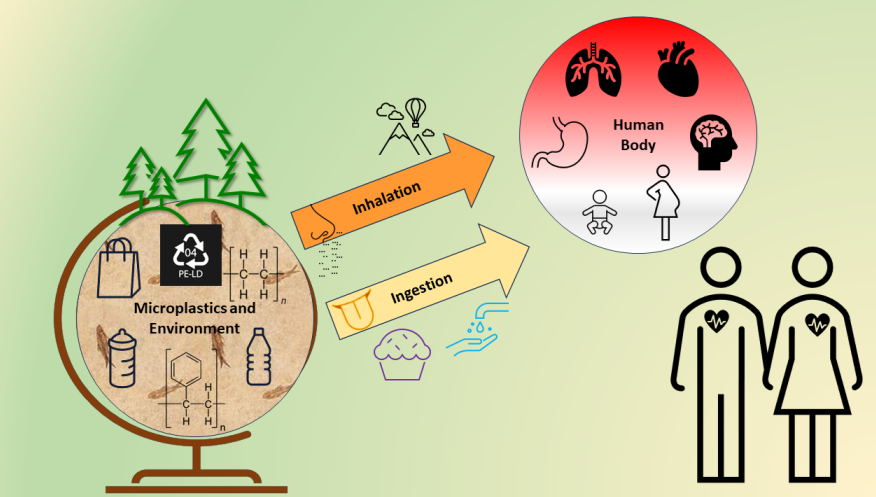
1. Introduction
The production of plastics worldwide has increased from just 2 million tons in 1950 to over 390 million tons in 2021. This is due to the high demand for the material, which was commonly believed to be the best choice for durability, adaptability, and usage in consumer products [
1]. Hence, plastic products can be found everywhere in our modern lives and are a very popular material in construction, textiles, consumer goods, transportation, electronics, and machine parts. The largest application for plastics, though, is as packaging material. One of the biggest concerns and challenges with plastics is that they can degrade to microplastic through weathering, e.g., by mechanical, microbial or photodegradation. The term "microplastics" (MPs) refers to particles that are smaller than 5 mm, and it is estimated that over 170 trillion of these microplastic particles afloat in the world’s oceans [
2]. MPs found in the sea can be ingested by marine animals and then accumulate and end up in humans through the food chain. Indeed, there are several reports of MPs in the environment and in food [
3], predominantly in sea salt, seafood [
4] and in drinking water [
5]. Their effect on human health is yet unknown but plastics often contain additives, such as stabilizers or flame-retardants, and other possibly toxic chemical substances that may be harmful to the organism ingesting them [
6,
7]. Because MPs are pervasive in the environment, human exposure is unavoidable, mostly through three major paths: consumption, breathing, and skin contact. MPs can traverse cell membranes after being internalized. They are seen as unknown substances by the body and hence generate regional immune responses [
8]. Given that the amount, incertitude, and variations of MP exposure concentrations and its associated incorporation kinetics still remain uncertain, the potential risks that MPs represent to human health can consequently result controversial [
7].
A previous review [
9] has reported on human exposure assessments for MPs by estimating total MP intake present in different sources (i.e., table salt, drinking water and air). Nonetheless, the analytical detection approach has proven to be challenging, as the distribution of global MP intake rates is not necessarily well represented when using only single exposure estimates based on average exposure rates. The review provides solid quantitative data highlighting that the most significant MPs intake is through inhalation (estimated to be 1.9 × 10
3 to 1.0 × 10
5 items·year
−1 indoor air; compared to 0 − 3.0 × 10
7 items·year
−1 outdoor air). Furthermore, they conclude that long-term MP exposure in human, as well as the fate and transport of MPs upon entering an organism through absorption and excretion, is vague [
10].
2. Characteristics of Micro- and Nanoplastics
Common plastic polymers like high- and low-density polyethylene, polyvinyl chloride, polyethylene terephthalate, polypropylene, and polystyrene are widely used [
4]. Industries produce primary plastic particles used as essential components, e.g., in manufacturing plastic goods, for biomedical applications, and as cosmetic additives. Secondary plastic particles are formed from the degradation of larger plastics in natural environments, e.g., by mechanical, microbial and/or photodegradation, as mentioned above. Primary MPs are intentionally produced for specific purposes, including preproduction resin pellets, microbeads in personal care products, powders for textile coatings, and drug delivery systems. Similarly, nanoplastics (NPs) are increasingly manufactured for products such as paints, adhesives, drug delivery vehicles, and electronics. These fragmented particles are the main source of MPs/NPs found in terrestrial and aquatic environments. Due to their limited biodegradability, they can persist for extended periods, spanning hundreds of years, in marine and terrestrial environments [
11].
Defining the size of MPs and NPs has lacked universal agreement. MPs are generally smaller than 5 mm, while NPs have less defined boundaries, but are typically below 1 µm or even below 100 nm [
11,
12,
13]. To provide context regarding human exposure, it is worth noting that viruses, which can easily enter human cells, have a diameter of approximately 100 nm. Bacteria, which can be engulfed by immune cells like macrophages, have diameters between 1 and 5 μm. Parasitic worms, larger than eukaryotic cells (10-100 μm), can cause chronic diseases in humans. Therefore, the size of MPs/NPs is comparable to pathogens that commonly infect humans [
13]. MPs and NPs exist in various shapes, including spherical, angular, irregular, and fibrous forms [
14]. Recent findings suggest that MPs can travel up to 100 km in the atmosphere, maybe even farther, exhibiting true free tropospheric MP transport and high-altitude MP particles <50 µm [
15,
16]. Therefore, it should not come as a surprise that MP particles are now found in a large variety of ecosystems, including in Arctic Sea ice [
17].
3. Routes of Exposure
As both MPs and NPs are present widely in food, water and air, the most common routes of exposure are ingestion and inhalation. Bottled water serves as a prevalent source of exposure, potentially originating from both the water itself and the plastic packaging it comes in [
18]. According to a study on human consumption, between 74,000 and 121,000 microplastic particles are consumed yearly per person in the United States [
19]. MPs have been found in human feces, which is a strong proof that they were consumed through the food chain and exposed to the stomach [
20]. MPs and NPs can be incorporated into the lungs and gastrointestinal tract through processes including endocytosis and persorption after being inhaled or ingested and eventually reach the circulation [
21]. An important development is the recent detection and quantification of plastic polymer particles even in human blood [
17,
22].
