossRef]
- Green R, Miller JW. Vitamin B12 deficiency. Vitam Horm. 2022; 119:405-439.
- Guéant JL, Guéant-Rodriguez RM, Alpers DH. Vitamin B12 absorption and malabsorption. Vitam Horm. 2022; 119:241-274. [CrossRef]
- Avci Z, Turul T, Aysun S, Unal I. Involuntary movements and magnetic resonance imaging findings in infantile cobalamine (vitamin B12) deficiency. Pediatrics. 2003;112(3 Pt 1):684-686. [CrossRef]
- Rasmussen SA, Fernhoff PM, Scanlon KS. Vitamin B12 deficiency in children and adolescents. J Pediatr. 2001;138(1):10-17. [CrossRef]
- Stabler SP, Allen RH. Vitamin B12 Deficiency As a Worldwide Problem. Annu Rev Nutr. 2004;24(1):299-326. [CrossRef]
- McLean E, de Benoist B, Allen LH. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull. 2008; 29 (2): 38-51. [CrossRef]
- Ramussen SA, Fernboff PM, Scanlon KS. Vitamin B12 deficiency in children and adolescents. J Pediat. 2001; 138: 7-10.
- Reynolds EH. The neurology of folic acid deficiency. Handb Clin Neurol. 2014;120:927-943. [CrossRef]
- Crellin R, Bottiglieri T, Reynolds EH. Folates and psychiatric disorders. Clinical potential. Drugs. 1993;45(5):623-636. [CrossRef]
- Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5(11):949-960.
- Goraya JS, Kaur S, Mehra B. Neurology of Nutritional Vitamin B12 Deficiency in Infants: Case Series From India and Literature Review. J Child Neurol 2015;30:1831–7.
- Bahadir A, Reis PG, Erduran E. Oral vitamin B12 treatment is effective for children with nutritional vitamin B12 deficiency. J Paediatr Child Health 2014;50:721–5. [CrossRef]
- Minet JC, Bissé E, Aebischer CP, Beil A, Wieland H, Lütschg J. Assessment of vitamin B-12, folate, and vitamin B-6 status and relation to sulfur amino acid metabolism in neonates. Am J Clin Nutr. 2000;72(3):751-757. [CrossRef]
- Monsen AL, Refsum H, Markestad T, Ueland PM. Cobalamin status and its biochemical markers methylmalonic acid and homocysteine in different age groups from 4 days to 19 years. Clin Chem. 2003;49(12):2067-2075. [CrossRef]
- Esnafoglu E, Ozturan DD. The relationship of severity of depression with homocysteine, folate, vitamin B12, and vitamin D levels in children and adolescents. Child Adolesc Ment Health. 2020;25(4):249-255. [CrossRef]
- Erden S, Akbaş İleri B, Sadıç Çelikkol Ç, Nalbant K, Kılınç İ, Yazar A. Serum B12, homocysteine, and anti-parietal cell antibody levels in children with autism. Int J Psychiatry Clin Pract. 2022;26(1):8-13.
- Yektaş Ç, Alpay M, Tufan AE. Comparison of serum B12, folate and homocysteine concentrations in children with autism spectrum disorder or attention deficit hyperactivity disorder and healthy controls. Neuropsychiatr Dis Treat. 2019;15:2213-2219. [CrossRef]
- Sharma TK, Vardey SK, Sitaraman S. Serum Homocysteine, Folate, and Vitamin B12 Levels in Carbamazepine Treated Epileptic Children. Clin Lab. 2016;62(7):1217-1224. [CrossRef]
- Altun H, Şahin N, Belge Kurutaş E, Güngör O. Homocysteine, Pyridoxine, Folate and Vitamin B12 Levels in Children with Attention Deficit Hyperactivity Disorder. Psychiatr Danub. 2018;30(3):310-316. [CrossRef]
- Verhoef P, Stampfer MJ, Buring JE, Gaziano JM, Allen RH, Stabler SP, et al. Homocysteine metabolism and risk of myocardial infarction: relation with vitamins B6, B12 and folate. Am J Epidemiol 1996;1;143:845-59. [CrossRef]
- Kuzminski AM, Del EJ, Allen RH, et al. Effective treatment of cobalamin deficiency with oral cobalamin. Blood 1998; 92:1191-1198.
