1. Introduction
Peste des petits ruminant (PPR) is a highly contagious, infectious viral disease of small ruminant species which is caused by the peste des petits ruminants virus (PPRV), the prototype member of the Morbillivirus genus in the Paramyxoviridae family [
1] The disease is currently endemic in most of Africa, the Middle East, South Asia and China [
2]. Despite strict control measures including statutory regulations along with the availability of vaccines and diagnostics, PPR remains a constant threat [
3]. Currently, PPR is not an endemic disease for the Republic of Kazakhstan. However, it should be noted that the results obtained by Lundervold et al. in 1997-1998, when conducting a study on the detection of antibodies in sheep, goats and cattle in Central Kazakhstan, it was shown that PPRV could circulate in the country unnoticed [
4]. Five years later, in 2003, an outbreak of PPR occurred in the Turkestan region among small agricultural ruminants [
4,
5]. Since then, until the end of 2014, the OIE has not received official reports of cases of infection with the PPRV from Kazakhstan. However, more than 10 years later, PPR caused three outbreaks in individual farms at the end of 2014 in Southern Kazakhstan (Zhambyl region). Based on partial N gene sequencing, the identified strains showed high similarity to Chinese strains from 2013/2014, suggesting PPR trans boundary spread between the two countries. The three outbreaks did not have any obvious epidemiological linkage, suggesting that PPRV may have been persistently present at a subclinical level despite vaccination efforts [
6,
7]. These data are of the greatest concern because Kazakhstan is home to the three largest populations of
Saiga antelope in the world, which are susceptible to the PPR virus. Although, a serological study conducted in Kazakhstan in the period from 2012 to 2014 did not reveal seropositive
Saiga antelopes for PPR [
6].
To date, due to the outbreak of PPR in Mongolia, the regions of Eastern Kazakhstan are at risk of PPR infection.
To achieve PPR eradication, which is targeted for 2030, a PPR Global Control and Eradication Strategy (PPR GCES) was developed, based on a progressive reduction in PPR incidence and spread through targeted vaccination [
8].
On the territory of the Republic of Kazakhstan, annual routine vaccination of small ruminants against PPR is carried out in border regions in order to prevent the introduction of PPR from neighboring countries that are disadvantaged by this disease. In Kazakhstan, live attenuated vaccines are mainly used for the prevention of PPR, which create 1-year immunity against PPR.
To increase the immunogenicity of the PPR vaccine, we have developed a live vaccine based on the Nigeria/75/1 strain. The live vaccine from the Nigeria/75/1 strain is the most widely used PPR vaccine approved by the OIE [
9]. As is known, the Nigeria 75/1 strain of the PPRV causes persistent immunity in once-immunized animals for up to 3 years [
10].
There are few reports on the duration of persistence of maternal antibodies in lambs/kids born from ewes vaccinated against PPR [
11]. Maternal antibodies transmitted to lambs from vaccinated ewes through colostrum protect newborn lambs from infection for a certain period. However, maternal antibodies can negatively affect the effectiveness of vaccination. Therefore, before starting vaccination, it is recommended to check the state of immunity of lambs.
To avoid negative consequences when immunizing young lambs, we study the persistence of maternal antibodies in lambs born from ewes of Kazakh breed fine-fleeced vaccinated against PPR, and to determine the optimal age of lambs for vaccination against PPR.
2. Materials and Methods
2.1. Animals
In the southern regions of Kazakhstan, sheep mating occurs more often in October and November months, and lambing occurs in March and April months. In this regard, in September, we purchased sheep of Kazakh breed fine-fleeced of either sex (3 males and 15 females aged 12-12.5 months). All animals were dewormed by oral administration of albendazole. Food and water were available in unlimited quantities throughout the experiment. The animals were labeled and kept in isolation in the RIBSP quarantine zone for 30 days. Body temperature was regularly measured in animals and blood serum was collected to determine the presence of specific antibodies to PPRV. Detection of antibodies to PPRV in sheep sera was performed using a virus neutralization test (VNT) [
12]. The animals were not found to have specific antibodies to the PPRV, and they had not previously been vaccinated against this disease.
Ewes of the third trimester of pregnancy were used for the research. Pregnancy periods in ewes were determined using ultrasonography. Estrus synchronization in ewes was not done therefore, as soon as the lambs were born; they were included in experimental studies. Newborn lambs were kept together with their mother so that they could suck colostrum freely.
Experimental studies were conducted in compliance with international and national ethical standards. The protocol was approved by the Ethics Committee for Animal Experimentation at the RIBSP (Permission nmber: 2908/22).
2.2. Vaccination
Out of 15 females, 10 females became pregnant. Ewes (n=6) of the 3rd trimester of pregnancy were immunized with PPR vaccine (Nigeria 75/1 strain) produced by the RIBSP, Kazakhstan. Ewes were immunized subcutaneously with a single field dose of the vaccine (1.0 × 103 TCID 50/ml). Four healthy ewes of the 3rd trimester of pregnancy were used as control animals (unvaccinated). All the vaccinated and unvaccinated ewes were monitored daily for clinical signs of PPR and rectal temperature was measured for 21 days post vaccination (dpv).
2.3. Blood Sample Collection
Blood samples of vaccinated and control ewes taken on days 0, 7, 14 and 21 were tested for the presence of antibodies in blood sera. Blood samples were taken from newborn lambs at the age of one week with an interval of 7 days for 18 weeks. The obtained blood serums of experimental animals were inactivated at a temperature of 56 °C for 30 min and placed in freezers with a storage temperature of -20 °C.
2.4. Serology Tests
2.4.1. VNT
PPRV antibodies were detected using VNT [
12] and ELISA [
13]. The titer of virus neutralizing antibodies (VNA) was calculated by the Reed and Muench method [
14]. The viral neutralizing activity of the serum was expressed in the neutralization index, which is the difference in the logarithms of the titer of the virus in the presence of specific and normal serum.
The highest serum dilution was taken as the antibody titer, which was able to suppress the activity of the virus injected at the specified dose in 50% of the infected culture.
VNT was carried out in two repetitions, and the average value of the two tests was used when analyzing the results of the study.
2.4.2. Competitive ELISA (c-ELISA)
Blood serums of experimental animals were additionally examined for the presence of antibodies in c-ELISA. As an additional test for detecting anti-PPRV antibodies to nucleoprotein (NP) in the blood serum of experimental animals, a c-ELISA kit (ID Screen®PPR Competition (PPRC-4P), ID.vet, Montpellier, France) was used [
13].The ELISA test was performed according to the manufacturer's instructions. The results of the ELISA analysis were read using a spectrophotometer at a wavelength of 450 nm. The test results were considered reliable if the average value of the
ODc-> 0.7 and the ratio of the average values of the
ODc+/ODc-< 0.3. The S/N percentage (S/N%)value was calculated for each sample using the formula sample
OD/ODc- ×100%. Samples showing a ratio of S/N% = 50% were considered positive, whereas S/N > 60% were considered negative. Samples with a ratio of 50% < S/N% ≤ 60% were considered doubtful.