Thus far, only a limited number of studies have assessed these MPs/NPs exposures to humans. Based on consumption of foods and drinking water in the United States, a study from Cox et al., in 2019, estimated daily MP exposure at 203 particles for girls and 223 particles for boys. Furthermore, the combination of ingestion and inhalation of MPs yields total exposure estimates of approximately 81000 and 74000 particles ⋅ year
-1, for boys and girls, respectively. Nonetheless, a thorough description of the children population is missing, only to be inferred that individuals are less than 18 years old [
23]. The authors used different analytical methods, including Fourier-transform infrared spectroscopy (FTIR), inductively coupled plasma mass spectrometry (ICP-MS), Raman spectroscopy, and Rose Bengal stain to determine MP concentration in several samples, including seafood, honey, salt, sugar, and water. Another research study assessed the daily consumption rates of 553 particles for children and projected the lifelong accumulation of MPs using a physiologically based pharmacokinetic model [
7]. According to more child-focused studies, however, these figures may be "dramatic underestimates". Cox’s et al. (2019) analysis on estimates of American consumption of MPs could be underestimated by a factor of at least 1 × 10
5 [
23]. They state that around 15% of an average male adult’s caloric intake is interrelated with the consumption of up to 52000 MP particles per year, unfortunately the remaining 85% of calories cannot be extrapolated to a specific number of MPs consumed. In another example, Li
et al. [
24] examined the potential exposure of infants to MP from consuming formula prepared in polypropylene infant feeding bottles. The bottles were able to release up 16.2 × 10
6 MPs per liter. This wide range underscores the significant uncertainty surrounding human exposure to both NP and MP, especially during infancy, as well as the difficult analytical tasks involved in quantifying them [
24].
In recent studies, the analysis of MPs and NPs has expanded to include various food products such as honey, chicken, beer, sugar, salt, teabags, milk, seaweed, and certain fruits and vegetables. Plastic teabags, bottled water, and seafood are identified as major sources of exposure to MPs/NPs. Particularly high levels of MPs have been reported in fruits and vegetables. Despite some estimates of daily exposure to MPs/NPs from certain sources, the actual extent of human exposure remains unknown [
11].
Most MPs and NPs that have the potential to be harmful to humans are primarily made of polyethylene terephthalate (PET), polystyrene (PS), and polyvinyl chloride (PVC) [
25]. In laboratory studies, these plastics have been found to negatively affect cell viability and trigger the expression of inflammatory genes by releasing compounds that could potentially cause cancer. Plastics, including MPs/NPs, can contain hazardous substances and endocrine-disrupting compounds like phthalates and bisphenol A. These substances have been associated with various health issues in humans, such as epigenetic changes, reproductive toxicity, insulin resistance, type II diabetes, obesity, skeletal abnormalities, allergies, asthma, and cancer [
26]. MPs/NPs may consist of different types of plastics and other chemicals, and their combined effects could lead to more severe health risks [
11].
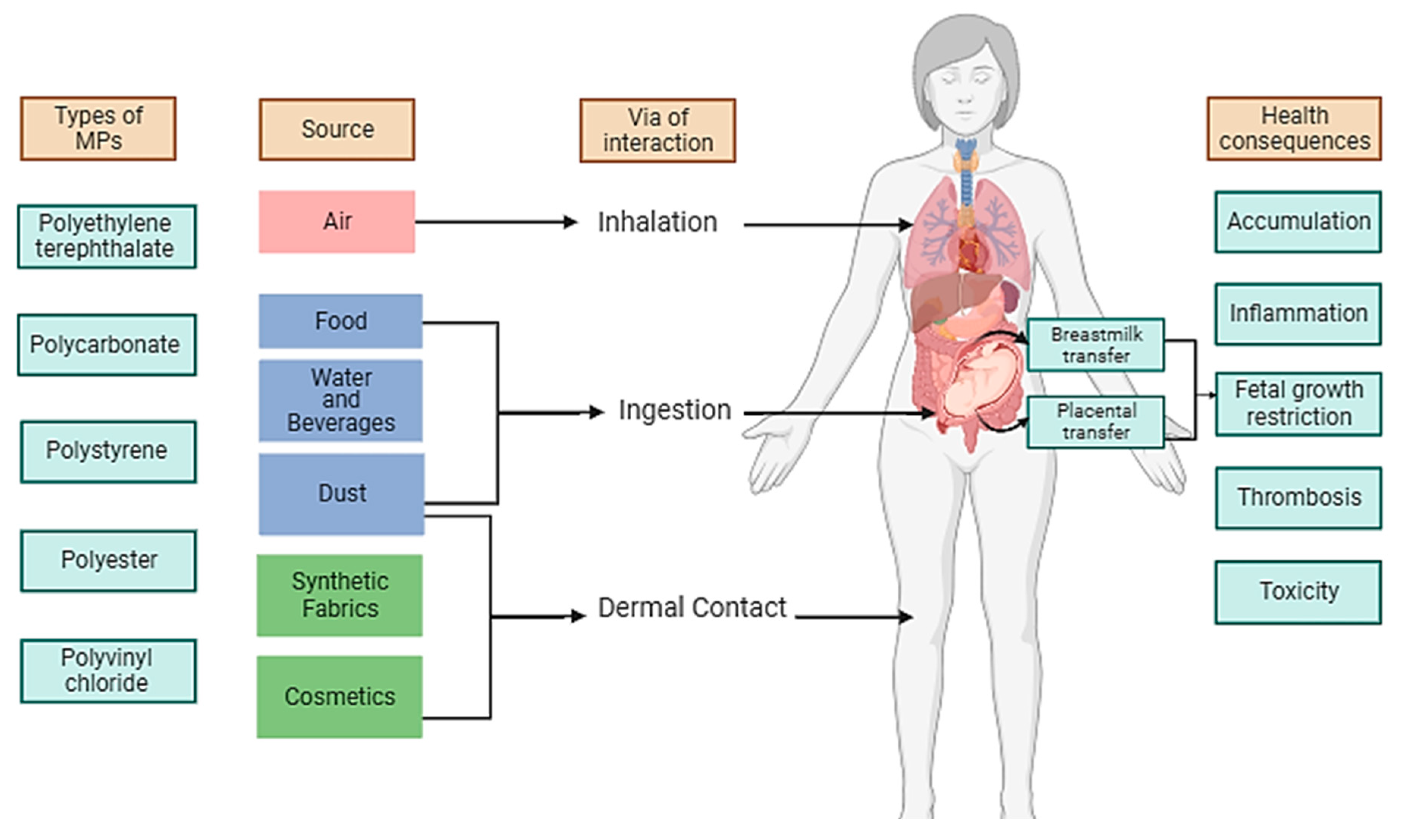
4. Transport routes for MPs in the human body
Inhalation, cutaneous contact, and ingestion are the three main ways that MP can be exposed as recorded in the literature; the latter is the most important and is thought to result in annual intakes in the range of 39,000 – 52,000 mg per individual [
7,
27]. After being swallowed or breathed, MPs can be incorporated in tissues. In the digestive tract, they can bypass the epithelial tissue by either endocytosis processes or by diffusion paracellularly before being transported by means of dendritic cells into both the circulatory and lymphatic system [
23]. According to reports, once MPs enter the body, they might gather and then cause localized damage by triggering or amplifying immune reactions, which weakens the body's defenses against infections [
18]. These MPs may enter the placenta through para-cellular or M cell-mediated endocytosis pathways from the respiratory or digestive systems. The most plausible method of transport for MPs is through particle absorption and translocation, which has already been described for internalization from the gastrointestinal system. [
18]. A recent study showed that the presence of MPs was at higher concentrations in indoor air and dust (from homes and offices) compared to outdoors [
28]. As an example, in a coastal area of California, airborne MPs in indoor air (3.3 ± 2.9 fibers and 12.6 ± 8.0 fragments per m
3) was higher than in outdoor air (0.6 ± 0.6 fibers and 5.6 ± 3.2 fragments per m
3). This was ascribed to several potential factors, such as interior MPs sources (such as furniture and textiles), greater atmospheric MP deposition indoors, and less atmospheric mixing and dilution in comparison to outdoor air [
28].