- Lee GR, Foerster J, Lukens J, et all: Wintrobe’s clinical Hematology; Pernicious anemia and other causes of vitamin B12 (cobalamin) deficiency. Lippincott Williams & Wilkins Philadelphia, 2004; 947-978.
- Schenck UV, Bender-Götze C, Koletzko B: Persistance of neurological damage induced by dietary vitamin B12 deficiency in infancy. Arch Dis Child 1997; 77:137-139.
- Guerra-Shinohar EM, Paiva AA, Rondo PH, Yamasaki K, Terzi CA, D’Almeida V. Relationship between total homocystein and folate levels in pregnant women and their newborn babies according to maternal serum levels of vitamin B12. BJOG 2002;109:784-91. [CrossRef]
- Karademir F, Suleymanoglu S, Ersen A, Aydinoz S, Gultepe M, Meral C, Ozkaya H, Gocmen I. Vitamin B12, folate, homocystein and urinary methlymalonic acid levels in infants. J Int Med Res 2007;35:384-8.
- Onal H, Adal E, Oner T, Onal Z, Aydın A. An important problem in developing countries: maternal and neonatal vitamin B12 deficiency. Turk Arc Ped 2010;45:242-5.
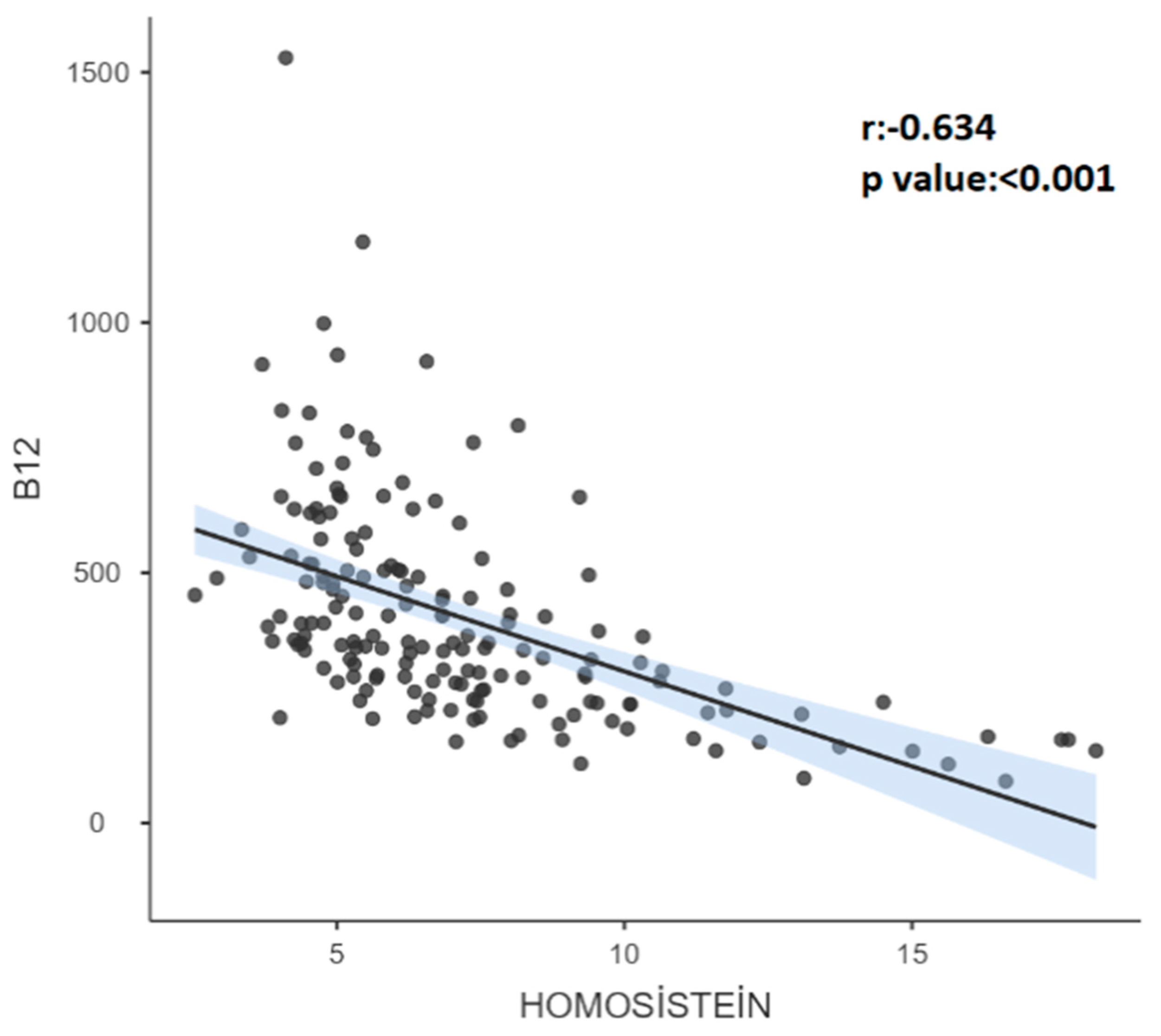
| 6-11 |
12-23 |
24-47 |
≥48 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All groups | |||||||||||
| n | % | n | % | n | % | n | % | n | % | p | |
| GENDER | |||||||||||
| Boys | 95(55,9%) | 26(54,2%) | 28(53,8%) | 19(52,8%) | 22(64,7%) | 0,715* | |||||
| Girls | 75(44,1%) | 22(45,8%) | 24(46,2%) | 17(47,2%) | 12(35,3%) | ||||||
| TERM_D | |||||||||||
| Term | 159(93,5%) | 46(95,8%) | 48(92,3%) | 34(94,4%) | 31(91,2%) | 0,881† | |||||
| Preterm | 11(6,5%) | 2(4,2%) | 4(7,7%) | 2(5,6%) | 3(8,8%) | ||||||
| NUTRITION | |||||||||||
| Breastfed | 45(26,5%) | 12(25,0%) | 16(30,8%) | 10(27,8%) | 7(20,6%) | ||||||
| Breast milk + formula | 35(20,6%) | 15(31,3%) | 8(15,4%) | 6(16,7%) | 6(17,6%) | 0,173† | |||||
| Breast milk + supplementary food | 58(34,1%) | 15(31,3%) | 22(42,3%) | 12(33,3%) | 9(26,5%) | ||||||
| Formula and/or supplementary food | 32(18,8%) | 6(12,5%) | 6(11,5%) | 8(22,2%) | 12(35,3%) | ||||||
| VITAMIN SUPLEMENT | 30(17,6%) | 12 (25,0%) a | 16 (30,8%) a | 1 (2,8%) b | 1(2,9%) b | <0,001† | |||||
| Fish oil | 2(1,2%) | 0(0,0%) | 0(0,0%) | 1(2,8%) | 1(2,9%) | 0,247† | |||||
