1. Introduction
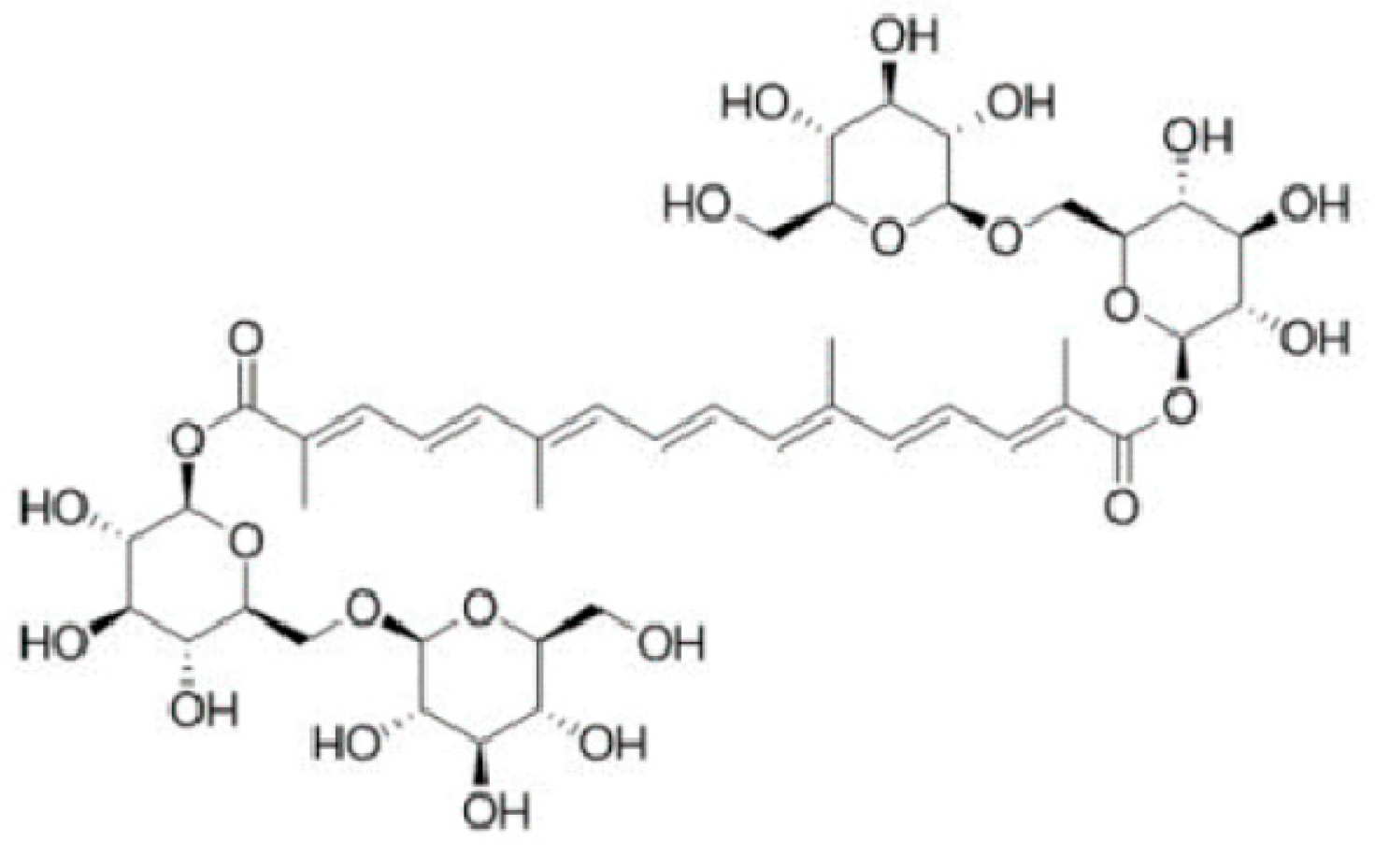
Crocin (all-trans crocetin di-β-D-gentiobiosyl ester) is a glycoside carotenoid (
Figure 1) endowed with several health benefits including antioxidant, antitumor, antidepressant, anxiolytic, cardioprotective and neuroprotective activity [
1]. In particular, crocin belongs to the “crocin” family consisting of hydrophilic carotenoids in which D-glucose and/or D-gentiobiose residues form either mono- or di-glycosyl polyene esters of crocetin. It is noteworthy that the high glycosyl content makes crocins unusual water-soluble carotenoids [
2].
The main sources of these natural compounds are the dried stigmas of
Crocus sativus L. (commonly named saffron) and the fruits of
Gardenia jasminoides E.
Crocus sativus L. is a perennial herb, belonging to the
Iridaceae family, and widely cultivated in the Mediterranean area and Western Asia, being Iran its main producer [
3,
4]. Due to its properties, saffron is one of the world’s most expensive and popular spices, widely used to add deep flavor and color to all kinds of dishes [
5].
Apart from its use as food supplement, nowadays, saffron is being widely investigated for its potential therapeutic applications. Many studies highlighted the ability of saffron extracts to act as antioxidant, anti-inflammatory, anticancer, wound healing, anti-ageing agents [
6,
7,
8,
9,
10,
11,
12,
13]. Clinical trials highlighted the efficiency of saffron capsules in ameliorating depression symptoms in comparison to placebo [
14] or conventional drugs such as imipramine [
15] and fluoxetine [
16]. Similarly, clinical studies performed on patients with mild to moderate Alzheimer’s disease showed significantly better outcome on cognitive function after administering saffron capsules in comparison to placebo [
17] while the same effectiveness was observed when saffron capsules were compared to donezepril [
18]. Studies carried out on different animal models pointed out the efficiency of saffron and its active ingredients crocin and safranal in the treatment of pathological conditions such as atherosclerosis [
19,
20], cancer [
21,
22,
23], hyperglycaemia and glucose uptake/metabolism [
24]. It is interesting to note that Shirali et al. [
25] reported the ability of crocin to lower significantly the formation of advanced glycation end-products, in addition to the levels of serum glucose, triglycerides, and total cholesterol in the diabetic rats. Goyal et al. [
26] suggested the feasibility of using crocin as cardioprotective agent due to the improvement of cardiac functions in Wistar albino rats after crocin pretreatment. At cutaneous level, crocin has been reported to inhibit the NF-κB signaling pathway, thus suppressing Th2 chemokines in TNF-α/INF-γ stimulated human epidermal keratinocyte HaCaT cell line [
27]. In addition, Das et al. [
28] reported the inhibition of skin papilloma formation after oral administration of saffron extracts in mice while topical application of a saffron extract cream on burn wounds in rats provided increased re-epithelialization and accelerated wound healing, thus suggesting the involvement of saffron in anti-inflammatory and antioxidant processes [
29]. Recently, crocin was endowed with the modulation of the expression of NF-kB and glycosylation related genes in keratinocytes cell cultures, supporting its potential usefulness in the prevention of aging related processes [30 old].