2.5. Molecular Test
2.5.1. Real-Time RT-PCR
Total RNA of the virus was isolated from the collected blood samples and swabs using a kit (ID Gene May Fact Extraction Kit (QIAGEN, Hilden, Germany). Aliquoting of RNA was stored in a freezer at a temperature of -70 °C until tested.
All types of samples were analyzed by real-time RT-PCR to detect the presence of viral nucleic acid using the RT-qPCR kit) (ID Gene Tempeste des Petits Ruminants Duplex, IDvet genetics, Grabels, France), using the Applied Biosystems 7500 system (Carlsbad, CA, USA).
2.6. Evaluation of the Protective Effectiveness of Passive Immunity
To assess the effectiveness of passive immunity, 2 lambs were used, born first at the age of 14 weeks with maternal antibodies (MAs) in the blood serum at a titer of 1:8. One of them was subcutaneously immunized with a vaccine at a dose (1.0×103 TCID50/ml). On 0, 7, 14 dpv, blood samples were collected to determine the titer of serum antibodies.
The second 14-week-old lamb was used to assess protective passive immunity.
As control (unvaccinated) animals, 2 lambs of 14 weeks of age were used, born first from unvaccinated ewes.
2.7. Challenge Study
On 14 dpv, 2 experimental lambs (one unvaccinated and one vaccinated) born with maternal antibodies and 2 control lambs were inoculated with the control strain of PPRV (Kentau-7) subcutaneously into the subscapular region in a volume of 1.0 ml (10
4 TCID50). After control infection, experimental and control animals were observed for 14 days with daily measurement of body temperature, sampling of swabs (nasal, ocular, oral and rectal) and blood, as well as the identification of clinical signs of PPR. Clinical signs of PPR detected in all animals were evaluated in points [
15,
16]. After the end of the control studies, the non-viable (not curable) lambs were humanely euthanized.
2.8. Data Analysis
During the statistical analysis, GraphPad Prism software (version 8.0.1.) was used. Differences between antibody titers and between temperature and clinical parameters of experimental and control animals after immunization and infection with a control virus were determined using bilateral ANOVA tests. The value of P ≤ 0.05 indicated that there was a significant difference between the data obtained. The difference in efficiency between the groups was compared using a one-sided Fisher exact criterion for two proportions at an alpha level of <0.05.
3. Results
3.1. Adverse Reactions Monitoring
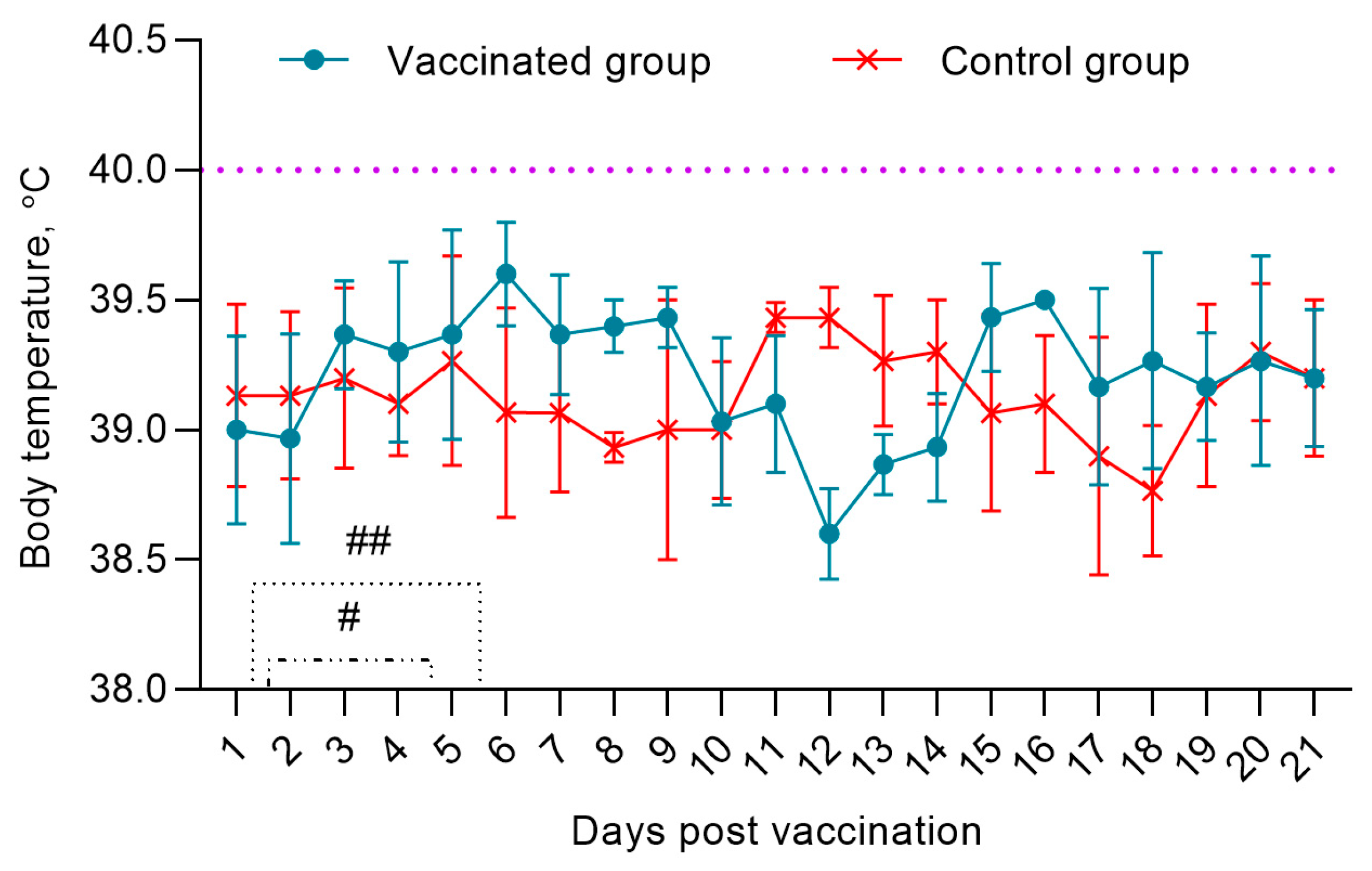
Post vaccination, the physiological state of pregnant ewes was within normal limits. The pregnancy of the ewes proceeded without any complications. On the 2nd dpv, 2 ewes had a local reaction in the form of swellings with a diameter of 0.6 cm2, which disappeared within 4-5 days. During the observation period (21 dpv), the body temperature of the ewes fluctuated in the range of 38.5-39.8 °C (
Figure 1).
3.2. Post-Vaccination Titers of Neutralizing Antibodies to the PPRV in Pregnant Ewes
Before vaccination (day 0), all pregnant ewes were seronegative for PPRV antibodies. In the first week post immunization, the average titers of neutralizing antibodies (NAs) increased to 1.6 log2. On 14 dpv, the level of NAs to the PPRV in pregnant ewes was 5.6 log2, the indicated antibody titer increased to 7.2 log2 on the 21st day of vaccination (
Figure 2). Control ewes were seronegative to the PPRV (
Figure 2).