| Vitamin D | 21(12,4%) | 11(22,9%) | 10(19,2%) | 0(0,0%) | 0(0,0%) | <0,001† | |||||
| Iron | 15(8,8%) | 5(10,4%) | 10 (19,2%) | 0(0,0%) | 0(0,0%) | <0,001† | |||||
| Vitamin B | 2(1,2%) | 2(4,2%) | 0(0,0%) | 0(0,0%) | 0(0,0%) | 0,161† | |||||
| Education mother | |||||||||||
| Elementary Education | 27(15,9%) | 6(12,5%) | 5(9,6%) | 6(16,7%) | 10(29,4%) | 0,068† | |||||
| High School/associate degree | 72(42,4%) | 24(50,0%) | 17(32,7%) | 17(47,2%) | 14(41,2%) | ||||||
| Bachelor and above | 71(41,8%) | 18(37,5%) | 30(57,7%) | 13(36,1%) | 10(29,4%) | ||||||
| Education father | |||||||||||
| Elementary Education | 27(15,9%) | 6(12,5%) | 7(13,5%) | 8(22,2%) | 6(17,6%) | 0,721† | |||||
| High School/associate degree | 79(46,5%) | 24(50,0%) | 24(46,2%) | 13(36,1%) | 18(52,9%) | ||||||
| Bachelor and above | 64(37,6%) | 18(37,5%) | 21(40,4%) | 15(41,7%) | 10(29,4%) | ||||||
| B12 (pg/mL) | |||||||||||
| <200 | 19(11,20%) | 12 (25%) a | 3 (5,8%) b | 1 (2,8%) b | 3a,b(8,8%) | <0.001† | |||||
| 200-299 | 40(23,50%) | 16(33%) | 15(28,8%) | 6(16,7%) | 3(8,8%) | ||||||
| ≥300 | 111(65,30%) | 20 (41,70%) a | 34 (65,4%) a,b | 29 (80,6%) b | 28 (82,4%) b | ||||||
| B12 (pg/mL) | |||||||||||
| <300 | 59(34,7%) | 28 (58,3%) a | 18a,b(34,6%) | 7(19,4%) b | 6 (17,6%) b | <0.001* | |||||
| ≥300 | 111(65,30%) | 20 (41,70%) a | 34a,b(65,4%) | 29(80,6%) b | 28 (82,4%) b | ||||||
| *: chi-square test; †: Fisher exact test Different superscript letters indicate groups with significant differences. | |||||||||||
| All Groups | 6-11 | 12-23 | 24-47 | 48-72 | |||
| Mean±std | Mean±std | Mean±std | Mean±std | p | |||
| Age (Month) | Mean±std | 26.34±21.61 | 8.58±1.35 | 14.58±3.54 | 31.47±7.77 | 63.94±11.98 | - |
| Median(Q1-Q3) | 17(10-36) | 9(8-10) | 12(12-17) | 30.5(24-36) | 62(53-72) | ||
| Height (cm) | Mean±std | 86.05±17.38 | 69.92±3.94 | 78.14±5.65 | 92.92±8.14 | 113.66±9.6 | - |
| Median(Q1-Q3) | 79(72.5-97) | 70.25(66-72.75) | 78(75-81) | 94.5(88.5-99) | 113.65(108-120) | ||
| Weight (kg) | Mean±std | 12.84±5.13 | 8.69±1.09 | 10.85±1.47 | 13.91±2.42 | 20.58±5.39 | - |
| Median(Q1-Q3) | 11(9.4-15.3) | 8.6(8-9.35) | 10.78(9.85-11.4) | 14.05(11.9-16) | 19(17-22.7) | ||