However, it is interesting to note that these activities have been ascribed to the saffron extract
in toto or only to specific components. In particular, three main metabolites have been identified in saffron extracts: a) picrocrocins, responsible for the bitter taste, b) safranal, a volatile oil responsible for aroma, c) crocins, which give saffron the typical color [
2].
As the quality of saffron and its content of phytochemicals can be strongly affected by cultivation, territorial and climatic conditions [
31,
32], it is crucial to evaluate the concentration of each specific active ingredient according to the different saffron variety.
As crocin is regarded as one of the main antioxidants in saffron [
2], in this work, we assessed the crocin content and the in vitro antioxidant activity of saffron extracts obtained from three varieties (Greek, Sicilian, and Iranian). In vitro antioxidant activity of crocin has been already reported by others using different methods such as DPPH (2,2′-diphenylpicryl hydrazyl free radical) radical scavenging assay [
33], ethylene assay, and squalene peroxidation assay [
30]. As DPPH is hydrophobic and requires the use of organic solvents to perform the test [
34], such method could not be regarded as the most suitable to evaluate the antioxidant activity of crocin and/or saffron extracts. The ethylene assay and the squalene peroxidation assay assess hydroxyl radical and singlet oxygen scavenging activity, respectively [
30]. Both these methods were developed to point out the antioxidant ability of topical formulations [
35,
36] and they were not validated on plant extracts. Therefore, we assessed the antioxidant activity of saffron extracts using the oxygen radical absorbance capacity (ORAC) assay, which is regarded as a reliable tool for screening antioxidants [
34]. Additionally, in vitro nitric oxide (NO) radical scavenging ability of the saffron extracts under investigation was evaluated. As an oxidative stress may result in the formation of advanced glycation end products (AGEs), a further aim of this study was to determine the anti-glycation activity of saffron extracts using the Maillard reaction.
2. Results and Discussion
2.1. Crocin Determination in Saffron Extracts
According to the literature [
1], different extraction methods could be used for obtaining all components, including crocin, from saffron dried stigmas. Therefore, we carried out preliminary experiments to set out the optimal extraction conditions (data not shown). The content of crocin in each saffron extract was determined by ultra-performance liquid chromatography coupled with mass spectrometry (UPLC-MS/MS) analysis and reported in
Table 1. The Sicilian and the Iranian saffron contained similar amounts of crocin that were significantly higher (p<0.05) than that of the Greek variety.
According to the literature [
1], both geographical location and processing methods could affect the quality of saffron samples with regard to color, flavor and bitterness. For instance, Greek saffron has been reported to contain a higher concentration of active ingredients compared to Indian saffron due to different drying processes and storage, which could lead to increased concentrations of glycoside carotenoids [
37].
Additionally, as previously mentioned, environmental factors such as altitude, temperature and soil may affect the crocin content of saffron [
31,
32], as well as the development of secondary metabolites during the plant growth [
38].
Crocus Sativus L. grows well in Mediterranean and continental climates. The plant is capable of resisting temperatures ranging from -15 to 40 °C [
31]. Altitude is a key factor in determining the content of crocin in saffron stigmas [
39]: the higher the sun exposure and altitude, the higher the crocin content. In Greece, saffron is produced in the region of Kozani [
40], which has an altitude of 600 m. In contrast, the Sicilian saffron comes from the city of Maletto, in the Etna area, located at an altitude of 1000 m. In Iran, saffron is typical of the Qaen region, with an altitude between 1400 and 1500 m [
41]. Crocin content is strongly influenced by the composition of the soil as well;
Crocus Sativus L. prefers well-drained calcareous soils. To prevent water stagnation, it is recommended to cultivate on sloping soils to ensure good water runoff. In Iran, saffron is cultivated on sandy-textured calcareous soils, whereas in Greece, it grows on sandy clay soil with a pH of 7.4, and a high calcium carbonate content [
42]. The amount of organic matter is extremely poor both in the Iranian and Greek territories. On the contrary, in the Etna area, the soil is volcanic, fertile, and rich in nutrients, organic matter and minerals (nitrates, phosphates, magnesium, potassium, and calcium).
The environmental characteristics, taken together, could explain the different crocin content observed in the variety of saffron under investigation.