Figure 1 summarizes the different pathways that MP may encounter with the human body. The following subsections will expand to our current understanding of specific transport routes involving the placenta and breast milking, inhalation, ingestion, cutaneous contact, and circulatory system.
4.1. Placenta and breastmilk
As with other foreign substances, MPs can enter the tissue in depth once they have achieved the maternal side of the placenta through several, yet poorly known active and passive transport modes. Trans-placental transit of MPs sized 5–10 µm can be influenced by a variety of physiological factors and inherited traits. This may help to explain the various locations and properties of the particles found in the current investigation, as well as the patients' varied dietary and lifestyle habits and the lack of MPs in 2 out of 6 placentas that were examined in a recent study by Ragusa
et al. [
29]. Nonconforming chemokine receptors that control the communication between the fetus and the mother, motioning between the womb and the embryo, as well as transferring of uterine immune cells like the dendritic, natural killer, T cells, and macrophages during a characteristic gestation are just a few of the cellular regulatory pathways that MPs may potentially alter in the placenta. Preeclampsia and fetal growth restriction are two possible unfavorable pregnancy outcomes that might result as consequence [
19]. Breastmilk also serves as the finest nutritional standard for newborns, providing nutrients and strengthening their immune system, hence it is crucial to make sure breastfeeding is as pure as possible. The occurrence of MPs in placenta, which symbolizes interactions amongst the fetuses and the mothers that are exposed to the environment, was confirmed by Braun
et al. [
30]. Recent research has shown that MPs circulate throughout every region of the body due to the existence of plastic particles in the blood [
31]. Both immune cell-dependent pathways and mammary epithelial cell-dependent pathways have been proposed as potential routes for exogenous particles to go from the circulation to breast milk in the mammary glands, with the latter being more pertinent in inhaled particles [
13,
32,
33,
34].
4.2. Inhalation
Since MPs are consistently found at high quantities in both indoor and outdoor air, inhalation has lately become a significant pathway for human exposure to MPs [
35,
36]. The importance of the inhalation route was underlined by Cox
et al. (2019) in a meta-analysis of 26 research articles on human exposure to MPs with a special focus on the American population [
23]. Based on the findings, the average concentration was determined to be 9.8 MPs per m
3. Adult males and females were estimated to inhale 170 and 132 MPs per day, respectively, while children inhaled 110 and 97 MPs per day. These estimates accounted for approximately 50% or more of the total daily exposure to MPs through all routes, indicating that inhalation is the primary pathway of human exposure to MPs. Another recent study conducted in Australian homes reported an average inhalation intake of 0.2 mg per kg of body weight per year, equivalent to approximately 12,891 MP fibers per year, with higher intake observed in young children (≤0.5 years of age) at 0.31 mg per kg of body weight per year [
37]. Previous studies estimated individual inhalation exposure to be between 26 and 132 MPs per day [
27], while a study by Vianello
et al. [
38] reported an average inhalation of 272 MPs per day for adult males engaged in light activity. Notably, a recent study highlighted the increased risk of MPs inhalation associated with wearing various types of face masks during the COVID-19 pandemic. Fibers and spheres were the most commonly detected types of MPs, with activated carbon masks showing the highest levels of MPs inhalation and N95 masks the lowest [
28]. Another significant matrix for human exposure to MPs has emerged as indoor dust. High concentrations of MPs in samples of indoor dust were found, with children being more sensitive due to increased rates of dust consumption. Higher exposure was noted inside compared to outdoors, with an estimated daily intake of MPs via indoor dust ranging from 6500 to 89,700 ng per kg of body weight per day. Adults were found to have lower exposure levels due to variations in body weight and dust intake rates, whilst infants were found to have the greatest exposure levels. This emphasizes how important it is to take indoor dust into account as a possible cause of exposure, especially for children [
28].
4.3. Ingestion
Recent research has shown the paths through which people are exposed to MPs, revealing the importance of both nutrition and indoor dust as channels. MPs have been identified in a variety of foods, including fish, shellfish, table salt, sugar, honey, milk, and beer, therefore diet is an important factor. According to studies calculating food exposure, daily MP consumption for various demographic groups ranged from 106 to 142 particles per day [
23]. Another recognized source of exposure is the release of MPs from plastic packaging, such as teabags [
39] and baby feeding bottles, as discussed earlier. MPs have also been discovered in branded milk containers, meat items [
40], and bottled drinks. These results highlight the necessity of thorough research to comprehend the scope and dangers of food exposure. Another significant matrix for human exposure to MPs has emerged as indoor dust. Studies have found high concentrations of MPs in samples of indoor dust, with children being more sensitive due to increased rates of dust consumption.
