Submitted:
03 September 2023
Posted:
05 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
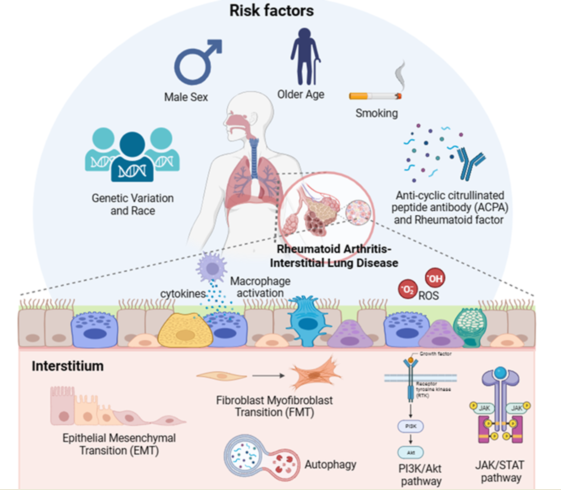
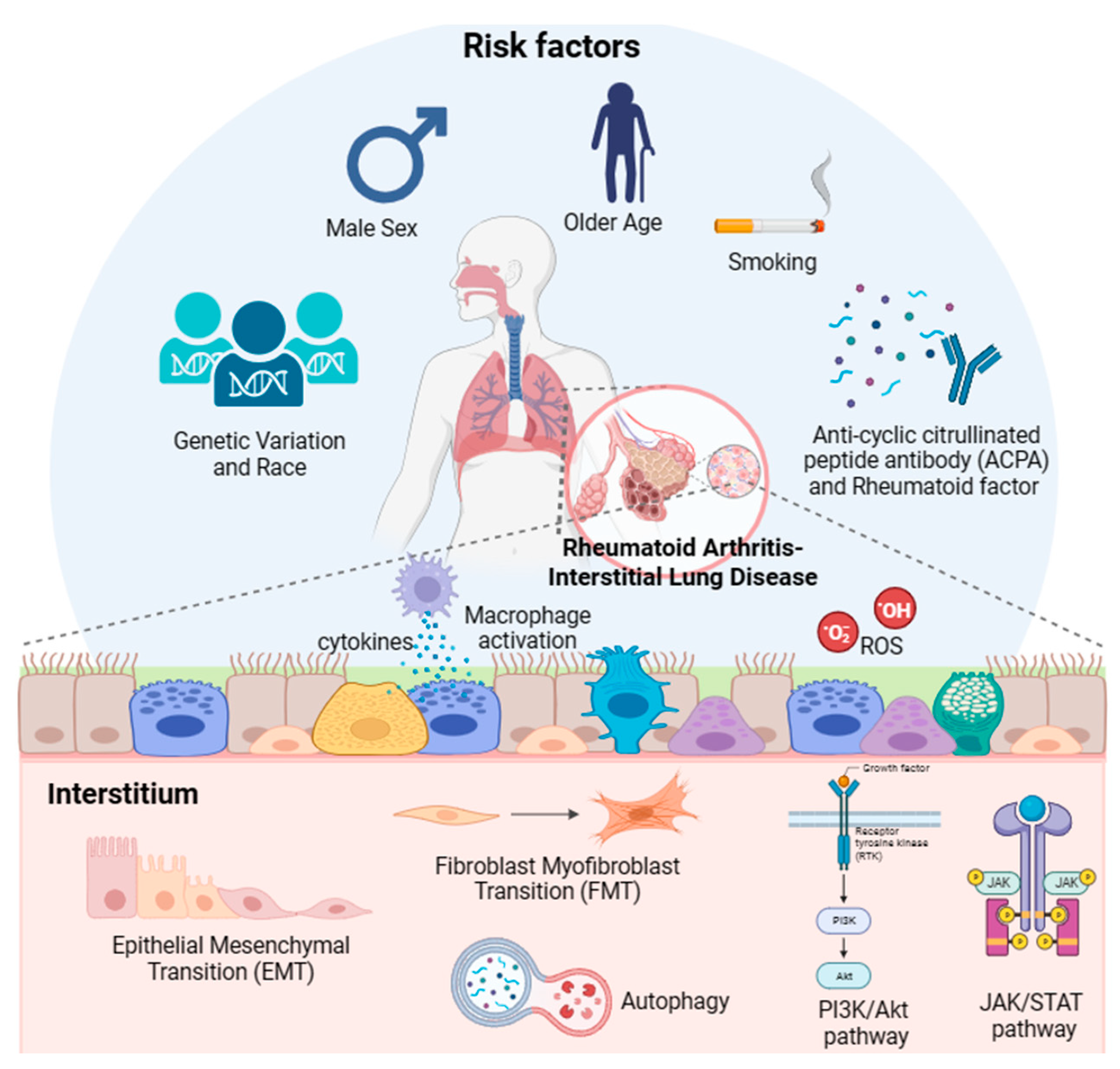
2. Risk Factors Associated with RA-ILD
2.1. Genetic factors
2.2. Age
2.3. Sex
2.4. Race
2.5. Smoking
2.6. Pollutants
2.7. Anti-cyclic citrullinated peptide antibody
2.8. Disease activity of RA
2.9. RF
2.10. Combination of factors
3. Pathogenesis of RA-ILD
3.1. FMT
3.2. EMT
3.3. Immunological pathways for production of different cytokines
3.4. Oxidative stress
3.5. Autophagy
3.6. Janus kinase/signal transducers and activators of transcription pathway
3.7. Phosphoinositide-3-kinase/protein kinase B pathway
4. Histopathological type of RA
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RA | rheumatoid arthritis |
| ILD | interstitial lung disease |
| CT | computed tomography |
| NSIP | nonspecific interstitial pneumonia |
| UIP | usual interstitial pneumonia |
| OP | organizing pneumonia |
| FPF | familial pulmonary fibrosis |
| SNP | single nucleotide polymorphism |
| PPFIBP2 | PPFIA binding protein 2 |
| HLA | human leukocyte antigen |
| GWAS | genome-wide association studies |
| OR | odds ratio |
| SE | shared epitope |
| PAD | peptidylarginine deiminase |
| AD | airway disease |
| SO2 | sulfur dioxide |
| NO2 | nitrogen dioxide |
| ACPA | anti-cyclic citrullinated peptide antibody |
| CI | confidence interval |
| IPF | idiopathic pulmonary fibrosis |
| CCP | cyclic citrullinated peptide |
| RF | rheumatoid factor |
| MCV | mutated citrullinated vimentin antibody |
| DAS28 | disease activity score in 28 joints |
| CDAI | clinical disease activity index |
| HR | hazard ratio |
| Ig | immunoglobulin |
| BRASS | Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study |
| ACR | American college of rheumatology |
| ECM | extracellular matrix |
| BLM | bleomycin |
| CIA | collagen-induced arthritis |
| FMT | fibroblast to myofibroblast transition |
| TGF | transforming growth factor |
| SSc | systemic sclerosis |
| HLF | human lung fibroblasts |
| MEF | mouse embryonic fibroblast |
| DOCK2 | dedicator of cytokinesis 2 |
| STAT | signal transducers and activators of transcription |
| ATF3 | activating transcription factor 3 |
| COX-2 | cyclooxygenase-2 |
| PGE2 | and prostaglandin E2 |
| EMT | epithelial-mesenchymal transition |
| PDGF | platelet derived growth factor |
| TIMP | tissue inhibitor |
| MMP | matrix metalloproteinase |
| DLCO | diffusing capacity for carbon monoxide |
| FVC | forced vital capacity |
| PFT | pulmonary function test |
| IL | interleukin |
| TNF | tumor necrosis factor |
| GSEA | gene set enrichment analysis |
| iBALT | inducible bronchial-associated lymphoid tissue |
| MDSCs | myeloid-derived suppressor cells |
| sPD-1 | soluble programmed death molecule-1 |
| HSPs | heat shock proteins |
| IFN | interferon |
| MDA | malondialdehyde |
| TBARS | thiobarbituric acid reactive substances |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| LPO | lipid peroxide |
| 8-OhdG | 8-hydroxydeoxyguanosine |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| BALF | bronchoalveolar lavage fluid |
| FoxO3a | forkhead transcription factor O subfamily member 3a |
| JAK | janus kinase |
| RANKL | receptor activator of nuclear factors κB ligand |
| CTD | connective tissue disease |
| PI3K | phosphoinositide-3-kinase |
| Akt | protein kinase B |
| AIA | antigen-induced arthritis |
| SDC2 | syndecan-2 |
| RIP1 | receptor-interacting serine-threonine kinase 1 |
| BAX | bcl2-associated X protein |
| HRCT | high resolution computed tomography |
References
- Cassone, G.; Manfredi, A.; Vacchi, C.; Luppi, F.; Coppi, F.; Salvarani, C.; Sebastiani, M. Treatment of rheumatoid arthritis-associated interstitial lung disease: lights and shadows. J. Clin. Med. 2020, 9, 1082. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, M.; Cojocaru, I.M.; Silosi, I.; Vrabie, C.D.; Tanasescu, R. Extra-articular manifestations in rheumatoid arthritis. Maedica 2010, 5, 286. [Google Scholar]
- Wilsher, M.; Voight, L.; Milne, D.; Teh, M.; Good, N.; Kolbe, J.; Williams, M.; Pui, K.; Merriman, T.; Sidhu, K. Prevalence of airway and parenchymal abnormalities in newly diagnosed rheumatoid arthritis. Respir. Med. 2012, 106, 1441–1446. [Google Scholar] [CrossRef]
- Norton, S.; Koduri, G.; Nikiphorou, E.; Dixey, J.; Williams, P.; Young, A. A study of baseline prevalence and cumulative incidence of comorbidity and extra-articular manifestations in RA and their impact on outcome. Rheumatology 2013, 52, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Chansakul, T.; Dellaripa, P.F.; Doyle, T.J.; Madan, R. Intra-thoracic rheumatoid arthritis: Imaging spectrum of typical findings and treatment related complications. Eur. J. Radiol. 2015, 84, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Ellman, P.; Ball, R. “Rheumatoid disease” with joint and pulmonary manifestations. BMJ 1948, 2, 816. [Google Scholar] [CrossRef]
- Bongartz, T.; Nannini, C.; Medina-Velasquez, Y.F.; Achenbach, S.J.; Crowson, C.S.; Ryu, J.H.; Vassallo, R.; Gabriel, S.E.; Matteson, E.L. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010, 62, 1583–1591. [Google Scholar] [CrossRef]
- Olson, A.L.; Swigris, J.J.; Sprunger, D.B.; Fischer, A.; Fernandez-Perez, E.R.; Solomon, J.; Murphy, J.; Cohen, M.; Raghu, G.; Brown, K.K. Rheumatoid arthritis–interstitial lung disease–associated mortality. Am. J. Respir. Crit. Care Med. 2011, 183, 372–378. [Google Scholar] [CrossRef]
- Yoshinouchi, T.; Ohtsuki, Y.; Fujita, J.; Yamadori, I.; Bandoh, S.; Ishida, T.; Ueda, R. Nonspecific interstitial pneumonia pattern as pulmonary involvement of rheumatoid arthritis. Rheumatol. Int. 2005, 26, 121–125. [Google Scholar] [CrossRef]
- Tanaka, N.; Kim, J.S.; Newell, J.D.; Brown, K.K.; Cool, C.D.; Meehan, R.; Emoto, T.; Matsumoto, T.; Lynch, D.A. Rheumatoid arthritis–related lung diseases: CT findings. Radiology 2004, 232, 81–91. [Google Scholar] [CrossRef]
- Lee, H.-K.; Kim, D.S.; Yoo, B.; Seo, J.B.; Rho, J.-Y.; Colby, T.V.; Kitaichi, M. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest 2005, 127, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.K. Roger S. Mitchell Lecture. Rheumatoid Lung Disease. Proc. Am. Thorac. Soc. 2007, 4, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Collard, H.R.; King Jr, T.E. Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest 2009, 136, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.; Collins, B.F.; Ho, L.A.; Raghu, G. Rheumatoid arthritis-associated lung disease. Eur. Respir. Rev. 2015, 24, 1–16. [Google Scholar] [CrossRef]
- Marigliano, B.; Soriano, A.; Margiotta, D.; Vadacca, M.; Afeltra, A. Lung involvement in connective tissue diseases: a comprehensive review and a focus on rheumatoid arthritis. Autoimmun. Rev. 2013, 12, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Cavagna, L.; Monti, S.; Grosso, V.; Boffini, N.; Scorletti, E.; Crepaldi, G.; Caporali, R. The multifaceted aspects of interstitial lung disease in rheumatoid arthritis. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- de Lauretis, A.; Veeraraghavan, S.; Renzoni, E. Review series: aspects of interstitial lung disease: connective tissue disease-associated interstitial lung disease: how does it differ from IPF? How should the clinical approach differ? Chron Respir Dis 2011, 8, 53–82. [Google Scholar] [CrossRef]
- Raimundo, K.; Solomon, J.J.; Olson, A.L.; Kong, A.M.; Cole, A.L.; Fischer, A.; Swigris, J.J. Rheumatoid arthritis–interstitial lung disease in the United States: prevalence, incidence, and healthcare costs and mortality. J. Rheumatol. 2019, 46, 360–369. [Google Scholar] [CrossRef]