3.3. Post-Vaccination Titers of Antibodies to the PPRV in ELISA in Pregnant Ewes
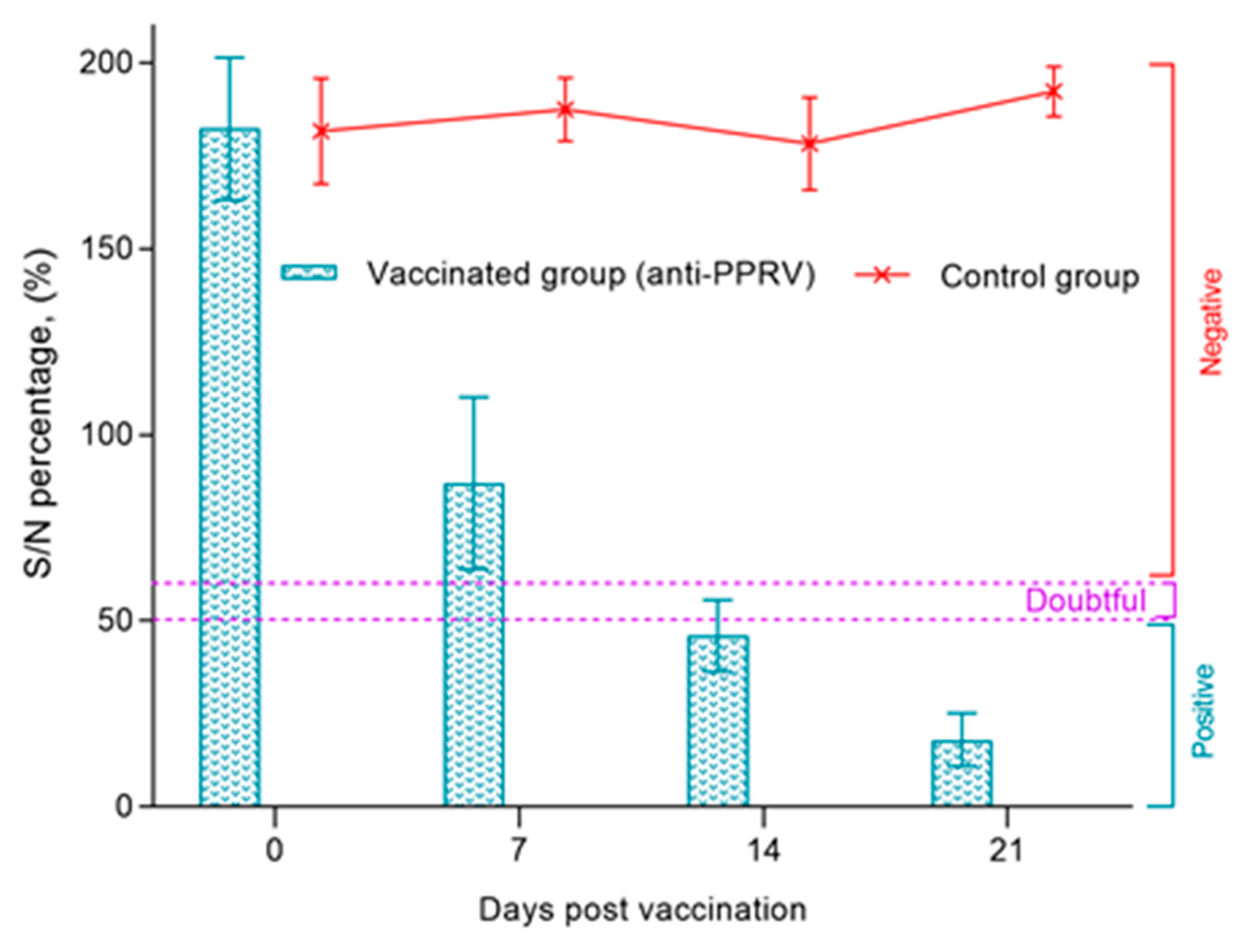
All samples of blood sera of pregnant ewes were negative before immunization with the vaccine against PPRV and had a ratio of S/N>150%. Within 7 dpv, the S/N ratio in pregnant ewes was 87%. Protective titers of antibodies to the PPRV were found in 43% of animals. In 32% of animals, the titer of the produced antibodies exceeded the permissible limit of the S/N value (60%). In the remaining 25% of pregnant ewes, the results of c-ELISA were doubtful (50%-60%). On 14 dpv, judging by the antibody titers, 92% of pregnant ewes were protected from PPRV, the S/N ratio was 46%. The lowest S/N% value in vaccinated ewes (18%) was recorded on 21 dpv (
Figure 3).
3.4. Titers of Maternal Antibodies in Lambs Born from Vaccinated Ewes
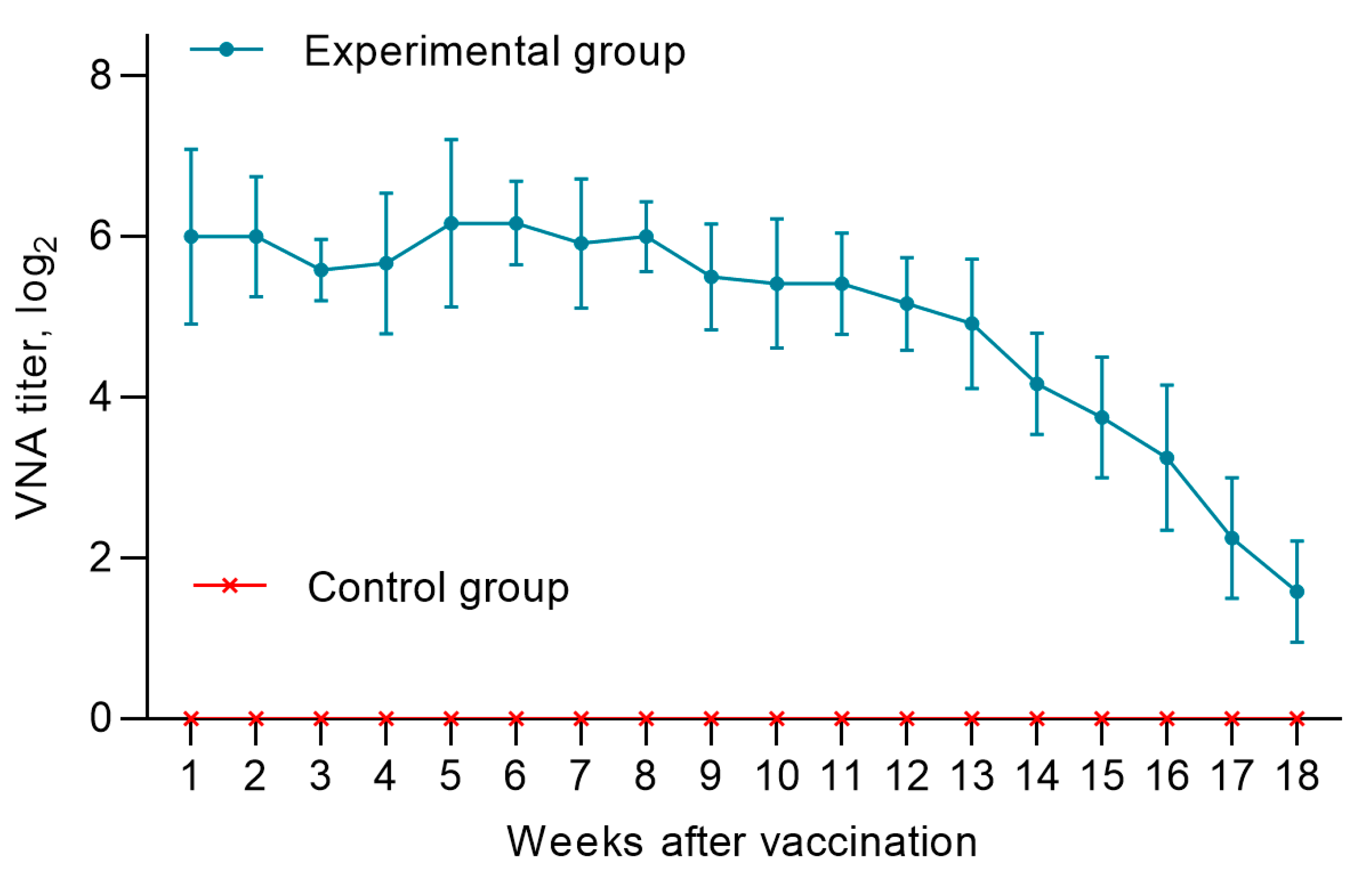
A total, 10 lambs were born from vaccinated ewes at different times. In all lambs (n=6) born from vaccinated pregnant ewes, the level of MAs produced was in the range of 5.5 log2 and 7.2 log2, and provided 100% humoral protection of the offspring from the PPRV up to the 14th week. Starting from the 14th week, there was a gradual decrease in MAs titers below the protective level (<1:8), and by the end of the 18th week, the MAs titer in lambs was in the range from 1.0 to 2.25 log2 (
Figure 4). Control lambs (n=4) were seronegative to the PPRV (
Figure 4).