4.4. Dermal Contact
As summarized in
Figure 1, the dermal contact includes cosmetic use and the use of synthetic fibers. Studies explicitly evaluating human skin exposure to MPs and its dangers are scarce. However, it is logical to think about dermal contact as a potential route for human exposure to MPs given the widespread presence of MPs in indoor dust, atmospheric deposition from both indoor and outdoor air, the use of microbeads in cosmetics, and the ongoing degradation of microfibers from textiles, as discussed earlier. Microbeads, which generally have a diameter of less than 1 mm, are frequently found in toothpaste, denture fillings, and treatments for washing and exfoliating the skin. A limited number of studies tried to calculate the amount of microbeads that each person uses in various personal care products [
41]. For instance, research on face scrubs conducted in the UK discovered that the MPs content ranged from 10 to 100 g per liter, translating to intake of 40.5 to 215 mg per capita per day [
42]. Another study calculated that the US population has an intake of 2.4 mg of MPs per person per day on average, using liquid soaps [
43].
These few studies do not offer a thorough knowledge of human cutaneous exposure to MPs, but they do show that it is important to consider this route of exposure. Research indicates that NPs may immediately overcome the dermal barrier, even though human skin serves as an efficient barrier against the entry of bigger particles. The transdermal penetration of bigger particles, however, may also occur by other pathways, including hair follicles, sweat glands, or open skin wounds. Additionally, skin damage brought on by inflammation and oxidative stress has been linked to dermal exposure to MPs. Determining human skin exposure to MPs through interaction with cosmetics, settling dust particles, fabric fibers, and other sources requires more investigation. The relevance of this exposure pathway and any potential health concerns involved must be further considered [
28].
4.5. Circulatory System
Regarding the circulatory system, inhaled MPs are more likely to pass via the lower respirational region, which has a fine coating of secretion, and diffuse into the circulation via both cellular absorption and paracellular distribution [
44]. Growing scientific evidence supports the existence of MPs in individuals. Ibrahim
et al. revealed the predominance of MPs in specimens from colectomy whereas Schwabl
et al. stated the occurrence of MPs in feces from humans, demonstrating that they can only partially cut across the intestinal membrane [
45,
46]. Medical studies on both rodents and humans have shown that particles of PVC [
47] and PS [
48] less than 150 µm translocated from the gut cavity to their lymph and circulatory systems. The mussel
Mytilus edulis was used to investigate ingestion, translocation, and accumulation of MPs. After ingestion, MPs accumulated in the gut and then translocated to the circulatory system within 3 days and persisted for over 48 days. This long persistence of MP particles in the hemolymph of
M. edulis has further implications for predators (i.e., birds, crabs, starfish, and humans) [
49]. Another publication shows that NPs can induce thrombosis, here hamsters were injected with 60 nm particles of polystyrene, showing up later in the blood stream [
50]. Consequently, MPs in the circulatory system can potentially restrict the blood flow, ergo damaging vascular tissues and causing changes in cardiac activity [
51].
As stated by Persiani
et al [
51], different MP types have been demonstrated to accumulate in the heart, explained by a trophic transfer via the bloodstream. It has been shown in mammals, that MP presence impaired heart contractility, neonatal cardiomyocyte apoptosis, and activation of fibrotic processes. MPs/NPs negatively interact with developing hearts, impairing cardiac function (including loss of function, failure of cardiac morphogenesis in early stages, arrhythmia, or reduced contractility, in developing and likely adult hearts).
In another study by Yang et al. [
52], the existence of MPs in the human heart and its surrounding tissues was examined using laser-based infrared chemical imaging and scanning electron microscopy. Microplastic samples, comprising diverse tissue types and blood samples, were gathered from a range of heart surgery patients. Even while not all tissue samples had MPs, nine different forms were found in five different types of tissues, with the biggest being 469 µm in diameter. Blood samples taken before and after surgery revealed the presence of MPs, the largest of which had a diameter of 184 µm. After surgery, there was a shift in the kinds and sizes of MPs in the blood. The study demonstrated conclusive proof of MPs in heart surgery patients' tissues, excluding surgical accident as the source. Further study is necessary to understand how certain types of MPs are introduced during surgery and any possible impact they may have on human health [
52].
5. Placental translocation and effect on fetus
According to research conducted over the past few decades, the windows of sensitivity to environmental toxins are during pregnancy and childhood [
53]. Even little amounts of early exposure to harmful substances can have long-term effects on a person's health because of child-specific activities including crawling and hand-to-mouth action [
54]. Children are exposed to the world differently than adults [
55]. The key development of the immunological, metabolic, cardiovascular, and other vital bodily systems coincides with these greater exposures.
The placenta carefully controls the fetal-maternal milieu and, indirectly, the external environment, operating as a critical interface through several complex systems [
14,
17]. MPs may harm embryo development by affecting the ability to distinguish self from non-self. Knowledge in this area is limited and clarification is needed regarding the associated effects [
56]. In a study done by Ragusa
et al. [
13], MPs were found inside the placental cord of healthy females with a normal gestation and childbirth; therefore, it is most likely that the mothers inhaled or ingested the particles. MPs were also discovered in the barrier that the fetus develops inside the placenta, as well as on the maternal and fetal sides of the placenta [
13].
In another study by Ragusa
et al., Raman Microspectroscopy revealed 58 MP particles in samples from human placentas taken from six individuals [
29]. A total of 12 MP pieces were discovered in four placentas (3 in the chorioamniotic membranes, 4 on the maternal side, and 5 in the fetal side). Furthermore, milk samples from 34 patients were collected and evaluated using Raman Microspectroscopy, to verify the existence of microplastic contamination in breastmilk and to evaluate a different MP exposure pathway in the particularly sensitive subset of babies. Out of the 34 samples of breast milk analyzed by Ragusa
et al. [
29], 26 exhibited MP contamination. The types of MPs identified in the breast milk samples included polyethylene, polyvinyl chloride; polypropylene; polyvinyl alcohol; poly (ethylene-co-vinyl acetate); poly (ethyl methacrylate); polyester, and polycarbonate [
13]. Additionally, most of the MPs that were detected were colored (~90%), with orange/yellow and blue being the highly prevalent hues (about 36% and 17%, respectively).
Age, usage of hygiene items containing plastic (such as lotions, cleansers, and toothpaste), consumption of seafood, drinks in plastic containers, and meals in plastic containers in the seven days prior to and seven days after the expected date of delivery were all factors that were reflected.