| BMI (kg/m2) | Mean±std | 17.03±2.24 | 17.81±2.09 | 17.82±2.04 | 16.07±1.62 | 15.72±2.35 | - |
| Median(Q1-Q3) | 16.86(15.72-18.01) | 17.4(16.46-19.07) | 17.44(16.4-19.1) | 16.22(14.92-16.85) | 15.28(14.31-16.58) | ||
| Dairy products (…day/week) | Mean±std | 5.86±2.27 | 5.23±2.87 | 5.88±2.13 | 6.31±2.01 | 6.26±1.58 | 0,199¶ |
| Median(Q1-Q3) | 7(7-7) | 7(3-7) | 7(7-7) | 7(7-7) | 7(7-7) | ||
| Meat consumption (…day/week) | Mean±std | 2.31±1.73 | 1.67±1.56 | 2.71±1.92 | 2.43±1.59 | 2.49±1.62 | 0,051¶ |
| Median(Q1-Q3) | 2(1-3) | 2(0-3) | 2(1.75-3.75) | 2(2-3) | 2(1.5-3) | ||
| B12 (pg/mL) | Mean±std | 411.99±215.58 | 322.23±179.08 | 430.13±233.84 | 491.11±219.41 | 427.21±193.51 | 0,001¶ |
| Median(Q1-Q3) | 360.5(264-506) | 282(201.5-405.5)a | 361.5(272-532)b | 459.5(332.5-635)b | 395(317-514)b | ||
| Homocysteine(μmol/L) | Mean±std | 7.12±3.03 | 8.46±3.83 | 6.99±2.91 | 5.75±1.75 | 6.86±2.23 | 0,002¶ |
| Median(Q1-Q3) | 6.34(5.01-8.16) | 7.38(5.62-9.4)a | 6.4(5.03-8.26)a,b | 5.34(4.64-6.49)b | 6.37(5.33-7.52)a,b | ||
| Folic acid(ng/mL) | Mean±std | 12.57±4.09 | 14.86±2.42 | 14.09±3.55 | 10.34±3.75 | 9.35±3.93 | <0,001Ͳ |
| Median(Q1-Q3) | 13(9.8-15.7) | 14.95(12.95-16.7)a | 14.3(11.55-15.9)a | 10.35(7-13.75)b | 8.3(6.2-12.7)b | ||
| Ferritin(mL/ng) | Mean±std | 30.44±24.13 | 36.99±30.59 | 25.01±19.36 | 27.85±20.52 | 32.23±22.45 | 0,181¶ |
| Median(Q1-Q3) | 22.31(14.21-40.24) | 29.88(15.28-48.15) | 19.94(12.03-32.18) | 19.24(13.29-43.44) | 23.12(19.09-39.54) | ||
| Leukocyte(x103/µL) | Mean±std | 10.36±3.04 | 10.58±3.1 | 11.13±3.03 | 9.38±2.52 | 9.9±3.23 | 0,037¶ |
| Median(Q1-Q3) | 10.1(8.31-12.14) | 9.9(8.91-11.8)a,b | 10.48(9.14-12.75)a | 9.32(7.41-10.82)b | 9.54(7.75-12.14)a,b | ||
| Neutrophil(103/µL) | Mean±std | 3.66±1.06 | 3.78±1.14 | 3.74±0.99 | 3.64±0.91 | 3.41±1.17 | 0,432Ͳ |
| Median(Q1-Q3) | 3.7(2.91-4.32) | 3.86(2.95-4.45) | 3.9(3.18-4.39) | 3.69(3.1-4.21) | 3.17(2.57-4.2) | ||
| Lymphocyte(103/µL) | Mean±std | 2.66±1.14 | 2.6±1.05 | 2.65±1.25 | 2.51±0.83 | 2.9±1.34 | 0,831¶ |
| Median(Q1-Q3) | 2.44(1.9-3.2) | 2.53(1.8-3.2) | 2.18(1.89-3.35) | 2.4(2.09-2.97) | 2.69(2-3.9) | ||