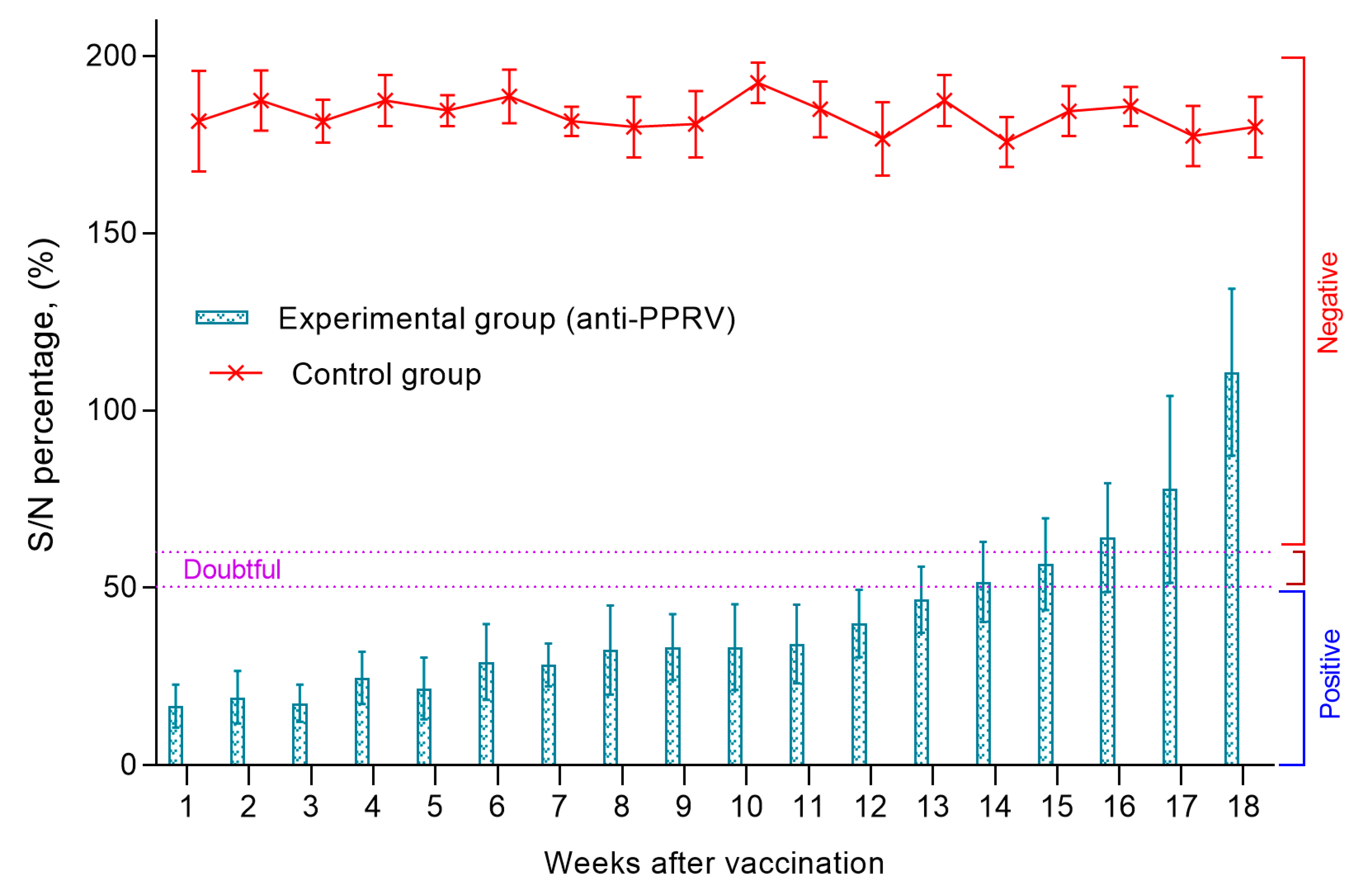
3.5. Titers of Antibodies to the PPRV in ELISA in Lambs Born from Vaccinated Ewes
Blood serum samples were collected from all lambs a week after birth to assess the level of MAs. In all collected samples of lambs' blood serum, the S/N ratio was lower than <50%, which indicated a high level of passive immunity in newborn lambs.
The level of MAs reproduced in kids persisted until the 14th week, while there was no significant difference between antibody titers (P>0.05). At the same time, the average value of S/N was in the range of 20-55% (
Figure 5). The blood sera of control lambs were negative, since all samples had a ratio of S/N >150% (
Figure 5). From the 14th week, there was a gradual increase in the S/N ratio, which increased to 150% at the end of the experiment (week 18). Accordingly, by the end of the experiment, the level of produced antibodies in all lambs exceeded >60%, which indicated a decrease in MAs titers in lambs below the protective level. The serum samples of the control lambs were negative, since all samples had an S/N ratio >150% (
Figure 5).
3.6. Post-Vaccination Titer of Neutralizing Antibodies to the PPRV in a Lamb Born with MAs
Before vaccination (day 0), the titer of NAs in the vaccinated lamb was 4.0 log2. In the first week of vaccination, the titer of NAs increased to 4.7 log2. 2 weeks post vaccination, the level of NAs was 5.4 log2. PPRV antibodies were not found in the blood of the control lambs.