- Iqbal, K.; Kelly, C. Treatment of rheumatoid arthritis-associated interstitial lung disease: a perspective review. Ther. Adv. Musculoskelet. Dis. 2015, 7, 247–267. [Google Scholar] [CrossRef]
- Hyldgaard, C.; Hilberg, O.; Pedersen, A.B.; Ulrichsen, S.P.; Løkke, A.; Bendstrup, E.; Ellingsen, T. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann. Rheum. Dis. 2017, 76, 1700–1706. [Google Scholar] [CrossRef]
- Johnson, C. Recent advances in the pathogenesis, prediction, and management of rheumatoid arthritis-associated interstitial lung disease. Curr. Opin. Rheumatol. 2017, 29, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Minhas, R.; Shankar, S.; Taha, O. Rheumatoid arthritis-associated interstitial lung disease. QJM 2019, 112, 815–816. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Kaneko, Y. Pathogenesis, clinical features, and treatment strategy for rheumatoid arthritis-associated interstitial lung disease. Autoimmun. Rev. 2022, 21, 103056. [Google Scholar] [CrossRef] [PubMed]
- Redente, E.F.; Aguilar, M.A.; Black, B.P.; Edelman, B.L.; Bahadur, A.N.; Humphries, S.M.; Lynch, D.A.; Wollin, L.; Riches, D.W. Nintedanib reduces pulmonary fibrosis in a model of rheumatoid arthritis-associated interstitial lung disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L998–L1009. [Google Scholar] [CrossRef] [PubMed]
- Fragoulis, G.E.; Conway, R.; Nikiphorou, E. Methotrexate and interstitial lung disease: controversies and questions. A narrative review of the literature. Rheumatology 2019, 58, 1900–1906. [Google Scholar] [CrossRef]
- Juge, P.-A.; Lee, J.S.; Lau, J.; Kawano-Dourado, L.; Serrano, J.R.; Sebastiani, M.; Koduri, G.; Matteson, E.; Bonfiglioli, K.; Sawamura, M. Methotrexate and rheumatoid arthritis associated interstitial lung disease. Eur. Respir. J. 2021, 57. [Google Scholar] [CrossRef]
- Juge, P.-A.; Borie, R.; Kannengiesser, C.; Gazal, S.; Revy, P.; Wemeau-Stervinou, L.; Debray, M.-P.; Ottaviani, S.; Marchand-Adam, S.; Nathan, N. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef]
- Wheeler, A.M.; Baker, J.F.; Poole, J.A.; Ascherman, D.P.; Yang, Y.; Kerr, G.S.; Reimold, A.; Kunkel, G.; Cannon, G.W.; Wysham, K.D. Genetic, social, and environmental risk factors in rheumatoid arthritis-associated interstitial lung disease. In Proceedings of the Seminars in arthritis and rheumatism; 2022; p. 152098. [Google Scholar]
- Juge, P.-A.; Lee, J.S.; Ebstein, E.; Furukawa, H.; Dobrinskikh, E.; Gazal, S.; Kannengiesser, C.; Ottaviani, S.; Oka, S.; Tohma, S. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med 2018, 379, 2209–2219. [Google Scholar] [CrossRef]
- Palomäki, A.; Palotie, A.; Koskela, J.; Eklund, K.K.; Pirinen, M.; Ripatti, S.; Laitinen, T.; Mars, N.; Group, F.R.C.E. Lifetime risk of rheumatoid arthritis-associated interstitial lung disease in MUC5B mutation carriers. Ann. Rheum. Dis. 2021, 80, 1530–1536. [Google Scholar] [CrossRef]
- Hayashi, S.; Matsubara, T.; Fukuda, K.; Maeda, T.; Funahashi, K.; Hashimoto, M.; Takashima, Y.; Kikuchi, K.; Fujita, M.; Matsumoto, T. A genome-wide association study identifying single nucleotide polymorphisms in the PPFIBP2 gene was predictive for interstitial lung disease in rheumatoid arthritis patients. Rheumatol. Adv. Pract. 2022, 6, rkac088. [Google Scholar] [CrossRef]
- Furukawa, H.; Oka, S.; Shimada, K.; Sugii, S.; Ohashi, J.; Matsui, T.; Ikenaka, T.; Nakayama, H.; Hashimoto, A.; Takaoka, H. Association of human leukocyte antigen with interstitial lung disease in rheumatoid arthritis: a protective role for shared epitope. PloS one 2012, 7, e33133. [Google Scholar] [CrossRef]
- Migita, K.; Nakamura, T.; Koga, T.; Eguchi, K. HLA-DRB1 alleles and rheumatoid arthritis-related pulmonary fibrosis. J. Rheumatol. 2010, 37, 205–207. [Google Scholar] [CrossRef]
- Le Guen, P.; Borie, R.; Legendre, M.; Dupin, C.; Dunogeant, L.; Ottaviani, S.; Debray, M.-P.; Cazes, A.; Dieudé, P.; Kannengiesser, C. NKX2. 1 mutation revealed by a lymphoid interstitial pneumonia in an adult with rheumatoid arthritis. ERJ Open Research 2023, 9. [Google Scholar] [CrossRef]
- Shirai, Y.; Honda, S.; Ikari, K.; Kanai, M.; Takeda, Y.; Kamatani, Y.; Morisaki, T.; Tanaka, E.; Kumanogoh, A.; Harigai, M. Association of the RPA3-UMAD1 locus with interstitial lung diseases complicated with rheumatoid arthritis in Japanese. Ann. Rheum. Dis. 2020, 79, 1305–1309. [Google Scholar] [CrossRef]
- Higuchi, T.; Oka, S.; Furukawa, H.; Shimada, K.; Tsunoda, S.; Ito, S.; Okamoto, A.; Katayama, M.; Saisho, K.; Shinohara, S. Association of a FAM13A variant with interstitial lung disease in Japanese rheumatoid arthritis. RMD open 2023, 9, e002828. [Google Scholar] [CrossRef]
- Jönsson, E.; Ljung, L.; Norrman, E.; Freyhult, E.; Ärlestig, L.; Dahlqvist, J.; Rantapää-Dahlqvist, S. Pulmonary fibrosis in relation to genetic loci in an inception cohort of patients with early rheumatoid arthritis from northern Sweden. Rheumatology 2022, 61, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Murtha, L.A.; Morten, M.; Schuliga, M.J.; Mabotuwana, N.S.; Hardy, S.A.; Waters, D.W.; Burgess, J.K.; Ngo, D.T.; Sverdlov, A.L.; Knight, D.A. The role of pathological aging in cardiac and pulmonary fibrosis. Aging Dis. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Innala, L.; Berglin, E.; Möller, B.; Ljung, L.; Smedby, T.; Södergren, A.; Magnusson, S.; Rantapää-Dahlqvist, S.; Wållberg-Jonsson, S. Age at onset determines severity and choice of treatment in early rheumatoid arthritis: a prospective study. Arthrit. Res. Ther. 2014, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Koduri, G.; Norton, S.; Young, A.; Cox, N.; Davies, P.; Devlin, J.; Dixey, J.; Gough, A.; Prouse, P.; Winfield, J. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology 2010, 49, 1483–1489. [Google Scholar] [CrossRef]
- Lai, N.-L.; Jia, W.; Wang, X.; Luo, J.; Liu, G.-Y.; Gao, C.; Li, X.-F.; Xie, J.-F. Risk factors and changes of peripheral NK and T cells in pulmonary interstitial fibrosis of patients with rheumatoid arthritis. Can. Respir. J. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Assayag, D.; Lubin, M.; Lee, J.S.; King, T.E.; Collard, H.R.; Ryerson, C.J. Predictors of mortality in rheumatoid arthritis-related interstitial lung disease. Respirology 2014, 19, 493–500. [Google Scholar] [CrossRef]
- Saag, K.G.; Cerhan, J.R.; Kolluri, S.; Ohashi, K.; Hunninghake, G.W.; Schwartz, D.A. Cigarette smoking and rheumatoid arthritis severity. Ann. Rheum. Dis. 1997, 56, 463–469. [Google Scholar] [CrossRef]
- Restrepo, J.F.; Del Rincón, I.; Battafarano, D.F.; Haas, R.W.; Doria, M.; Escalante, A. Clinical and laboratory factors associated with interstitial lung disease in rheumatoid arthritis. Clin. Rheumatol. 2015, 34, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Klester, E.; Klester, K.; Shoykhet, Y.; Elykomov, V.; Yarkova, V.; Berdyugina, A.; Mukhtarova, E. Risk factors of interstitial lung diseases in patients with rheumatoid arthritis. Eur Respiratory Soc 2019, 54. [Google Scholar]
- Aubart, F.; Crestani, B.; Nicaise-Roland, P.; Tubach, F.; Bollet, C.; Dawidowicz, K.; Quintin, E.; Hayem, G.; Palazzo, E.; Meyer, O. High levels of anti-cyclic citrullinated peptide autoantibodies are associated with co-occurrence of pulmonary diseases with rheumatoid arthritis. J. Rheumatol. 2011, 38, 979–982. [Google Scholar] [CrossRef]