| HGB(g/dL) | Mean±std | 11.73±1 | 11.35±0.93 | 11.68±1 | 11.83±0.94 | 12.23±0.97 | 0,001Ͳ |
| Median(Q1-Q3) | 11.8(11-12.4) | 11.4(10.6-12.05)a | 11.8(11.05-12.3)a | 11.9(11.35-12.3)a,b | 12.55(11.4-12.8)b | ||
| HCT(%) | Mean±std | 35.06±2.58 | 34.07±2.47 | 35.04±2.72 | 35.14±2.18 | 36.39±2.4 | 0,001Ͳ |
| Median(Q1-Q3) | 34.95(33.2-37) | 33.8 (32.45-35.65)a | 34.8 (33.15-36.55)a,b | 34.9 (33.65-36.65)a,b | 37.1(34.3-37.9)b | ||
| PLT(x103/mL) | Mean±std | 344.52±89.01 | 356.31±101.39 | 343.58±94.64 | 341.08±81.79 | 332.94±68.13 | 0,691Ͳ |
| Median(Q1-Q3) | 341.5(288-399) | 352(295-406.5) | 343(273.5-402) | 336(289.5-380.5) | 330(290-370) | ||
| MCV(fL) | Mean±std | 76.21±4.76 | 75.59±4.55 | 75.14±4.99 | 77.17±4.05 | 77.71±5.01 | 0,003¶ |
| Median(Q1-Q3) | 76.9(74.2-79.4) | 76.15(74.1-78.2)a | 76(72.8-78.6)a | 77.5(75.5-80.3)a,b | 78.65(76.4-80.6)b | ||
| MCH(pg) | Mean±std | 25.92±4.75 | 25.7±4.4 | 25.9±6.92 | 25.99±2.84 | 26.18±2.28 | 0,013¶ |
| Median(Q1-Q3) | 25.9(24.3-27.1) | 25.45(24.15-26.5)a | 25.2(24-27.05) a,b | 26.4(25.4-27.35) a,b | 26.6(25.4-27.5)b | ||
| MCHC(g/L) | Mean±std | 33.49±1.41 | 33.31±1.17 | 33.35±1.59 | 33.64±1.42 | 33.81±1.38 | 0,307¶ |
| Median(Q1-Q3) | 33.6(32.7-34.5) | 33.35(32.5-34.05) | 33.5(32.3-34.4) | 33.7(33.05-34.65) | 33.8(33-34.8) | ||
| NLR | Mean±std | 1.68±1.06 | 1.69±0.88 | 1.71±0.86 | 1.8±1.56 | 1.51±0.93 | 0.638¶ |
| Median(Q1-Q3) | 1.57(1.05-2.06) | 1.59(1.13-2.05) | 1.6(1.14-2.08) | 1.41(1.07-1.98) | 1.33(0.71-2.11) | ||
| PLR | Mean±std | 153.95±80.22 | 160.13±86.03 | 154.34±76.78 | 157.89±86.88 | 140.44±70.99 | 0.746¶ |
| Median(Q1-Q3) | 135.91(101.44-181.48) | 144.27(108.24-185.09) | 128.94(94.75-188.33) | 146.34(104.96-183.55) | 125.31(82.86-174) | ||
| SII | Mean±std | 573.85±384.37 | 597.37±375.9 | 580.78±348.19 | 606.44±502.3 | 495.55±303.36 | 0.620¶ |
| Median(Q1-Q3) | 507.82(326.63-696) | 547.07(361-668.45) | 520.04(344.03-729.66) | 506.7(306.21-706.75) | 419.08(223.89-711.74) | ||
| ¶: Kruskal-Wallis test;Ͳ:One-way ANOVA | |||||||
| B12 Groups | |||||||
| <200 | 200-299 | ≥300 | |||||
| n | % | n | % | n | % | p | |
| Gender | |||||||
| Boys | 11(57,9%) | 23(57,5%) | 61(55,0%) | 0,972* | |||