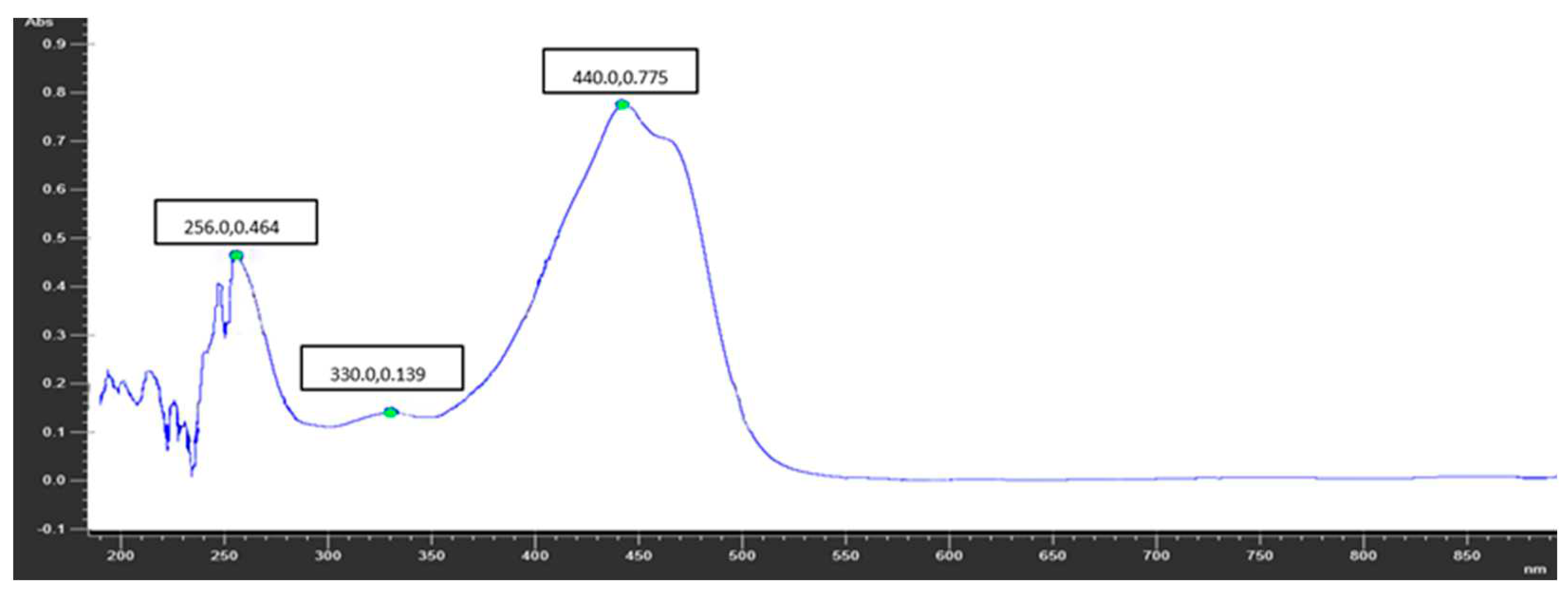
As the coloring of saffron is related to the presence of carotenoids, especially crocin, the ISO 3632-1:2011 standard (last reviewed and confirmed in 2017 and currently in force) establishing specifications for dried saffron obtained from the pistils of Crocus sativus L. flowers, was applied to obtain information on crocin conten in the extracts from different variety of saffron. As this is a qualitative method based on coloring, it was coupled to UPLC-MS/MS analysis to quantify the amount of crocin in the sample. The obtained results showed that the Sicilian saffron (
Figure 2) had the highest crocin content among the tested variety. This data support the correlation between the carotenoid content and the cultivation area as carotenoid concentration rises with increasing sun exposure and altitude [
43,
44].
2.2. Antioxidant and NO Scavenging Activity of Saffron Extracts
The ORAC assay has been widely accepted as a suitable
in vitro test to measure the antioxidant activity of natural active compounds [
34] while the NO scavenger assay provides useful information about the efficiency in counteracting an overproduction of NO radicals, which has been associated with various pathological conditions, involving oxidative stress and DNA mutations.
As illustrated in
Table 2, all tested samples showed an excellent antioxidant activity compared to the reference standard Trolox. The ability of the different saffron extracts to inhibit the spontaneous production of NO from a sodium nitroprusside solution, determined by NO scavenger assay, decreased in the order Greek> Sicilian > Iranian.
According to the data reported in
Table 1, Sicilian and Iranian saffron extracts were expected to provide the highest antioxidant activity due to their higher crocin content. On the contrary, the Sicilian variety was the least effective in the ORAC assay, thus suggesting that other extract components were involved in determining the radical scavenging efficiency. According to the literature [
45], the main active ingredients of saffron are carotenoids (crocetins, crocins, α-carotene, β-carotene, lycopene, zeaxanthin, mangicrocin, xanthone-carotenoid), monoterpene aldehydes (picrocrocin, safranal and its isomers), monoterpenoids (crocusatins) and flavonoids (kaempferol derivatives). As spices containing phenolic and flavonoid compounds show antioxidant activities [
46,
47,
48], the radical scavenging properties of saffron could be ascribed both to its phenolic content and to its active ingredients such as safranal, crocin, crocetin, and carotene, whose antioxidant efficiency has been widely investigated [
48]. A study by Makhlouf et al. [
49] supported the importance of the phenolic content of saffron extracts in determining the protective effect against radical species for the different organs such as liver and heart.
Therefore, in the attempt to explain the obtained results, the total phenolic content of each extract was determined. As reported in
Table 2, the extract of Greek saffron showed a value of total phenolic content (0.55 ± 0.12 mg GAE/100 g) about two-fold greater than that of the other two extracts, Sicilian and Iranian, whose values were almost similar (0.29 ± 0.10 and 0.23 ± 0.08 mg GAE/100 g, respectively). As natural extracts are made up of hundreds of components, a full characterization is hard to achieve. However, we attempted to identify at least some of the most abundant ingredients, apart from crocin. In
Table 3, the molecules identified by UPLC-MS/MS analyses in each investigated sample are listed.
As a quantitative determination of each ingredient contained in saffron extracts was out of the scope of this work, to compare the relative abundancy of each molecule in the different extracts, we reported the peak area calculated from the UPLC-MS/MS spectrum. It is noteworthy that none of the identified active ingredients in the extract of Greek saffron was more abundant than in the Sicilian and Iranian saffron extracts.