3.7. Assessment of Viral Genomic Load in Blood and Swabs in Lambs
PPRV genome was not detected in blood samples and smears of lambs born with MAs (
Figure 6). Similar results were obtained when testing all types of samples collected from 14-week-old lambs (1 vaccinated and 1 unvaccinated), used to assess the protective effectiveness of passive immunity (
Figure 6).
On the contrary, according to the results of RT-qPCR analysis, all types of samples taken from unvaccinated lambs turned out to be positive (
Figure 6). In control lambs, PPRV genome was detected from 2-3 days in nasal swabs, from 4-5 days in blood and ocular swabs, from 5-6 days in oral swabs and from 6-7 days in rectal swabs.
3.8. Evaluation of the Resistance of Experimental and Control Lambs against Inoculation with the Field PPRV
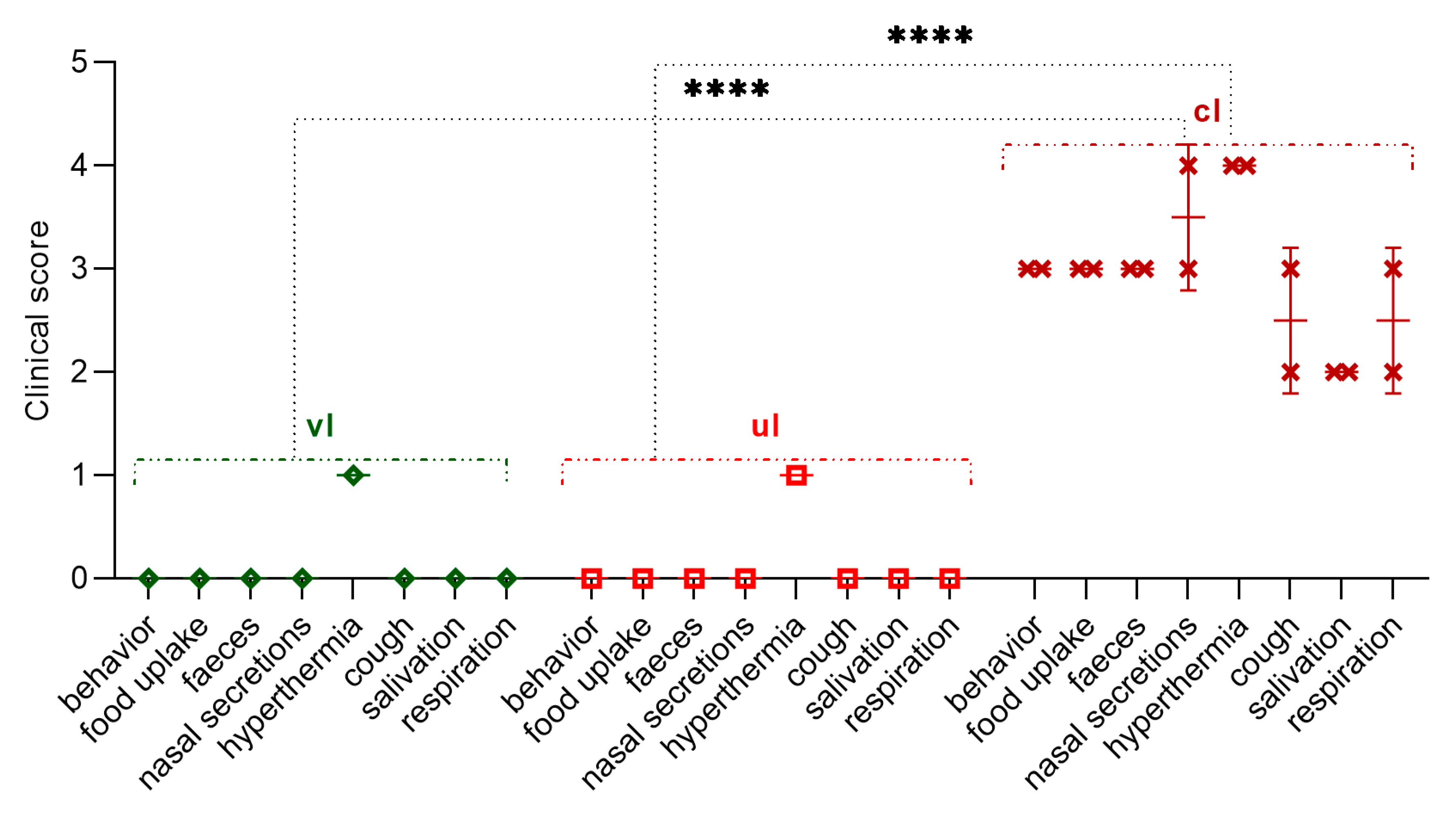
Vaccinated at 14 weeks of age, the lamb was not ill with the manifestation of clinical symptoms of the PPRV. However, within 2 days (on the 3rd-4th day), he had an increase in body temperature to 40.0 °C, which then normalized (
Figure 7); at the same time, the level of clinical indicators increased to 1 point (
Figure 8). Also, the lamb had pink spot of irregular rounded shape with a diameter of 0.2 cm2 at the injection site of the vaccine, which resolved within 4 dpc. However, the results of the RT-qPCR analysis were negative.
In an unvaccinated 14-week-old lamb born with MAs post challenge with the control strain, had an increase in rectal temperature to 40.2 °C on the 3rd dpc, which normalized on the 6th day of the control test; at this stage (
Figure 7), lamb's clinical score reached 1 (
Figure 8). Also, a pink swelling of irregular rounded shape with a diameter of 0.3 cm2 was observed in the lamb at the injection site of the vaccine, which resolved within 6 dpc. All types of samples and swabs collected from an unvaccinated lamb as a result of PCR analysis were negative.
On 3 dpc, control lambs had an increase in rectal temperature, which reached up to 40.8 °C on 5 dpc (
Figure 7). Pyrexia in infected lambs lasted for 8 days. On the 10th day of the control trial, pyrexia was observed in lambs equal to 4 clinical score (41.1 °C) (
Figure 7 and
Figure 8).