- Kelly, C.A.; Saravanan, V.; Nisar, M.; Arthanari, S.; Woodhead, F.A.; Price-Forbes, A.N.; Dawson, J.; Sathi, N.; Ahmad, Y.; Koduri, G. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics—a large multicentre UK study. Rheumatology 2014, 53, 1676–1682. [Google Scholar] [CrossRef]
- Chen, N.; Diao, C.-Y.; Gao, J.; Zhao, D.-B. Risk factors for the progression of rheumatoid arthritis-related interstitial lung disease: clinical features, biomarkers, and treatment options. In Proceedings of the Seminars in Arthritis and Rheumatism; 2022; p. 152004. [Google Scholar]
- Huang, S.; Kronzer, V.L.; Dellaripa, P.F.; Deane, K.D.; Bolster, M.B.; Nagaraja, V.; Khanna, D.; Doyle, T.J.; Sparks, J.A. Rheumatoid arthritis–associated interstitial lung disease: current update on prevalence, risk factors, and pharmacologic treatment. Curr. Treat. Options Rheumatol. 2020, 6, 337–353. [Google Scholar] [CrossRef]
- Gaik, O.S.; Jen, D.H.; Hamid, Z.A.; Aziz, A.A.; Wong, N.I. Predictors and radiological characteristics of rheumatoid arthritis-associated interstitial lung disease in a multi-ethnic Malaysian cohort. Med J Malaysia 2022, 77, 293. [Google Scholar]
- Karlson, E.W.; Mandl, L.A.; Hankinson, S.E.; Grodstein, F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis?: Results from the Nurses' Health Study. Arthritis Rheum. 2004, 50, 3458–3467. [Google Scholar] [CrossRef]
- Brun, J.; Nilssen, S.; Kvåle, G. Breast feeding, other reproductive factors and rheumatoid arthritis. A prospective study. Rheumatology 1995, 34, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Jeganathan, N.; Nguyen, E.; Sathananthan, M. Rheumatoid arthritis and associated interstitial lung disease: mortality rates and trends. Ann. Am. Thorac. Soc. 2021, 18, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- Peschken, C.A.; Hitchon, C.A.; Robinson, D.B.; Smolik, I.; Barnabe, C.R.; Prematilake, S.; El-Gabalawy, H.S. Rheumatoid arthritis in a north american native population: longitudinal followup and comparison with a white population. J. Rheumatol. 2010, 37, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Azuma, A.; Kudoh, S. High prevalence of drug-induced pneumonia in Japan. Japan Med. Assoc. J. 2007, 50, 405. [Google Scholar]
- Jo, H.E.; Corte, T.J. Nintedanib for idiopathic pulmonary fibrosis in the Japanese population. Respirology 2017, 22, 630–631. [Google Scholar] [CrossRef]
- Tekaya, A.B.; Mokaddem, S.; Athimini, S.; Kamoun, H.; Mahmoud, I.; Abdelmoula, L. Risk factors for rheumatoid arthritis-associated interstitial lung disease: a retrospective study. Multidiscip. Respir. Med. 2022, 17. [Google Scholar]
- Sparks, J.A.; Karlson, E.W. The roles of cigarette smoking and the lung in the transitions between phases of preclinical rheumatoid arthritis. Curr. Rheumatol. Rep. 2016, 18, 15. [Google Scholar] [CrossRef]
- Gizinski, A.M.; Mascolo, M.; Loucks, J.L.; Kervitsky, A.; Meehan, R.T.; Brown, K.K.; Holers, V.M.; Deane, K.D. Rheumatoid arthritis (RA)-specific autoantibodies in patients with interstitial lung disease and absence of clinically apparent articular RA. Clin. Rheumatol. 2009, 28, 611–613. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Wu, N.; Dong, X.; Zheng, Y. Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clin. Rheumatol. 2017, 36, 817–823. [Google Scholar] [CrossRef]
- Saag, K.G.; Kolluri, S.; Koehnke, R.K.; Georgou, T.A.; Rachow, J.W.; Hunninghake, G.W.; Schwartz, D.A. Rheumatoid arthritis lung disease. Determinants of radiographic and physiologic abnormalities. Arthritis Rheum. 1996, 39, 1711–1719. [Google Scholar] [CrossRef]
- Kronzer, V.L.; Huang, W.; Dellaripa, P.F.; Huang, S.; Feathers, V.; Lu, B.; Iannaccone, C.K.; Gill, R.R.; Hatabu, H.; Nishino, M. Lifestyle and clinical risk factors for incident rheumatoid arthritis-associated interstitial lung disease. J. Rheumatol. 2021, 48, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Erlandsson Harris, H.; Ulfgren, A.K.; Rantapää-Dahlqvist, S. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA–DR (shared epitope)–restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006, 54, 38–46. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, W.; Yu, Y.; Hu, S. Rheumatoid arthritis–associated interstitial lung disease: an overview of epidemiology, pathogenesis and management. Clin. Rheumatol. 2021, 40, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Makrygiannakis, D.; Hermansson, M.; Ulfgren, A.-K.; Nicholas, A.P.; Zendman, A.J.; Eklund, A.; Grunewald, J.; Skold, C.M.; Klareskog, L.; Catrina, A.I. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann. Rheum. Dis. 2008, 67, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Koga, Y.; Sugimoto, M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir. Med. 2012, 106, 1591–1599. [Google Scholar] [CrossRef]
- Liu, B.; Sun, G.; Liu, Y.; Hou, Y. Observational studies: Ambient air pollution and hospitalization for RA-ILD in a heavily polluted city in China. Medicine 2022, 101. [Google Scholar] [CrossRef]
- Yahya, A.; Bengtsson, C.; Larsson, P.; Too, C.L.; Mustafa, A.N.; Abdullah, N.A.; Muhamad, N.A.; Klareskog, L.; Murad, S.; Alfredsson, L. Silica exposure is associated with an increased risk of developing ACPA-positive rheumatoid arthritis in an Asian population: evidence from the Malaysian MyEIRA case–control study. Mod. Rheumatol. 2014, 24, 271–274. [Google Scholar] [CrossRef]
- Stolt, P.; Yahya, A.; Bengtsson, C.; Källberg, H.; Rönnelid, J.; Lundberg, I.; Klareskog, L.; Alfredsson, L.; Group, E.S. Silica exposure among male current smokers is associated with a high risk of developing ACPA-positive rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 1072–1076. [Google Scholar] [CrossRef]
- Stolt, P.; Källberg, H.; Lundberg, I.; Sjögren, B.; Klareskog, L.; Alfredsson, L. Silica exposure is associated with increased risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann. Rheum. Dis. 2005, 64, 582–586. [Google Scholar] [CrossRef]
- Chen, H.-H.; Yong, Y.-M.; Lin, C.-H.; Chen, Y.-H.; Chen, D.-Y.; Ying, J.-C.; Chao, W.-C. Air pollutants and development of interstitial lung disease in patients with connective tissue disease: a population-based case–control study in Taiwan. Bmj Open 2020, 10, e041405. [Google Scholar] [CrossRef]
- Sack, C.; Vedal, S.; Sheppard, L.; Raghu, G.; Barr, R.G.; Podolanczuk, A.; Doney, B.; Hoffman, E.A.; Gassett, A.; Hinckley-Stukovsky, K. Air pollution and subclinical interstitial lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA) air–lung study. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
- Conti, S.; Harari, S.; Caminati, A.; Zanobetti, A.; Schwartz, J.D.; Bertazzi, P.A.; Cesana, G.; Madotto, F. The association between air pollution and the incidence of idiopathic pulmonary fibrosis in Northern Italy. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.B.; Li, W.; Schwartz, J.; Di, Q.; Kloog, I.; Koutrakis, P.; Gold, D.R.; Hallowell, R.W.; Zhang, C.; O'connor, G. Ambient air pollution exposure and risk and progression of interstitial lung abnormalities: the Framingham Heart Study. Thorax 2019, 74, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Al-Aly, Z.; Zheng, B.; van Donkelaar, A.; Martin, R.V.; Pineau, C.A.; Bernatsky, S. Fine particulate matter components and interstitial lung disease in rheumatoid arthritis. Eur. Respir. J. 2022, 60. [Google Scholar] [CrossRef] [PubMed]
- Van Venrooij, W.J.; Van Beers, J.J.; Pruijn, G.J. Anti-CCP antibodies: the past, the present and the future. Nat. Rev. Rheumatol. 2011, 7, 391–398. [Google Scholar] [CrossRef]