Therefore, these results suggest that the antioxidant activity of the investigated extracts could not be related to a specific component but it could be ascribed to the complex mixture of phytochemicals that naturally occur in each extract. A greater activity of saffron in comparison to crocin has already been reported by Asdaq and Inamdar [
50] studying their antihyperlipidemic and antioxidant potential after oral administration in rats. The authors concluded that other components, apart from crocin, were involved in determining the synergistic antihyperlipidemic and antioxidant potential of saffron, suggesting that the flavonoid content could be responsible for the better efficacy of saffron compared to crocin.
2.3. Antiglycation Activity
In vivo accumulation of advanced glycation end products (AGEs), resulting from non-enzymatic reactions between proteins and reducing sugars (glycation) , may lead to different cell and tissue damages [
51,
52]. As recent reports pointed out the effectiveness of several polyphenols in counteracting AGEs formation [
53], we thought it would be interesting to evaluate the anti-glycation activity of the saffron extracts under investigation. As shown in
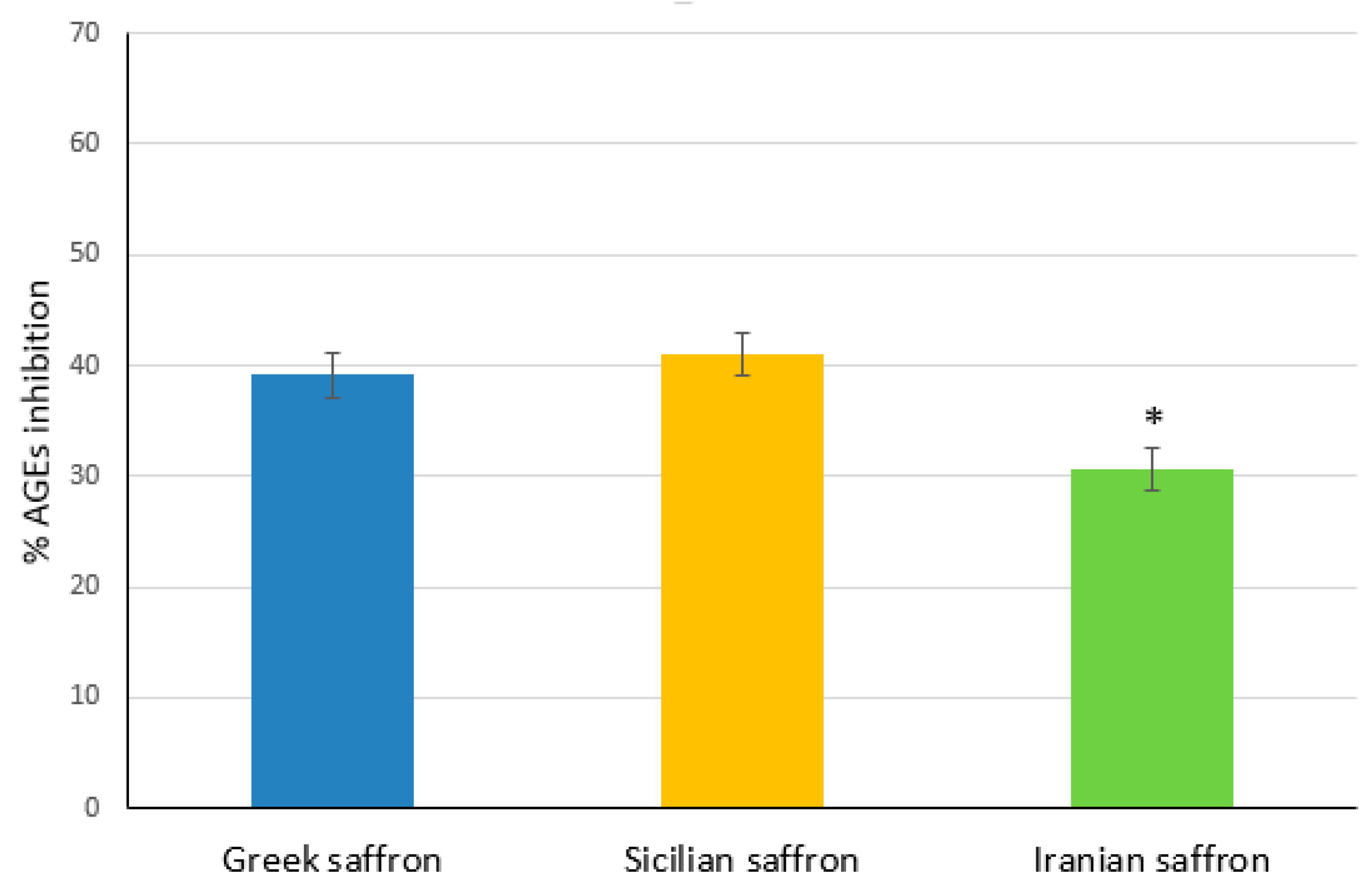
Figure 3, the extracts of Sicilian and Greek saffron had similar activities against AGEs formation, providing about 40% inhibition, while the Iranian saffron was significantly (p<0.05) less effective (30% inhibition). As saffron extracts contained several different polyphenols (see
Table 3), the mechanisms underlying the observed anti-glycation activity were quite difficult to elucidate. As reported in the literature [
53], some polyphenols, such as quercetin, are able to prevent AGEs production by inhibiting methylglyoxal formation while others, such as phenolic acids, can act as ROS inhibitors. Due to the presence of different types of polyphenols (e.g. quercetin, caffeic acid, p-coumaric acid etc.) in the investigated saffron extracts, concurrent mechanisms could be hypothesized in determining the observed anti-glycation activity.
3. Materials and Methods
3.1. Chemicals
All solvents (ethanol, methanol LC/MS grade, water LC/MS grade, acetonitrile LC/MS grade, formic acid) and chemical compounds crocetin digentiobiose ester (crocin); fluorescein (FL); (2,2’-Azobis(2-methylpropionamidine) dihydrochloride (AAPH); 6-Hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid (Trolox); aminoguanidine carbonate (AMG); bovine serum albumin (BSA); d-fructose; sodium nitroprusside; Folin & Ciocalteu’s phenol reagent and Griess reagent (1% dihydrochloride sulphanylamide) were of analytical grade and purchased from Merck (Milan, Italy).