| Girls | 8(42,1%) | 17(42,5%) | 50(45,0%) | ||||
| Birth | |||||||
| Term | 18(94,7%) | 38(95,0%) | 103(92,8%) | 1† | |||
| Preterm | 1(5,3%) | 2(5,0%) | 8(7,2%) | ||||
| Age (month) | |||||||
| ≤11 | 12 (63.2%)a | 16 (40.0%)a | 20 (18.0%)b | ||||
| 12-23 | 3 (15.8%) | 15 (37.5%) | 34 (30.6%) | <0.001† | |||
| 24-47 | 1 (5.3%) | 6 (15.0%) | 29 (26.1%) | ||||
| ≥48 | 3 (15.8%) | 3 (7.5%) | 28 (25.2%) | ||||
| Nutrition | |||||||
| breastfed | 6(31,6%) | 10(25,0%) | 29(26,1%) | ||||
| breast milk + formula | 5(26,3%) | 9(22,5%) | 21(18,9%) | 0,026† | |||
| breast milk + supplementary food | 8(42,1%) | 18(45,0%) | 32(28,8%) | ||||
| formula and/or supplementary food | 0 (0,0%) a | 3 (7,5%) a | 29 (26,1%) b | ||||
| Vitamin Supplement | 5(26,3%) | 9(22,5%) | 16(14,4%) | 0,256† | |||
| Fish oil | 0(0,0%) | 0(0,0%) | 2(1,8%) | 1† | |||
| Vitamin D | 4(21,1%) | 6(15,0%) | 11(9,9%) | 0,304† | |||
| Iron | 3(15,8%) | 5(12,5%) | 7(6,3%) | 0,181† | |||
| Vitamin B | 0(0,0%) | 1(2,5%) | 1(0,9%) | 0,575† | |||
| Education mother | |||||||
| Elementary Education | 1(5,3%) | 9(22,5%) | 17(15,3%) | ||||
| High School/Associate Degree | 7(36,8%) | 18(45,0%) | 47(42,3%) | 0,346† | |||
| Bachelor and above | 11(57,9%) | 13(32,5%) | 47(42,3%) | ||||
| Education father | |||||||
| Elementary Education | 2(10,5%) | 6(15,0%) | 19(17,1%) | ||||
| High School/associate degree | 10(52,6%) | 22(55,0%) | 47(42,3%) | 0,675† | |||
| Bachelor and above | 7(36,8%) | 12(30,0%) | 45(40,5%) | ||||
| B12 Groups | |||||||
| <200 (pg/mL) | 200-299 (pg/mL) | >=300 (pg/mL) | |||||
| Mean±std | Median(Q1-Q3) | Mean±std | Median(Q1-Q3) | Mean±std | Median(Q1-Q3) | p | |
| Age (month) | 20,53±24,2 | 9(8-12)a | 19,58±18,18 | 12,5(9-22,5)a | 29,77±21,68 | 24(12-48)b | <0,001¶ |
| BMI (kg/m2) | 17,86±2,62 | 17,3(15,49-20)a,b | 17,5±1,87 | 17,29(16,55-18,61)a | 16,71±2,24 | 16,4(15,28-17,75)b | 0,016¶ |
| Dairy products (…day/week) | 4,74±3,12 | 7(1-7)a | 5,35±2,53 | 7(3-7)a,b | 6,24±1,9 | 7(7-7)b | 0,012¶ |
| Meat consumption (…day/week) | 1,76±1,77 | 2(0-2,5) | 2,39±1,95 | 2(1-3) | 2,38±1,64 | 2(1,5-3) | 0,273¶ |
| Homocysteine (μmol/L) | 12,6±3,72 | 12,35(8,92-16,32)a | 8,03±2,36 | 7,46(6,35-9,47)b | 5,85±1,65 | 5,45(4,64-6,84)c | <0,001¶ |