- Kamiya, H.; Panlaqui, O.M. Systematic review and meta-analysis of the risk of rheumatoid arthritis-associated interstitial lung disease related to anti-cyclic citrullinated peptide (CCP) antibody. BMJ open 2021, 11, e040465. [Google Scholar] [CrossRef]
- Zeng, X.; Ai, M.; Tian, X.; Gan, X.; Shi, Y.; Song, Q.; Tang, F. Diagnostic value of anti-cyclic citrullinated Peptide antibody in patients with rheumatoid arthritis. J. Rheumatol. 2003, 30, 1451–1455. [Google Scholar]
- Rodríguez-Mahou, M.; López-Longo, F.J.; Sánchez-Ramón, S.; Estecha, A.; García-Segovia, A.; Rodríguez-Molina, J.J.; Carreño, L.; Fernández-Cruz, E. Association of anti–cyclic citrullinated peptide and anti-Sa/citrullinated vimentin autoantibodies in rheumatoid arthritis. Arthritis Care Res 2006, 55, 657–661. [Google Scholar] [CrossRef]
- Yang, J.A.; Lee, J.S.; Park, J.K.; Lee, E.B.; Song, Y.W.; Lee, E.Y. Clinical characteristics associated with occurrence and poor prognosis of interstitial lung disease in rheumatoid arthritis. Korean J Med 2019, 34, 434. [Google Scholar] [CrossRef]
- Doyle, T.J.; Patel, A.S.; Hatabu, H.; Nishino, M.; Wu, G.; Osorio, J.C.; Golzarri, M.F.; Traslosheros, A.; Chu, S.G.; Frits, M.L. Detection of rheumatoid arthritis–interstitial lung disease is enhanced by serum biomarkers. Am. J. Respir. Crit. Care Med. 2015, 191, 1403–1412. [Google Scholar] [CrossRef]
- Natalini, J.G.; Baker, J.F.; Singh, N.; Mahajan, T.D.; Roul, P.; Thiele, G.M.; Sauer, B.C.; Johnson, C.R.; Kawut, S.M.; Mikuls, T.R. Autoantibody seropositivity and risk for interstitial lung disease in a prospective male-predominant rheumatoid arthritis cohort of US veterans. Ann. Am. Thorac. Soc. 2021, 18, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.T.; Danoff, S.K.; Sokolove, J.; Wagner, C.A.; Winchester, R.; Pappas, D.A.; Siegelman, S.; Connors, G.; Robinson, W.H.; Bathon, J.M. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann. Rheum. Dis. 2014, 73, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhou, Y.; Chen, X.; Li, J. A metaanalysis of the increased risk of rheumatoid arthritis-related pulmonary disease as a result of serum anticitrullinated protein antibody positivity. J. Rheumatol. 2014, 41, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- López-Longo, F.J.; Oliver-Miñarro, D.; de la Torre, I.; González-Díaz de Rábago, E.; Sánchez-Ramón, S.; Rodríguez-Mahou, M.; Paravisini, A.; Monteagudo, I.; González, C.M.; GarCía-Castro, M. Association between anti–cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Care Res 2009, 61, 419–424. [Google Scholar] [CrossRef]
- Liao, K.P.; Gunnarsson, M.; Källberg, H.; Ding, B.; Plenge, R.M.; Padyukov, L.; Karlson, E.W.; Klareskog, L.; Askling, J.; Alfredsson, L. Specific association of type 1 diabetes mellitus with anti–cyclic citrullinated peptide–positive rheumatoid arthritis. Arthritis Rheum. 2009, 60, 653–660. [Google Scholar] [CrossRef]
- Alexiou, I.; Germenis, A.; Koutroumpas, A.; Kontogianni, A.; Theodoridou, K.; Sakkas, L.I. Anti-cyclic citrullinated peptide-2 (CCP2) autoantibodies and extra-articular manifestations in Greek patients with rheumatoid arthritis. Clin. Rheumatol. 2008, 27, 511–513. [Google Scholar] [CrossRef]
- Gerli, R.; Bocci, E.B.; Sherer, Y.; Vaudo, G.; Moscatelli, S.; Shoenfeld, Y. Association of anti-cyclic citrullinated peptide antibodies with subclinical atherosclerosis in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 724–725. [Google Scholar] [CrossRef]
- Solomon, J.J.; Matson, S.; Kelmenson, L.B.; Chung, J.H.; Hobbs, S.B.; Rosas, I.O.; Dellaripa, P.F.; Doyle, T.J.; Poli, S.; Esposito, A.J. IgA antibodies directed against citrullinated protein antigens are elevated in patients with idiopathic pulmonary fibrosis. Chest 2020, 157, 1513–1521. [Google Scholar] [CrossRef]
- Kim, J.-W.; Lee, H.; Choe, J.-Y.; Hwang, J.H.; Park, S.-H.; Lee, H.-S.; Kim, S.-K. Factors associated with airway disease and interstitial lung disease in rheumatoid arthritis. J Rheum Dis 2016, 23, 101–108. [Google Scholar] [CrossRef]
- Yin, Y.; Liang, D.; Zhao, L.; Li, Y.; Liu, W.; Ren, Y.; Li, Y.; Zeng, X.; Zhang, F.; Tang, F. Anti-cyclic citrullinated peptide antibody is associated with interstitial lung disease in patients with rheumatoid arthritis. PloS one 2014, 9, e92449. [Google Scholar] [CrossRef]
- Samara, K.D.; Trachalaki, A.; Tsitoura, E.; Koutsopoulos, A.V.; Lagoudaki, E.D.; Lasithiotaki, I.; Margaritopoulos, G.; Pantelidis, P.; Bibaki, E.; Siafakas, N.M. Upregulation of citrullination pathway: from autoimmune to idiopathic lung fibrosis. Respir. Res. 2017, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-X.; Du, C.-G. A retrospective study of clinical characteristics of interstitial lung disease associated with rheumatoid arthritis in Chinese patients. Med Sci Monit 2015, 21, 708. [Google Scholar] [PubMed]
- Turesson, C.; Jacobsson, L.; Sturfelt, G.; Matteson, E.; Mathsson, L.; Rönnelid, J. Rheumatoid factor and antibodies to cyclic citrullinated peptides are associated with severe extra-articular manifestations in rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 59–64. [Google Scholar] [CrossRef]
- Zrour, S.H.; Touzi, M.; Bejia, I.; Golli, M.; Rouatbi, N.; Sakly, N.; Younes, M.; Tabka, Z.; Bergaoui, N. Correlations between high-resolution computed tomography of the chest and clinical function in patients with rheumatoid arthritis: prospective study in 75 patients. Jt. Bone Spine 2005, 72, 41–47. [Google Scholar] [CrossRef]
- Bongartz, T.; Cantaert, T.; Atkins, S.; Harle, P.; Myers, J.; Turesson, C.; Ryu, J.; Baeten, D.; Matteson, E. Citrullination in extra-articular manifestations of rheumatoid arthritis. Rheumatology 2007, 46, 70–75. [Google Scholar] [CrossRef]
- Tsoyi, K.; Esposito, A.J.; Sun, B.; Bowen, R.G.; Xiong, K.; Poli, F.; Cardenas, R.; Chu, S.G.; Liang, X.; Ryter, S.W. Syndecan-2 regulates PAD2 to exert antifibrotic effects on RA-ILD fibroblasts. Sci. Rep. 2022, 12, 2847. [Google Scholar] [CrossRef]
- Tian, F.; Li, J.; Tuo, H.; Ling, Q.; Zeng, S.; Wen, Z.; Luo, X. The anti-mutated citrullinated vimentin antibody as a potential predictor for rheumatoid arthritis associated interstitial lung diseases. Int J Clin Exp Med 2016, 9, 6813–6818. [Google Scholar]
- Inui, N.; Enomoto, N.; Suda, T.; Kageyama, Y.; Watanabe, H.; Chida, K. Anti-cyclic citrullinated peptide antibodies in lung diseases associated with rheumatoid arthritis. Clin. Biochem. 2008, 41, 1074–1077. [Google Scholar] [CrossRef]
- Jearn, L.-H.; Kim, T.-Y. Level of anticitrullinated peptide/protein antibody is not associated with lung diseases in rheumatoid arthritis. J. Rheumatol. 2012, 39, 1493–1494. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and management of rheumatoid arthritis: a review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Solomon, D.; Reed, G.; Kremer, J.; Curtis, J.; Farkouh, M.; Harrold, L.; Hochberg, M.; Tsao, P.; Greenberg, J. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol. 2015, 67, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.A.; He, X.; Huang, J.; Fletcher, E.A.; Zaccardelli, A.; Friedlander, H.M.; Gill, R.R.; Hatabu, H.; Nishino, M.; Murphy, D.J. Rheumatoid arthritis disease activity predicting incident clinically apparent rheumatoid arthritis–associated interstitial lung disease: a prospective cohort study. Arthritis Rheumatol. 2019, 71, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Paulin, F.; Doyle, T.J.; Mercado, J.F.; Fassola, L.; Fernández, M.; Caro, F.; Alberti, M.L.; Espíndola, M.E.C.; Buschiazzo, E. Development of a risk indicator score for the identification of interstitial lung disease in patients with rheumatoid arthritis. Reumatol. Clin. 2021, 17, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, J.; Lama, S.; Knapp, K.; Gutierrez, C.; Lovett, K.; Thai, S.; Craig, G.L. Epidemiology and clinical characteristics of interstitial lung disease in patients with rheumatoid arthritis from the JointMan database. Sci. Rep. 2023, 13, 11678. [Google Scholar] [CrossRef] [PubMed]