6. Microplastics in our daily life
The feasting of seafood, drinks in plastics, plastic wrapped food, and the practice of using hygiene products encompassing plastic particles in the seven days formerly and subsequently the anticipated day of delivery were evaluated as possible relationships between the occurrence of MPs in the breastmilk and data about moms' lifestyles. However, no links amongst MP existence or amount and any of the aforementioned facts were discovered [
57].
The lack of an association between utilizing personal care products and exposure to contaminants is mostly justified by the fact that dermal touch has little influence as an exposure pathway, since only particles smaller than 100 nm may get through the dermal barrier [
58]. Contrarily, it is more difficult to explain why there is not a connection between the absence of lima relationship and mothers' dietary preferences, given that ingesting food is the main way that MPs are exposed. Fish, shellfish, and essential everyday items for humans such as bottled water, honey, milk, salt, sugar, teabags, and, to a lesser amount, synthetic kitchenware, plates, and containers have all been shown to contain MPs [
59]. Furthermore, as per a study done by Liu
et al. [
60], sixteen varieties of MPs were detected, where polyamide and polyurethane dominated. It was shown that the intake of water and the use of toothpaste or soap can be exposure sources for expectant females. Additionally, nursing, the use of nursing bottles, and the use of synthetic toys may expose babies. Due to MPs' ubiquitous presence in the environment and inherent vulnerability, it is difficult to pinpoint their particular origin amid the complex array of confronted interactions.
7. Crossing the Blood Brain Barrier
The function of the biomolecular corona in the blood-brain barrier (BBB) breaching of MPs and NPs has been discussed in the work by Kopatz
et al. [
61]. A critical defensive system, the BBB works to keep dangerous chemicals from entering the brain. In the study, mice were given oral doses of polystyrene micro/nanoparticles of different sizes (9.55, 1.14 and 0.293 µm) for short-term uptake tests. The findings demonstrated that whereas bigger particles could not cross the BBB, nanometer-sized particles could do so within two hours after consumption. In another study by Shan
et al. [
62] the effects of PS-NPs (polystyrene nanoparticles) were investigated. When administered to mice, PS-NPs increased the permeability of the blood-brain barrier (BBB) and accumulated in the brain in a dose-dependent manner. PS-NPs were found to activate microglia and cause damage to neurons. In vitro studies using human brain endothelial cells showed that PS-NPs were internalized by the cells, leading to the production of reactive oxygen species, inflammation, disruption of tight junctions, and cell death. PS-NPs also activated murine microglia cells and their culture medium caused damage to murine neurons. Overall, these findings suggest that PS-NPs can cross the BBB, induce neurotoxicity, and activate microglia.
Since rodents and humans share many genetic and biological traits, they are frequently employed as study models. After oral or intravenous injection, these investigations have demonstrated that MPs/NPs bioaccumulate in several organs, including the liver, spleen, kidney, brain, gut, and placenta. The size of the particles ranged from 40 nm to 50 µm, however due to the dearth of investigations, there is little knowledge regarding the link between particle size and results [
11]. Upcoming studies must, however, account for field circumstances and evaluate a wide variety of plastic compositions, forms, and sizes. The estimation of daily exposure in human populations, the identification of populations with low and high exposures, a robust analysis of the characteristics of the MPs/NPs that humans are exposed to, as well as the development of more advanced fast throughput analytical tools to measure these particles in the environment and in human tissues, are challenges that must be urgently addressed, and human studies are a priority.
8. Towards the potential standardization of techniques for quantifying microplastics in biological samples
There are currently no established techniques for calculating the amount of MPs present in biological samples. This makes it difficult to compare study results and restricts our ability to draw generalizations about the health concerns associated with exposure to MP. The dearth of epidemiological research on the possible consequences of MPs on human health is another gap. Animal models or in vitro studies have been used for most research up to this point. To comprehend the possible health consequences linked to human exposure to MP, more extensive epidemiological studies are required. Furthermore, we still have a limited understanding of the degree and modes of human exposure to MPs.
Most environmental toxicology tests involving MPs use procedures that involve chemical digestion of biological samples to extract, identify, and quantify the MPs. Various methods using alkaline agents, acids, oxidants, enzymes, or combinations of these agents have been employed. These procedures aim to remove organic matter and separate it from plastic particles. The specific concentrations of reagents, digestion time, and temperature can affect extraction efficiency, making some protocols more suitable than others based on study conditions. After digestion, post-digestion procedures commonly involve filtration of the digested solution using filters like fiberglass, cellulose nitrate, or cellulose acetate membranes, followed by washing to identify and quantify MPs. Some studies have also reported scraping membranes post-filtration to isolate plastic particles for further analysis [
63]. The success of these procedures relies on the effectiveness of reagents in detaching particles from the filtered membranes. If MPs remain attached to the membranes, their subsequent identification and quantification may be underestimated. Inefficiencies in the washing procedures for these membranes can compromise the accuracy of MP identification and quantification. It has been observed that small fragments may attach to the membrane pores, and pellets can aggregate within the membrane’s layers after filtration. Therefore, thorough washing of the membranes is crucial to ensure analytical accuracy in the detection and quantification of MPs.
An increase in the diversity of methods and techniques for the identification and quantification of MPs has also been remarked [
64], both, in environmental samples (e.g., water [
64,
65], soil [
66,
67], sediment [
68] and air [
69]) and in biological samples [
63,
70]. However, the comparison between the various studies has become more difficult, with analytical accuracy being a key issue in recent publications [
71,
72], which can be attributed to the lack of standardization of the methods and techniques used [
73].
Table 1 summarizes the most common techniques used, their action range and major advantages and drawbacks [
74].
9. Bridging Gaps
According to a recent report by the World Health Organization (WHO) on plastic particles in drinking water, there is currently no conclusive evidence available in the public domain that directly associates plastic particles with negative health effects in humans [
75]. However, the report emphasizes that this conclusion is primarily due to a lack of extensive research rather than concrete evidence supporting the safety of plastic particles. It underscores the urgent need for rigorous research focused on human populations to better understand the potential risks associated with plastic particles [
11]. The standardization of techniques for calculating and quantifying the presence of MPs in biological samples is one of these gaps. Although some scientists have demonstrated that MPs can have harmful impacts on animal models' health, we are yet unsure exactly how much of a risk MP exposure poses to human health. Finally, there is a significant lack of long-term research on how MPs could affect human health. Further studies are required to understand the possible health hazards linked to chronic, long-term exposure to MPs because most studies have concentrated on short-term exposure so far. To safeguard human health and guide policy decisions, it is essential to fill in these knowledge gaps about the possible dangers linked to exposure to MPs. In 2019, the Norwegian Food Safety Authority conducted a systematic analysis and discovered just three studies that were pertinent to human health, concluding that it was presently impossible to evaluate the health hazards of NMPs [
76]. This is consistent with past evaluations made by the European Food Safety Authority [
77], the United Nations Food and Agriculture Organization [
78], and the European Academies' Science Advice for Policy [
79].