In addition, the control lambs had clinical signs characteristic of PPRV (liquid transparent discharge from the eyes and nose), and a seals with a diameter of 2.0 cm2 was observed at the site of vaccine administration. On the 5th-6th dpc in intact lambs, nasal discharge became thick, purulent yellow-greenish in color. In both control lambs, the gums became hyperemic. On the 7th-8th dpc wheezing was heard in the breasts of the lambs, while they had no appetite, they were apathetic and sluggish, liquid pus accumulated in the corners of the eyes (acute conjunctivitis). In addition, both lambs had loose stools, estimated at 3 clinical points (
Figure 8). At 10 dpc, due to the deterioration of the general physiological condition of both control lambs, a decision was made on immediate humane euthanasia. The total clinical score in intact lambs before euthanasia was 41 points (
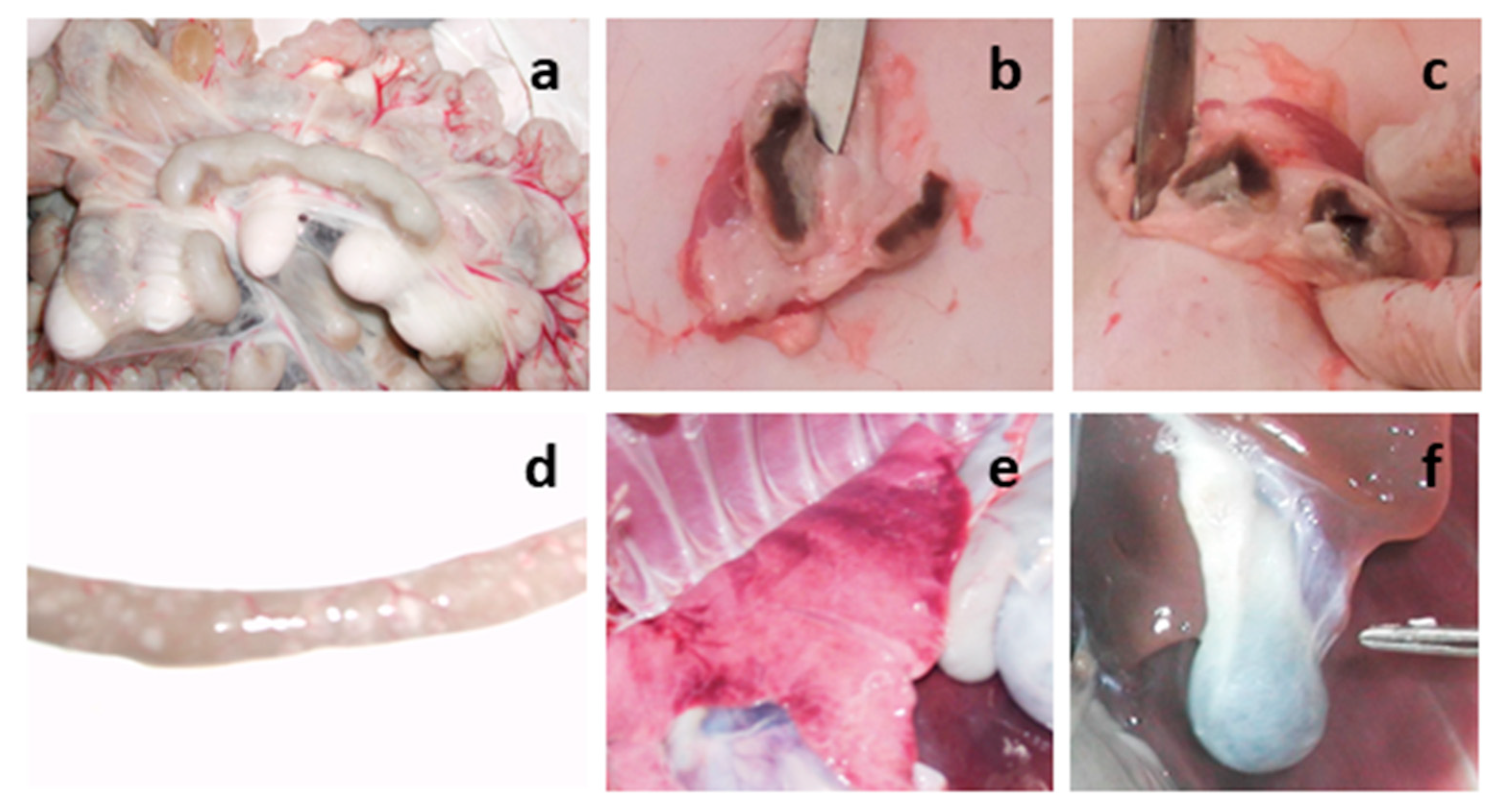
Figure 8). After euthanasia, both lambs were subjected to necropsy (
Figure 9).
3.9. Necropsy Euthanized Lambs
During the necropsy and examination of the internal organs of the euthanized lambs, changes were found in the lymph nodes (mesenteric, pre-scapular, patellar), small intestine and lungs. The lymph nodes were enlarged (
Figure 9a–c). Catarrhal inflammation and hemorrhages were observed in the mucous membrane of the small intestine (
Figure 9d). Multiple dark red hemorrhages were observed under the pleura of the lungs (
Figure 9e). The gallbladder was filled (
Figure 9f).
4. Discussion
It is known that proper vaccination of ewes before mating is of great importance for the immune system of the offspring, since vaccines stimulate the production of MAs, which then pass into colostrum and provide newborn lambs with additional passive protection.
Conversely, vaccination of newborn lambs causes an insignificant immune response, because their immune system is not yet fully developed and cannot produce antibodies until the third or fourth week of life. In addition, it is known that vaccines administered to lambs up to 2 weeks of age bind MAs, and the young body remains defenseless.
Therefore, determining the optimal age for vaccination of young animals, which will come at a time when the level of MAs will significantly decrease and the young body will be able to develop its own protective adaptive immunity in response to the introduction of the vaccine with the formation of immunological memory, is of great importance. It should be noted that the formation of maternal immunity in offspring largely depends on the breed of the animal and the effectiveness of the strain used in the manufacture of a vaccine applicable for the immunization of pregnant animals.
Previously, in Kazakhstan, a live attenuated vaccine from the G45-MK strain was used to prevent PPR, which protected immunized animals from PPR for 1 year. Due to the short duration of immunity in vaccinated animals after immunization with a vaccine against PPR from the G-45MK strain, RIBSP employees decided to use a highly immunogenic strain (Nigeria 75/1) as the main agent for the development of a vaccine against PPR.
Although the Nigeria 75/1 strain is the main agent recommended by the OIE for the development of a vaccine against PPR, there is currently limited information on about the optimal age of lambs at which they should be vaccinated with this vaccine.
These studies were devoted to assessing the duration of maternal immunity in lambs born from pregnant ewes of Kazakh breed fine-fleeced, immunized with a vaccine from the attenuated Nigeria 75/1 strain.
The post-vaccination results of pregnant ewes obtained by us prove the effectiveness of the vaccine used for sheep of the Kazakh breed fine-fleeced, since immunized pregnant ewes had protective antibody titers formed on day 14 (SN≥ 1:8) to the PPRV (
Figure 2 and
Figure 3). In previous studies, the authors reported that protective antibodies to the PPRV were formed in Kano brown goats a week post vaccination with a similar vaccine [
17]. This difference in the periods of formation protective antibody titers is probably due to the difference in the type of experimental animals.
Lambs born from sheep of Kazakh breed fine-fleeced immunized with a vaccine from the Nigeria 75/1 strain after receiving a sufficient amount of colostrum had protective levels of maternal antibodies against PPR for 14 weeks. From week 14 to the end of the experiment (18 weeks), a decrease in antibody activity was observed, and at week 18, all lambs had titers lower than <1:8 (
Figure 4 and
Figure 5). In this connection, lambs of the Kazakh breed fine-fleeced are proposed to be immunized at the age of 14 weeks or older, when the maternal immunity is sufficiently weakened and the lamb can develop its own immune response to the introduction of the vaccine.
Similar results were given in a previously published work by Bodo et al. (2006), where the authors recommend immunizing lambs born from Djallonké ewes in the interval from 11 to 14 weeks after birth [
18].