- Severo, C.R.; Chomiski, C.; Valle, M.B.d.; Escuissato, D.L.; Paiva, E.d.S.; Storrer, K.M. Assessment of risk factors in patients with rheumatoid arthritis-associated interstitial lung disease. J Bras Pneumol 2022, 48. [Google Scholar]
- Rojas-Serrano, J.; Mejía, M.; Rivera-Matias, P.A.; Herrera-Bringas, D.; Pérez-Román, D.I.; Pérez-Dorame, R.; Mateos-Toledo, H. Rheumatoid arthritis-related interstitial lung disease (RA-ILD): a possible association between disease activity and prognosis. Clin. Rheumatol. 2022, 41, 1741–1747. [Google Scholar] [CrossRef]
- Tyker, A.; Ventura, I.B.; Lee, C.T.; Strykowski, R.; Garcia, N.; Guzy, R.; Jablonski, R.; Vij, R.; Strek, M.E.; Chung, J.H. High-titer rheumatoid factor seropositivity predicts mediastinal lymphadenopathy and mortality in rheumatoid arthritis-related interstitial lung disease. Sci. Rep. 2021, 11, 22821. [Google Scholar] [CrossRef]
- Kadura, S.; Raghu, G. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur Respir Rev 2021, 30. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.-I.; Kim, H.-O. Recent advances in basic and clinical aspects of rheumatoid arthritis-associated interstitial lung diseases. J Rheum Dis 2022, 29, 61–70. [Google Scholar] [CrossRef]
- Wu, E.K.; Ambrosini, R.D.; Kottmann, R.M.; Ritchlin, C.T.; Schwarz, E.M.; Rahimi, H. Reinterpreting evidence of rheumatoid arthritis-associated interstitial lung disease to understand etiology. Curr. Rheumatol. Rev. 2019, 15, 277–289. [Google Scholar] [CrossRef]
- Doyle, T.J.; Lee, J.S.; Dellaripa, P.F.; Lederer, J.A.; Matteson, E.L.; Fischer, A.; Ascherman, D.P.; Glassberg, M.K.; Ryu, J.H.; Danoff, S.K. A roadmap to promote clinical and translational research in rheumatoid arthritis-associated interstitial lung disease. Chest 2014, 145, 454–463. [Google Scholar] [CrossRef]
- Bao, L.; Ye, J.; Liu, N.; Shao, Y.; Li, W.; Fan, X.; Zhao, D.; Wang, H.; Chen, X. Resveratrol Ameliorates Fibrosis in Rheumatoid Arthritis-Associated Interstitial Lung Disease via the Autophagy–Lysosome Pathway. Molecules 2022, 27, 8475. [Google Scholar] [CrossRef]
- Gabbiani, G.; Ryan, G.; Majno, G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971, 27, 549–550. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Gabbiani, G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb. Haemost. 2003, 90, 993–1002. [Google Scholar]
- Shu, D.Y.; Lovicu, F.J. Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis. Prog. Retin. Eye Res. 2017, 60, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Conforti, F.; Hill, C.; Bell, J.; Drawater, L.; Li, J.; Liu, D.; Xiong, H.; Alzetani, A.; Chee, S.J. Paracrine signalling during ZEB1-mediated epithelial–mesenchymal transition augments local myofibroblast differentiation in lung fibrosis. Cell Death Differ. 2019, 26, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Hansel, C.; Jendrossek, V.; Klein, D. Cellular senescence in the lung: the central role of senescent epithelial cells. Int. J. Mol. Sci. 2020, 21, 3279. [Google Scholar] [CrossRef]
- Györfi, A.H.; Matei, A.-E.; Distler, J.H. Targeting TGF-β signaling for the treatment of fibrosis. Matrix Biol. 2018, 68, 8–27. [Google Scholar] [CrossRef]
- Guillotin, D.; Taylor, A.R.; Platé, M.; Mercer, P.F.; Edwards, L.M.; Haggart, R.; Miele, G.; McAnulty, R.J.; Maher, T.M.; Hynds, R.E. Transcriptome analysis of IPF fibroblastic foci identifies key pathways involved in fibrogenesis. Thorax 2021, 76, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, G.; Harari, S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev 2015, 24, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Gabasa, M.; Royo, D.; Molina-Molina, M.; Roca-Ferrer, J.; Pujols, L.; Picado, C.; Xaubet, A.; Pereda, J. Lung myofibroblasts are characterized by down-regulated cyclooxygenase-2 and its main metabolite, prostaglandin E2. PloS one 2013, 8, e65445. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Yettella, R.R.; Kim, J.; Kwon, K.; Kim, M.; Min, D.B. Effects of grilling and roasting on the levels of polycyclic aromatic hydrocarbons in beef and pork. Food Chem. 2011, 129, 1420–1426. [Google Scholar] [CrossRef]
- Scotton, C.J.; Chambers, R.C. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest 2007, 132, 1311–1321. [Google Scholar] [CrossRef]
- Tanner, L.; Bergwik, J.; Bhongir, R.K.; Egesten, A. Zoledronic acid targets the mevalonate pathway causing reduced cell recruitment and attenuation of pulmonary fibrosis. bioRxiv 2021. 2021.2012. 2001.470755. [Google Scholar]
- Guo, X.; Adeyanju, O.; Sunil, C.; Mandlem, V.; Olajuyin, A.; Huang, S.; Chen, S.-Y.; Idell, S.; Tucker, T.A.; Qian, G. DOCK2 contributes to pulmonary fibrosis by promoting lung fibroblast to myofibroblast transition. Am. J. Physiol. Cell Physiol. 2022, 323, C133–C144. [Google Scholar] [CrossRef]
- Pedroza, M.; Le, T.T.; Lewis, K.; Karmouty-Quintana, H.; To, S.; George, A.T.; Blackburn, M.R.; Tweardy, D.J.; Agarwal, S.K. STAT-3 contributes to pulmonary fibrosis through epithelial injury and fibroblast-myofibroblast differentiation. FASEB J. 2016, 30, 129. [Google Scholar] [CrossRef]
- Chakraborty, D.; Šumová, B.; Mallano, T.; Chen, C.-W.; Distler, A.; Bergmann, C.; Ludolph, I.; Horch, R.E.; Gelse, K.; Ramming, A. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat. Commun. 2017, 8, 1130. [Google Scholar] [CrossRef]
- Pechkovsky, D.V.; Prêle, C.M.; Wong, J.; Hogaboam, C.M.; McAnulty, R.J.; Laurent, G.J.; Zhang, S.S.-M.; Selman, M.; Mutsaers, S.E.; Knight, D.A. STAT3-mediated signaling dysregulates lung fibroblast-myofibroblast activation and differentiation in UIP/IPF. Am. J. Pathol. 2012, 180, 1398–1412. [Google Scholar] [CrossRef]
- Milara, J.; Hernandez, G.; Ballester, B.; Morell, A.; Roger, I.; Montero, P.; Escrivá, J.; Lloris, J.M.; Molina-Molina, M.; Morcillo, E. The JAK2 pathway is activated in idiopathic pulmonary fibrosis. Respiratory research 2018, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Lin, H.; Zhang, X. Inhibitory effects of pirfenidone on fibroblast to myofibroblast transition in rheumatoid arthritis-associated interstitial lung disease via the downregulation of activating transcription factor 3 (ATF3). Int. Immunopharmacol. 2019, 74, 105700. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.H.; Li, T.; Wang, H.; Wu, Y.N.; Wang, S.P.; Zhao, Y.Y.; Zhang, G.Q.; Duan, J. IL-17A-producing T cells exacerbate fine particulate matter-induced lung inflammation and fibrosis by inhibiting PI3K/Akt/mTOR-mediated autophagy. J. Cell. Mol. Med. 2020, 24, 8532–8544. [Google Scholar] [CrossRef] [PubMed]
- Konen, J.M.; Rodriguez, B.L.; Padhye, A.; Ochieng, J.K.; Gibson, L.; Diao, L.; Fowlkes, N.W.; Fradette, J.J.; Peng, D.H.; Cardnell, R.J. Dual inhibition of MEK and AXL targets tumor cell heterogeneity and prevents resistant outgrowth mediated by the epithelial-to-mesenchymal transition in NSCLC. Cancer Res. 2021, 81, 1398–1412. [Google Scholar] [CrossRef] [PubMed]
- Salazar, Y.; Zheng, X.; Brunn, D.; Raifer, H.; Picard, F.; Zhang, Y.; Winter, H.; Guenther, S.; Weigert, A.; Weigmann, B. Microenvironmental Th9 and Th17 lymphocytes induce metastatic spreading in lung cancer. J. Clin. Investig. 2020, 130, 3560–3575. [Google Scholar] [CrossRef]