3.2. Plant Material and Preparation of the Extracts
Sicilian saffron was purchased from the Capizzi Agricultural Company that provided a certificate claiming Etna area as the origin of saffron. Greek saffron from Kozani region, and Iranian saffron from Qaen region were provided from a local herbalist shop that certified their origin. Samples were stored at -5 to 5 C°, in the dark. Each extract was obtained from 10 g of stigmas in 200 mL of methanol at room temperature (RT) for 48 h. After filtration through Whatman® Grade 1 filter paper (Whatman, UK), the obtained extracts were evaporated at 25 °C using a rotatory evaporator (Stuart RE300) under reduced pressure, to obtain 0.98310 g (±0.1 mg) of dry extracts.
3.3. Determination of Crocin Content
Ultra-performance liquid chromatography coupled with Mass Spectrometry (UPLC-Ms/Ms) (Perkin-Elmer/AB SCIEX API 2000TM) was performed to analyze the content of crocin in saffron extracts. The separation was performed using water: acetonitrile with 0.1% formic acid as mobile phase with a gradient elution as follows: 0-3 min 95:5 (v/v); 3-6 min 70:30 (v/v); 6-10 min 50:50 (v/v); 10-13 min 5:95 (v/v); 13-30 min 95:5 (v/v). The elution rate was 300 µL/min for 30 min into a C18 column (Phenomenex Kinetex® 2,6 µm C18 100 Å, 100 x 2,1 mm, with a volume of injection of 10 µL. To assess comprehensively the analyzed spectrum, ESI-Ms/Ms was used with positive and negative polarities, for detailed spectrum analysis, with the equipment settings as previously reported [
54]. In order to quantify the crocin content in each variety of saffron, crocin was properly diluted in water and a calibration curve was constructed in the range 8,000 ng/mL - 150,000 ng/mL using the software's "Quantitation Wizard" function. The limit of detection (LOD) of the analytical method was 0.812 ng/mL while the limit of quantitation (LOQ) was 2.143 ng/mL.
As the coloring of saffron is related to presence of carotenoids, especially crocin, saffron samples were additionally analyzed according to the ISO 3632-1:2011 standard. Firstly, homogenization of the stigmas (250 mg) with 500 mL of deionized water was carried out for 1 h. 10 mL of the obtained mixture was diluted 10-fold with deionized water and filtered through a 0.45 µm filter. The absorbance was measured at 440 nm using a Perkin-Elmer Lambda 25 UV-Vis (Perkin Elmer, Waltham, MA, USA). This method was coupled to UPLC-MS/MS analysis to quantify the crocin content.
3.4. Determination of Total Phenolic Content
The polyphenol content of three saffron varieties was determined by the Folin-Ciocalteau method according to Aiyegoro and Okoh [
55], with slight modifications. Firstly, samples (125 mg of dry stigma extract for each saffron variety) were prepared by adding a hydroalcoholic solution (50:50). 5 mL of each sample was mixed with 2.5. mL of Folin-Ciocalteu reagent (diluted 10-fold with deionized water) and 2.5 mL of sodium bicarbonate (10% w/v). After incubation at 45°C for 15 min, the absorbance of the samples was measured at 765 nm using UV–vis spectrophotometer (Thermo Scientific Genesys 10 Scanning UV-Visible Spectrophotometer, Santa Clara, CA). The standard calibration curve was prepared by dissolving gallic acid in water at the following concentrations: 0, 0.05, 0.1, 0.15, 0.2, and 0.25 mg·mL
−1 . The total phenolic content (TPC) was expressed as milligram of gallic acid equivalents (GAE)/g of extract.
3.5. ORAC Assay
The antioxidant activity of saffron extracts was determined by the Oxygen Radical Absorbance Capacity (ORAC) assay as previously described [
56,
57]. In particular, this assay measures the loss of fluorescence over time due to peroxyl-radical formation by the breakdown of AAPH (2,2′-azobis-2-methyl-propanimidamide, dihydrochloride). Trolox, a water-soluble vitamin E analogue, is used as a positive control, inhibiting fluorescein decay in a dose-dependent manner. Experiments were carried out using AAPH as peroxyl radical generator, while Trolox (12.5 µM) and phosphate buffer (pH 7.0) were used as standard and blank, respectively. 50 µL of each extract properly diluted (1 mg/mL), Trolox or buffer were placed in a 96-multiwell-plate, and the fluorescein solution (12 nM) was added. After preincubation for 30 min at 37 °C, AAPH solution (100 mM) was added in each well. Fluorescence was monitored by VICTOR Wallac 1420 Multilabel Counters fluorimeter (Perkin Elmer, Boston, MA, USA) where excitation and emission wavelengths were 540 and 575 nm, respectively. Experiments were performed in triplicate. ORAC values were calculated using Origin® 7 software (Origin Lab Corporation). ORAC Unit were calculated according to Equation (1) and expressed as µmol Trolox/µg sample:
where K is the sample dilution factor, S the area under the fluorescence decay curve of sample, trolox or blank. Each experiment was performed in triplicate and data were expressed as mean ± SD.
3.6. NO Scavenger Assay
NO radical scavenging activity of saffron extracts was determined using a well-established procedure [
58]. This colorimetric assay measures the antioxidant activity of a compound by evaluating its ability to inhibit the spontaneous production of NO from a sodium nitroprusside solution.
The reaction mixture, containing the sample under investigation (1mg/mL of saffron dried extract), phosphate buffer, an aqueous solution of sodium nitroprusside (20 mM) was incubated at 25°C for 150 min. After incubation, Griess reagent was added and the sample absorbance was measured at 540 nm with a spectrophotometer (Multiskan® EX, Thermo Scientific, Waltham, MA, USA). The percent of inhibition of NO radical production was obtained according to Equation (2):
where A
0 is the absorbance of blank, while A
1 is the absorbance of the sample.