Conclusions and Future Perspectives
The prevalence of MPs in maternal breastmilk and placenta is especially problematic as it impacts a vulnerable population of neonates. Chemicals present in foodstuff, drinks, and items for personal hygiene consumed by nursing mothers might well be transferred on to their infants, possibly harming them. Steadily increasing scientific research efforts are necessitated to increase consciousness about the possible health implications of MP internalization and buildup, particularly in infants and the placenta. It is also important to assess innovative, effective methods for minimizing exposure to these contaminants during lactation and pregnancy.
The effects of plastic pollution on the environment have been well researched, but it is still unclear how ingesting plastic by mammals, including humans, may affect their health. These latest discoveries about the mechanics of plastic particle transfer provide a crucial foundation for further study and regulatory measures intended to lessen their harmful impact on human health. We can create practical methods and recommendations to reduce the dangers associated with plastic use and protect human health by better understanding the underlying processes of plastic particle toxicity.
MPs are pervasive in the environment and have several entry points into the body, including ingestion, inhalation, and skin contact (Fig. 1). It is crucial to understand the extent and modes of human exposure to MPs to assess the health risks associated with them. These plastic fragments may include dangerous materials and pathogens that have a detrimental impact on human health. Determining the long-term health impacts of MP exposure may be done by studying the biopersistence of MPs and their possible accumulation in different tissues. To limit exposure to MPs and promote ecologically sustainable practices, legislative changes and consumer behavior may be influenced by raising public knowledge of the possible health hazards related to MPs. Because the health of ecosystems is directly correlated with human health, microplastic contamination can have serious negative effects on the environment.
Every effort should be made to minimize the manufacture and use of plastics, as well as to boost recycling and ecologically safe disposal of plastics, together with the development of technologies that remove MPs from our environment. This should be done until these issues are overcome to restrict the potential damage MPs and NPs might cause on our health.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Mannheim University of Applied Sciences for enabling access to several bibliographical sources. This review is an enhanced outcome from the Master course Scholarly Communication for the Biotechnology Faculty, we thank Prof. Dr. L. Greiner and J. Clear for their kind suggestions and advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jadhav, E.B.; Sankhla, M.S.; Bhat, R.A.; Bhagat, D. Microplastics from food packaging: An overview of human consumption, health threats, and alternative solutions. Environ. Nanotechnology, Monit. Manag. 2021, 16, 100608. [Google Scholar] [CrossRef]
- Eriksen, M.; Cowger, W.; Erdle, L.M.; Coffin, S.; Villarrubia-Gómez, P.; Moore, C.J.; Carpenter, E.J.; Day, R.H.; Thiel, M.; Wilcox, C. A growing plastic smog, now estimated to be over 170 trillion plastic particles afloat in the world’s oceans—Urgent solutions required. PLOS ONE 2023, 18, e0281596. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.M.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.; Lundebye, A.-K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total. Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total. Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef]
- Nor, N.H.M.; Kooi, M.; Diepens, N.J.; Koelmans, A.A. Lifetime Accumulation of Microplastic in Children and Adults. Environ. Sci. Technol. 2021, 55, 5084–5096. [Google Scholar] [CrossRef]
- Schymanski, D.; Goldbeck, C.; Humpf, H.-U.; Fürst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A Review of Microplastics in Table Salt, Drinking Water, and Air: Direct Human Exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef]
- Lim, X. Microplastics are everywhere — but are they harmful? Nature 2021, 593, 22–25. [Google Scholar] [CrossRef]
- O’neill, S.M.; Lawler, J. Knowledge gaps on micro and nanoplastics and human health: A critical review. Case Stud. Chem. Environ. Eng. 2021, 3, 100091. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C.; Sivanesan, S.; Ogunkanmi, A.L.; Krishnamurthi, K. Micro(nano)-plastics in the environment and risk of carcinogenesis: Insight into possible mechanisms. J. Hazard. Mater. 2021, 416, 126143. [Google Scholar] [CrossRef]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef]
- Käppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Baladima, F.; Phoenix, V.R.; Thomas, J.L.; Le Roux, G.; Sonke, J.E. Evidence of free tropospheric and long-range transport of microplastic at Pic du Midi Observatory. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Jiménez, P.D.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Medley, E.A.; Spratlen, M.J.; Yan, B.; Herbstman, J.B.; Deyssenroth, M.A. A Systematic Review of the Placental Translocation of Micro- and Nanoplastics. Curr. Environ. Heal. Rep. 2023, 10, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Smith, D.J.; Leal, L.G.; Mitragotri, S.; Shell, M.S. Nanoparticle transport across model cellular membranes: when do solubility-diffusion models break down? J. Phys. D: Appl. Phys. 2018, 51, 294004. [Google Scholar] [CrossRef]
- Ilekis, J.V.; Tsilou, E.; Fisher, S.; Abrahams, V.M.; Soares, M.J.; Cross, J.C.; Zamudio, S.; Illsley, N.P.; Myatt, L.; Colvis, C.; et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am. J. Obstet. Gynecol. 2016, 215, S1–S46. [Google Scholar] [CrossRef]
- Jones, J.I.; Vdovchenko, A.; Cooling, D.; Murphy, J.F.; Arnold, A.; Pretty, J.L.; Spencer, K.L.; Markus, A.A.; Vethaak, A.D.; Resmini, M. Systematic Analysis of the Relative Abundance of Polymers Occurring as Microplastics in Freshwaters and Estuaries. Int. J. Environ. Res. Public Heal. 2020, 17, 9304. [Google Scholar] [CrossRef] [PubMed]