- Zeisberg, M.; Kalluri, R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J. Mol. Med. 2004, 82, 175–181. [Google Scholar] [CrossRef]
- Marmai, C.; Sutherland, R.E.; Kim, K.K.; Dolganov, G.M.; Fang, X.; Kim, S.S.; Jiang, S.; Golden, J.A.; Hoopes, C.W.; Matthay, M.A. Alveolar epithelial cells express mesenchymal proteins in patients with idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L71–L78. [Google Scholar] [CrossRef]
- Namba, T.; Tanaka, K.; Ito, Y.; Hoshino, T.; Matoyama, M.; Yamakawa, N.; Isohama, Y.; Azuma, A.; Mizushima, T. Induction of EMT-like phenotypes by an active metabolite of leflunomide and its contribution to pulmonary fibrosis. Cell Death Differ. 2010, 17, 1882–1895. [Google Scholar] [CrossRef]
- Ba, X.; Wang, H.; Huang, Y.; Yan, J.; Han, L.; Lin, W.; Shen, P.; Huang, Y.; Yang, S.; Qin, K. Simiao pill attenuates collagen-induced arthritis and bleomycin-induced pulmonary fibrosis in mice by suppressing the JAK2/STAT3 and TGF-β/Smad2/3 signalling pathway. J. Ethnopharmacol. 2023, 309, 116274. [Google Scholar] [CrossRef]
- Miura, Y.; Ohkubo, H.; Niimi, A.; Kanazawa, S. Suppression of epithelial abnormalities by nintedanib in induced-rheumatoid arthritis-associated interstitial lung disease mouse model. ERJ Open Res. 2021, 7. [Google Scholar] [CrossRef]
- Kolahian, S.; Fernandez, I.E.; Eickelberg, O.; Hartl, D. Immune mechanisms in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2016, 55, 309–322. [Google Scholar] [CrossRef]
- Przybylo, J.A.; Radisky, D.C. Matrix metalloproteinase-induced epithelial–mesenchymal transition: tumor progression at Snail's pace. Int. J. Biochem. Cell Biol. 2007, 39, 1082–1088. [Google Scholar] [CrossRef]
- Kollar, B.; Uffing, A.; Borges, T.J.; Shubin, A.V.; Aoyama, B.T.; Dagot, C.; Haug, V.; Kauke, M.; Safi, A.-F.; Talbot, S.G. MMP3 is a non-invasive biomarker of rejection in skin-bearing vascularized composite allotransplantation: a multicenter validation study. Front. immunol. 2019, 10, 2771. [Google Scholar] [CrossRef]
- Yamashita, C.M.; Dolgonos, L.; Zemans, R.L.; Young, S.K.; Robertson, J.; Briones, N.; Suzuki, T.; Campbell, M.N.; Gauldie, J.; Radisky, D.C. Matrix metalloproteinase 3 is a mediator of pulmonary fibrosis. Am J Pathol 2011, 179, 1733–1745. [Google Scholar] [CrossRef]
- McKeown, S.; Richter, A.G.; O'Kane, C.; McAuley, D.F.; Thickett, D. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur. Respir. J. 2009, 33, 77–84. [Google Scholar] [CrossRef]
- Xue, J.; Hu, W.; Wu, S.; Wang, J.; Chi, S.; Liu, X. Development of a risk nomogram model for identifying interstitial lung disease in patients with rheumatoid arthritis. Front. immunol. 2022, 13, 823669. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Doyle, T.J.; Liu, Y.; Aggarwal, R.; Wang, X.; Shi, Y.; Ge, S.X.; Huang, H.; Lin, Q.; Liu, W. Biomarkers of rheumatoid arthritis–associated interstitial lung disease. Arthritis Rheumatol. 2015, 67, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, J.S.; Im Yoon, Y.; Chang, S.H.; Lee, Y.A.; Ha, Y.-J.; Kang, E.H.; Park, Y.-B.; Lee, H.; Choe, J.-Y. Association of serum biomarkers with pulmonary involvement of rheumatoid arthritis interstitial lung disease: from KORAIL cohort baseline data. J Rheum Dis 2021, 28, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Holers, V.M.; Demoruelle, M.K.; Kuhn, K.A.; Buckner, J.H.; Robinson, W.H.; Okamoto, Y.; Norris, J.M.; Deane, K.D. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat. Rev. Rheumatol. 2018, 14, 542–557. [Google Scholar] [CrossRef]
- O'Dwyer, D.N.; Armstrong, M.E.; Cooke, G.; Dodd, J.D.; Veale, D.J.; Donnelly, S.C. Rheumatoid arthritis (RA) associated interstitial lung disease (ILD). Eur. J. Intern. Med. 2013, 24, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Mena-Vázquez, N.; Godoy-Navarrete, F.J.; Lisbona-Montañez, J.M.; Redondo-Rodriguez, R.; Manrique-Arija, S.; Rioja, J.; Mucientes, A.; Ruiz-Limón, P.; Garcia-Studer, A.; Ortiz-Márquez, F. Inflammatory Biomarkers in the Diagnosis and Prognosis of Rheumatoid Arthritis–Associated Interstitial Lung Disease. Int. J. Mol. Sci. 2023, 24, 6800. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, S. TNF inhibitor therapy for rheumatoid arthritis. Biomed Rep 2013, 1, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lin, W.; Chen, Z.; Wang, Y.; Huang, Y.; Tu, S. Effect of tumor necrosis factor inhibitors on interstitial lung disease in rheumatoid arthritis: angel or demon? Drug Des Devel Ther 2019, 2111–2125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, S.; Lau, J.; Roden, A.C.; Matteson, E.L.; Sun, J.; Luo, F.; Tschumperlin, D.J.; Vassallo, R. IL-23 amplifies the epithelial-mesenchymal transition of mechanically conditioned alveolar epithelial cells in rheumatoid arthritis-associated interstitial lung disease through mTOR/S6 signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 321, L1006–L1022. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, G.E.; Stock, C.J.; Shi-Wen, X.; Leoni, P.; Sestini, P.; Howat, S.L.; Bou-Gharios, G.; Nicholson, A.G.; Denton, C.P.; Grutters, J.C. Microarray profiling reveals suppressed interferon stimulated gene program in fibroblasts from scleroderma-associated interstitial lung disease. Respir. Res. 2013, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, G.; Ren, Q.; Wu, J.; Gu, B.; Su, D.; Shen, M. Increased interleukin-11 associated with disease activity and development of interstitial lung disease in patients with rheumatoid arthritis. Clin. Exp. Rheumatol 2021. [Google Scholar] [CrossRef]
- Chung, S.-J.; Kwon, Y.-J.; Park, M.-C.; Park, Y.-B.; Lee, S.-K. The correlation between increased serum concentrations of interleukin-6 family cytokines and disease activity in rheumatoid arthritis patients. Yonsei Med. J. 2011, 52, 113–120. [Google Scholar] [CrossRef]
- Gasse, P.; Riteau, N.; Vacher, R.; Michel, M.-L.; Fautrel, A.; Di Padova, F.; Fick, L.; Charron, S.; Lagente, V.; Eberl, G. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PloS one 2011, 6, e23185. [Google Scholar] [CrossRef]
- Yuan, H.; Jiao, L.; Yu, N.; Duan, H.; Yu, Y.; Bai, Y. Histone deacetylase 3-mediated inhibition of microRNA-19a-3p facilitates the development of rheumatoid arthritis-associated interstitial lung disease. Front. physiol. 2020, 11, 549656. [Google Scholar] [CrossRef]
- Yang, G.; Lyu, L.; Wang, X.; Bao, L.; Lyu, B.; Lin, Z. Systemic treatment with resveratrol alleviates adjuvant arthritis-interstitial lung disease in rats via modulation of JAK/STAT/RANKL signaling pathway. Pulm. Pharmacol. Ther. 2019, 56, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jeong, Y.; de Frías, S.P.; Easthausen, I.; Hoffman, K.; Oromendia, C.; Taheri, S.; Esposito, A.J.; Arias, L.Q.; Ayaub, E.A. Serum proteomic profiling of rheumatoid arthritis–interstitial lung disease with a comparison to idiopathic pulmonary fibrosis. Thorax 2022, 77, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Sendo, S.; Saegusa, J.; Okano, T.; Takahashi, S.; Akashi, K.; Morinobu, A. CD 11b+ Gr-1dim Tolerogenic Dendritic Cell–Like Cells Are Expanded in Interstitial Lung Disease in SKG Mice. Arthritis Rheumatol. 2017, 69, 2314–2327. [Google Scholar] [CrossRef] [PubMed]
- Sendo, S.; Saegusa, J.; Yamada, H.; Nishimura, K.; Morinobu, A. Tofacitinib facilitates the expansion of myeloid-derived suppressor cells and ameliorates interstitial lung disease in SKG mice. Arthritis Res. Ther. 2019, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jiang, L.; Nie, L.; Zhang, S.; Liu, L.; Du, Y.; Xue, J. Soluble programmed death molecule 1 (sPD-1) as a predictor of interstitial lung disease in rheumatoid arthritis. BMC Immunol. 2021, 22, 1–10. [Google Scholar] [CrossRef]