Markus et al. (2019) and Abdollahi et al. (2023), who conducted similar studies, recommend immunizing kids born from Kano brown and Saanen goats vaccinated with the Nigeria 75/1 strain at the age of 10 weeks [
17,
19], while Olushola S. Olaolu et al. (2021) suggest vaccinating Yankasa lambs at the age of 9 weeks [
20]. In these studies, differences in the age of kids at which they should be vaccinated may be related to the type and breed of experimental animals used in the studies. It has previously been proven that the same PPR vaccine can cause different immune responses in different breeds of goats [
21].
It is known that for reliable protection of animals from PPR, the level of VNA in the blood serum of animals should not be lower ≥1:8 [
22,
23]. Since the lambs were born at different dates, 2 lambs of 14 weeks of age with a serum antibody titer of 1:8, born first from vaccinated ewes and 2 lambs of the same age, born first from unvaccinated ewes, were used to evaluate the effectiveness of passive immunity in lambs. At the same time, in the blood serum of the vaccinated lamb, the antibody titer increased above 1:16 for 14 days, and the lamb was 100% protected from the control virus (
Figure 7). An unvaccinated lamb of 14 weeks of age, born with maternal antibodies was also fully protected during active infection with the control PPRV (
Figure 7).
In a similar study conducted by Balamurugan et al. (2012) in a lamb immunized with the Sungri vaccine strain, protective titers of antibodies to the PPRV began to increase from the 21st day of vaccination (21 dpv) [
11]. It is possible that the difference in the period of the formation of immunity in kids in these similar studies is due to the difference in the vaccine strains used in the two live vaccines, and also the possible influence of the type and breed of experimental animals on the obtained research results is not excluded.
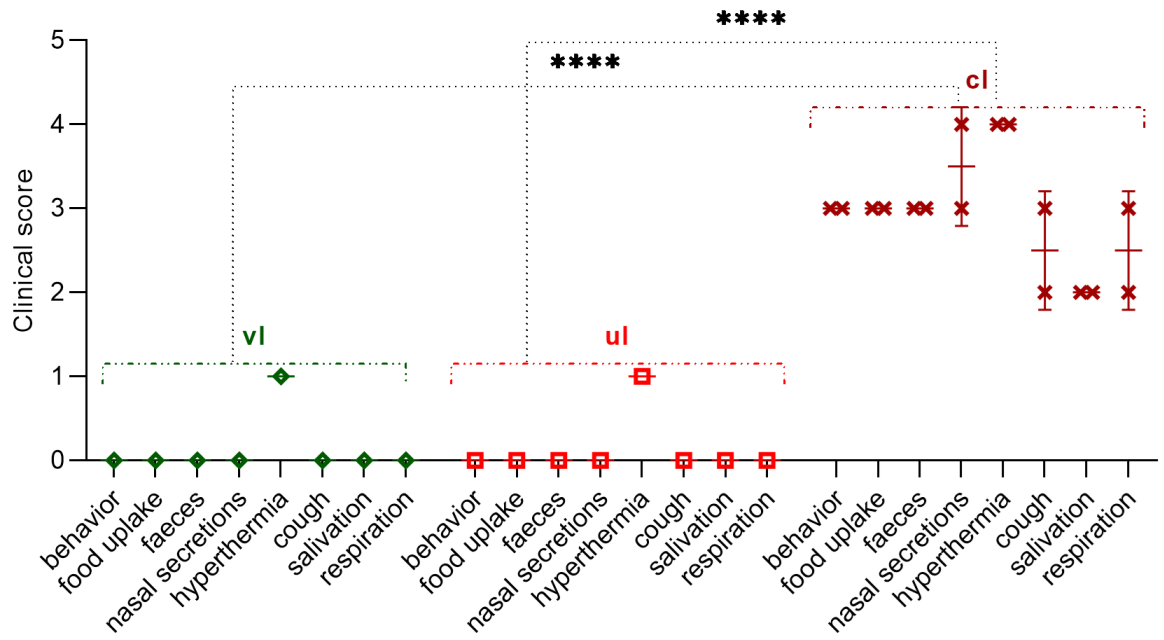
The total clinical score calculated by us during the control trial using the field strain PPRV was for the experimental and control groups of lambs 2 and 41 (
Figure 8), respectively.
At the same time, the lambs of the experimental group had a two-day increase in body temperature and pink spots on the site of the introduction of the vaccine, which disappeared by themselves within 4 dpc (
Figure 7).
However, the results of RT-qPCR analysis turned out to be negative when examining blood samples and swabs collected from an experimental group of lambs after vaccination and control infection (
Figure 6).
The control lambs showed pronounced clinical signs of PPRV after the control test. In addition, all swabs and blood samples collected from the control group of lambs were positive when examined using RT-qPCR (
Figure 6). Due to the deterioration of the general condition, both lambs of the control group were euthanized using an injectable drug belonging to the barbiturate group (
Figure 7).
The results of this study once again prove the safety and effectiveness of the vaccine from the Nigeria 75/1 strain for pregnant ewes, since the vaccine did not affect the course of pregnancy in ewes of the Kazakh breed fine-fleeced, while all ewes were protected from PPR from 2 weeks of vaccination. The maternal immunity formed in lambs born from vaccinated sheep of the Kazakh breed fine-fleeced persisted until 14 weeks after birth with slight fluctuations in antibody titers (P>0.05). Although a minimum number of lambs were used to determine the effectiveness of the vaccine, the results obtained indicate that lambs born from a fine-fleeced Kazakh breed should be vaccinated from the age of 14 weeks or older.
5. Conclusions
Analyzing the results we obtained in this study, we came to the conclusion that during routine vaccination against PPR lambs born from sheep of Kazakh breed fine-fleeced should be immunized from the age of 14 weeks or older to avoid a period of susceptibility of lambs to the PPRV. Since the same vaccine against PPR can cause different immune reactions in different breeds of sheep and goats, it is necessary to conduct further studies on other breeds of sheep and goats living in Kazakhstan in order to determine the appropriate period of immunization of small cattle with a vaccine from the Nigeria 75/1 strain.
Author Contributions
Conceptualization, Zhanat Amanova; Data curation, Zhanat Amanova and Yerbol Bulatov; Formal analysis, Zhanat Amanova; Funding acquisition, Aslan Kerimbaev; Investigation Zhanat Kondybaeva, Zhanna Sametova, Sholpan Turyskeldy; Abdurakhman Usembai; Methodology, Zhanat Amanova, Zhanat Kondybaeva, Sholpan Turyskeldy; Project administration, Aslan Kerimbaev; Resources, Zhanna Sametova and Abdurakhman Usembai; Supervision, Yerbol Bulatov; Validation, Zhanat Amanova; Visualization, Zhanat Amanova, Zhanat Kondybaeva, Zhanna Sametova, Sholpan Turyskeldy; Abdurakhman Usembai; Writing – original draft, Zhanat Amanova; Writing – review & editing, Yerbol Bulatov and Zhanat Amanova.
Funding
This work was supported by the Ministry of Agriculture of the Republic of Kazakhstan: for No04/8-21-29 "Program-targeted financing of scientific research and activities" 2021-2023, carried out within the framework of the scientific and technical program "Biological safety of the Republic of Kazakhstan: threat assessment, scientific and technical basis for their prevention and elimination" for 2021-2023.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee for Animal Experimentation at the RIBSP, RK (Permission number: 2908/22)
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Ye. Zh. Alimbayev for his help during the animal experiments. We also thank the computer equipment maintenance operator for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funder MA, RK had no role in the development of the study; in the collection, analysis and interpretation of data; in the writing of the report; and in deciding whether to submit the article for publication.