- Bajt, O. From plastics to microplastics and organisms. FEBS Open Bio 2021, 11, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shi, Y.; Yang, L.; Xiao, L.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food 2020, 1, 746–754. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.; Nogueira, H.S.; Marques, J.; Gonçalves, A.M. Impacts of plastic products used in daily life on the environment and human health: What is known? Environ. Toxicol. Pharmacol. 2019, 72, 103239. [Google Scholar] [CrossRef]
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Ageel, H.K.; Harrad, S.; Abdallah, M.A.-E. Occurrence, human exposure, and risk of microplastics in the indoor environment. Environ. Sci. Process. Impacts 2021, 24, 17–31. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2020, 146, 106274. [Google Scholar] [CrossRef]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Llorca, M.; Farré, M.; Picó, Y.; Teijón, M.L.; Álvarez, J.G.; Barceló, D. Infant exposure of perfluorinated compounds: Levels in breast milk and commercial baby food. Environ. Int. 2010, 36, 584–592. [Google Scholar] [CrossRef] [PubMed]
- LaKind, J.S.; Verner, M.-A.; Rogers, R.D.; Goeden, H.; Naiman, D.Q.; Marchitti, S.A.; Lehmann, G.M.; Hines, E.P.; Fenton, S.E. Current Breast Milk PFAS Levels in the United States and Canada: After All This Time, Why Don’t We Know More? Environ. Heal. Perspect. 2022, 130, 25002. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zang, X.; Wu, Z.; Liu, J.; Wang, D. Translocation of transition metal oxide nanoparticles to breast milk and offspring: The necessity of bridging mother-offspring-integration toxicological assessments. Environ. Int. 2019, 133, 105153. [Google Scholar] [CrossRef]
- Torres-Agullo, A.; Karanasiou, A.; Moreno, T.; Lacorte, S. Overview on the occurrence of microplastics in air and implications from the use of face masks during the COVID-19 pandemic. Sci. Total. Environ. 2021, 800, 149555–149555. [Google Scholar] [CrossRef]
- Ahmad, M.; Chen, J.; Khan, M.T.; Yu, Q.; Phairuang, W.; Furuuchi, M.; Ali, S.W.; Nawab, A.; Panyametheekul, S. Sources, analysis, and health implications of atmospheric microplastics. Emerg. Contam. 2023, 9, 100233. [Google Scholar] [CrossRef]
- Soltani, N.S.; Taylor, M.P.; Wilson, S.P. Quantification and exposure assessment of microplastics in Australian indoor house dust. Environ. Pollut. 2021, 283, 117064. [Google Scholar] [CrossRef]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic contamination of packaged meat: Occurrence and associated risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Anagnosti, L.; Varvaresou, A.; Pavlou, P.; Protopapa, E.; Carayanni, V. Worldwide actions against plastic pollution from microbeads and microplastics in cosmetics focusing on European policies. Has the issue been handled effectively? Mar. Pollut. Bull. 2021, 162, 111883. [Google Scholar] [CrossRef]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Gouin, T.; Roche, N.; Lohmann, R.; Hodges, G. A Thermodynamic Approach for Assessing the Environmental Exposure of Chemicals Absorbed to Microplastic. Environ. Sci. Technol. 2011, 45, 1466–1472. [Google Scholar] [CrossRef]
- Mowat, A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003, 3, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.S.; Anuar, S.T.; A Azmi, A.; Khalik, W.M.A.W.M.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of microplastics in human colectomy specimens. JGH Open 2021, 5, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Volkheimer, G. HEMATOGENOUS DISSEMINATION OF INGESTED POLYVINYL CHLORIDE PARTICLES. Ann. New York Acad. Sci. 1975, 246, 164–171. [Google Scholar] [CrossRef]
- Hussain, N.; Jaitley, V.; Florence, A.T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliv. Rev. 2001, 50, 107–142. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested Microscopic Plastic Translocates to the Circulatory System of the Mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoylaerts, M.F.; Hoet, P.H.; Vermylen, J.; Nemery, B. Size effect of intratracheally instilled particles on pulmonary inflammation and vascular thrombosis. Toxicol. Appl. Pharmacol. 2003, 186, 38–45. [Google Scholar] [CrossRef]
- Persiani, E.; Cecchettini, A.; Ceccherini, E.; Gisone, I.; Morales, M.A.; Vozzi, F. Microplastics: A Matter of the Heart (and Vascular System). Biomedicines 2023, 11, 264. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, E.; Du, Z.; Peng, Z.; Han, Z.; Li, L.; Zhao, R.; Qin, Y.; Xue, M.; Li, F.; et al. Detection of Various Microplastics in Patients Undergoing Cardiac Surgery. Environ. Sci. Technol. 2023, 57, 10911–10918. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Etzel, R.A. “Children’s Environmental Health—A New Branch of Pediatrics.” In Oxford University Press EBooks, 2–17. 17. [CrossRef]
- Amran, N.H.; Zaid, S.S.M.; Mokhtar, M.H.; Manaf, L.A.; Othman, S. Exposure to Microplastics during Early Developmental Stage: Review of Current Evidence. Toxics 2022, 10, 597. [Google Scholar] [CrossRef] [PubMed]
- Moya, J.; Bearer, C.F.; Etzel, R.A. Children’s Behavior and Physiology and How It Affects Exposure to Environmental Contaminants. Pediatrics 2004, 113, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Prabhudas, M.; Bonney, E.; Caron, K.; Dey, S.; Erlebacher, A.; Fazleabas, A.; Fisher, S.; Golos, T.; Matzuk, M.; McCune, J.M.; et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat. Immunol. 2015, 16, 328–334. [Google Scholar] [CrossRef]
- Jin, M.; Wang, X.; Ren, T.; Wang, J.; Shan, J. Microplastics contamination in food and beverages: Direct exposure to humans. J. Food Sci. 2021, 86, 2816–2837. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total. Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the mass of microplastics ingested – A pivotal first step towards human health risk assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef]
- Liu, S.; Guo, J.; Liu, X.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: A pilot prospective study. Sci. Total. Environ. 2023, 854, 158699. [Google Scholar] [CrossRef]