- Greisen, S.; Rasmussen, T.; Stengaard-Pedersen, K.; Hetland, M.L.; Hørslev-Petersen, K.; Hvid, M.; Deleuran, B. Increased soluble programmed death-1 (sPD-1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand. J. Rheumatol. 2014, 43, 101–108. [Google Scholar] [CrossRef]
- Guo, Y.; Walsh, A.M.; Canavan, M.; Wechalekar, M.D.; Cole, S.; Yin, X.; Scott, B.; Loza, M.; Orr, C.; McGarry, T. Immune checkpoint inhibitor PD-1 pathway is down-regulated in synovium at various stages of rheumatoid arthritis disease progression. PloS one 2018, 13, e0192704. [Google Scholar] [CrossRef]
- Tsan, M.-F.; Gao, B. Heat shock proteins and immune system. J. Leukoc. Biol. 2009, 85, 905–910. [Google Scholar] [CrossRef]
- Tanaka, K.-I.; Tanaka, Y.; Namba, T.; Azuma, A.; Mizushima, T. Heat shock protein 70 protects against bleomycin-induced pulmonary fibrosis in mice. Biochem. Pharmacol. 2010, 80, 920–931. [Google Scholar] [CrossRef]
- Namba, T.; Tanaka, K.-I.; Hoshino, T.; Azuma, A.; Mizushima, T. Suppression of expression of heat shock protein 70 by gefitinib and its contribution to pulmonary fibrosis. PloS one 2011, 6, e27296. [Google Scholar] [CrossRef]
- Chen, J.; Song, S.; Liu, Y.; Liu, D.; Lin, Y.; Ge, S.; Ascherman, D.P. Autoreactive T cells to citrullinated HSP90 are associated with interstitial lung disease in rheumatoid arthritis. Int. J. Rheum. Dis. 2018, 21, 1398–1405. [Google Scholar] [CrossRef]
- Harlow, L.; Rosas, I.O.; Gochuico, B.R.; Mikuls, T.R.; Dellaripa, P.F.; Oddis, C.V.; Ascherman, D.P. Identification of citrullinated Hsp90 isoforms as novel autoantigens in rheumatoid arthritis–associated interstitial lung disease. Arthritis Rheum. 2013, 65, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Sibinska, Z.; Tian, X.; Korfei, M.; Kojonazarov, B.; Kolb, J.S.; Klepetko, W.; Kosanovic, D.; Wygrecka, M.; Ghofrani, H.A.; Weissmann, N. Amplified canonical transforming growth factor-β signalling via heat shock protein 90 in pulmonary fibrosis. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef]
- Ramani, S.; Pathak, A.; Dalal, V.; Paul, A.; Biswas, S. Oxidative stress in autoimmune diseases: an under dealt malice. Curr. Protein Pept. Sci. 2020, 21, 611–621. [Google Scholar] [CrossRef]
- Mishra, R.; Singh, A.; Chandra, V.; Negi, M.P.; Tripathy, B.C.; Prakash, J.; Gupta, V. A comparative analysis of serological parameters and oxidative stress in osteoarthritis and rheumatoid arthritis. Rheumatol. Int. 2012, 32, 2377–2382. [Google Scholar] [CrossRef]
- Aryaeian, N.; Djalali, M.; Shahram, F.; Jazayeri, S.; Chamari, M.; Nazari, S. Beta-carotene, vitamin E, MDA, glutathione reductase and arylesterase activity levels in patients with active rheumatoid arthritis. Iran. J. Public Health 2011, 40, 102. [Google Scholar] [PubMed]
- Veselinovic, M.; Barudzic, N.; Vuletic, M.; Zivkovic, V.; Tomic-Lucic, A.; Djuric, D.; Jakovljevic, V. Oxidative stress in rheumatoid arthritis patients: relationship to diseases activity. Mol. Cell. Biochem. 2014, 391, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Crapo, J. Oxidative stress as an initiator of cytokine release and cell damage. Eur. Respir. J. 2003, 22, 4s–6s. [Google Scholar] [CrossRef] [PubMed]
- Kinnula, V.L.; Fattman, C.L.; Tan, R.J.; Oury, T.D. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am. J. Respir. Crit. Care Med. 2005, 172, 417–422. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Javad-Moosavi, S.A.; Reiter, R.J.; Yarahmadi, R.; Ghaznavi, H.; Mehrzadi, S. Oxidative/nitrosative stress, autophagy and apoptosis as therapeutic targets of melatonin in idiopathic pulmonary fibrosis. Expert Opin. Ther. Targets 2018, 22, 1049–1061. [Google Scholar] [CrossRef]
- Larios, J.M.; Budhiraja, R.; Fanburg, B.L.; Thannickal, V.J. Oxidative Protein Cross-linking Reactions Involvingl-Tyrosine in Transforming Growth Factor-β1-stimulated Fibroblasts. J. Biol. Chem. 2001, 276, 17437–17441. [Google Scholar] [CrossRef]
- Fu, X.; Kassim, S.Y.; Parks, W.C.; Heinecke, J.W. Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin): an oxidative mechanism for restraining proteolytic activity during inflammation. J. Biol. Chem. 2003, 278, 28403–28409. [Google Scholar] [CrossRef]
- Nelson, K.K.; Melendez, J.A. Mitochondrial redox control of matrix metalloproteinases. Free Radic. Biol. Med. 2004, 37, 768–784. [Google Scholar] [CrossRef]
- Bergeron, A.; Soler, P.; Kambouchner, M.; Loiseau, P.; Milleron, B.; Valeyre, D.; Hance, A.; Tazi, A. Cytokine profiles in idiopathic pulmonary fibrosis suggest an important role for TGF-β and IL-10. Eur. Respir. J. 2003, 22, 69–76. [Google Scholar] [CrossRef]
- Waghray, M.; Cui, Z.; Horowitz, J.C.; Subramanian, I.M.; Martinez, F.J.; Toews, G.B.; Thannickal, V.J. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, V.K.; Lebrecht, D.; Nicholson, A.G.; Wells, A.; Bhayani, H.; Gazdhar, A.; Tamm, M.; Venhoff, N.; Geiser, T.; Walker, U.A. Mitochondrial DNA mutations and respiratory chain dysfunction in idiopathic and connective tissue disease-related lung fibrosis. Sci. Rep. 2019, 9, 5500. [Google Scholar] [CrossRef]
- Terasaki, Y.; Terasaki, M.; Kanazawa, S.; Kokuho, N.; Urushiyama, H.; Kajimoto, Y.; Kunugi, S.; Maruyama, M.; Akimoto, T.; Miura, Y. Effect of H2 treatment in a mouse model of rheumatoid arthritis-associated interstitial lung disease. J. Cell. Mol. Med. 2019, 23, 7043–7053. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xia, G.; Guan, X.; Wang, L.; Qin, L.; Fu, M. Expression of Nrf2 protein in serum of patients with rheumatoid arthritis: A novel indicator for disease activity and disease prognosis. Clin. Biochem. 2023, 113, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vasarmidi, E.; Sarantoulaki, S.; Trachalaki, A.; Margaritopoulos, G.; Bibaki, E.; Spandidos, D.A.; Tzanakis, N.; Antoniou, K. Investigation of key autophagy-and mitophagy-related proteins and gene expression in BALF cells from patients with IPF and RA-ILD. Mol. Med. Rep. 2018, 18, 3891–3897. [Google Scholar] [CrossRef]
- Hill, C.; Wang, Y. Autophagy in pulmonary fibrosis: friend or foe? GENES DISs 2022, 9, 1594–1607. [Google Scholar] [CrossRef]
- Araya, J.; Kojima, J.; Takasaka, N.; Ito, S.; Fujii, S.; Hara, H.; Yanagisawa, H.; Kobayashi, K.; Tsurushige, C.; Kawaishi, M. Insufficient autophagy in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L56–L69. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Hergert, P.; Nho, R.S. Reduced FoxO3a expression causes low autophagy in idiopathic pulmonary fibrosis fibroblasts on collagen matrices. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L552–L561. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Lin, L.; Geyer, A.; Haspel, J.A.; An, C.H.; Cao, J.; Rosas, I.O.; Morse, D. Autophagy in idiopathic pulmonary fibrosis. PloS one 2012, 7, e41394. [Google Scholar] [CrossRef] [PubMed]
- Tieyuan, Z.; Ying, Z.; Xinghua, Z.; Huimin, W.; Huagang, L. Piceatannol-mediated JAK2/STAT3 signaling pathway inhibition contributes to the alleviation of oxidative injury and collagen synthesis during pulmonary fibrosis. Int. Immunopharmacol. 2022, 111, 109107. [Google Scholar] [CrossRef]
- Sheng, H.; Lin, G.; Zhao, S.; Li, W.; Zhang, Z.; Zhang, W.; Yun, L.; Yan, X.; Hu, H. Antifibrotic mechanism of Piceatannol in bleomycin-induced pulmonary fibrosis in mice. Front. pharmacol. 2022, 13, 771031. [Google Scholar] [CrossRef]