3.7. Antiglycation Activity
The antiglycation activity of the saffron stigmas was determined by measuring their ability to inhibit the formation of fluorescent AGEs using the Maillard reaction [
58,
59]. Bovine serum albumin (BSA) (10 mg/mL) was incubated with D-fructose (0.5 M) in phosphate buffer 50 mM, pH 7.4 and NaN
3 0.02% w/v as positive controls. BSA alone was used as negative control as it did not provide any formation of fluorescent AGEs. Aminoguanidine (AMG) (3 mM) was used as the reference compound. Final glycated BSA solution (300 µL) alone and with three different extracts (1 mg/ mL) was incubated at 37 °C in 96-well microtiter closed with their silicon lids for 7 days. Inhibition rate was determined (λ
exc 370 nm; λ
em 440 nm) using a VICTOR Wallac 1420 Multilabel Counter fluorimeter (Perkin Elmer, Waltham, MA, USA). The results were expressed as relative fluorescence units (RFU) and calculated according to Equation (3):
3.8. Statistical Analysis
Data were expressed as mean ± standard deviation (±SD) of three replicates of three independent experiments. The statistical significance of these data was assessed by the one-way Anova test.
4. Conclusions
Crocin is regarded as the main antioxidant component in saffron extracts whose phytochemical composition could be affected by cultivation, territorial and climatic conditions. In the present work, the crocin content in extracts of three different variety of saffron (Greek, Sicilian, and Iranian) was analyzed, pointing out a higher concentration of this antioxidant in the Sicilian and Iranian extracts. However, the determination of the in vitro antioxidant activity of the above-mentioned extracts, assessed by the ORAC and NO scavenger assays, showed that the Greek saffron had a greater efficiency in scavenging peroxyl and NO radicals than Sicilian and Iranian ones. It is interesting to note that the Greek saffron extract showed the highest total phenolic content but a qualitative analysis of its main components did not provide information that could explain the obtained results. In addition to a good antioxidant activity, the above-mentioned extracts demonstrated an interesting in vitro anti-glycation activity, which was higher for Greek and Sicilian saffron extracts.
These results suggest that saffron extracts would deserve further investigations as supplement to prevent pathological processes induced by AGEs and radical species, considering that their effectiveness could be strongly affected by the saffron variety.
Author Contributions
Conceptualization, S.R. and A.P.; methodology, S.R. and A.P.; software, F.S., E.A.S.; validation, S.R. and A.P.; formal analysis, F.S.; investigation, F.S., E.A.S.; data curation, F.S., D.S.; writing—original draft preparation, D.S., F.S. and E.A.S; writing—review and editing, S.R., D.S. F.S., C.P., A.P., L.M.; visualization, C.P., A.P., S.R., L.M.; supervision, C.P., A.P., S.R., L.M.; project administration, S.R., A.P., C.P.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no consflict of interest.
Sample Availability
All samples are available from the authors.
References
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, E.; Kadoglou, N.P.; Kostomitsopoulos, N.; Valsami, G. Saffron: A natural product with potential pharmaceutical applications. J. Pharm. Pharmacol. 2015, 67, 1634–1649. [Google Scholar] [CrossRef]
- Ahmadian, Z.; Niazmand, R.; Pourfarzad, A. Microencapsulation of Saffron Petal Phenolic Extract: Their Characterization, In Vitro Gastrointestinal Digestion, and Storage Stability. J. Food Sci. 2019, 84, 2745–2757. [Google Scholar] [CrossRef]
- Esmaeili, N.; Ebrahimzadeh, H.; Abdi, K.; Safarian, S. Determination of some phenolic compounds in Crocus sativus L. corms and its antioxidant activities study. Pharmacogn. Mag. 2011, 7, 74–80. [Google Scholar]
- Vardakas, A.T.; Shikov, V.T.; Dinkova, R.H.; Mihalev, K.M. Optimisation of the enzyme-assisted extraction of polyphenols from saffron (Crocus sativus L.) petals. Acta Sci. Pol. Technol. Aliment. 2021, 20, 359–367. [Google Scholar] [CrossRef]
- Zeka, K.; Marrazzo, P.; Micucci, M.; Ruparelia, K.C.; Arroo, R.R.J.; Macchiarelli, G.; Nottola, A.S.; Continenza, M.A.; Chiarini, A.; Angeloni, C.; et al. Activity of Antioxidants from Crocus sativus L. Petals: Potential Preventive Effects towards Cardiovascular System. Antioxidants (Basel) 2020, 9, 1102. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Pignatello, R.; Fuochi, V.; Furneri, P.M.; Lauro, M.R.; Santonocito, D.; Cortesi, R.; Esposito, E. Lipid Nanoparticles and Active Natural Compounds: A Perfect Combination for Pharmaceutical Applications. Curr. Med. Chem. 2019, 26, 4681–4696. [Google Scholar] [CrossRef]
- Puglia, C.; Santonocito, D.; Musumeci, T.; Cardile, V.; Graziano, A.C.E.; Salerno, L.; Raciti, G.; Crascì, L.; Panico, A.M.; Puglisi, G. Nanotechnological Approach to Increase the Antioxidant and Cytotoxic Efficacy of Crocin and Crocetin. Planta Med. 2019, 85, 258–265. [Google Scholar] [CrossRef]
- Esposito, E.; Drechsler, M.; Huang, N.; Pavoni, G.; Cortesi, R.; Santonocito, D.; Puglia, C. Ethosomes and organogels for cutaneous administration of crocin. Biomed. Microdevices 2016, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Nile, S.H.; Zhang, Y.; Qin, L.; El-Seedi, H.R.; Daglia, M.; Kai, G. Novel Insight into Utilization of Flavonoid Glycosides and Biological Properties of Saffron (Crocus sativus L.) Flower. Byproducts. J. Agric. Food Chem. 2020, 68, 10685–10696. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Pharmacokinetic Properties of Saffron and its Active Components. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 383–390. [Google Scholar] [CrossRef]