| Folic Acid (ng/mL) | 14,53±2,91 | 14,8(13-17) | 12,06±4,12 | 11,9(9,8-14,3) | 12,41±4,18 | 13,3(9,2-15,8) | 0,075Ͳ |
| Ferritin (mL/ng) | 36,26±36,05 | 22,4(11,77-53,49) | 27,43±24,32 | 17,8(10,3-36,05) | 30,53±21,52 | 23,15(15,39-42,05) | 0,296¶ |
| Leukocyte (x103/µL) | 10,61±4,28 | 9,45(7,75-12,84) | 10,53±2,75 | 10,39(8,33-11,93) | 10,25±2,91 | 10,07(8,38-12,14) | 0,787¶ |
| Neutrophil (103/µL) | 3,44±1,16 | 3,1(2,41-4,5) | 3,77±1,13 | 3,86(2,96-4,55) | 3,67±1,01 | 3,7(3-4,2) | 0,53Ͳ |
| Lymphocyte (103/µL) | 2,53±0,91 | 2,3(2-3,1) | 2,66±1,12 | 2,6(1,95-3,2) | 2,68±1,18 | 2,37(1,9-3,2) | 0,982¶ |
| HGB (g/dL) | 11,56±1,14 | 11,4(10,6-12,5)a,b | 11,34±0,9 | 11,4(10,55-12)a | 11,9±0,98 | 12(11,3-12,5)b | 0,006Ͳ |
| HCT (%) | 34,88±2,86 | 35(32,9-38)a,b | 34,15±2,17 | 34,25(32,55-35,35)a | 35,41±2,61 | 35,4(33,4-37,5)b | 0,027Ͳ |
| PLT (x103/mL) | 357,42±99,37 | 349(316-426) | 350,58±76,74 | 354,5(295-406) | 340,13±91,69 | 335(281-388) | 0,655Ͳ |
| MCV (fL) | 75,03±5,2 | 76,4(72,1-78,6) a,b | 75,05±5,02 | 75,6(72,95-78,35)a | 76,83±4,51 | 77,3(74,9-79,9)b | 0,03¶ |
| MCH (pg) | 24,66±2,31 | 25(22,7-26,5) a,b | 25,15±2,62 | 25,3(24,15-26,55)a | 26,41±5,53 | 26,3(25-27,2)b | 0,012¶ |
| MCHC (g/L) | 33,13±1,16 | 33,3(32,2-34,2) a,b | 33,18±1,25 | 33,25(32,4-34)a | 33,67±1,48 | 33,7(33-34,6)b | 0,045¶ |
| NLR | 1.52±0.66 | 1.58(1.07-1.88) | 1.73±0.98 | 1.6(1-2.22) | 1.7±1.14 | 1.54(1.03-2.06) | 0.852¶ |
| PLR | 164.83±85.8 | 152.17(112.76-194.38) | 156.09±79.95 | 126.23(105.06-194.11) | 151.31±79.92 | 135.5(98.54-179.71) | 0.733¶ |
| SII | 528.97±267.95 | 446.81(295.63-737.32) | 611±419.88 | 554.96(329.17-691.75) | 568.15±389.67 | 506.45(326.63-709.23) | 0.841¶ |
| Height (cm) | 76,1±13,8 | 73,5(66-75)a | 80,74±15,35 | 75,5(70-89)b | 89,67±17,59 | 85(75-104)c | <0,001¶ |
| Weight (kg) | 10,40±3,43 | 9,5(8,1-11,20)a | 11,73±4,93 | 10,7(8,33-13,45)b | 13,65±5,26 | 11,8(9,9-16,5)c | <0,001¶ |
| ¶: Kruskal-Wallis test; Ͳ:One-way ANOVA Significant differences are shown with the letters a, b, c in the columns. | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