- Ahangari, F.; Price, N.L.; Malik, S.; Chioccioli, M.; Bärnthaler, T.; Adams, T.S.; Kim, J.; Pradeep, S.P.; Ding, S.; Cosmos Jr, C. microRNA-33 deficiency in macrophages enhances autophagy, improves mitochondrial homeostasis, and protects against lung fibrosis. JCI insight 2023, 8. [Google Scholar] [CrossRef]
- Hodge, J.A.; Kawabata, T.T.; Krishnaswami, S.; Clark, J.D.; Telliez, J.-B.; Dowty, M.E.; Menon, S.; Lamba, M.; Zwillich, S. The mechanism of action of tofacitinib-an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol 2016, 34, 318–328. [Google Scholar]
- Montero, P.; Milara, J.; Roger, I.; Cortijo, J. Role of JAK/STAT in interstitial lung diseases; molecular and cellular mechanisms. Int. J. Mol. Sci. 2021, 22, 6211. [Google Scholar] [CrossRef]
- Citera, G.; Mysler, E.; Madariaga, H.; Cardiel, M.H.; Castañeda, O.; Fischer, A.; Richette, P.; Chartrand, S.; Park, J.K.; Strengholt, S. Incidence rates of interstitial lung disease events in tofacitinib-treated rheumatoid arthritis patients: post hoc analysis from 21 clinical trials. J. Clin. Rheumatol. 2021, 27, e482. [Google Scholar] [CrossRef]
- Dowty, M.E.; Jesson, M.I.; Ghosh, S.; Lee, J.; Meyer, D.M.; Krishnaswami, S.; Kishore, N. Preclinical to clinical translation of tofacitinib, a Janus kinase inhibitor, in rheumatoid arthritis. J. Pharmacol. Exp. Ther. 2014, 348, 165–173. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Jesson, M.I.; Li, X.; Lee, J.L.; Ghosh, S.; Alsup, J.W.; Warner, J.D.; Tanaka, M.; Steward-Tharp, S.M.; Gadina, M. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J. Immunol. 2011, 186, 4234–4243. [Google Scholar] [CrossRef] [PubMed]
- Milici, A.J.; Kudlacz, E.M.; Audoly, L.; Zwillich, S.; Changelian, P. Cartilage preservation by inhibition of Janus kinase 3 in two rodent models of rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, R.; Liu, X.; Xie, J.; Si, K.; Duan, L. Effects of matrine on JAK-STAT signaling transduction pathways in bleomycin-induced pulmonary fibrosis. Afr J Tradit Complement Altern Med 2013, 10, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Garton, M.; Kelly, C. Is Janus Kinase Inhibition the Future of the Management of Rheumatoid Arthritis-associated Interstitial Lung Disease? touchREVIEWS in Respiratory & Pulmonary Diseases 2022, 7. [Google Scholar]
- Florescu, A.; Gherghina, F.L.; Mușetescu, A.E.; Pădureanu, V.; Roșu, A.; Florescu, M.M.; Criveanu, C.; Florescu, L.-M.; Bobircă, A. Novel Biomarkers, Diagnostic and Therapeutic Approach in Rheumatoid Arthritis Interstitial Lung Disease—A Narrative Review. Biomedicines 2022, 10, 1367. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, M.; Li, X.; Zhang, J.; Wang, F.; Zhang, C.; Roden, A.; Ryu, J.H.; Warrington, K.J.; Sun, J. Canonical and noncanonical regulatory roles for JAK2 in the pathogenesis of rheumatoid arthritis-associated interstitial lung disease and idiopathic pulmonary fibrosis. FASEB J. 2022, 36, e22336. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.S.; Taylor, P.C.; Choy, E.H.; Sebba, A.; Quebe, A.; Knopp, K.L.; Porreca, F. The Jak/STAT pathway: A focus on pain in rheumatoid arthritis. In Proceedings of the Semin. Arthritis Rheum. 2021; pp. 278–284. [Google Scholar]
- He, L.; Qin, Q.; He, J.; Wang, H.; Hu, Y.; He, W.; Xu, B.; Zhou, G.; Shan, H.; Yang, B. ErMiao San inhibits angiogenesis in rheumatoid arthritis by suppressing JAK/STAT signaling pathways. Evid. Based Complementary Altern. Med. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Moura, R.A.; Fonseca, J.E. JAK inhibitors and modulation of B cell immune responses in rheumatoid arthritis. Front. Med. 2021, 7, 607725. [Google Scholar] [CrossRef]
- Hosseini, A.; Gharibi, T.; Marofi, F.; Javadian, M.; Babaloo, Z.; Baradaran, B. Janus kinase inhibitors: A therapeutic strategy for cancer and autoimmune diseases. J. Cell. Physiol. 2020, 235, 5903–5924. [Google Scholar] [CrossRef]
- Ng, B.; Dong, J.; D’Agostino, G.; Viswanathan, S.; Widjaja, A.A.; Lim, W.-W.; Ko, N.S.; Tan, J.; Chothani, S.P.; Huang, B. Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci. Transl. Med. 2019, 11, eaaw1237. [Google Scholar] [CrossRef]
- Ng, B.; Dong, J.; Viswanathan, S.; Widjaja, A.A.; Paleja, B.S.; Adami, E.; Ko, N.S.; Wang, M.; Lim, S.; Tan, J. Fibroblast-specific IL11 signaling drives chronic inflammation in murine fibrotic lung disease. FASEB J. 2020, 34, 11802–11815. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.; Cook, S.A.; Schafer, S. Interleukin-11 signaling underlies fibrosis, parenchymal dysfunction, and chronic inflammation of the airway. Exp. Mol. Med. 2020, 52, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Camps, M.; Rückle, T.; Ji, H.; Ardissone, V.; Rintelen, F.; Shaw, J.; Ferrandi, C.; Chabert, C.; Gillieron, C.; Françon, B. Blockade of PI3Kγ suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat. Med. 2005, 11, 936–943. [Google Scholar] [CrossRef]
- Randis, T.M.; Puri, K.D.; Zhou, H.; Diacovo, T.G. Role of PI3Kδ and PI3Kγ in inflammatory arthritis and tissue localization of neutrophils. Eur. J. Immunol. 2008, 38, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Gruen, M.; Rose, C.; König, C.; Gajda, M.; Wetzker, R.; Bräuer, R. Loss of phosphoinositide 3-kinase γ decreases migration and activation of phagocytes but not T cell activation in antigen-induced arthritis. BMC Musculoskelet. Disord. 2010, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-H.; Gelinas, R.; Wang, K.; Etheridge, A.; Piper, M.G.; Batte, K.; Dakhlallah, D.; Price, J.; Bornman, D.; Zhang, S. Systems biology of interstitial lung diseases: integration of mRNA and microRNA expression changes. BMC Medical Genom. 2011, 4, 1–20. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Abdirahman, S.M.; Putoczki, T.L. Emerging roles for Interleukin-11 in disease. Growth Factors 2019, 37, 1–11. [Google Scholar] [CrossRef]
- Xu, D.H.; Zhu, Z.; Wakefield, M.R.; Xiao, H.; Bai, Q.; Fang, Y. The role of IL-11 in immunity and cancer. Cancer Lett. 2016, 373, 156–163. [Google Scholar] [CrossRef]
- Chung, J.H.; Cox, C.W.; Montner, S.M.; Adegunsoye, A.; Oldham, J.M.; Husain, A.N.; Vij, R.; Noth, I.; Lynch, D.A.; Strek, M.E. CT features of the usual interstitial pneumonia pattern: differentiating connective tissue disease–associated interstitial lung disease from idiopathic pulmonary fibrosis. Am. J. Roentgenol. 2018, 210, 307–313. [Google Scholar] [CrossRef]
- Dawson, J.; Fewins, H.; Desmond, J.; Lynch, M.; Graham, D. Predictors of progression of HRCT diagnosed fibrosing alveolitis in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2002, 61, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, D.S.; Park, I.-N.; Jang, S.J.; Kitaichi, M.; Nicholson, A.G.; Colby, T.V. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease–related subtypes. Am. J. Respir. Crit. Care Med. 2007, 175, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Takayanagi, N.; Sugiura, H.; Miyahara, Y.; Tokunaga, D.; Kawabata, Y.; Sugita, Y. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur. Respir. J. 2011, 37, 1411–1417. [Google Scholar] [CrossRef]
- Nakamura, Y.; Suda, T.; Kaida, Y.; Kono, M.; Hozumi, H.; Hashimoto, D.; Enomoto, N.; Fujisawa, T.; Inui, N.; Imokawa, S. Rheumatoid lung disease: prognostic analysis of 54 biopsy-proven cases. Respir. Med. 2012, 106, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Suhara, K.; Miyazaki, Y.; Okamoto, T.; Ishizuka, M.; Tsuchiya, K.; Inase, N. Fragmented gelsolins are increased in rheumatoid arthritis-associated interstitial lung disease with usual interstitial pneumonia pattern. Allergol. Int. 2016, 65, 88–95. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