- Moshiri, M.; Vahabzadeh, M.; Hosseinzadeh, H. Clinical Applications of Saffron (Crocus sativus) and its Constituents: A Review. Drug Res (Stuttg) 2015, 65, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Tahmacebi-Pour, N.; Noorbala, A.A.; Amini, H.; Fallah-Pour, H.; Jamshidi, A.H.; Khani, M. Crocus sativus L. in the treatment of mild to moderate depression: A double blind, randomized and placebo controlled trial. Phytother. Res. 2005, 19, 148–151. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Fallah-Pour, H.; Afkham, K.; Jamshidi, A.H.; Khalighi-Cigaroudi, F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: A pilot double-blind randomized trial. BMC Complement. Altern. Med. 2004, 4, 12. [Google Scholar] [CrossRef]
- Akhondzadeh, Basti, A.; Moshiri, E.; Noorbala, A.A.; Jamshidi, A.H.; Abbasi, S.H.; Akhondzadeh, S. Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: A pilot doubleblind randomized trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 439–442. [CrossRef]
- Alimardani, R.; Jamshidi, A.; Rezazadeh, S.A.; Yousefi, A.; Zare, F.; Moradi, A.; Vossoughi, A. A 22-week multicenter, randomized, doubleblind controlled trial of Crocus sativus in the treatment of mild-tomoderate Alzheimer’s disease. Psychopharmacology 2010, 207, 637–643. [Google Scholar]
- Akhondzadeh, S.; Sabet, M.S.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Sh Hejazi, S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; Zare, F.; et al. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: A 16-week, a randomized and placebo controlled trial. In J. Clin. Pharm. Ther.; 2010; Volume 35, pp. 581–585. [Google Scholar]
- He, S.; Qian, Z.Y.; Tang, F.T.; Wen, N.; Xu, G.L.; Sheng, L. Effect of crocin on experimental atherosclerosis in quails and its mechanisms. Life Sci. 2005, 77, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Abd Rahim, I.N.; Mohd Kasim, N.A.; Isa, M.R.; Nawawi, H. A Systematic Review on the Effect of Saffron Extract on Lipid Profile in Hyperlipidaemic Experimental Animal Models. Malays. J. Med. Sci. 2022, 29, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.C.; Pannikar, B.; Panikkar, K.R. Antitumour activity of saffron (Crocus sativus). Cancer Lett. 1991, 57, 109–114. [Google Scholar] [CrossRef]
- Bathaie, S.Z.; Miri, H.; Mohagheghi, M.A.; Mokhtari-Dizaji, M.; Shahbazfar, A.A.; Hasanzadeh, H. Saffron aqueous extract inhibits the chemically induced gastric cancer progression in the Wistar albino rat. Iran. J. Basic. Med. Sci. 2013, 16, 27–38. [Google Scholar] [PubMed]
- Garcia-Olmo, D.C.; Riese, H.H.; Escribano, J.; Ontañón, J.; Fernandez, J.A.; Atiénzar, M.; García-Olmo, D. Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L.): An experimental study in the rats. Nutr. Cancer 1999, 35, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Kianbakht, S.; Hajiaghaee, R. Antihyperglycemic effetcs of saffron and its active constituents, crocin and safranal in alloxan-induced diabetic rats. J. Med. Plants 2011, 10, 82–89. [Google Scholar]
- Shirali, S.; Zahra Bathaie, S.; Nakhjavani, M. Effect of crocin on the insulin resistance and lipid profile of streptozotocin-induced diabetic rats. Phytother. Res. 2013, 27, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Arora, S.; Sharma, A.K.; Joshi, S.; Ray, R.; Bhatia, J.; Kumari, S.; Arya, D.S. Preventive effect of crocin of Crocus sativus on hemodynamic, biochemical, istopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine 2010, 17, 227–232. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, K.Y.; Park, B.; Yoon, J. Suppression of Th2 chemokines by crocin via blocking of ERK-MAPK/NF-κB/STAT1 signalling pathways in TNF-α/IFN-γ-stimulated human epidermal keratinocytes. Exp Dermatol. 2015, 24, 634–636. [Google Scholar] [CrossRef]
- Das, I.; Das, S.; Saha, T. Saffron suppresses oxidative stress in DMBA-induced skin carcinoma: A histopathological study. Acta Histochem. 2010, 112, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, G.; Hosseinimehr, S.J.; Zamani, P.; Ghasemi, M.; Ahmadi, A. The effect of saffron (Crocus sativus) extract for healing of second-degree burn wounds in rats. Keio J. Med. 2008, 57, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Fagot, D.; Pham, D.M.; Laboureau, J.; Planel, E.; Guerin, L.; Nègre, C.; Donovan, M.; Bernard, B.A. Crocin, a natural molecule with potentially beneficial effects against skin ageing. Int. J. Cosmet. Sci. 2018, 40, 388–400. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, V.; Devi, K.; Sharma, M.; Singh, M.K.; Ahuja, P.S. State of Art of Saffron (Crocus sativus L.) Agronomy: A Comprehensive Review. Food Rev. Int. 2008, 25, 44–85. [Google Scholar] [CrossRef]
- Zarinkamar, F.; Tajik, S.; Soleimanpour, S. Effects of Altitude on Anatomy and Concentration of Crocin, Picrocrocin and Safranal in “Crocus sativus” L. Aust. J. Crop Sci. 2011, 5, 831–838. [Google Scholar]