- Kopatz, V.; Wen, K.; Kovács, T.; Keimowitz, A.S.; Pichler, V.; Widder, J.; Vethaak, A.D.; Hollóczki, O.; Kenner, L. Micro- and Nanoplastics Breach the Blood–Brain Barrier (BBB): Biomolecular Corona’s Role Revealed. Nanomaterials 2023, 13, 1404. [Google Scholar] [CrossRef]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef] [PubMed]
- Malafaia, G.; da Luz, T.M.; Araújo, A.P.d.C.; Ahmed, M.A.I.; Rocha-Santos, T.; Barceló, D. Novel methodology for identification and quantification of microplastics in biological samples. Environ. Pollut. 2022, 292, 118466. [Google Scholar] [CrossRef] [PubMed]
- Kiran, B.R.; Kopperi, H.; Mohan, S.V. Micro/nano-plastics occurrence, identification, risk analysis and mitigation: challenges and perspectives. Rev. Environ. Sci. Bio/Technology 2022, 21, 169–203. [Google Scholar] [CrossRef]
- Rodríguez-Narvaez, O.M.; Goonetilleke, A.; Perez, L.; Bandala, E.R. Engineered technologies for the separation and degradation of microplastics in water: A review. Chem. Eng. J. 2021, 414, 128692. [Google Scholar] [CrossRef]
- Thomas, D.; Schütze, B.; Heinze, W.M.; Steinmetz, Z. Sample Preparation Techniques for the Analysis of Microplastics in Soil—A Review. Sustainability 2020, 12, 9074. [Google Scholar] [CrossRef]
- Yang, H.; Chen, G.; Wang, J. Microplastics in the Marine Environment: Sources, Fates, Impacts and Microbial Degradation. Toxics 2021, 9, 41. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Wu, J.; Wang, J.; Zhang, C.; Wu, J. Fate of land-based antibiotic resistance genes in marginal-sea sediment: Territorial differentiation and corresponding drivers. Chemosphere 2021, 288, 132540. [Google Scholar] [CrossRef]
- Beaurepaire, M.; Dris, R.; Gasperi, J.; Tassin, B. Microplastics in the atmospheric compartment: a comprehensive review on methods, results on their occurrence and determining factors. Curr. Opin. Food Sci. 2021, 41, 159–168. [Google Scholar] [CrossRef]
- Vandermeersch, G.; Van Cauwenberghe, L.; Janssen, C.R.; Marques, A.; Granby, K.; Fait, G.; Kotterman, M.J.; Diogène, J.; Bekaert, K.; Robbens, J.; et al. A critical view on microplastic quantification in aquatic organisms. Environ. Res. 2015, 143, 46–55. [Google Scholar] [CrossRef]
- Bessa, F.; Frias, J.; Knögel, T.; Lusher, A.; Andrade, J.; Antunes, J.C.; Sobral, P.; Pagter, E.; Nash, R.; O'Connor, I.; et al. “Harmonized Protocol for Monitoring Microplastics in Biota.” HAL (Le Centre Pour La Communication Scientifique Directe). French National Centre for Scientific Research. [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- López-Rosales, A.; Andrade, J.; Fernández-González, V.; López-Mahía, P.; Muniategui-Lorenzo, S. A reliable method for the isolation and characterization of microplastics in fish gastrointestinal tracts using an infrared tunable quantum cascade laser system. Mar. Pollut. Bull. 2022, 178, 113591. [Google Scholar] [CrossRef]
- Baruah, A., Sharma, A., Sharma, S., & Nagraik, R. (2022). An insight into different microplastic detection methods. International Journal of Environmental Science and Technology, 19(6), 5721-5730.
- World Health Organization 2019 Water, Sanitation, Hygiene and Health. 2019. “Microplastics in Drinking-Water.” Geneva, Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/publications/i/item/9789241516198.
- Skåre, Janneche Utne. 2019. “Microplastics; Occurrence, Levels and implications for Environment and Human Health Related to Food. Opinion of the Steering Committee of the Norwegian Scientific Committee for Food And.” October 12, 2019. https://munin.uit.no/handle/10037/16566.
- EFSA Panel on Contaminants in the Food Chain (Contam). Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Vázquez-Rowe, I.; Ita-Nagy, D.; Kahhat, R. Microplastics in fisheries and aquaculture: implications to food sustainability and safety. Curr. Opin. Green Sustain. Chem. 2021, 29, 100464. [Google Scholar] [CrossRef]
- Koelmans, B., Pahl, S., Backhaus, T., Bessa, F., van Calster, G., Contzen, N., Cronin, R., Galloway, T., Hart, A., Henderson, L., Kalcikova, G., Kelly, F., Kolodziejczyk, B., Marku, E., Poortinga, W., Rillig, M., van Sebille, E., Steg, L., Steinhorst, J., Steidl, J., Syberg, K., Thompson, R., Wagner, M., van Wezel, A., Wyles, K. und Wright, S. and SAPEA, Science Advice for Policy by European Academies (2019) “A Scientific Perspective on Microplastics in Nature and Society”. Open Access . SAPEA, Berlin, 173 pp https://oceanrep.geomar.de. [CrossRef]
- Turner, A.; Holmes, L. Occurrence, distribution and characteristics of beached plastic production pellets on the island of Malta (central Mediterranean). Mar. Pollut. Bull. 2011, 62, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef]
- Guo, X.; Lin, H.; Xu, S.; He, L. Recent Advances in Spectroscopic Techniques for the Analysis of Microplastics in Food. J. Agric. Food Chem. 2022, 70, 1410–1422. [Google Scholar] [CrossRef]
- Renner, G.; Sauerbier, P.; Schmidt, T.C.; Schram, J. Robust Automatic Identification of Microplastics in Environmental Samples Using FTIR Microscopy. Anal. Chem. 2019, 91, 9656–9664. [Google Scholar] [CrossRef]
- El Hadri, H.; Gigault, J.; Mounicou, S.; Grassl, B.; Reynaud, S. Trace element distribution in marine microplastics using laser ablation-ICP-MS. Mar. Pollut. Bull. 2020, 160, 111716. [Google Scholar] [CrossRef]
- Trujillo, C.; Pérez-Arantegui, J.; Lobinski, R.; Laborda, F. Improving the Detectability of Microplastics in River Waters by Single Particle Inductively Coupled Plasma Mass Spectrometry. Nanomaterials 2023, 13, 1582. [Google Scholar] [CrossRef]
- Laborda, F.; Trujillo, C.; Lobinski, R. Analysis of microplastics in consumer products by single particle-inductively coupled plasma mass spectrometry using the carbon-13 isotope. Talanta 2021, 221, 121486. [Google Scholar] [CrossRef]
- Collard, F.; Gilbert, B.; Eppe, G.; Parmentier, E.; Das, K. Detection of Anthropogenic Particles in Fish Stomachs: An Isolation Method Adapted to Identification by Raman Spectroscopy. Arch. Environ. Contam. Toxicol. 2015, 69, 331–339. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).