References
- Mantip, S.E.; Shamaki, D.; Farougou, S. Peste des petits ruminants in Africa: Meta-analysis of the virus isolation in molecular epidemiology studies. Onderstepoort J Vet Res. 2019, 86, e1–e15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Maherchandani, S.; Kashyap, S.K.; Singh, S.V.; Sharma, S.; Chaubey, K.K.; Ly, H. Peste des petits ruminants virus infection of small ruminants: a comprehensive review. Viruses. 2014, 6, 2287–327. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, V.; Vinod Kumar, K.; Dheeraj, R.; Kurli, R.; Suresh, K.P.; Govindaraj, G.; Shome, B.R.; Roy, P. Temporal and Spatial Epidemiological Analysis of Peste Des Petits Ruminants Outbreaks from the Past 25 Years in Sheep and Goats and Its Control in India. Viruses. 2021, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Lundervold, M.; Milner-Gulland, E. J.; O'Callaghan, C. J.; Hamblin, C.; Corteyn, A.; Macmillan, A. P. A serological survey of ruminant livestock in Kazakhstan during post-Soviet transitions in farming and disease control. Acta veterinaria Scandinavica 2004, 45, 211. [Google Scholar] [CrossRef] [PubMed]
- Abduraimov, Y.O.; Yershebulov, Z.D.; Zhugunisov, K.D.; Ye, A.B.; Nurgaziyev, R.Z.; Ye, D.K. The study of immunobiological properties of the vaccine against ovine rinderpest. Bull ASAU 2016, 2, 121–125. (In Russian) [Google Scholar]
- Legnardi, M.; Raizman, E.; Beltran-Alcrudo, D.; Cinardi, G.; Robinson, T.; Falzon, L.C.; Djomgang, H.K.; Okori, E.; Parida, S.; Njeumi, F.; et al. Peste des Petits Ruminants in Central and Eastern Asia/West Eurasia: Epidemiological Situation and Status of Control and Eradication Activities after the First Phase of the PPR Global Eradication Programme (2017–2021). Animals 2022, 12, 2030. [Google Scholar] [CrossRef]
- Kock, R.A.; Orynbayev, M.B.; Sultankulova, K.T.; Strochkov, V.M.; Omarova, Z.D.; Shalgynbayev, E.K.; Rametov, N.M.; Sansyzbay, A.R.; Parida, S. Detection and Genetic Characterization of Lineage IV Peste Des Petits Ruminant Virus in Kazakhstan. Transbound. Emerg. Dis. 2015, 62, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Benfield, C.T.O.; Legnardi, M.; Mayen, F.; Almajali, A.; Cinardi, G.; Wisser, D.; Chaka, H.; Njeumi, F. Peste Des Petits Ruminants in the Middle East: Epidemiological Situation and Status of Control and Eradication Activities after the First Phase of the PPR Global Eradication Program (2017–2021). Animals 2023, 13, 1196. [Google Scholar] [CrossRef] [PubMed]
- Shatar M, Khanui B, Purevtseren D, Khishgee B, Loitsch A, Unger H, Settypalli TBK, Cattoli G, Damdinjav B, Dundon WG First genetic characterization of peste des petits ruminants virus from Mongolia. Arch Virol 2017, 162, 3157–3160. [CrossRef] [PubMed]
- Amanova, Z.; Zhugunissov, K.; Barakbayev, K.; Kondybaeva, Z.; Sametova, Z.; Shayakhmetov, Y.; Kaissenov, D.; Dzhekebekov, K.; Zhunushov, A.; Abduraimov, Y.; et al. Duration of Protective Immunity in Sheep Vaccinated with a Combined Vaccine against Peste des Petits Ruminants and Sheep Pox. Vaccines 2021, 9, 912. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, V., Sen, A., Venkatesan, G., Rajak, K. K., Bhanuprakash, V., & Singh, R. K. Study on passive immunity: Time of vaccination in kids born to goats vaccinated against Peste des petits ruminants. Virologica Sinica 2012, 27, 228–233. [CrossRef]
- OIE Peste des petits ruminants (infection with peste des petits ruminant’s virus). Testerial Manual 2018; OIE: Paris, France, 2018; Chapter 3.7.9; pp. 1–16. [Google Scholar]
- Libeau, G.; Préhaud, C.; Lancelot, R.; Colas, F.; Guerre, L.; Bishop, D.H.; Diallo, A. Development of a competitive ELISA for detecting antibodies to the Peste des Petits Ruminants virus using a recombinant nucleoprotein. Res Vet Sci. 1995, 58, 50–5. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Bamouh, Z.; Fakri, F.; Jazouli, M.; et al. Peste des petits ruminants pathogenesis on experimental infected goats by the Moroccan 674 2015 isolate. BMC Vet Res 2019, 15, 452. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, J.; Bamouh, Z.; Jazouli, M.; et al. Experimental infection of indigenous North African goats with goatpox virus. Acta Vet Scand 2021, 63, 9. [Google Scholar] [CrossRef] [PubMed]
- Markus, T. P.; Adamu, J.; Kazeem, H. M.; Olaolu, O. S.; Woma, T. Y. Assessment of Peste des petits ruminants antibodies in vaccinated pregnant Kano brown does from Nigeria and subsequent maternal immunity in their kids. Small Ruminant Research 2019, 174, 53–56. [Google Scholar] [CrossRef]
- Bodjo, S.C.; Couacy-Hymann, E.; Koffi, M.Y.; Danho, T. Assessment of the duration of maternal antibodies specific to the homologous peste des petits ruminant vaccine “Nigeria 75/1” in Djallonké lambs. Biokemistri 2006, 18, 99–103. [Google Scholar] [CrossRef]
- Abdollahi, M.; Lotfi, M.; Lotfollahzadeh, S.; Adibi, M.; Kamalzadeh, M.; Firuzyar, S. Determining the decreasing trend of maternal immunity against small ruminant morbillivirus in goat kids. Veterinary medicine and science 2023, 9, 1818–1823. [Google Scholar] [CrossRef]
- Оlaolu, O.; Kazeem, H.; Adamu, J.; Markus, T.; Woma, T. Assessment of Peste Des Petits Ruminants Antibodies in Vaccinated Yankasa Pregnant Ewes from Nigeria and the Duration of Maternal Immunity in Their Lambs. vacres 2021, 8, 47–51. [Google Scholar]
- Begum, S.S.; Mahato, G.; Sharma, P.; Hussain, M.; Saleque, A. Assessment of immune response to a lyophilized peste-des-petits-ruminants virus vaccine in three different breeds of goats. Veterinary world 2016, 9, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Rossitter, P.B.; Jessett, D.M.; Taylor, W.P. Microneutralisation systems for use with different strains of peste des petits ruminants virus and rinderpest virus. Trop Anim Health Prod 1985, 17, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Sen, A.; Balamurugan, V.; et al. Comparative efficacy of peste des petits ruminants (PPR) vaccines. Biologicals 2010, 38, 479–485. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).