- Amin, A.; Hamza, A.A.; Bajbouj, K.; Ashraf, S.S.; Daoud, S. Saffron: A potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology 2011, 54, 857–867. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Reprint of “Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays”. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Galey, J.B.; Millecamps, F.; Nguyen, Q.L. Ethylene formation from methionine as a method to evaluate oxygen free radical scavenging and metal inactivation by cosmetics. Int. J. Cosmet. Sci. 1991, 13, 65–78. [Google Scholar] [CrossRef]
- Pham, D.M.; Boussouira, B.; Moyal, D.; Nguyen, Q.L. Oxidization of squalene, a human skin lipid: A new and reliable marker of environmental pollution studies. Int. J. Cosmet. Sci. 2015, 37, 357–365. [Google Scholar] [CrossRef]
- Caballero-Ortega, H.; Pereda-Miranda, R.; Abdullaev, F.I. HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources. Food Chem. 2007, 100, 1126–1131. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Zarinkamar, F.; Tajik, S.; Soleimanpour, S. Effects of Altitude on Anatomy and Concentration of Crocin, Picrocrocin and Safranal in “Crocus sativus” L. Aust. J. Crop Sci. 2011, 5, 831–838. [Google Scholar]
- Mitsopoulou, T.; Tsimidou, M. Morphological characteristics of greek saffron stigmas from kozani region. Acta Hortic. 2004, 189–193. [Google Scholar] [CrossRef]
- Rahimi, H.; Shokrpour, M.; Raeini, T.L.; Esfandiari, E. A study on the effects of environmental factors on vegetative characteristics and corm yield of saffron (Crocus sativus). Iranian J. Hortic. Sci. 2017, 48, 45–52. [Google Scholar]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Scrano, L.; Cicco, N.; Candido, V. The Influence of Soil Physical and Chemical Properties on Saffron (Crocus sativus L.) Growth, Yield, and Quality. Agronomy 2020, 10, 1154. [Google Scholar] [CrossRef]
- Vardakas, A.T.; Shikov, V.T.; Dinkova, R.H.; Mihalev, K.M. Optimisation of the enzyme-assisted extraction of polyphenols from saffron (Crocus sativus L.) petals. Acta Sci. Pol. Technol. Aliment. 2021, 20, 359–367. [Google Scholar] [CrossRef]
- D'Archivio, A.A.; Giannitto, A.; Maggi, M.A.; Ruggieri, F. Geographical classification of Italian saffron (Crocus sativus L.) based on chemical constituents determined by high-performance liquid-chromatography and by using linear discriminant analysis. Food Chem. 2016, 212, 110–116. [Google Scholar] [CrossRef]
- Bathaie, S.Z.; Mousavi, S.Z. New applications and mechanisms of action of saffron and its important ingredients. Crit. Rev. Food Scu Nutr. 2010, 50, 761–786. [Google Scholar] [CrossRef] [PubMed]
- Rahaiee, S.; Gharibzahedi, S.M.T.; Razavi, S.H.; Jafari, S.M. Recent developments on new formulations based on nutrient-dense ingredients for the production of healthy-functional bread: A review. J. Food Sci. Technol. 2014, 51, 2896–2906. [Google Scholar] [CrossRef]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Jaafar, H.Z.E. Evaluation of Crocus sativusL. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules 2010, 15, 6244–6256. [Google Scholar] [PubMed]
- Martinez-Tome, M.; Jimenez, A.M.; Ruggieri, S.; Frega, N.; Strabbioli, R.; Murcia, M.A. Antioxidant properties of Mediterranean spices compared with common food additives. J. Food Prot. 2001, 64, 1412–1419. [Google Scholar] [CrossRef]
- Makhlouf, H.; Saksouk, M.; Habib, J.; Chahine, R. Determination of antioxidant activity of saffron taken from the flower of Crocus sativus grown in Lebanon. Afr. J. Biotechnol. 2011, 10, 8093–8100. [Google Scholar]
- Asdaq, S.M.B.; Inamdar, M.N. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl. Biochem. Biotechnol. 2010, 162, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Galiniak, S.; Krawczyk-Mar´c, I.; Sek-Mastej, A.; Leksa, N.; Biesiadecki, M.; Orkisz, S. Clinical aspects of protein glycation. Eur. J. Clin. Exp. Med. 2017, 15, 263–267. [Google Scholar] [CrossRef]
- Yeh, W.J.; Hsia, S.M.; Lee, W.H.; Wu, C.H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef] [PubMed]
- D’Angeli, F. , Guadagni, F., Genovese, C., Nicolosi, D., Salinaro Trovato, A., Spampinato, M., Mannino, G., Lo Furno, D., Petronio, G., Ronsisvalle, S., et al. Anti-Candidal Activity of the Parasitic Plant Orobanche crenata Forssk. Antibiotics 2021, 10, 1373. [Google Scholar] [CrossRef] [PubMed]
- Aiyegoro, O.A.; Okoh, A.I. Phytochemical screening and polyphenolic antioxidant activity of aqueous crude leaf extract of Helichrysum pedunculatum. Int. J. Mol. Sci. 2009, 10, 4990–5001. [Google Scholar] [CrossRef] [PubMed]
- Intagliata, S.; Spadaro, A.; Lorenti, M.; Panico, A.; Siciliano, E.A.; Barbagallo, S.; Macaluso, B.; Kamble, S.H.; Modica, M.N.; Montenegro, L. In Vitro Antioxidant and Anti-Glycation Activity of Resveratrol and Its Novel Triester with Trolox. Antioxidants 2021, 10, 12. [Google Scholar] [CrossRef]
- Santonocito, D.; Granata, G.; Geraci, C.; Panico, A.; Siciliano, E.A.; Raciti, G.; Puglia, C. Carob. Seeds: Food Waste or Source of Bioactive Compounds? Pharmaceutics 2020, 12, 1090. [Google Scholar]
- Ronsisvalle, S.; Panarello, F.; Longhitano, G.; Siciliano, E.A.; Montenegro, L.; Panico, A. Natural Flavones and Flavonols: Relationships among Antioxidant Activity, Glycation, and Metalloproteinase Inhibition. Cosmetics 2020, 7, 71. [Google Scholar] [CrossRef]
- Crascì, L.; Lauro, M.R.; Puglisi, G.; Panico, A. Natural antioxidant polyphenols on inflammation management: Anti-glycation activity vs metalloproteinases inhibition. Crit. Rev. Food Sci. Nutr. 2018, 58, 893–904. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).